Abstract
The seemingly inexorable decline in insulin independence after islet transplant alone (ITA) has raised concern about its clinical utility. We hypothesized that induction immunosuppression therapy determines durability of insulin independence. We analyzed the proportion of insulin independent patients following final islet infusion in four groups of ITA recipients according to induction immunotherapy: University of Minnesota recipients given FcR nonbinding anti-CD3 antibody alone or T cell depleting antibodies (TCDAb) and TNF-α inhibition (TNF-α-i) (Group 1;n=29); recipients reported to the Collaborative Islet Transplant Registry (CITR) given TCDAb+TNF-α-i (Group 2; n=20); CITR recipients given TCDAb without TNF-α-i (Group 3;n=43); and CITR recipients given IL-2 receptor antibodies (IL-2RAb) alone (Group 4,n=177). Results were compared with outcomes in pancreas transplant alone (PTA) recipients reported to the Scientific Registry of Transplant Recipients (Group 5;n=677). 5-yr insulin independence rates in Group 1 (50%) and Group 2 (50%) were comparable to outcomes in PTA (Group 5: 52%; p>>0.05) but significantly higher than in Group 3 (0%; p=0.001) and Group 4 (20%; p=0.02). Induction immunosuppression was significantly associated with 5-year insulin independence (p=0.03), regardless of maintenance immunosuppression or other factors. These findings support potential for long-term insulin independence after ITA using potent induction therapy, with anti-CD3 Ab or TCDAb+TNF-α-i.
Keywords: Islet transplant, Anti-thymocyte globulin, CD3, T-cell, Alemtuzumab, OKT3
INTRODUCTION
Type 1 diabetes mellitus (T1D) continues to present therapeutic challenges. Intensive insulin therapy reduces the risk of microvascular complications but is limited by an increased risk of life-impacting hypoglycemia (1). Some patients with long-standing T1D will develop unawareness of hypoglycemia, preventing achievement of goal glycemic control. Successful intraportal allogeneic islet transplantation can substantially reduce glycemic variability, eliminate severe hypoglycemia, re-establish hypoglycemia awareness, and permit insulin independence by restoring endogenous insulin secretion (2–5).
A majority of islet transplant recipients at experienced centers achieve insulin independence within the first year after transplant (2–4). Recipients are protected from severe hypoglycemia, the most common indication for islet transplant. However, early studies suggest a rapid loss of insulin independence beyond 1 year in many patients (2,3). Identifying transplant strategies which minimize the decline in insulin independence is critical to developing islet transplant as an effective therapeutic intervention for T1D.
Proposed factors contributing to islet graft decline include alloimmune rejection, autoimmune recurrence (6), immunosuppressive drug toxicity (7), and non-immunologic factors including exhaustion of a marginal beta cell mass (8). It is unclear whether manipulation of immune modulating therapies can prolong insulin independence. Most islet transplant recipients in the recent era received IL-2 receptor antibodies (IL-2RAb; daclizumab or basiliximab) for induction (2). Within this population, insulin independence declines significantly after one year (2,3). Another approach to induction therapy involves the administration of potent induction immunosuppression (PII) regimens, with either FcR-nonbinding anti-CD3 monoclonal antibody or the novel combination of anti-thymocyte globulin (ATG) or alemtuzumab combined with tumor necrosis factor-alpha inhibition (TNF-α-i) (9,10). These PII protocols target T-cells, while preserving or expanding regulatory T cells and their function, and minimizing cytokine toxicity on transplanted islets.
The mechanism by which these induction immunosuppression regimens may exert benefit is two-fold. First, PII may provide better early posttransplant protection of islets from innate and alloimmunity, thereby increasing the engrafted beta cell mass (11). Second, we speculate that these regimens may mitigate recurrent autoimmunity directed at transplant islets. This is supported by preclinical observations that T-cell depletion therapy reverses diabetes in NOD mice (12,13), and clinical studies documenting prolonged C-peptide production in type 1 diabetics receiving short-term monotherapy with anti-CD3 (14,15).
In the present study, we investigate the impact of induction immunosuppression on long-term insulin independence in islet transplant recipients at the University of Minnesota (UMN) and in the Collaborative Islet Transplant Registry (CITR), which represents the largest collection of islet transplant data available in the world to date.. Those patients receiving anti-CD3 monoclonal antibody alone, or ATG or alemtuzumab plus TNF-α-i exhibited greater long-term insulin independence compared to patients treated with IL-2RAb. Outcomes are comparable to that seen with pancreas transplant alone (based on UNOS/SRTR data).
MATERIALS AND METHODS
Transplant cohorts
Islet allograft recipients meeting the following criteria were included in the analysis: 1) last islet infusion between 2002 to 2008, 2) at least one year of follow-up, and 3) at least 50% of primary endpoint data reported (based on November 2010 data lock). We analyzed outcomes in four groups of islet transplant alone ITA recipients according to induction immunotherapy: University of Minnesota recipients enrolled in single-center clinical trials (9,10,16) given FcR nonbinding anti-CD3 antibody teplizumab alone or the T cell depleting antibody (TCDAb) ATG and TNF-α-i with etanercept, with or without daclizumab (Group 1; n=29); recipients reported to the Collaborative Islet Transplant Registry (CITR) given TCDAb + TNF-α-i (Group 2; n=20); CITR recipients given TCDAb without TNF-α-i (Group 3; n=43); and CITR recipients given IL-2RAb alone (Group 4, n=177). Each individual allograft recipient was analyzed in only one of the four groups. The patients from the University of Minnesota included in group 1 were excluded from groups 2 or 3 (CITR groups).
Results were compared with outcomes in pancreas transplant alone (PTA) recipients reported to the Scientific Registry of Transplant Recipients (Group 5; n=677), obtained for the UNOS/SRTR 2002–2007 5-year cohort from the 2009 SRTR Annual Report (http://optn.transplant.hrsa.gov/ar2009/data_tables_section6.htm).
ITA recipients received 1 to 6 donor infusions (1–3 in 98%). Selection of immunosuppression was at the discretion of the transplant center. Immunosuppression agents were coded as ever or never given and grouped in categories according to mode of action, over all infusions for a recipient. Maintenance immunosuppression, unless stated otherwise, consisted of a low-dose calcineurin inhibitor (tacrolimus, cyclosporine) with either mTOR inhibitor therapy (everolimus, sirolimus) or mycophenolate acid/mycophenolate mofetil.
Outcome Measures
The proportion of insulin independent patients (defined by no exogenous insulin use for ≥14 consecutive days) was determined at 1, 3, and 5 years following the final islet infusion in groups 1–4. Rates of insulin independence following PTA were obtained from UNOS/SRTR for pancreas graft survival, equivalent to euglycemia without the need for exogenous insulin therapy (UNOS/SRTR 2009 Annual Report Table11) (17). Fasting C-peptide to glucose ratios (calculated as C-peptide (ng/ml) × 100 divided by glucose (mg/dl)) were evaluated at 28 days posttransplant, to reflect functional beta cell mass in the early post-transplant period. Autoantibody status at pretransplant baseline was defined by the following antibodies: glutamic acid decarboxylase antibody, insulin autoantibody, and islet cell antibody. Other outcome measures included complete graft failure (C-peptide<0.3 ng/mL) and absence of severe hypoglycemic episodes (SHE) defined as requiring assistance by another person to restore euglycemia.
Statistical Analysis
Baseline characteristics were compared across the four groups. Continuous measures were compared by the Wilcoxon test and discrete measures by the chi-square test.
Insulin independence and severe hypoglycemia at follow up were analyzed as the percent of patients meeting the endpoint annually post last infusion; missing data was considered missing at random (i.e., deducted from both numerator and denominator). The data was analyzed by generalized estimating equation (GEE) models with repeated measures within subject to determine which factors were associated with insulin independence over time. Recipient, donor, and islet characteristics, plus transplant center and immunosuppression categories (cumulative for all infusions) were first analyzed univariately. Factors significant at p<0.10 were included in multivariate models, and then stepped down at p<0.05. Hazard ratios are reported for significant factors in final models.
RESULTS
Comparison of long-term insulin independence in ITA recipients treated with differing induction immunosuppression
Patient, donor, and islet graft characteristics are outlined in Table 1. The islet transplant groups did not differ in terms of baseline insulin use, weight, or BMI. Total IEQ transplanted was slightly lower and islet autoantibodies were more often positive in group 1.
Table 1.
Baseline recipient characteristics, concomitant immunosuppression, and transplant characteristics
| Group 1 UMN |
Group 2 CITR:TCD+TNF-α-i |
Group 3 CITR:TCD−TNF-α-i |
Group 4 CITR:IL2RAb |
PTA | p§ | |
|---|---|---|---|---|---|---|
| N (%) or Mean ± SE |
N (%) or Mean ± SE |
N (%) or Mean ± SE |
N (%) or Mean ± SE |
N (%) or Mean ± SE |
||
| Era | 29 | 22 | 41 | 177 | 1104 | |
| 1999–2003 | 16 (55%) | 3 (14%) | 3 (7%) | 86 (46%) | 341# | <0.01 |
| 2004–2006 | 4 (14%) | 11 (50%) | 10 (24%) | 78 (44%) | 555 | |
| 2007–2009 | 9 (31%) | 8 (36%) | 28 (68%) | 18 (10%) | 208 | |
| Patient characteristics | ||||||
| Age at transplant (yrs) | 40.6 ± 1.4 | 45.2 ± 1.5 | 44.3 ± 1.6 | 44.1 ± 0.8 | 33.3 ± 7.1 | NS |
| BMI (kg/m2) | 23.6 ± 0.5 | 23.6 ± 0.5 | 23.2 ± 0.5 | 23.0 ± 0.2 | - | NS |
| Weight (kg) | 64.2 ± 1.7 | 63.6 ± 1.6 | 65.0 ± 1.6 | 64.9 ± 0.8 | - | NS |
| Insulin Use (u/day) | 0.5 ± 0.01 | 0.6 ± 0.01 | 0.5 ± 0.01 | 0.5 ± 0.01 | - | NS |
| Female | 23 (79%) | 14 (64%) | 28 (68%) | 104 (59%) | 670 (60.7%) | NS |
| Presence of SHE | 29 (100%) | 17 (77%) | 31 (76%) | 137 (77%) | - | NS |
| Immunosuppression, induction | ||||||
| Anti-thymocyte globulin | 18 (62%) | 14 (64%) | 36 (88%) | .. | 537 (48.6%) | ** |
| Alemtuzumab | - | 6 (27%) | 5 (12%) | 267 (24.2%) | ||
| Anti-CD3 mAb | 11 (38%) | 2 (9%) | 36 (0.3%) | |||
| IL-2 receptor antagonists | 14 (48%) | 10 (46%) | 7 (17%) | 177 (100%) | 164 (14.9%) | |
| TNF alpha inhibitors | 15 (51%) | 22 (100%) | .. | 29 (16%) | - | |
| Other immunosuppression in first month post-transplant | ||||||
| IMDPH (mycophenolic acid) | 3 (10%) | 14 (64%) | 34 (83%) | 28 (16%) | (58.6%) | <0.01 |
| m-TOR inhibitors | 28 (97%) | 17 (77%) | 26 (63%) | 173 (98%) | (16.5%) | 0.03 |
| Calcineurin inhibitors | 25 (86%) | 22 (100%) | 21 (51%) | 176 (99%) | (91.6%) | 0.04 |
| Efalizumab | 3 (10%) | 5 (12%) | 12 (7%) | NS | ||
| Infusions | ||||||
| 1 | 21 (72%) | 8 (36%) | 14 (34%) | 36 (20%) | - | <0.01 |
| 2 | 7 (24%) | 8 (36%) | 18 (44%) | 81 (46%) | - | |
| 3 | 1 (3%) | 4 (18%) | 8 (20%) | 59 (33%) | - | |
| ≥4 | .. | 2 (9%) | 1 (2%) | 1 (0.6%) | - | |
| Cumulative IEQs (*100,000) | 614 ± 46 | 889 ± 88 | 908 ± 87 | 906 ± 32 | - | 0.01 |
| Donor/Graft Characteristics | ||||||
| Donor BMI | 34 ± 1.5 | 31.1 ± 1.1 | 29.7 ± 0.9 | 29.0 ± 0.4 | - | 0.003 |
| Donor age | 32 ± 3 | 45.1 ± 2.1 | 45 ± 3 | 43 ± 1 | - | <0.001 |
| Cold Ischemia Time | 6.0 ± 0.5 | 6.8 ± 0.4 | 6.8 ± 0.4 | 7.5 ± 0.2 | 12.2 ± 3.5 | NS |
| Packed cell volume | 5.0 ± 0.8 | 7.7 ± 0.9 | 7.7 ± 0.9 | 8.4 ± 0.4 | - | 0.02 |
The 2009 UNOS/SRTR Report did not include any transplants done in 2009.
Abbreviations: SHE= severe hypoglycemic episodes; IMDPH= ionosine monophosphate dehydrogenase, including mycophenolic acid and mycophenolate mofetil; m-TOR= mammalian target of rapamycin and includes sirolimus and everolimus. TNF-alpha= tumor necrosis factor-alpha; TNF-alpha inhibitors include etanercept and infliximab. Calcineurin inhibitors include tacrolimus and cyclosporine. T-cell depleting agents include anti-CD3 monoclonal antibodies, anti-thymocyte globulin, alemtuzumab.
These define the study categories, hence testing is not necessary.
p-values relate to differences among the islet transplant groups (do not include the PTA)
Results are shown here and in Figure 1 for TPAs done in 2002–2007, comprising the 5-year cohort of US Scientific Registry of Transplant Recipients, thus, TPAs transplanted in 1999–2001 are not included.
Observed rates of insulin independence in UMN patients receiving PII were 74% at 1 year, 50% at 3 years, and 50% at 5 years. Forty percent were insulin independent at 10 years, although the number of cases that have yet reached this time point is small (n=2/5). Mean hemoglobin A1c levels were normal or near-normal in insulin independent recipients (5.5±0.4% at 3 years, 5.6±0.5% at 5 years).
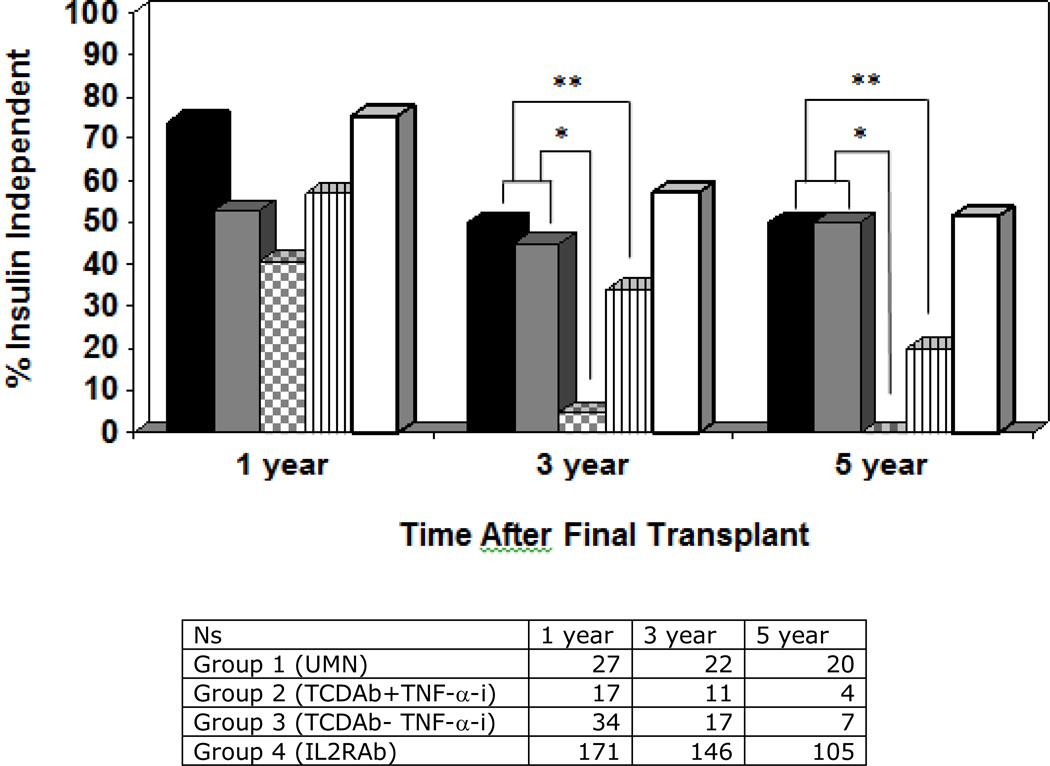
Insulin independence rates at 3 and 5 years post-last infusion (figure 1) are superior for the groups 1 and 2 (UMN and TCDAb+TNF-α-i) compared to group 3 (TCDAb without TNF-α-i) (p≤0.01 at 3 yrs and 0.001 at 5 yrs) and group 4 (IL2R-Ab recipients) (p=0.05 at 3 yrs and 0.002 at 5 yrs), a difference which was sustained within the subset of autoantibody positive patients. Observed rates of insulin independence in groups 1 and 2 were 50% and 45% respectively at 3 years and 50% in both groups at 5 years. The proportion of insulin independence at 3 and 5 years in these two groups was comparable to that of PTA (group 5), based on registry data from SRTR (p>>0.05).
Figure 1.
Observed insulin independence in pancreas transplant alone and in islet transplant alone recipients based on induction immunosuppression administered. Islet transplant groups are indicated as follows: group 1 (UMN recipients, solid black), group 2 (CITR recipients receiving TCDAb +TNF-α-i, solid grey), group 3 (CITR recipients receiving TCDAb without TNF-α-i, grey checkered), group 4 (CITR recipients receiving IL-2RAb, vertical lines), group 5 (pancreas transplant alone recipients, white). Insulin independence was superior in groups 1 and 2 compared to groups 3 and 4 at 3 and 5 years posttransplant; groups 1 and 2 did not differ statistically from pancreas transplant alone (group 5).
* p<0.01; ** p≤0.05
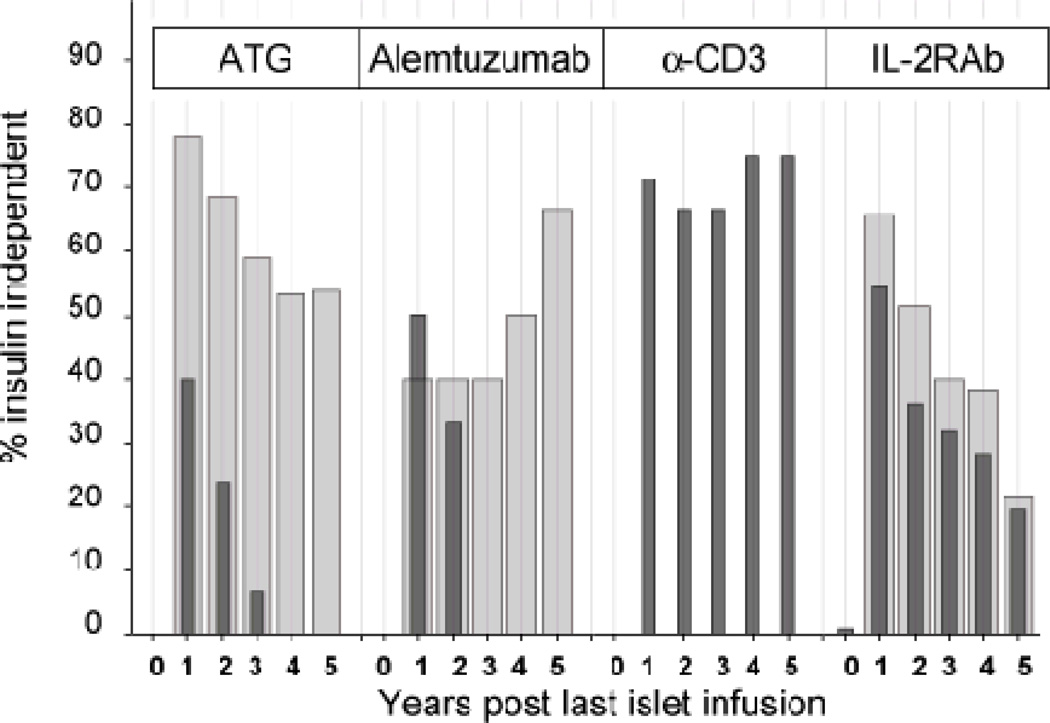
Insulin independence rates were significantly higher when TNF-α-i was given in the peritransplant period. TNF-α-i conferred substantial benefit with ATG or alemtuzumab induction (group 2), and marginal benefit with daclizumab (group 4) (Figure 2). TNF-α-i with anti-CD3 monoclonal antibody was never used.
Figure 2.
Insulin independence by induction agent, in the presence of TNF-alpha inhibitor therapy (wide bars) or absence of TNF-alpha inhibitor (narrow bars). Insulin independence was significantly lower at every time point in the ATG group in the absence of TNF-alpha inhibitor as compared to ATG with TNF-alpha inhibitor.
Virtually everyone in group 2 (TCDAb+TNF-α-i) received calcineurin inhibition (CNI), while only about half of group 3 (TCDAb without TNF-α-i) received CNI. However, when subdividing group 3 by presence or absence of CNI, both CNI +/− groups experience substantial graft function losses over 5 years relative to group 2. Outcomes were poor in group 3, regardless of CNI use-- no patient in the CNI +subgroup and fewer than 10% of patients in the CNI- subgroup were insulin independent at 3 years, and none were insulin independent at 5 years. Hence, there is no evidence that the superior outcome in group 2 is due to the confounding effect of CNI.
Comparison of early post-transplant islet graft function, measured by fasting C-peptide to glucose ratios
The fasting C-peptide to glucose ratio at day 28 post-transplant was higher in group 1 (1.2±0.1) as compared to group 3 (0.6±0.1) and group 4 (0.8±0.1; p≤0.001). There was a trend toward higher C-peptide to glucose ratios in group 2 (0.9±0.1) compared to group 3 (p=0.12) and group 4 (p=0.23). Long term retention of any graft function (C-peptide production≥0.3 ng/mL) was greater when TNF-α-i was administered with TCDAbs (data not shown).
Reduction in severe hypoglycemia post-transplant
Severe hypoglycemic episodes were uncommon in all ITA recipients, regardless of induction therapy. Over 62% of ITA recipients suffered from severe hypoglycemic episodes pre-first transplant. In contrast, over 80% of ITA recipients in all groups at 1– 3 years posttransplant reported absence of severe hypoglycemia.
Adjusted analysis of effect of TCDAb and TNF-α-i on insulin independence
An adjusted analysis was performed to determine the relative impact of induction immunosuppression when accounting for potential confounding variables, including other immunosuppression and baseline characteristics (Table 1). Insulin independence decreased over time in all groups. TCDAb or anti-CD3 (p=0.03), TNF-α-i (p=0.03), and length of follow up (p<0.0001) emerged as significant variables associated with insulin independence regardless of other recipient, donor, graft, or immunosuppression variables. None of the donor, islet or recipient pre-infusion characteristics included in the model blunted or explained the effects of T-cell depletion or TNF-α–i on insulin independence.
DISCUSSION
The decline in insulin independence rates beyond 1 year after alloislet transplant for T1D has raised concern about the therapeutic value of islet allografts. Proposed factors contributing to islet loss include non-immunologic injury such as amyloid-mediated islet apoptosis (18) and immunologic factors such as recurrent beta cell autoimmunity (6.19). We demonstrate here that patients receiving potent induction immunosuppression, with FcR non-binding anti-CD3 or either ATG or alemtuzumab with TNF-α inhibitors, are more than twice as likely to maintain long-term insulin independence for ≥5 years post-transplant compared to those receiving IL-2 receptor antagonists, despite the fact that only a single donor pancreas was utilized in over 70% of group 1 recipients. Such regimens may benefit long-term outcomes through improved engrafted islet mass and minimization of recurrent autoimmunity. Insulin independence rates in these recipients approach those seen in PTA. These data demonstrate the potential for sustained insulin independence after alloislet transplantation for T1D and highlight the importance in selection of induction immunosuppression.
Initial reports evaluating long-term insulin independence in islet allotransplant recipients receiving IL-2RAb suggested frequent return to insulin dependence after one year post-transplant, with ~10% of recipients insulin independent at 5 years (2). The present analysis shows half of patients treated with PII maintained insulin independence through 5 years, a proportion comparable to that seen in PTA recipients.
Although much research has focused on the immunologic factors contributing to islet loss, non-immunologic factors, in particular the exhaustion and decline of a marginal mass likely play a role (20). Constant overstimulation of an inadequately low islet mass causes endoplasmic reticulum stress-mediated islet apoptosis (21,22), a potential mediator of gradual islet loss. This is supported by recent observations of wide spread amyloid deposition in transplanted human islets (18), as well as observations of gradual loss of insulin independence in islet autotransplants (which are not subject to immune destruction) in canines (23) and in humans with a marginal islet mass (<2500 IEQ/kg) (24). Under this reasoning, the transplanted and engrafted islet mass in IL-2RAb-treated patients was borderline adequate to facilitate short-term insulin independence but insufficient to sustain long-term graft survival. In contrast, we hypothesize that sustained islet graft survival is achievable in patients receiving PII, through engraftment of a higher proportion of transplanted islets.
Early studies in alloislet transplant documented sustained insulin independence primarily in the context of multiple donor infusions to increase transplanted beta cell mass (3,5,25). Induction immunosuppression regimens that inhibit innate and adaptive immunity protect islets from immune-mediated destruction early posttransplant and thereby increase engrafted beta cell mass, insulin secretory capacity, and islet reserve. Single center trials have documented achievement of insulin independence with fewer islets and sustained insulin independence with a single islet donor when induction was comprised of ant-CD3 or TCDAb and TNF-α-I (9,10,26). Insulin secretory capacity in response to glucose-potentiated arginine (a surrogate marker of beta cell mass) is superior in patients receiving TCDAb + TNF-α-i compared to IL-2RAb induction immunosuppression (11).
We postulate that these PII regimens may have further benefit through their effect on the autoimmune process of T1D. Autoimmunity has been identified as one cause of pancreas transplant failure, as evidenced by autoreactive T cells and insulitis in pancreas allografts (27). In animal models, islets are even more susceptible to recurrent autoimmunity (28,29). In clinical islet transplant recipients, the presence of T-cell autoreactivity to β-cell antigens is associated with failure to achieve or delayed achievement of insulin independence (19). Whole organ are much less prone to recurrent autoimmune destruction than islet grafts in immunosuppressed recipients, presumably due to immunomodulatory effects of donor T cells co-transplanted with peripancreatic lymph nodes (29). Thus, islet transplant protocols must adequately protect from recurrent autoimmunity for optimal success.
Anti-CD3, ATG, and alemtuzumab may manifest a long-term benefit through restored self-tolerance. In clinical trials for new onset T1D, short-term monotherapy with anti-CD3 monoclonal antibody has resulted in sustained superior C-peptide production and lower insulin requirements (14,15). In contrast, no such benefit was observed in recent onset T1D patients receiving the IL-2RAb daclizumab plus mycophenolate mofetil (30). In pancreas transplant recipients, although daclizumab suppressed β-cell autoimmunity while sparing T cell memory responses to bacterial and viral antigens, the effect was short-lived, limited to the duration of drug action; in contrast, ATG resulted in prolonged alteration of the cellular autoreactivity, for >1 year after drug administration (31).
The mechanism for prolonged abrogation of autoimmunity likely relates to the potential of these agents to enhance T regulatory cells (Tregs) and shift the balance of Tregs and memory T-effector cells towards a more tolerant phenotype (13,32–36). Treatment with anti-CD3 mAb or anti-lymphocyte serum in NOD mice restores self-tolerance to beta-cell antigens and results in prolonged reversal of diabetes (12,13). Both agents can induce expansion of foxp3+ CD4+CD25+ and, in the case of anti-CD3, also CD8+CD25+ T-regs (13,32–34). This effect of ATG is preserved or even enhanced in the presence of tacrolimus (35). Islet transplant recipients treated with alemtuzumab had an increase in CD4+foxp3+Tregs that was not seen with daclizumab (36).
Co-administration of TNF-α-i at induction resulted in a greater likelihood of insulin independence in the multivariate model, particularly when ATG or alemtuzumab were used for induction. TNF-α-i may mediate this benefit by two proposed mechanisms. First, TNF-α-i may prevent the detrimental effects of cytokine exposure at the time of islet infusion. TNF-α exposure is known to be cytotoxic to human islets in culture (37), and innate immunity—including cytokine toxicity—in the peritransplant period is one proposed contributor to islet loss (38). In heart allograft models, TNF-α-i substantially reduces early leukocyte infiltration of the allograft and prolongs graft survival (39). The protective effect of TNF-α-i may be more important with an agent such as ATG, which is well known to induce a cytokine release syndrome in the recipient (40). Second, TNF-α-i may have a sustained impact on autoimmunity. The timing of TNF-α-I exposure is likely critical. TNF-α-i increases Tregs and completely prevents onset of autoimmune diabetes in NOD mice only if given in the neonatal period but not when administered later (41,42). Similarly, TNF-α-i may be particularly effective when given peritransplant (i.e. during the initial period of antigen exposure).
This is the largest study to date reporting such a sustained benefit of induction therapy in islet transplantation. One limitation is that results derive from a voluntary, although audited, registry rather than a controlled trial. In order to account for this limitation, we controlled for potential confounders in a multivariate analysis that included patient and islet graft characteristics, concurrent maintenance immunosuppression, and transplant center. We acknowledge that there are some limitations inherent in the registry despite the controlled analysis. Patients were categorized as ever treated with a particular immunosuppression agent over time, and were not able to account for changes in maintenance immunosuppression and/or adequacy of immunosuppression (trough levels). The number of patients, although large for the islet transplant field, is small within the subgroups and diminishes over time. In addition, mechanistic data, including cellular autoimmunity assays are not available for these recipients. Although autoantibody data is contained within the registry, autoantibodies are poor markers for the presence of active autoimmunity (19).
The comparison between the CITR islet transplant and the UNOS/SRTR whole pancreas alone data is limited by the use of two separate data registries, and results must be carefully interpreted within this context. While not randomized, this comparison is concurrent and based on the most complete data available. Both CITR and UNOS/SRTR collect similar data in transplanted type 1 diabetic recipients, particularly with regards to insulin independence, and provide the best opportunity for comparison of these two groups outside a clinical trial. Notably, this analysis was restricted to islet transplant alone versus solitary pancreas transplant (without kidney transplant) for this analysis, in order to maintain comparability.
In conclusion, these results highlight the potential for prolonged insulin independence after alloislet transplant when potent induction immunosuppression is used, and emphasize the critical impact of induction immunosuppression on long-term islet graft function. Prolonged insulin independence was superior in alloislet transplant recipients receiving PII, with either FcR non-binding anti-CD3 monoclonal antibody alone, or ATG or alemtuzumab plus TNF-α-i for induction immunosuppression compared to IL-2RAb induction. This benefit may in part be mediated by improved islet engraftment and mitigation of autoreactive T cell responses. These results show that with potent induction strategies, intraportal alloislet grafts can provide prolonged glycemic benefits in selected recipients, approaching those seen with solitary vascularized pancreas grafts, with a far less invasive impact.
ACKNOWLEDGEMENTS
The authors are indebted to the Investigators of the Collaborative Islet Transplant Registry (CITR) for their contribution of data and critical appraisal of the manuscript.
The CITR is funded by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) with supplemental funding by the Juvenile Diabetes Research Foundation. Dr. Melena Bellin is supported by a K23 grant from the NIDDK (1K23DK084315-01A1). The University of Minnesota islet transplant program is currently supported in part by a grant from the National Institutes of Health (5U01 AI-065193) and previously supported by grants from NIH (National Institute for Diabetes, Digestive and Kidney Diseases, DK56963; the National Center for Research Resources, MO1-RR00400 and U42 RR016598-01), and the Juvenile Diabetes Research Foundation (JDRF #4-1999-841 and 4-2008-386). The data and analyses reported in the 2009 Annual Report of the U.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients have been supplied by UNOS and Arbor Research under contract with HHS/HRSA. The authors alone are responsible for reporting and interpreting these data; the views expressed herein are those of the authors and not necessarily those of the U.S. Government.
Footnotes
DISCLOSURES:
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation
REFERENCES
- 1.Cryer PE. The barrier of hypoglycemia in diabetes. Diabetes. 2008 Dec;57(12):3169–3176. doi: 10.2337/db08-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ryan EA, Paty BW, Senior PA, Bigam D, Alfadhli E, Kneteman NM, et al. Five-year follow-up after clinical islet transplantation. Diabetes. 2005 Jul;54(7):2060–2069. doi: 10.2337/diabetes.54.7.2060. [DOI] [PubMed] [Google Scholar]
- 3.Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006 Sep 28;355(13):1318–1330. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- 4.Robertson RP. Islet transplantation a decade later and strategies for filling a half-full glass. Diabetes. 2010 Jun;59(6):1285–1291. doi: 10.2337/db09-1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000 Jul 27;343(4):230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 6.Monti P, Scirpoli M, Maffi P, Ghidoli N, De Taddeo F, Bertuzzi F, et al. Islet transplantation in patients with autoimmune diabetes induces homeostatic cytokines that expand autoreactive memory T cells. J Clin Invest. 2008 Apr 22; doi: 10.1172/JCI35197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nir T, Melton DA, Dor Y. Recovery from diabetes in mice by beta cell regeneration. J Clin Invest. 2007 Sep;117(9):2553–2561. doi: 10.1172/JCI32959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keymeulen B, Korbutt G, De Paepe M, Gorus F, Kloppel G, Pipeleers DG. Long-term metabolic control by rat islet grafts depends on the composition of the implant. Diabetes. 1996 Dec;45(12):1814–1821. doi: 10.2337/diab.45.12.1814. [DOI] [PubMed] [Google Scholar]
- 9.Hering BJ, Kandaswamy R, Harmon JV, Ansite JD, Clemmings SM, Sakai T, et al. Transplantation of cultured islets from two-layer preserved pancreases in type 1 diabetes with anti-CD3 antibody. Am J Transplant. 2004 Mar;4(3):390–401. doi: 10.1046/j.1600-6143.2003.00351.x. [DOI] [PubMed] [Google Scholar]
- 10.Hering BJ, Kandaswamy R, Ansite JD, Eckman PM, Nakano M, Sawada T, et al. Single-donor, marginal-dose islet transplantation in patients with type 1 diabetes. JAMA. 2005 Feb 16;293(7):830–835. doi: 10.1001/jama.293.7.830. [DOI] [PubMed] [Google Scholar]
- 11.Rickels MR, Chiou AJ, Fuller C, Dalton-Bakes C, Markmann E, Palanjian SA, et al. Beta-cell secretory capacity after human islet transplantation by the CIT07 vs Edmonton protocol: Preliminary results. American Journal of Transplantation. 2011;11(S2):79–80. [Google Scholar]
- 12.Maki T, Ichikawa T, Blanco R, Porter J. Long-term abrogation of autoimmune diabetes in nonobese diabetic mice by immunotherapy with anti-lymphocyte serum. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3434–3438. doi: 10.1073/pnas.89.8.3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chatenoud L. Immune therapy for type 1 diabetes mellitus-what is unique about anti-CD3 antibodies? Nat Rev Endocrinol. 2010 Mar;6(3):149–157. doi: 10.1038/nrendo.2009.275. [DOI] [PubMed] [Google Scholar]
- 14.Keymeulen B, Vandemeulebroucke E, Ziegler AG, Mathieu C, Kaufman L, Hale G, et al. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med. 2005 Jun 23;352(25):2598–2608. doi: 10.1056/NEJMoa043980. [DOI] [PubMed] [Google Scholar]
- 15.Herold KC, Gitelman S, Greenbaum C, Puck J, Hagopian W, Gottlieb P, et al. Treatment of patients with new onset Type 1 diabetes with a single course of anti-CD3 mAb Teplizumab preserves insulin production for up to 5 years. Clin Immunol. 2009 Aug;132(2):166–173. doi: 10.1016/j.clim.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bellin MD, Kandaswamy R, Parkey J, Zhang HJ, Liu B, Ihm SH, et al. Prolonged insulin independence after islet allotransplants in recipients with type 1 diabetes. Am J Transplant. 2008 Nov;8(11):2463–2470. doi: 10.1111/j.1600-6143.2008.02404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.2010 Annual Data Report of the U.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients. Rockville, MD: Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation; [Google Scholar]
- 18.Westermark GT, Westermark P, Berne C, Korsgren O Nordic Network for Clinical Islet Transplantation. Widespread amyloid deposition in transplanted human pancreatic islets. N Engl J Med. 2008 Aug 28;359(9):977–979. doi: 10.1056/NEJMc0802893. [DOI] [PubMed] [Google Scholar]
- 19.Huurman VA, Hilbrands R, Pinkse GG, Gillard P, Duinkerken G, van de Linde P, et al. Cellular islet autoimmunity associates with clinical outcome of islet cell transplantation. PLoS One. 2008 Jun 18;3(6):e2435. doi: 10.1371/journal.pone.0002435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith RN, Kent SC, Nagle J, Selig M, Iafrate AJ, Najafian N, et al. Pathology of an islet transplant 2 years after transplantation: evidence for a nonimmunological loss. Transplantation. 2008 Jul 15;86(1):54–62. doi: 10.1097/TP.0b013e318173a5da. [DOI] [PubMed] [Google Scholar]
- 21.Prentki M, Nolan CJ. Islet beta cell failure in type 2 diabetes. J Clin Invest. 2006 Jul;116(7):1802–1812. doi: 10.1172/JCI29103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonora E. Protection of pancreatic beta-cells: is it feasible? Nutr Metab Cardiovasc Dis. 2008 Jan;18(1):74–83. doi: 10.1016/j.numecd.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 23.Alejandro R, Cutfield RG, Shienvold FL, Polonsky KS, Noel J, Olson L, et al. Natural history of intrahepatic canine islet cell autografts. J Clin Invest. 1986 Nov;78(5):1339–1348. doi: 10.1172/JCI112720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sutherland DE, Radosevich D, Bland B, Beilman GJ, Dunn T, Vickers S, et al. Total pancreatectomy and islet autotransplant for chronic pancreatitis: Early outcomes in recent cases. [abstract] Pancreas. 2009;38(8):1051. [Google Scholar]
- 25.Warnock GL, Kneteman NM, Ryan E, Seelis RE, Rabinovitch A, Rajotte RV. Normoglycaemia after transplantation of freshly isolated and cryopreserved pancreatic islets in type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1991 Jan;34(1):55–58. doi: 10.1007/BF00404026. [DOI] [PubMed] [Google Scholar]
- 26.Gangemi A, Salehi P, Hatipoglu B, Martellotto J, Barbaro B, Kuechle JB, et al. Islet transplantation for brittle type 1 diabetes: the UIC protocol. Am J Transplant. 2008 Jun;8(6):1250–1261. doi: 10.1111/j.1600-6143.2008.02234.x. [DOI] [PubMed] [Google Scholar]
- 27.Vendrame F, Pileggi A, Laughlin E, Allende G, Martin-Pagola A, Molano RD, et al. Recurrence of type 1 diabetes after simultaneous pancreas-kidney transplantation, despite immunosuppression, is associated with autoantibodies and pathogenic autoreactive CD4 T-cells. Diabetes. 2010 Apr;59(4):947–957. doi: 10.2337/db09-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naji A, Silvers WK, Bellgrau D, Barker CF. Spontaneous diabetes in rats: destruction of islets is prevented by immunological tolerance. Science. 1981 Sep 18;213(4514):1390–1392. doi: 10.1126/science.6791286. [DOI] [PubMed] [Google Scholar]
- 29.Bartlett ST, Chin T, Dirden B, Quereshi A, Hadley G. Inclusion of peripancreatic lymph node cells prevents recurrent autoimmune destruction of islet transplants: evidence of donor chimerism. Surgery. 1995 Aug;118(2):392–397. doi: 10.1016/s0039-6060(05)80350-2. discussion 397–8. [DOI] [PubMed] [Google Scholar]
- 30.Gottlieb PA, Quinlan S, Krause-Steinrauf H, Greenbaum CJ, Wilson DM, Rodriguez H, et al. Failure to preserve beta-cell function with mycophenolate mofetil and daclizumab combined therapy in patients with new- onset type 1 diabetes. Diabetes Care. 2010 Apr;33(4):826–832. doi: 10.2337/dc09-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van de Linde P, Vd Boog PJ, Tysma OM, Elliott JF, Roelen DL, Claas FH, et al. Selective unresponsiveness to beta cell autoantigens after induction immunosuppression in pancreas transplantation with anti-interleukin-2 receptor antibody versus anti-thymocyte globulin. Clin Exp Immunol. 2007 Jul;149(1):56–62. doi: 10.1111/j.1365-2249.2007.03400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bisikirska B, Colgan J, Luban J, Bluestone JA, Herold KC. TCR stimulation with modified anti-CD3 mAb expands CD8+ T cell population and induces CD8+CD25+ Tregs. J Clin Invest. 2005 Oct;115(10):2904–2913. doi: 10.1172/JCI23961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lopez M, Clarkson MR, Albin M, Sayegh MH, Najafian N. A novel mechanism of action for anti-thymocyte globulin: induction of CD4+CD25+Foxp3+ regulatory T cells. J Am Soc Nephrol. 2006 Oct;17(10):2844–2853. doi: 10.1681/ASN.2006050422. [DOI] [PubMed] [Google Scholar]
- 34.Gurkan S, Luan Y, Dhillon N, Allam SR, Montague T, Bromberg JS, et al. Immune reconstitution following rabbit antithymocyte globulin. Am J Transplant. 2010 Sep;10(9):2132–2141. doi: 10.1111/j.1600-6143.2010.03210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sewgobind VD, van der Laan LJ, Kho MM, Kraaijeveld R, Korevaar SS, Mol W, et al. The calcineurin inhibitor tacrolimus allows the induction of functional CD4CD25 regulatory T cells by rabbit anti-thymocyte globulins. Clin Exp Immunol. 2010 Aug;161(2):364–377. doi: 10.1111/j.1365-2249.2010.04183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Toso C, Edgar R, Pawlick R, Emamaullee J, Merani S, Dinyari P, et al. Effect of different induction strategies on effector, regulatory and memory lymphocyte sub-populations in clinical islet transplantation. Transpl Int. 2009 Feb;22(2):182–191. doi: 10.1111/j.1432-2277.2008.00746.x. [DOI] [PubMed] [Google Scholar]
- 37.Rabinovitch A, Sumoski W, Rajotte RV, Warnock GL. Cytotoxic effects of cytokines on human pancreatic islet cells in monolayer culture. J Clin Endocrinol Metab. 1990 Jul;71(1):152–156. doi: 10.1210/jcem-71-1-152. [DOI] [PubMed] [Google Scholar]
- 38.Lai Y, Chen C, Linn T. Innate immunity and heat shock response in islet transplantation. Clin Exp Immunol. 2009 Jul;157(1):1–8. doi: 10.1111/j.1365-2249.2009.03899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ishii D, Schenk AD, Baba S, Fairchild RL. Role of TNFalpha in early chemokine production and leukocyte infiltration into heart allografts. Am J Transplant. 2010 Jan;10(1):59–68. doi: 10.1111/j.1600-6143.2009.02921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hardinger KL. Rabbit antithymocyte globulin induction therapy in adult renal transplantation. Pharmacotherapy. 2006 Dec;26(12):1771–1783. doi: 10.1592/phco.26.12.1771. [DOI] [PubMed] [Google Scholar]
- 41.Yang XD, Tisch R, Singer SM, Cao ZA, Liblau RS, Schreiber RD, et al. Effect of tumor necrosis factor alpha on insulin-dependent diabetes mellitus in NOD mice. I. The early development of autoimmunity and the diabetogenic process. J Exp Med. 1994 Sep 1;180(3):995–1004. doi: 10.1084/jem.180.3.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu AJ, Hua H, Munson SH, McDevitt HO. Tumor necrosis factor-alpha regulation of CD4+CD25+ T cell levels in NOD mice. Proc Natl Acad Sci U S A. 2002 Sep 17;99(19):12287–12292. doi: 10.1073/pnas.172382999. [DOI] [PMC free article] [PubMed] [Google Scholar]