Ligands play critical roles during the catalytic reactions in inorganic systems and in metalloproteins through bond formation/breaking, protonation/deprotonation, and electron/spin delocalization. There are well-defined element-specific spectroscopic handles, such as X-ray spectroscopy and EPR, to follow the chemistry of metal catalytic sites. However, directly probing particular ligand atoms like C, N, and O, especially in a large protein matrix, is challenging due to their abundance in the protein. FTIR/Raman and ligand-sensitive EPR techniques such as ENDOR and ESEEM have been applied to study metal-ligand interactions. X-ray absorption spectroscopy (XAS) can also probe the ligand environment; its element-specificity allows us to focus only on the catalytic metal site, and EXAFS and XANES provide metal-ligand distances, coordination numbers, and symmetry of ligand environments. However, the information is limited, because one cannot distinguish among ligand elements with similar atomic number (i.e. C, N. and O). As an alternative and a more direct method to probe the specific metal-ligand chemistry in the protein matrix, we investigated the application of X-ray emission spectroscopy (XES). Using this technique we have identified the oxo-bridging ligands of the Mn4Ca complex of photosystem II (PS II), a multisubunit membrane protein, that catalyzes the water oxidizing reaction in photosynthesis.[1] The catalytic mechanism has been studied intensively by Mn XAS.[2] The fundamental challenge, however, is to learn how the water molecules are ligated to the Mn4Ca cluster and how O-O bond formation occurs before the evolution of O2.[3-5] This implies that it is necessary to follow the chemistry of the oxygen ligands to understand the mechanism.
XES, which is a complementary method to XAS, has the potential to directly probe ligation modes.[6] Among the several emission lines, Kβ1,3 and Kβ′ lines originate from the metal 3p to 1s transition, and they have been used as an indicator of the charge and spin states on Mn in the oxygen-evolving complex (OEC) (Figure 1).[7, 8] The higher energy region corresponds to valence-to-core transitions just below the Fermi level and can be divided into the Kβ″ and the Kβ2,5 emission (Fig.1 left scheme). Kβ2,5 emission is predominantly from ligand 2p (metal 4p) to metal 1s, and the Kβ″ emission is assigned to a ligand 2s to metal 1s; both are referred to as cross-over transitions.[9-11] Therefore, only direct ligands to the metal of interest are probed with Kβ,2,5/Kβ″ emission; i.e. other C, N, and O atoms in the protein media do not contribute to the spectra. In this report, we focus on the Kβ″ spectral region to characterize metal-ligand interactions, in particular contributions from ligated oxygens. The energy of the Kβ″ transition depends on the difference between the metal 1s and ligand 2s binding energies, which reflects the environment of the ligand owing to orbital hybridization. Therefore the Kβ″ energy is affected by the charge density on the metal, the ligand protonation state, and changes in the coordination environment. The Kβ″ intensity is influenced by the spatial overlap between the wavefunction that describes the Mn 1s orbital and the molecular orbitals on the ligands. The Kβ″ intensity is affected by the metal-to-ligand distance, and the number of ligands per metal ion. Shorter distances (e.g. from higher bond order or deprotonation) result in increased Kβ″ intensity with an approximate exponential dependence on distance.[9] A spread of the molecular wavefunction over next-nearest neighbor atoms will decrease the Kβ″ spectral intensity. Therefore single-atom ligands such as oxo-bridges, or terminal oxo ligands bonded to Mn have predominant contributions to the spectra (see below). This combination of factors makes the Kβ″ spectrum a powerful tool for detection and characterization of oxo-bridges in the Mn4Ca cluster of PS II.
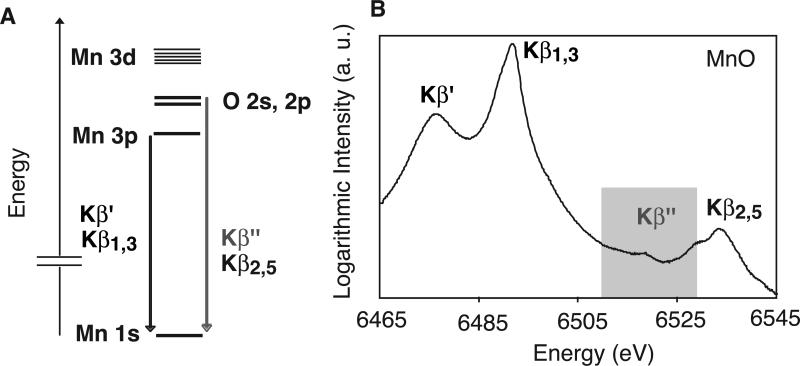
Figure 1.
(A) Energy diagram of Mn Kβ transitions in MnO. The Kβ″ and Kβ2,5 transitions are from valence molecular orbitals; Kβ″ is the O 2s to Mn 1s ‘cross-over’ transition. (B) Logarithmic plot of the MnO Kβ spectrum. The O-Mn cross-over Kβ″ transition is highlighted.
However, because of the weak intensity of the Kβ″ spectrum, obtaining such spectra from biological samples as dilute as PS II (800μM Mn) have been difficult. For O ligation in a typical model compound the signal is ~103 times weaker than that of Kα, and there is an additional large background from both the Kβ1,3 and the Kβ2,5 spectral features (Fig. 1). Furthermore the work is challenging because of the high sensitivity of the Mn4Ca cluster to radiation damage.[12] This study of PS II became possible by using a new high resolution spectrometer equipped with 8-14 analyzer crystals collecting over a large solid angle (see experimental section).
Fig. 2 shows the Kβ″ spectrum of a sample of PS II in the S1 state compared with a series of Mn oxide spectra. Each spectrum is normalized by the Kβ1,3 peak intensity which is proportional to the number of Mn atoms in the system. The peak position of the PS II S1 state falls between those of the MnIII and MnIV oxides. The energy of the Kβ″ feature may be influenced by charge screening effects that depend on the charge density on the Mn ion, i.e. the Mn oxidation state. However, the formal oxidation state, is only an approximation to the actual charge density.[13]
Figure 2.
Mn Kβ″ emission spectra from Mn oxides and the PS II S1 state. These spectra referred to as cross-over peaks are assigned to emission from ligand 2s2p levels to the Mn 1s core level.
Fig. 3 shows a comparison of the PS II S1 state spectrum with those of a series of Mn coordination compounds: MnV-oxo (a), di-μ-oxo bridged Mn2III,IV and Mn2IV (b, c), cubane-type Mn2III Mn2IV and MnIIIMn3IV (d, e), and a μ-alkoxide bridged Mn2II (f). These compounds have oxo-bridged Mn (except for MnV-oxo) with O or N/O terminal ligands. In Fig. 3, there is no detectable Kβ″ peaks from the μ-alkoxide/carboxylate bridged Mn2II complex (f). This is likely due to peak broadening originating from the delocalization of the oxygen 2s electron into a molecular orbital that is spread over the whole methyl/carboxylate group, i.e. atoms that are next-nearest to Mn, and to a lesser degree due to the longer Mn-ligand bridging interactions (~2.0-2.1 Å).[14, 15] Similarly, contributions from O and N terminal ligands from carboxylates or histidine/amines are weak, because the molecular orbitals that contain O and N 2s are strongly delocalized. Moreover, terminal ligands are generally at longer distances to Mn than the bridging ligands, and their contributions are smaller.[9] A similar effect has been observed in Fe cyanides.[6] Theoretical studies regarding X-ray emission spectra from transition metal complexes are emerging,[14, 15] yet at present the interpretation of the Kβ″ spectra has remained largely empirical. There is a strong peak in the MnV-oxo compound, indicating that the Kβ″ peak intensity is predominantly sensitive to single atom ligands and short metal ligand atom distances, namely, bridging O ligands and double/triple bonds which all have localized 2s orbitals. The sensitivity of the spectra to even one-electron changes is illustrated in Fig. 3. The spectra are different for the two binuclear and the two cubane molecules in which one of two or four Mn is oxidized from (III) to (IV). The Kβ″ peak of the PS II S1 state is relatively intense compared to other Mn2III,IV di-μ-oxo bridged compounds, which is suggestive that there are several μ-oxo bridged Mn-O bonds in the S1 state.[2]
Figure 3.
The Kβ″ emission spectra from a series of multinuclear Mn complexes with oxo-bridging groups, a MnV-oxo complex and PS II in the S1 state. The cross-over peak from the O ligand is prominent when short bridging Mn-O distances are present.
The involvement of bridging oxo groups[4] or the high valent MnIV=O• or MnV≡O species[3, 5] have all been implicated in the mechanism for the formation of the critical O-O bond in the water-oxidation reaction of PS II. As we have demonstrated here, it is now feasible to obtain such spectra for the Mn4Ca cluster in PS II, and the outlook for Kβ″ spectroscopy as a tool for studying the nature of the O ligand binding modes and therefore the mechanism of the water-splitting reaction seems promising. Finally, new powerful X-ray lasers such as the LINAC Coherent Light Source, Stanford, can be used for future real-time XES studies of the S-state cycle. In contrast to XAS, XES does not require scanning of the incident X-ray beam, and with a dispersive XES spectrometer, a full spectrum can be collected at once, and the time-evolution of the spectrum can be monitored.
Experimental Section
Mn Model compounds
The Mn model compounds used were the following: a bridging μ-alkoxide Mn2 compound ([Mn2II2(μ-RO)(μ–CH3CO2)](ClO4)2 (f),[16] two di-μ-oxo bridged Mn2 compounds ([Mn2III,IV O2bipy4](ClO4)3[17] (b), and [Mn2IV,IVO2terpy2(SO4)2].6H2O (c),[18] two Mn4 cubane compounds (hexakis(μ2-Diphenylphosphinato)-tetrakis(μ3-oxo)Mn4III,III,IV,IV (d), and [hexakis(μ2-Diphenylphosphinato)-tetrakis(μ3-oxo)Mn4III,IV,IV,IV]CF3SO3 (e),[19] and a macrocyclic MnV-oxo complex (a).[20] The compounds were prepared following published procedures.
PS II sample preparation
PS II membranes were prepared from fresh spinach.[21] The PS II sample holders (40 μL each, about 100) were designed to fit into both EPR and X-ray cryostats. After dark-adaptation for one hour at room temperature, the samples were predominantly in the S1 state.
Kβ XES measurement
Kβ emission spectra were recorded on beamline 6-2 at SSRL using a crystal array spectrometer. The samples were positioned at an angle of 45° between the surface of the sample and the incident X-ray beam. They were kept in an Oxford CF1208 cryostat at a temperature of ~10 K under an ambient pressure He atmosphere. The incident X-ray beam (beam size 1×2 mm2) had a flux of ~ 4×1012 photons/sec and an energy of 10.4 keV. The X-ray exposure time on the PS II samples was based on the published value for a radiation damage of less than 1%.[9] This was also confirmed by the XANES study carried out at BL 9-3 (SSRL) by irradiating PS II samples under conditions used for the Kβ XES measurements (X-ray energy of 10.4 keV, samples at ~10 K). A fast shutter which opened only during data collection protected the samples during all spectrometer and sample movements. This guaranteed a precise assessment of the X-ray dose to the samples. The model compound spectra shown in Fig. 3 were recorded with an 8-crystal device[10] using a single-element liq. N2 cooled Ge detector. The PS II S1 state spectra were recorded with a 14-crystal analyzer with spherically curved Si(440) crystals (10 cm diameter, 1 m radius of curvature) aligned on intersecting Rowland circles (Fig. 4). An energy-resolving Si drift detector (Vortex™) was used to reduce background mainly from elastic scattering.[22] The emission spectrum is recorded with ~1 eV resolution.
Figure 4.
Schematic drawing of the setup for X-ray emission spectroscopy (right) and the 14-crystal analyzer (left). Only 7 analyzers are shown in the schematic. 14 spherically curved Si(440) crystals , the detector and the sample are in an approximate Rowland circle geometry. The simultaneous vertical movement of the analyzer (d mm) and the detector (2d mm) changes the Bragg angle of the Si crystals with respect to the emission from the sample and thus the wavelength of detection.
All the spectra were normalized to the incident flux I0 measured with a gas filled ion chamber and calibrated to the published value of 6490.40 eV for the 1st moment (integrated from 6485 – 6495 eV) of Mn2O3.[11] In order to evaluate the relative intensities of the cross-over peaks, the spectra of all samples were normalized to the intensity of their respective Kβ1,3 peaks. Only a negligible difference was found when using the integrated versus peak intensity. The Kβ cross-over spectrum of PS II was collected between 6511 eV to 6529 eV at a step size of 0.32 eV. 1035 sweeps, each with 1 sec/step exposure (56 sec total/sweep/position), were added. Second order polynomial functions were used to fit the background of the Kβ cross-over region.
Footnotes
Acknowledgements: This work was supported by the NIH grant (GM 55302), and the DOE, Director, Office of Science, Office of Basic Energy Sciences (OBES), Chemical Sciences, Geosciences, and Biosciences Division, under Contract DE-AC02-05CH11231. Portions of this research were carried out at SSRL, operated by Stanford University for DOE, OBES. The SSRL SMB Program is supported by the DOE, OBER and by the NIH, NCRR. We thank Prof. Steve Cramer for the use of his emission spectrometer (NIH Grant EB-001962) for some of the work and Prof. Ken Sauer for many useful discussions.
Contributor Information
Yulia Pushkar, Physical Biosciences Division, Lawrence Berkeley National Laboratory Berkeley, CA 94720, USA.
Xi Long, Physical Biosciences Division, Lawrence Berkeley National Laboratory Berkeley, CA 94720, USA.
Pieter Glatzel, Physical Biosciences Division, Lawrence Berkeley National Laboratory Berkeley, CA 94720, USA.
Gary W. Brudvig, Department of Chemistry, Yale University New Haven, CT 06520, USA
G. Charles Dismukes, Department of Chemistry, Princeton University Princeton, NJ 08544, USA.
Terrence J. Collins, Department of Chemistry, Carnegie-Mellon University Pittsburgh, PA 15213, USA
Vittal K. Yachandra, Physical Biosciences Division, Lawrence Berkeley National Laboratory Berkeley, CA 94720, USA
Junko Yano, Physical Biosciences Division, Lawrence Berkeley National Laboratory Berkeley, CA 94720, USA.
Uwe Bergmann, SSRL, SLAC National Accelerator Laboratory Menlo Park, CA 94025, USA.
References
- 1.Wydrzynski T, Satoh S, editors. Photosystem II: The Light-Driven Water:Plastoquinone Oxidoreductase. Springer; Dordrecht: 2005. [Google Scholar]
- 2.Yano J, Yachandra VK. Inorg. Chem. 2008;47:1711. doi: 10.1021/ic7016837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Messinger J. Phys. Chem. Chem. Phys. 2004;6:4764. [Google Scholar]
- 4.Yachandra VK, Sauer K, Klein MP. Chem. Rev. 1996;96:2927. doi: 10.1021/cr950052k. [DOI] [PubMed] [Google Scholar]
- 5.McEvoy JP, Brudvig GW. Chem. Rev. 2006;106:4455. doi: 10.1021/cr0204294. [DOI] [PubMed] [Google Scholar]
- 6.Glatzel P, Bergmann U. Coord. Chem. Rev. 2005;249:65. [Google Scholar]
- 7.Bergmann U, Grush MM, Horne CR, DeMarois P, Penner-Hahn JE, Yocum CF, Wright DW, Dube CE, Armstrong WH, Christou G, Eppley HJ, Cramer SP. J. Phys. Chem. B. 1998;102:8350. [Google Scholar]
- 8.Messinger J, Robblee JH, Bergmann U, Fernandez C, Glatzel P, Visser H, Cinco RM, McFarlane KL, Bellacchio E, Pizarro SA, Cramer SP, Sauer K, Klein MP, Yachandra VK. J. Am. Chem. Soc. 2001;123:7804. doi: 10.1021/ja004307+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bergmann U, Horne CR, Collins TJ, Workman JM, Cramer SP. Chem. Phys. Lett. 1999;302:119. [Google Scholar]
- 10.Bergmann U, Glatzel P, Robblee JH, Messinger J, Fernandez C, Cinco R, Visser H, McFarlane K, Bellacchio E, Pizarro S, Sauer K, Yachandra VK, Klein MP, Cox BL, Nealson KH, Cramer SP. J. Synchrotron Rad. 2001;8:199. doi: 10.1107/s0909049500016484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bergmann U, Bendix J, Glatzel P, Gray HB, Cramer SP. J. Chem. Phys. 2002;116:2011. [Google Scholar]
- 12.Yano J, Kern J, Irrgang K-D, Latimer MJ, Bergmann U, Glatzel P, Pushkar Y, Biesiadka J, Loll B, Sauer K, Messinger J, Zouni A, Yachandra VK. Proc. Natl. Acad. Sci. USA. 2005;102:12047. doi: 10.1073/pnas.0505207102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glatzel P, Bergmann U, Yano J, Visser H, Robblee JH, Gu WW, de Groot FMF, Christou G, Pecoraro VL, Cramer SP, Yachandra VK. J. Am. Chem. Soc. 2004;126:9946. doi: 10.1021/ja038579z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Groot F. Chem. Rev. 2001;101:1779. doi: 10.1021/cr9900681. [DOI] [PubMed] [Google Scholar]
- 15.Smolentsev G, Soldatov AV, Messinger J, Merz K, Weyermuller T, Bergmann U, Pushkar Y, Yano J, Yachandra VK, Glatzel P. J. Am. Chem. Soc. 2009;131:12451. doi: 10.1021/ja808526m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pessiki PJ, Dismukes GC. J. Am. Chem. Soc. 1994;116:898. [Google Scholar]
- 17.Limburg J, Vrettos JS, Chen HY, de Paula JC, Crabtree RH, Brudvig GW. J. Am. Chem. Soc. 2001;123:423. doi: 10.1021/ja001090a. [DOI] [PubMed] [Google Scholar]
- 18.Plaksin PM, Stoufer RC, Mathew M, Palenik GJ. J. Am. Chem. Soc. 1972;94:2121. [Google Scholar]
- 19.Ruettinger WF, Ho DM, Dismukes GC. Inorg. Chem. 1999;38:1036. doi: 10.1021/ic981145y. [DOI] [PubMed] [Google Scholar]
- 20.Workman JM, Powell RD, Procyk AD, Collins TJ, Bocian DF. Inorg. Chem. 1992;31:1548. [Google Scholar]
- 21.Berthold DA, Babcock GT, Yocum CF. FEBS Lett. 1981;134:231. [Google Scholar]
- 22.Feng L, Iwanezyk JS, Patt BE, Barkan S, Tull CR. Proc. SPIE. 2004;5198:103. [Google Scholar]