Abstract
Relapse after autologous stem cell transplantation for low-grade B-cell lymphoma is common secondary to ineffective conditioning and/or tumor autograft contamination. We investigated high-dose cyclophosphamide and rituximab without stem cell rescue as first-line or salvage-therapy in lymphomas. After establishing safety, accrual was increased to evaluate event-free survival (EFS). 81 adults received rituximab [375mg/mm days 1, 4, 8, 11, 45, 52], cyclophosphamide [50mg/kg days 15-18] and pegfilgrastim (day 20). Forty-two patients had low-grade B-cell lymphoma [grade I/II follicular (69%), transformed lymphoma (17%), other (15%)]: 45% were treated without measurable disease. Thirty-nine patients had mantle cell lymphoma: 82% were treated without measurable disease. All achieved hematopoietic recovery; 46% required brief hospitalizations. The 5 year EFS and overall survival (OS) for low-grade B-cell and transformed patients was 40% and 72%, respectively. The 5 year EFS and OS for the MCL patients was 39% and 62%, respectively. This low-toxicity therapeutic approach obviates the need for stem cell products and establishes a platform for future therapies.
Keywords: Cyclophosphamide, NHL, Mantle Cell Lymphoma
Introduction
As conventional cytotoxic therapy often results in a complete response only to be followed by near universal disease relapse, high-dose chemotherapy to eradicate residual disease has been extensively studied in low grade B-cell lymphomas. Although numerous autologous blood and marrow stem cell transplantation (autoSCT) trials have shown a progression-free and even overall-survival advantage, most patients with low grade B-cell lymphomas eventually relapse.[1-5] Relapses result from lymphoma cells surviving the conditioning regimen and/or disease re-introduction at the time of stem cell rescue -- the relative contribution of each are unknown.
In 2003, we undertook a feasibility study testing whether patients with low grade B-cell hematologic malignancies could achieve full hematopoietic recovery after high-dose consolidative therapy and rituximab in-vivo B-cell purging without stem cell rescue. The theoretic advantage to this high-dose therapy treatment scheme is that high-dose cyclophosphamide (HDC) does not require autologous stem cell rescue, thus eliminating the possibility of tumor reinfusion. Normal hematopoietic stem cells express high levels of aldehyde dehydrogenase (ALDH1), which confers protection against the activity of cyclophosphamide.[6] Given universal hematopoietic recovery, the study was later amended for additional accrual as a phase I/II trial.
Patients, Materials, and Methods
Patient eligibility
Patients with the following lymphomas were eligible: follicular (grades I-II) including associated transformations, marginal zone, small lymphocytic (SLL)/chronic lymphocytic leukemia (CLL), and mantle cell lymphoma. Eligibility criteria included age ≥ 18 years, platelet count ≥75,000/mm3, WBC greater ≥3,000/mm3, ≤ 10% lymphomatous bone marrow involvement, at least a partial response to the most recent therapy, a serum creatinine ≤ 2.0mg/dL, total bilirubin ≤2mg/dL, left ventricular cardiac ejection fraction ≥45%, DLCO >50% predicted, and an ECOG performance status of 0 or 1. Seventy-eight patients consented for, and participated in, this Institutional Review Board (IRB) approved research study. The IRB allowed three additional patients into this analysis who had received the identical treatment: these patients had agreed to participate but could not secondary to medical insurance limitations. The patients were treated consecutively between October 2003 and September 2009. Patients who chose not to participate in the protocol would have been considered for conventional autologous stem cell transplantation. As patients were actively receiving treatment, many of the eligibility criteria were known before protocol participation was offered; these included: histologic diagnosis, age, peripheral blood counts, chemotherapy responsiveness, serum chemistries and ECOG performance. Screening established cardiac ejection fraction, DLCO and percentage marrow involvement. Analysis was locked in October 2010. The national trial registry number for this study is NCT00278161.
Treatment schema
Treatment consisted of rituximab (375 mg/m2, days 1, 4, 8, 11, 45 and 52), cyclophosphamide (50 mg/kg/day, days 15–18), mesna (10 mg/kg/dose, 15 minutes before, 3, 6, and 8 hours after cyclophosphamide) and pegfilgrastim (6 mg, 24-48 hours after last dose of cyclophosphamide [day 19 or 20]). Rituximab was dosed according to actual body weight; the cyclophosphamide and mesna were dosed based on the lower of actual or ideal body weight. Supportive care was administered as per institutional standards including anti-emetics, prophylactic antimicrobials and transfusions of red blood cells and platelets. All treatment was planned as outpatient. Disease status was assessed at baseline and every 3-4 months after treatment.
Statistical analysis
The estimated total accrual for the initial safety phase of this trial was 32 patients. The primary safety variable of interest was achievement of count recovery within 30 days. A response of 90% was expected and a response of 75% would have been acceptable to continue investigation of this treatment combination. An additional safety endpoint was mortality. It was anticipated the mortality associated with this treatment to be less than 5%. If more than 1 of the first 32 patients died as a result of the treatment combination, the study would have been considered for termination.
When the initial safety phase of the trial was completed without stopping for one of the safety stopping rules, the study accrual was increased to estimate efficacy. The trial was expanded to 80 patients to better estimate the event-free survival (EFS).
The primary statistical end points were EFS, defined as the interval between the first dose of rituximab and relapse or death from any cause, and overall survival (OS), defined as the interval between the first dose of rituximab and the date of death. Event time distributions were estimated with the method of Kaplan and Meier[7] and compared using the log-rank statistic,[8] or the Cox proportional hazards regression model.[9] Computations were performed using the Statistical Analysis Software,[10] version 9.1 (SAS Institute, Carry, NC).
Results
Patient characteristics
81/94 (86%) of patients screened were proceeded with protocol participation. Of the 13 patients screened who did not proceed with protocol participation, 9 did not meet eligibility criteria: 6 had less than a partial response, 1 patient had a reduced ejection fraction, 1 patinet had a low hemoglobin and 2 patients were found to have other concurrent active malignancies; three screened patients did not wish to participate.
Of the 81 participating patients, 42 had low grade B lymphomas (Table 1). Most of these patients (29/42, 69%) had grade I/II follicular lymphomas; seven (17%) had large cell transformations; two (5%) had SLL/CLL; two (5%) had marginal zone lymphoma, and two (5%) had grade III follicular lymphoma. Their median age at time of protocol participation was 54 (range 24-71) years. Of these patients, 15 (36%) received protocol treatment as consolidation after first response: eight (19%) in first partial remission (PR1) and seven (17%) in first complete remission (CR1). Nineteen (45%) patients received protocol treatment without residual disease (CR1, CR2, or CR3), and 23 (55%) participated after achieving only a PR to initial therapy or to salvage therapy. Of the 15 patients who received treatment immediately after first response (CR1 or PR1), 14 (93%) patients received one regimen with a median of 6 (range, 2-8) cycles; one (7%) patient received single agent rituximab therapy and 13 (87%) received R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone). The 27 (64%) patients with relapsed disease received a median of 3 (range: 1-5) regimens. Their median disease duration between initial diagnosis and protocol participation was 3.5 years (range, 1.3-14.4 years). One patient with relapsed grade I/II follicular lymphoma did not receive additional therapy before receiving protocol therapy.
Table 1.
Patient Charateristics
| Patients | ||||
|---|---|---|---|---|
| No. | % | |||
| Total | 81 | - | ||
| Low grade B cell lymphomas |
42 | 100 | ||
| Median age | 54 | |||
| Range | 24-71 | |||
| Grade I/II Follicular | 29 | 69 | ||
| Transformed | 7 | 17 | ||
| Chronic lymphocytic leukemia | 2 | 5 | ||
| Marginal Zone | 2 | 5 | ||
| Grade III Follicular | 2 | 5 | ||
| Tx in First Response (CR1 or PR) |
15 | 36 | ||
| Tx in CR1 | 7 | 17 | ||
| Tx without measurable disease (CR1, CR2, or CR3) |
19 | 45 | ||
| PR to initial or salvage therapy | 23 | 55 | ||
| Mantle Cell | 39 | 100 | ||
| Median age | 58 | |||
| Range | 40-76 | |||
| ≥65 yoa | 10 | 25.6 | ||
| Tx in First Response (CR1 or PR) |
38 | 97 | ||
| Tx in CR1 | 32 | 82 | ||
Thirty-nine patients had mantle cell lymphoma/leukemia (Table 1). Their median age at time of protocol participation was 58 (range, 40-76) years, including 10 (25.6%) patients > 65 years of age. All but one of the mantle cell patients received protocol therapy after initial cytotoxic therapy, most (32, 82%) in CR1. One patient was treated after a PR to salvage therapy. Thirty-six (92%) received R-CHOP as sole induction therapy, one patient received HyperCVAD (cyclophosphamide, hydroxydaunomycin, vincristine, dexamethasone, cytarabine and methotrexate) and 1 patient received R-EPOCH (rituximab, etoposide, prednisone, vincristine, and hydroxydaunomycin, and prednisone) as initial therapy with a median of 6 (range: 3-8) cycles. The relapsed patient received R-ICE [rituximab, ifosfamide, carboplatin, and etoposide] for progression after R-CHOP.
Toxicities
High-dose cyclophosphamide (HDC) induced the expected pancytopenia with an absolute neutrophil count (ANC) of <100/μL in all patients; most patients also required red cell and platelet transfusions (Table 2). All patients experienced full hematopoietic recovery. The median time to an ANC ≥500/μL from the initiation of high-dose cyclophosphamide was 15 (range, 11 to 32) days. The median time to an untransfused platelet count of ≥ 20 × 109/L from the initiation of cyclophosphamide was 15 (range, 0-43) days; 15 (18.5%) patients never experiencing a platelet count <20 × 109/L and did not receive platelet transfusions.
Table 2.
Treatment Related Cytopenias and Hospitalizations
| Event | Number (%) | Median Duration i | |
|---|---|---|---|
| Time to neutrophil count >500/μL from initiation of cyclophosphamide |
80 (99%) | 9 (range, 4 to 6) days | |
| Time to untransfused platelet count of > 20 × 109/L from initiation of cyclophosphamide |
66 (81.5%) | 15 (range, 0-43) days | |
| Patients Hospitalized | 35 (43%) | ||
| Cause | Neutropenic fever |
27 events | |
| Non- infectious |
10 events | ||
There were no treatment related deaths and no unexpected life-threatening toxicities. Fewer than half (35/81 or 43%) of the patients required hospitalization; two patients were hospitalized twice. Neutropenic fever resulted in admission for 27 patients: 14 were associated with bacteremia; 2 admissions were secondary to Parainfluenza 3 infection; no identifiable infection was found in 11 patients. Ten hospitalizations resulted from non-febrile complications included: two admissions for dehydration/hypotension, one episode each of: aseptic arthritis, atrial fibrillation that lasted <24 hours, mild epistaxis, anxiety induced delirium, tenosynovitis, transient hyponatremia, a syncopal episode from anti-nausea medications resulting in a fibular fracture, and poor nutritional intake requiring percutaneous endoscopic gastrostomy tube placement. One patient did not receive post cyclophosphamide rituximab secondary to elevated liver enzymes. No patient has developed a secondary leukemia or myelodysplastic syndrome.
Survival Outcomes
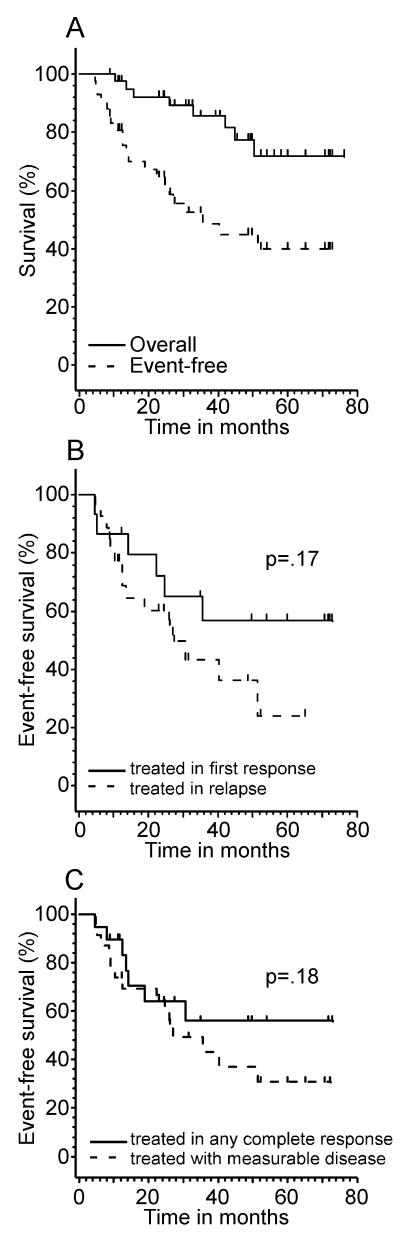
Of 42 patients with low-grade B cell tumors, 41 (98%) completed protocol therapy. Twenty patients have relapsed resulting in 5 deaths from progressive lymphoma. The median follow-up of the 34 surviving patients is 3.9 years (range, 0.7 – 6.4 years). Their respective EFS at 2, 3, and 5 years were 65%, 49%, and 40% and their respective overall survival at 2, 3, and 5 years were 92%, 86% and 72%. (Table 3, Figure 1A). The median EFS in this group was 35.6 months. Those treated in first response had an EFS of 72% at 24 months as compared to those treated after first relapse who had an EFS of 60% at 24 months, p=0.17 (Figure 1B). For those receiving therapy in any complete response, the median EFS has not been reached and is 56% at 36 months, while those starting protocol therapy not in complete remission had a median EFS of 24 months, p=0.18 (figure 1C). Of the 20 relapsed patients, 7 underwent non-myeloablative allogeneic bone marrow transplantation and 4 are alive and in complete remission from 12.2 to 27.5 months.
Table 3.
Event Free and Overall Survival for Low Grade B and Mantle Cell Lymphoma
| Histology | Time (months) | Event Free Survival |
95% Confidence interval |
Overall Survival |
95% Confidence Interval |
|---|---|---|---|---|---|
| Low Grade B Cell Lymphoma |
|||||
| 24 | 65% | 0.51, 0.82 | 92% | 0.84, 1.00 | |
| 36 | 49% | 0.35, 0.69 | 86% | 0.75, 0.98 | |
| 60 | 40% | 0.26, 0.62 | 72% | 0.56, 0.92 | |
| Mantle Cell Lymphoma |
|||||
| 24 | 73% | 0.60 , 0.90 | 100% | 1.00, 1.00 | |
| 36 | 57% | 0.41, 0.79 | 92% | 0.82, 1.00 | |
| 60 | 39% | 0.22, 0.68 | 62% | 0.38, 1.00 |
Figure 1.
Survival Curves for low grade B non mantle cell lymphoma patients
1A: Overall event free and survival curves 1B: Event free survival by treatment in first response versus treatment after first relapse 1C: Event free survival by treatment in any complete response versus treatment with measurable disease
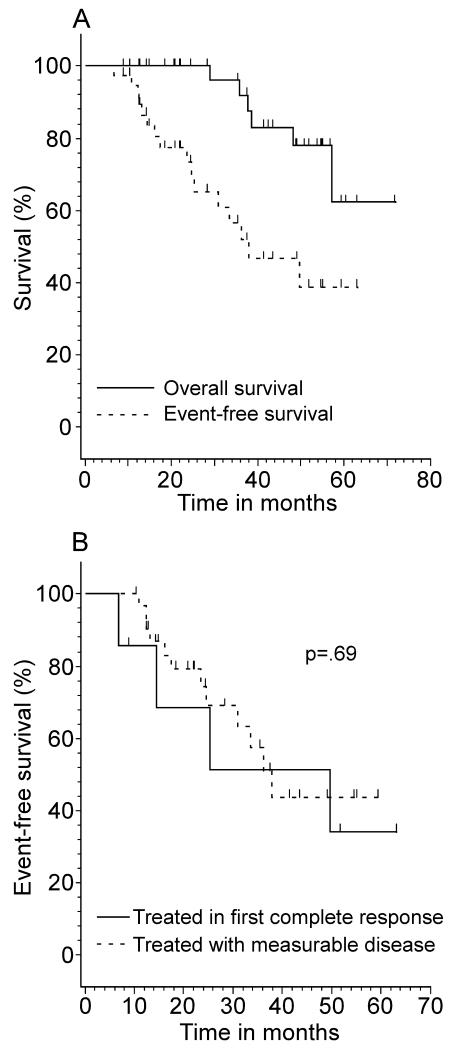
Of 39 patients with mantle cell histology, 16 patients relapsed resulting in 6 deaths from progressive lymphoma. The median follow-up for the 33 surviving patients with mantle cell histology was 3.5 years (range, 0.7 - 6 years). The respective EFS at 2, 3 and 5 years were 73%, 57%, and 39% and the respective overall survival at 2, 3 and 5 years were 100%, 92% and 62% (Table 3, Figure 2A). The median EFS for mantle cell patients treated in first complete response was 38 months and 50 months Of forthe with only a partial response before patients 16 protocol initiation, p=0.69 (Figure 2B). relapsed patients, 12 (75%) underwent allogeneic BMT of whom half remain in remission from 4.9-36.3 months.
Figure 2.
Survival Curves for Mantle Cell Lymphoma Patients
2A: Overall and event free survival for Mantle Cell Lymphoma Patients 2B: Event free survival by treatment in first complete response versus treatment with measurable disease
Discussion
We explored the feasibility of high-dose cyclophosphamide and rituximab in-vivo B-cell purging without stem cell rescue as an alternative to conventional autoSCT in patients with low grade B-cell lymphomas. After documenting universal hematopoietic recovery, this study was expanded from a safety trial to an exploratory efficacy trial in two cohorts: low-grade B cell lymphomas and mantle cell lymphoma.
A potential advantage of this approach is the ability to avoid reinfusion of lymphoma cells with the autologous stem cell graft. The concern for autograft graft contamination is not just theoretic: tumor cells can be found circulating in the blood of most patients with lymphoid malignancies as well as in autologous stem cell grafts.[11-13] Moreover, autograft contamination as a contributor to relapse may explain the lower relapse rates observed in syngeneic and purged autologous SCT as compared to unmanipulated autologous SCT.[2,3,14,15]
The therapy was well tolerated with no unexpected adverse events. All patients achieved rapid and full hematologic recovery, similar to that seen with autologous transplantation and the toxicities of stem cell mobilization were avoided.[16,17] Moreover, adverse events were mild: fewer than a third of patients developed febrile neutropenia, fewer than half required hospitalization, nearly a fifth did not require platelet transfusion support and no deaths occurred.
Despite the lack of a myeloablative preparative regimen, these results are similar to previous autoSCT reports for low grade lymphoma. Patients with low-grade, non-MCL histologies exhibited a 5 year EFS and OS of 40% and 72%, respectively. Comparatively, in 693 follicular lymphoma patients (43% with ex-vivo manipulation) Montoto et al, reported a 5 year PFS and OS of 44% and 64% after autoSCT.[5] Similarly, Bierman et al, reported a 5 year PFS and OS of 46% and 62%, in 160 patients who received a purged autoSCT and a 5 year PFS and OS of 37% and 50%, in 427 patients who received an unpurged autoSCT for low grade NHL.[2]
The 5 year EFS and OS was 39% and 62%, respectively, in our 39 mantle cell patients. While our current study was neither powered nor designed to be compared to other reports, contemporary approaches to MCL requires comment. No consensus exists on the optimal treatment paradigm for MCL. In fact, dichotomous recommendations from deferring initial therapy to high-dose induction therapy followed by autologous hematopoietic progenitor cell transplantation (HSCT) in first remission have been published in 2009.[18,19] However, a single institutional report generated at the MD Anderson Cancer Center supports early intensive treatment. In their a non-randomized experience, fifty patients who underwent an autologous HSCT in first remission had a median progression free survival of 42 months and overall survival of 93 months.[18] The Nordic Lymphoma group, reported in 2008, a 6-year EFS and OS of 56% and 70% with a non-relapse mortality rate of 5% in 160 consecutive, untreated MCL patients younger than 66 years treated with Maxi-RCHOP-21 ×3 alternating with rituximab high-dose cytarabine x3 followed by rituximab in-vivo purged autologous transplant.[20] Both of these trials suggest that a more aggressive pre-transplant treatment than CHOP, as used in our patients, is critical to the improved results with autoSCT.[18,20] While these results argue for immediate and aggressive intervention, Martin et al, reported on 31 patients with MCL who had a median pre-treatment observation time of 12 months (range: 4 to 128 months), concluding some patients can be observed and even suggested these patients may have an improved overall survival compared with those who receive early treatment.[19] As a whole, these results suggest patient selection and lead-time bias can greatly affect treatment result interpretation in mantle cell lymphoma.
Our data suggest high-dose cyclophosphamide with in-vivo rituximab purging may have a role as an alternative to autoSCT in multiple subtypes of low grade B-cell lymphoma. Compared to autoSCT, this treatment is simpler, less labor-intensive, and avoids the costs and risks of stem cell mobilization and processing. Furthermore, the treatment was well tolerated by older individuals: neither mortality nor unexpected or excessive toxicities were observed in the 17 patients who were 65 years or older, suggesting this approach may also be especially well suited for individuals not candidates for intensive cytoreduction followed by autoSCT. Moreover, this treatment approach does not preclude relapsed patients from salvage allogeneic transplant, as occurred in 19 patients of whom 10 remain disease-free. HDC with in-vivo rituximab purging is a well tolerated consolidative therapy that can provide a platform for the integration of novel molecularly targeted compounds.
Footnotes
Co-Authors:
Javier Bolaños-Meade: Sidney Kimmel Comprehensive Cancer Center
Carol Ann Huff: Sidney Kimmel Comprehensive Cancer Center
Marianna Zahurak: Johns Hopkins University: Division of Biostatistics
Ian Flinn: Tennessee Oncology
Ivan Borrello: Sidney Kimmel Comprehensive Cancer Center
Leo Luznik: Sidney Kimmel Comprehensive Cancer Center
Ephraim Fuchs: Sidney Kimmel Comprehensive Cancer Center
Yvette Kasamon: Sidney Kimmel Comprehensive Cancer Center
William Matsui: Sidney Kimmel Comprehensive Cancer Center
Jonathan Powell: Sidney Kimmel Comprehensive Cancer Center
Hyam Levitsky: Sidney Kimmel Comprehensive Cancer Center
Robert A. Brodsky: Johns Hopkins University: Division of Hematology
Richard Ambinder: Sidney Kimmel Comprehensive Cancer Center
Richard Jones: Sidney Kimmel Comprehensive Cancer Center
Lode J. Swinnen Sidney Kimmel Comprehensive Cancer Center
References
- 1.Oliansky DM, Gordon LI, King J, Laport G, Leonard JP, McLaughlin P, Soiffer RJ, van Besien KW, Werner M, Jones RB. The role of cytotoxic therapy with hematopoietic stem cell transplantation in the treatment of follicular lymphoma: An evidence-based review. Biol Blood Marrow Transplant. doi: 10.1016/j.bbmt.2010.01.008. others. [DOI] [PubMed] [Google Scholar]
- 2.Bierman PJ, Sweetenham JW, Loberiza FR, Jr., Taghipour G, Lazarus HM, Rizzo JD, Schmitz N, van Besien K, Vose JM, Horowitz M. Syngeneic hematopoietic stem-cell transplantation for non-Hodgkin’s lymphoma: a comparison with allogeneic and autologous transplantation--The Lymphoma Working Committee of the International Bone Marrow Transplant Registry and the European Group for Blood and Marrow Transplantation. J Clin Oncol. 2003;21:3744–53. doi: 10.1200/JCO.2003.08.054. others. [DOI] [PubMed] [Google Scholar]
- 3.van Besien K, Loberiza FR, Jr., Bajorunaite R, Armitage JO, Bashey A, Burns LJ, Freytes CO, Gibson J, Horowitz MM, Inwards DJ. Comparison of autologous and allogeneic hematopoietic stem cell transplantation for follicular lymphoma. Blood. 2003;102:3521–9. doi: 10.1182/blood-2003-04-1205. others. [DOI] [PubMed] [Google Scholar]
- 4.Schouten HC, Qian W, Kvaloy S, Porcellini A, Hagberg H, Johnson HE, Doorduijn JK, Sydes MR, Kvalheim G. High-dose therapy improves progression-free survival and survival in relapsed follicular non-Hodgkin’s lymphoma: results from the randomized European CUP trial. J Clin Oncol. 2003;21:3918–27. doi: 10.1200/JCO.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 5.Montoto S, Canals C, Rohatiner AZ, Taghipour G, Sureda A, Schmitz N, Gisselbrecht C, Fouillard L, Milpied N, Haioun C. Long-term follow-up of high-dose treatment with autologous haematopoietic progenitor cell support in 693 patients with follicular lymphoma: an EBMT registry study. Leukemia. 2007;21:2324–31. doi: 10.1038/sj.leu.2404850. others. [DOI] [PubMed] [Google Scholar]
- 6.Emadi A, Jones RJ, Brodsky RA. Cyclophosphamide and cancer: golden anniversary. Nat Rev Clin Oncol. 2009;6:638–47. doi: 10.1038/nrclinonc.2009.146. [DOI] [PubMed] [Google Scholar]
- 7.Kaplan E, Meier P. Nonparametric estimator from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 8.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–48. [PubMed] [Google Scholar]
- 9.Cox D. Regression models and life tables (with discussion) J Roy Statist Soc. 1972;34:187–220. [Google Scholar]
- 10.Inc. SI . SAS User’s Guide: Statistics. 5 ed Cary: 1985. [Google Scholar]
- 11.Jones RJ, Gocke CD, Kasamon YL, Miller CB, Perkins B, Barber JP, Vala MS, Gerber JM, Gellert LL, Siedner M. Circulating clonotypic B cells in classic Hodgkin lymphoma. Blood. 2009;113:5920–6. doi: 10.1182/blood-2008-11-189688. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsui W, Wang Q, Barber JP, Brennan S, Smith BD, Borrello I, McNiece I, Lin L, Ambinder RF, Peacock C. Clonogenic multiple myeloma progenitors, stem cell properties, and drug resistance. Cancer Res. 2008;68:190–7. doi: 10.1158/0008-5472.CAN-07-3096. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hardingham JE, Kotasek D, Sage RE, Dobrovic A, Gooley T, Dale BM. Molecular detection of residual lymphoma cells in peripheral blood stem cell harvests and following autologous transplantation. Bone Marrow Transplant. 1993;11:15–20. [PubMed] [Google Scholar]
- 14.Gribben JG, Freedman AS, Neuberg D, Roy DC, Blake KW, Woo SD, Grossbard ML, Rabinowe SN, Coral F, Freeman GJ. Immunologic purging of marrow assessed by PCR before autologous bone marrow transplantation for B-cell lymphoma. N Engl J Med. 1991;325:1525–33. doi: 10.1056/NEJM199111283252201. others. [DOI] [PubMed] [Google Scholar]
- 15.Kasamon YL, Jones RJ, Gocke CD, Blackford AL, Seifter EJ, Davis-Sproul JM, Gore SD, Ambinder RF. Extended Follow-Up of Autologous Bone Marrow Transplantation with 4-Hydroperoxycyclophosphamide (4-HC) Purging for Indolent or Transformed Non-Hodgkin’s Lymphomas. Biol Blood Marrow Transplant. doi: 10.1016/j.bbmt.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dreger P, Rieger M, Seyfarth B, Hensel M, Kneba M, Ho AD, Schmitz N, Pott C. Rituximab-augmented myeloablation for first-line autologous stem cell transplantation for mantle cell lymphoma: effects on molecular response and clinical outcome. Haematologica. 2007;92:42–9. doi: 10.3324/haematol.10608. [DOI] [PubMed] [Google Scholar]
- 17.Hoerr AL, Gao F, Hidalgo J, Tiwari D, Blum KA, Mathews V, Adkins DR, Blum W, Devine S, Vij R. Effects of pretransplantation treatment with rituximab on outcomes of autologous stem-cell transplantation for non-Hodgkin’s lymphoma. J Clin Oncol. 2004;22:4561–6. doi: 10.1200/JCO.2004.05.035. others. [DOI] [PubMed] [Google Scholar]
- 18.Tam CS, Bassett R, Ledesma C, Korbling M, Alousi A, Hosing C, Kebraei P, Harrell R, Rondon G, Giralt SA. Mature results of the MD Anderson Cancer Center risk-adapted transplantation strategy in mantle cell lymphoma. Blood. 2009 doi: 10.1182/blood-2008-10-184200. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin P, Chadburn A, Christos P, Weil K, Furman RR, Ruan J, Elstrom R, Niesvizky R, Ely S, Diliberto M. Outcome of deferred initial therapy in mantle-cell lymphoma. J Clin Oncol. 2009;27:1209–13. doi: 10.1200/JCO.2008.19.6121. others. [DOI] [PubMed] [Google Scholar]
- 20.Geisler CH, Kolstad A, Laurell A, Andersen NS, Pedersen LB, Jerkeman M, Eriksson M, Nordstrom M, Kimby E, Boesen AM. Long-term progression-free survival of mantle cell lymphoma after intensive front-line immunochemotherapy with in vivo-purged stem cell rescue: a nonrandomized phase 2 multicenter study by the Nordic Lymphoma Group. Blood. 2008;112:2687–93. doi: 10.1182/blood-2008-03-147025. others. [DOI] [PMC free article] [PubMed] [Google Scholar]