Abstract
Functional performance, a child’s ability to perform the tasks of daily living and to fulfill expected social roles, is now recommended in follow-up of preterm children. This study examined neonatal, preschool health, and motor effects on functional performance at age 4. The sample of 155 infants, classified by perinatal morbidity and birth weight, was assessed during a home visit. Neonatal illness, socioeconomic status, preschool health, and motor predictors explained 44% of the variance in functional performance. Functional performance is a useful clinical measure to understand how well preterm children perform age-expected daily activities as well as the family burden of preterm sequelae.
Technological advances have resulted in improved survival rates for pre-term infants; yet, this is moderated by a growing concern for a greater prevalence of neurodevelopmental morbidity on the daily lives of these children (Aylward, 2005; Hack & Fanaroff, 1999; Vohr & Msall, 1997). Functional assessment, primarily used with adults, has been recommended in preterm follow-up as a measure of the overall burden of preterm morbidity (Saigal et al., 1994; Vohr & Msall, 1997). However, functional assessment of neonatal intensive care unit (NICU) survivors has received little empirical study (Hack, Taylor, Klein, & Minich, 2000; Msall, Tremont, & Ottenbacher, 2001). The concept of functional performance refers to the child’s ability to perform the tasks of daily living and to fulfill expected social roles (McCabe & Granger, 1990; Vohr & Msall, 1997). In children, these tasks include feeding, dressing, bathing, toileting, moving inside and outdoors, communicating, playing, remembering routines, and interacting with others (Vohr & Msall, 1997). Social roles include peer group activities, school attendance and achievement, and community participation. In preterm children, functional performance represents the junction of a child’s abilities and limitations and, thus, can provide critical data about the pathways to their assets and challenges and variation in outcomes (Msall & Tremont, 2000; Msall et al., 2001). Common sequelae of prematurity, motor delays, and subnormal health may affect functional performance. For example, a child who is clumsy or uncoordinated in gross and/or fine motor movement may not be able to button, zip, toilet independently, or play outdoors in developmentally appropriate sports activities. Thus, we can anticipate an additive effect of health status and motor abilities on functional performance.
PURPOSE
In this study, we examine functional performance as an outcome and explore its relationships with health and motor outcomes for preschool preterm children born with varying birth weights and perinatal morbidities. We test the effect of perinatal morbidity (birth weight and neonatal illness) and preschool (age 4) health and motor development on functional performance. We hypothesize that preterm children with medical and/or neurological neonatal illness will have poorer functional performance and less optimal health and motor development skills compared with full-term children at age 4. Also, we hypothesize a longitudinal effect of perinatal morbidity with concurrent preschool variables on functional performance. Applying a bioecological framework, socioeconomic status (SES) is a marker variable for family resources, parental education, and occupation, representing the child’s environmental context.
LITERATURE REVIEW
Low birth weight has adverse consequences for health and functional outcomes, and evidence shows that the percentage of children with functional limitations may be increasing (Hack et al., 2000; Vohr & Msall, 1997). Birth weight is the common predictor in preterm follow-up studies where decreasing birth weight is associated with more developmental sequelae, such that the smallest babies are likely to have later problems (Aylward, 2002a). However, not all extremely low birth weight (ELBW; <1,000 g) children have developmental impairments, whereas some heavier birth weight (≥1,500 and <2,500 g) children can have substantial motor and multiple impairments. This suggests that other perinatal factors in addition to birth weight have a role to play in subsequent developmental outcomes.
The brains of infants at younger gestational ages are particularly vulnerable to hypotensive and hypoxic perinatal injury with the pattern of small lesion injury involving fibers to the limbs and motor pathways. Brain structure abnormalities, decreased white matter, and increased ventricular size in preterm children have been identified in imaging studies (Peterson et al., 2000). Perinatal infection and inflammatory cytokines associated with infection may exacerbate brain injury and higher rates of neurodevelopmental impairment (Dammann & Leviton, 2001; O’Shea, 2002). Volpe (2001) contends that nondisabling degrees of periventricular leukomalacia interfere with cell migration to the cortex, disrupting cortical plate neurons, leading to abnormal patterns of development of cortical structures. This suggests that the neurodevelopmental delay reported in preterm follow-up studies has a neurophysiological basis. What is not clear is how perinatal morbidity, which affects health and motor outcomes, impacts children’s functional performance at preschool age.
Taylor, Klein, Schatschneider, and Hack (1998) found that the rate of substandard adaptive function for children at age 6 1/2 with more than three neonatal illnesses is twice that for children with less than three neonatal morbidities. Msall et al. (2004) reported that preservation of favorable visual status was associated with higher functional status at age 5 1/2 and that both were significant predictors of special education placement and community participation at age 8. In the Midwest Newborn Lung Project, Palta, Sadek-Badawi, Evans, Weinstein, and McGuiness (2000) followed up 425 very low birth weight (<1,500 g) survivors to age 5. They demonstrated that Pediatric Evaluation of Disability Inventory functional performance scores were lowest in those with the lowest birth weight (600–799 g) and in those with neurosonographic abnormalities such as intraventricular hemorrhage (IVH 3–4). Among children without cerebral palsy (CP), 5.2% had self-care functional disability and 7.6% had social disability. Among those with CP, 57% had self-care disability and 32% had social disability. Overall, 79% of children without CP were within 2 SD of peers compared with 11.3% of children with CP. Additionally, both IVH 3–4 and bronchopulmonary dysplasia (BPD) predicted CP.
Motor function impairments remain as one of the predominant preterm sequelae despite NICU advances and reductions in IVH (Botting, Powls, Cooke, & Marlow, 1997; Hack & Fanaroff, 1999). In empirical studies, preterm motor problems do not appear to improve with age (Marlow, Roberts, & Cooke, 1993; Powls, Botting, Cooke, & Marlow, 1995). Of particular concern is the potential prevalence of visual–spatial and eye–hand coordination delays (Schraeder, Heverly, O’Brien, & McEvoy-Shields, 1992). Visual–spatial coordination and eye–hand coordination are important to developmentally appropriate preschool-age tasks such as dressing and fastening clothes. Thus, functional performance may be a marker of problems with an integrative function and direct the “scaffolding” needed from parents, teachers, and coaches. Just as a scaffold is a supporting framework for a building, parenting behaviors, community resources, and school services can support a child’s development and functional performance.
THEORETICAL FRAMEWORK
The theoretical model for the study is derived from the bioecological model of development (Bronfenbrenner & Morris, 1998). In this model, the biopsychological organism represents the newborn infant who may have experienced prematurity and perinatal morbidity (medical illness, neurological illness, or both). The experiences of the preterm infant during the neonatal period are influenced by the processes that occur in the immediate environment called proximal processes. In turn, what happens in the environment is influenced by the infant characteristics and experiences. The environment is not simply viewed as a stimulus but forms a dynamic interaction with the child that operates over time. Proximal processes are the primary mechanisms that produce development. Thus, functional performance, health, and motor development at age 4 are influenced by earlier infancy processes (prematurity, birth weight, perinatal morbidity, and neonatal illness severity) within proximal processes and environmental context.
METHOD
Sample
The sample of 155 children, born between April 1996 and March 1999, was enrolled into one of four a priori perinatal groups according to neonatal illness and birth weight: 41 preterm infants with birth weight <1,000 g and medical illness (BPD, respiratory distress syndrome, necrotizing enterocolitis, sepsis; medical preterm group 1 [MPT1]); 39 preterm infants with birth weight ≥1,000 g and medical illness (medical preterm group 2 [MPT2]); 32 preterm infants with severe neurological illness (meningitis, hydrocephalus, IVH 3 or IVH 4; neurological preterm group [NPT]). Forty-three healthy full-term infants (FT), whose mothers did not experience complications in pregnancy, labor, and delivery, were also recruited. All infants were born at the same hospital where the preterm infants were treated in the Level III NICU.
Sample recruitment occurred in three steps. First, all possible subjects were identified from the hospital’s medical records database using the sampling inclusion criteria for birth dates, birth weight, gestational age, diagnosis of neonatal illness, and maternal health status. To stratify SES, we used health insurance coverage as a proxy. From this list, approximately 250 medical charts were randomly selected using computer-generated random numbers. Next, the maternal and infant hospital medical records were reviewed by two qualified nurse practitioners or by the Principal Investigator (PI) to assure that sample criteria were met. If questions arose during the review, expert opinion was sought from a second NICU nurse practitioner or one of two consulting pediatricians. Third, families were then invited to participate by mail followed by a personal telephone call. Throughout the study recruitment, families were very willing to participate, with only 9 of 164 potential subjects not seen because they refused to participate (n = 2), we were unable to track them, or they moved from the region (n = 5). Two families of FTs refused participation due to the child’s recent diagnoses (one FT with celiac disease; one FT with multiple developmental delays). An additional 15% were recruited to account for attrition within the groups, for a total sample of 155. We computed power using the tables provided by Cohen (1988) using an α level of .01 for main effects and interactions and .05 for simple main effects. We tested the power to detect effects that explain 10% of the variance of the dependent variable. Univariate analyses of variance (ANOVAs) had greater than 90% power to detect significant main effects and interactions.
For the preschool assessment, children and parents were visited at home at age 4 (M = 49.1 months; SD = 1.3; range = 46–54 months) with no correction for prematurity. Once the children were enrolled in the study and informed consent was obtained, the demographic variables estimated from the medical chart review were verified. SES measured both maternal and paternal education level and occupation (Hollingshead, 1975). There were no significant perinatal group differences in SES, χ2(1, 12) = 16.28; p = .17. Neonatal illness severity was measured using the Score for Neonatal Acute Physiology (SNAP II; Richardson, 1999). The correlation between the SNAP II and birth weight was −.67 (p = .0001). Tables 1 and 2 show the significant differences across perinatal groups for the neonatal variables.
Table 1.
Neonatal Characteristics by Perinatal Groups (N = 155)
| FT (n = 43) | MPT1 (n = 41) | MPT2 (n = 39) | NPT (n = 32) | Duncan Post Hoc Test | |
|---|---|---|---|---|---|
| Birth weight* | |||||
| M (SD) | 3,664.0 (354) | 811.7 (140) | 1,294.9 (260) | 1,021.0 (425) | FT > MPT2 > NPT > MPT1 |
| Range | 3,060–4,690 | 503–995 | 1,000–2,330 | 565–2,470 | |
| Gestational age* | |||||
| M (SD) | 39.9 (0.45) | 26.6 (2.07) | 29.1 (1.8) | 26.8 (3.0) | FT > MPT2 > NPT, MPT1 |
| Range | 39–41 | 23–33 | 27–36 | 23–36 | |
| Apgar score (5 minutes)* | |||||
| M (SD) | 8.5 (0.71) | 6.6 (1.5) | 7.3 (2) | 5.7 (2.5) | FT > MPT1, MPT2 > NPT |
| Range | 6–9 | 4–9 | 1–9 | 1–9 | |
| Total days hospitalized* | |||||
| M (SD) | 2.8 (1.1) | 78.6 (21.8) | 46.6 (15.8) | 78.4 (30.3) | NPT, MPT1 > MPT2 > FT |
| Range | 1–5 | 30–130 | 9–68 | 9–125 | |
| Neonatal illness severity*† | |||||
| M (SD) | 1.9 (5.3) | 41.6 (18.1) | 17.4 (12.9) | 44.2 (27.0) | NPT, MPT1 > MPT2 > FT |
| Range | 0–18 | 5–83 | 0–66 | 0–108 | |
| n/Total (%) | n/Total (%) | n/Total (%) | n/Total (%) | χ2 | |
|
| |||||
| Male gender | 25/43 (58) | 19/41 (46) | 23/39 (56) | 20/32 (62) | ns |
Table 2.
Frequency of Neonatal Illness for Preterm Groups (N = 112)
| MPT1 (n = 41) | MPT2 (n = 39) | NPT (n = 32) | p | |
|---|---|---|---|---|
| <1,000 g | 41 (100) | 0 (0) | 18 (56.3) | .0001 |
| ROP | 19 (46.3) | 9 (23) | 20 (62.5) | .01 |
| BPD | 24 (58.5) | 6 (15.4) | 23 (71.9) | .0001 |
| RDS | 41 (100) | 36 (92.3) | 31 (97) | .0001 |
| Pneumonia | 4 (9.8) | 0 (0) | 2 (6.3) | ns |
| Sepsis | 8 (19.5) | 4 (10.3) | 12 (37.5) | .05 |
| PDA | 8 (19.5) | 2 (5.1) | 10 (31.3) | .01 |
| NEC | 3 (7.3) | 3 (7.7) | 7 (21.9) | ns |
| IVH 1 and 2 | 16 (39) | 23 (59) | 8 (25) | .0001 |
| IVH 3 and 4 | 0 (0) | 0 (0) | 21 (65.6) | |
| Hydrocephalus with shunt | 0 (0) | 0 (0) | 8 (25) | .0001 |
| Seizures | 0 (0) | 0 (0) | 6 (18.8) | .0001 |
| Meningitis | 0 (0) | 0 (0) | 2 (6.3) | ns |
| PVL | 0 (0) | 0 (0) | 7 (22) | .0001 |
Note: Data are expressed as n (%). ROP = retinopathy of prematurity, RDS = respiratory distress syndrome, PDA = patent ductus arteriosis, NEC = necrotizing enterocolitis, IVH = intraventricular hemorrhage grades using Papile et al. (1978), PVL = periventricular leukomalacia, ns = not significant.
Measures
Functional Performance
The Functional Independence Measure for Children (WeeFIM) comprehensively assesses functional performance in children and adolescents with congenital, developmental, or acquired disabilities across settings (Msall, DiGaudio, Duffy, et al., 1994; Msall, DiGaudio, & Rogers, et al., 1994). It is a discipline-free measure of consistent performance of functional skills that allows pediatric professionals to describe consistent basic performance in daily routines.
The WeeFIM is an 18-item, 7-point ordinal scale with the following domains: self-care, sphincter control, transfer, mobility–locomotion, communication, and social cognition for children 6 months through 8 years of age or 16 years of age with disabilities. The test yields six domain scores and a total score. The mobility–locomotion quotient assesses motor performance in relation to predetermined criterion. In each domain, Levels 1 and 2 indicate complete dependency, Levels 3 to 5 indicate assistance required, and Levels 6 and 7 complete independence. Any reliable informant who has observed daily living task items can provide responses in a 15- to 20-minute interview. Parents were interviewed for this study.
The normative sample included 417 children in primary care or day care, a sample of 705 children with disabilities, and a convenience sample of 116 children in health and preschool settings. The test–retest score was .99 and interrater reliability was .95. Interrater agreement across four conditions showed κ values of .44–.82 and intraclass correlation coefficients of .73–.98 (Ottenbacher et al., 1997). Concurrent validity was established with the use of the Vineland Adaptive Behavior Scale, the Battelle Developmental Inventory, and the Pediatric Evaluation of Disability Inventory (Ottenbacher et al., 1999). Previous studies with ELBW survivors showed that the total WeeFIM score at kindergarten entry correlates with use of special education resources and parents’ rating of health status.
Health Status
Preschool age health status data were gathered from parental interview and review of pediatric records including hospitalizations, surgeries, emergency room visits, incidence of chronic disease, acute illnesses by systems, conditions of disability, and impairment. The health status classification was based on Prechtel and Beitema (1967) and the Collaborative Perinatal Study (Niswander & Gordon, 1972) used in the preterm follow-up clinic at Women & Infants Hospital. Health status was classified as normal (no physical or neurological abnormalities), suspect (referral for decreased hearing; orthopedic problems; difficulties with vision; deviations of tone, posture, movement patterns, reflexes, cranial nerves, head growth, chronic respiratory distress, cardiac conditions, questionable allergies, myringotomy tubes), or abnormal (asthma, diabetes, subnormal growth, CP, shunted hydrocephalus, blindness, deafness, uncontrolled seizures).
Motor
The motor assessment included general motor (fine motor and gross motor) and visual motor integration. The motor scale of the McCarthy Scales of Children’s Abilities (MSCA; McCarthy, 1972) assesses coordination in a variety of gross and fine motor tasks for children ages 2 1/2 to 8. The MSCA has been standardized on a national sample stratified by age, gender, race, geographic region, and father’s occupation. For the MSCA motor scale, the average reliability coefficients were .79 (SEM = 4.7) and .78 (SEM = 4.6) for age 4. Stability coefficients ranged from .75 to .78. Correlation with other MSCA scales for age 4 ranged from .41 to .66. There has been extensive use of the MSCA in preterm samples.
The MSCA has gross motor items (arm coordination [6 items], leg coordination [6 items], and imitative action [4 items]) and fine motor items (draw-a-design [9 items] and draw-a-child [10 items]). The motor scale, with a mean of 50 (SD = 10), takes 15–20 minutes to administer and yields a raw score, standard score, and age-equivalent scores. Several items on the MSCA are used to assess laterality, providing a way to evaluate a child in his or her neurological development of cortical hemispheric dominance. In the standardization sample, 70% of the children had established hand dominance by age 3. Test–retest reliability for the laterality assessment was .71.
Visual motor integration was measured with the Beery Developmental Test of Visual–Motor Integration (VMI), which assesses the degree to which visual perception and motor behavior are integrated for ages 3 to adult (Beery, 1997). The VMI and supplemental tests were normed on 2,614 children from 3 to 18 years of age, from five major sections of the United States. The Rasch–Wright results indicate high content reliability for the VMI, as its total group item separation was 1.0 and its total group person separation was .96. Concurrent validity correlated with the Developmental Test of Vision Perception and Drawing subtest of the Wide Range Assessment of Visual Motor Abilities. The VMI test is a developmental sequence of geometric forms to be copied using paper and pencil. It is considered virtually culture free because of its use of geometric shapes instead of varying numbers and letters. The test is administered in 15 to 25 minutes. The VMI yields standard scores, percentile ranks, stanines, and age-equivalent scores.
Procedure
We contacted families by mail followed by a telephone call when the child participant was close to our target window of age 4 years ± 2 months. An appointment was made for two research personnel (PI and research nurse or assistant) to make a home visit. During the visit, informed consent was obtained, followed by administration of the motor tests and parent interviews for functional performance and health. Demographic data were completed by the parent, usually the mother, and used to calculate the Hollingshead Four-Factor Index score for SES. Parents gave us permission to request their child’s pediatric record to verify health interview data. The parent and child were reimbursed US$25. Training on the protocols had been previously conducted and continued until an interrater reliability of 90% agreement was achieved. Rater agreement was checked every 2 months and maintained at 90% agreement or above throughout the study.
Data Analysis
Pearson correlation coefficients were examined to assess the relationships among the following pre-school variables: functional performance, health, and motor. Next, partial correlation, controlling for birth weight and neonatal illness acuity, was examined to assess the relationship among the preschool outcomes with neonatal variables controlled. Cohen conventions were used to determine effect sizes (Cohen, 1988). ANOVA with Duncan post hoc tests tested the first hypothesis, the effect of perinatal morbidity group on preschool outcomes of functional performance, health, general motor, and visual motor integration. For the second hypothesis, the hierarchical regression model was guided by the theoretical model to examine child-environment effects on functional performance at age 4. Birth weight and neonatal illness severity were entered on Step 1, and environment effects represented by SES were entered on Step 2. On Step 3, preschool health status and motor (general motor and visual motor integration) scores were entered to examine their unique contribution beyond neonatal and environmental effects.
RESULTS
Correlations across preschool measures of functional performance and motor scores were moderate in effect (total functional performance and motor: r = .58, p = .0001; total functional performance and visual motor integration: r = .57, p = .0001; general motor and visual motor integration: r = .55; p = .0001). Correlations of functional performance and motor scores with health status were negative, small in effect, but significant (total functional performance: r = −.21, p = .01; general motor: r = −.20, p = .01; and visual motor integration: r = −.16, p = .04). When birth weight and neonatal illness severity (SNAP II) were controlled, the partial correlation coefficients for functional performance and general motor remained significant (r = .49–.52). However, the correlation between functional performance and preschool health status was not significant (r = −.09 to −.13).
The ANOVA models of perinatal group effects for functional performance outcomes at age 4 were significant, total score: F(3, 154) = 13.5, p = .0001; self-care: F(3, 154) = 15.36, p = .0001; mobility: F(3, 154) = 8.54, p = .0001; cognition: F(3, 154) = 7.1, p = .0001, as shown in Table 3. Significant differences were also found for each of the eight self-care scale items (eating, grooming, bathing, dressing upper, dressing lower, toileting, bladder control, bowel control), the five mobility scale items (level of independence for the following: on/off chair, on/off toilet, tub/shower, walking, stairs), and four of the five cognition scale items (comprehension, social interaction, problem solving, memory). Only the expression item in the cognition scale was not significant (p = .08).
Table 3.
ANOVA for Preschool Functional Performance, Health, and Motor Outcomes
| FT (n = 43) | MPT1 (n = 41) | MPT2 (n = 39) | NPT (n = 32) | Duncan Post Hoc Test | |
|---|---|---|---|---|---|
| Functional performance | |||||
| Self-care* | |||||
| M (SD) | 44.9 (6.1) | 40.3 (8.5) | 39.8 (7.8) | 30.9 (12.9) | FT > MPT1, MPT2 > NPT |
| Range | 31–56 | 8–56 | 18–53 | 8–52 | |
| Mobility* | |||||
| M (SD) | 34.0 (1.1) | 32.9 (3.5) | 32.5 (3.6) | 29.0 (7.7) | FT, MPT1, MPT2 > NPT |
| Range | 31–35 | 20–35 | 17.35 | 5–35 | |
| Cognition* | |||||
| M (SD) | 27.8 (5.6) | 25.8 (6.5) | 24.6 (6.2) | 21.0 (7.5) | FT > MPT2 > NPT; FT, MPT1 > NPT |
| Range | 15–35 | 8–35 | 11–35 | 5–34 | |
| Total WeeFIM* | |||||
| M (SD) | 107.2 (11.5) | 99.8 (16.3) | 97.2 (15.1) | 81.5 (26.1) | FT > MPT2 > NPT; FT, MPT1 > NPT |
| Range | 82–128 | 36–124 | 54–122 | 18–117 | |
| Motor scores | |||||
| MSCA* | |||||
| M (SD) | 53.7 (9.1) | 44.6 (11.3) | 48.6 (12.4) | 35.6 (11.8) | FT > MPT1, MPT2 > NPT |
| Range | 38–79 | 21–66 | 21–75 | 21–60 | |
| VMI* | |||||
| M (SD) | 102.7 (12.4) | 92.2 (13.4) | 98.7 (13.5) | 80.0 (25.2) | FT, MPT2 > MPT1, NPT; MPT1 > NPT |
| Range | 84–144 | 61–122 | 61–135 | −1 to 107 | |
| Health, n (%) | |||||
| Normal | 39 (90.7) | 30 (73.2) | 32 (84.6) | 18 (56.3) | χ2 = 14.2; p = .02 |
| Suspect | 1 (2.3) | 5 (12.2) | 2 (5.1) | 5 (15.6) | |
| Abnormal | 3 (7) | 6 (14.6) | 4 (10.3) | 9 (28.1) | |
p = .0001.
Using the WeeFIM standardized, age-specific norms (WeeFIM System Clinical Guide: Version 5, 1998), the MPT1 and MPT2 group scores, although within 1 SD of the standardized norms, were lower than the FT on all four scales. The NPT group scores were significantly lower than the scores of the other groups. Their scores were 2 SD below the standardized norm on the self-care, mobility, and the total score scales and 1 SD below the standardized norm on the cognition scale. Although the larger birth weight MPT2 group scores were lower than the smaller birth weight MPT1 group on all WeeFIM scores, these were not statistically significant.
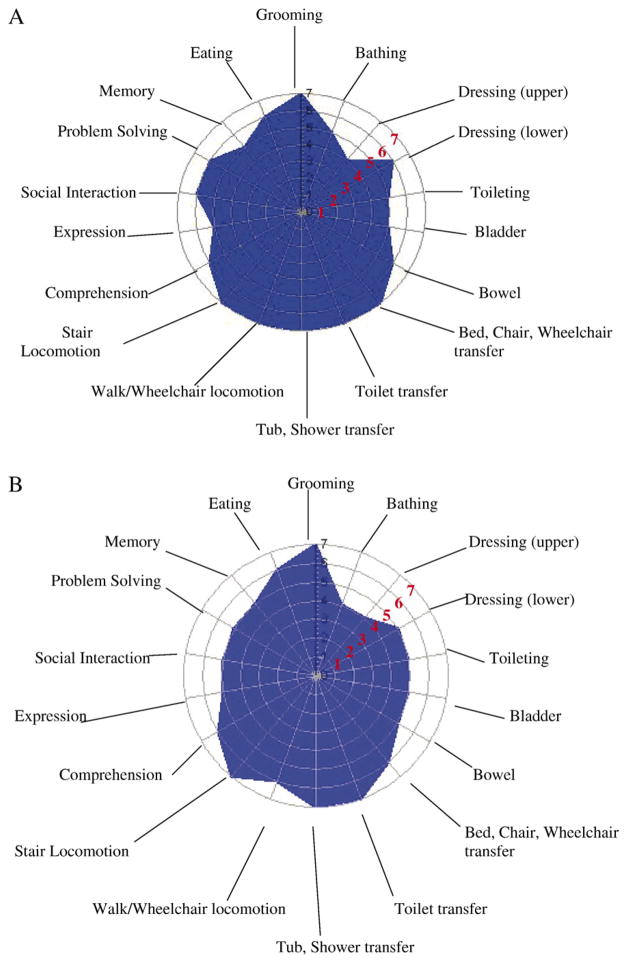
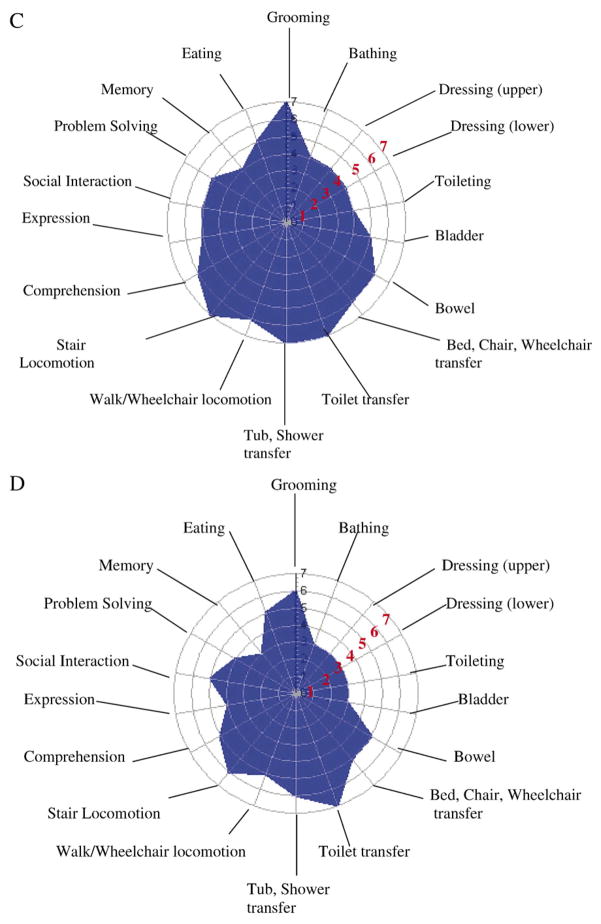
The polar graphs in Figure 1A–D illustrate the mean scores for each WeeFIM item by perinatal morbidity group. The larger the blue space is, the greater the functional performance level for the perinatal group. The WeeFIM items are shown on the circle perimeter; each radius/concentric circle is marked with the WeeFIM score of 0–7. A 0 score (child is unable to do this functional task) is at the center of the graph, whereas a score of 7 (child independently completes task) is marked at the outermost circle. Values of 3.5–4.2 are the average score for 4-year-old children. Figure 1A shows the mean item scores for the FT group with scores of 7 on the mobility items (bed, chair transfer, toilet transfer, tub/shower transfer, walk locomotion, stair locomotion). Lower scores, although age appropriate, are seen for the cognition scale items of expression and memory and for the self-care items of bathing and dressing upper body. In contrast, Figure 1D shows the smaller blue space for the NPT group for the WeeFIM items. High mean function scores are shown for toilet transfer (M = 6.9), stair locomotion (M = 6.7), and tub/shower transfer (M = 5.9). The lowest mean scores are seen in the self-care items of bathing (M = 2.8) and dressing (upper: M = 3.2; lower M = 2.8) and in the cognition items of memory (M = 4.9) and problem solving (M = 3.0). For the MPT groups, the blue spaces in Figure 1B and C are smaller compared with the FT group, with high mean scores for the mobility items and lower scores for the self-care items. In contrast to the MPT1 group, the MPT2 group had lower scores on toileting (M = 4.7), memory (M = 5.4), and eating (M = 6.2), as shown in Figure 1C.
Figure 1.
(A) Mean WeeFim item scores for the FT group. (B) Mean WeeFim item scores for MPT1. (C) Mean WeeFim item scores for MPT2. (D) Mean WeeFim item scores for the NPT group. Item scoring: Levels 1 and 2 indicate complete dependency; Levels 3–5 indicate assistance required; Levels 6 and 7 indicate complete independence.
Perinatal morbidity had a significant effect on preschool health, χ2(6) = 14.2, p = .02, as shown at the bottom of Table 3. Ninety percent of the FT preschool children had normal health status, compared with 56% of children in the NPT group who, as preterm infants, had neurological morbidity. Of the children who had medical morbidities as infants, 15% of the heavier birth weight MPT2 group and almost 27% of the <1,000-g MPT1 group had suspect or abnormal health status at age 4.
Perinatal morbidity had a significant effect on preschool motor outcomes, general motor: F(3, 154) = 16.9, p = .0001; visual motor integration: F(3, 154) = 12.0, p = .0001, as shown in Table 3. The FT group mean scores were in the normative range for general motor and visual motor integration. For both motor measures, the FT group had the highest score and the NPT group had the lowest score (1.5 SD below the mean). Although the mean scores for the MPT1 group were lower than those for the MPT2 group in general motor, the difference was not significant. In contrast, the MPT1 group had significantly lower visual motor integration scores than the MPT2 group. Thus, support was found for the first hypothesis.
Before testing the second hypothesis, Pearson correlation coefficients were examined. Correlations among the WeeFIM subscales were large (r = .61–.74); thus, the WeeFIM total score was used for the regression analysis. The hierarchical regression model was significant, F(6, 154) = 19.4, p = .0001, and is shown in Table 4. Birth weight and neonatal illness severity scores were entered on Step 1, F(2, 154) = 9.8, p = .0001, and explained 11.5% of the variance (R2 adjusted = .103). The addition of SES at Step 2 was significant, F change (1, 151) = 5.28, p = .02. The entry of preschool health status and motor scores on Step 3 was significant, F change (3, 149) = 28.9, p = .0001, explaining 44% of the total variance (R2 adjusted = .422). In the full model, general motor (t = 4.38, p = .0001) and visual motor integration (t = 4.59, p = .0001) were significant standardized beta weights. The analysis supported the theoretical framework that neonatal events, the child’s environment, and current health and motor skill contribute to functional performance outcomes at age 4.
Table 4.
Hierarchical Regression Model for Preschool Functional Performance
| Step | R | R2 | R2 (adjusted) | R2 change | F change | F |
|---|---|---|---|---|---|---|
| 1. Birth weight, neonatal illness severity | .339 | .115 | .103 | 9.86 | 9.86 | 9.8* |
| 2. SES | .381 | .145 | .128 | 5.28 | 5.28 | 8.5* |
| 3. Preschool health status, general motor, visual motor integration | .664 | .441 | .418 | 26.13 | 26.13 | 19.4* |
Note: Model: functional performance = X + birth weight (.018) + neonatal illness severity (−.065) + SES (.088) + preschool health (−.055) + general motor (.342) + visual motor (.35) + error.
p = .0001.
To examine the relative effect of each neonatal variable on functional performance, we tested two separate stepwise regression models. The first model entered birth weight followed by the neonatal illness severity (SNAP II) scores; then, the order was reversed for the second model. Both models were significant, F(2, 154) = 9.8, p = .0001. The two variables were significant when birth weight was entered first (birth weight: R2 change = .084, p = .0001; SNAP II R2 change = .03, p = .02). However, when the order was reversed, birth weight was not significant (SNAP II: R2 change = .106, p = .0001; birth weight: R2 change = .009, p = .213). Thus, neonatal illness severity added significantly more explained variance than the effect of birth weight alone in estimating preschool functional performance.
DISCUSSION
We found moderate correlations between functional performance, health status, and motor scores. These interrelationships suggest that lower functional performance is associated with poorer motor performance and less optimal (suspect or abnormal) health status. Developmentally, a 4-year-old is expected to perform most self-care tasks with minimal assistance, such as self-toileting, dressing both upper and lower body, and hand washing. The child should also have acquired salient preschool skills in the cognitive domain including communication (understanding and use), participation in play with peers and social situations, and memory. These skills are often expected of a 4-year-old for age-appropriate preschool placement. These results add to the growing evidence that the health of children born prematurely impacts their daily tasks of living and participation in age-appropriate activities and suggests an ongoing burden of care on the families of these children.
The effect of perinatal morbidity was demonstrated in significant differences between the four perinatal groups for the 18 WeeFIM items. The multiple ANOVAs may increase the Type I error and, thus, must be viewed as exploratory. The polar graphs of Figure 1 visually illustrate these group differences. The expression item, not different across groups, asks about communicating basic needs and ideas verbally, expressions of intent in gestures and manual signs, or use of a communication device. These data illustrate that the preterm children with various morbidities were capable of understandable communication for basic needs and ideas.
The FT group had the statistically best scores on three of the four functional performance scales, and the NPT group had the lowest on all four scales. The MPT1 and MPT2 groups had equivalent functional performance scores on two of the four scales. The MPT1 group, those with perinatal medical morbidities and birth weights below 1,000 g, had lower functional performance scores, although these were not statistically different from the FT group on the cognition and WeeFIM total score.
In this study, birth weight and perinatal morbidity continue to exert an effect 4 years later on functional performance, health status, and motor outcomes. The four-perinatal-group study design addresses a consistent limitation of follow-up studies, which is a lack of appreciation for the heterogeneity of prematurity. An additional comparison group of preterm infants without perinatal morbidity would have helped to determine whether the lower functional performance for the preterm groups was due to birth weight alone without perinatal morbidity. Understanding the pathways of perinatal morbidity and outcome must incorporate birth weight, as the lowest birth weight infants are often at greatest risk for poor outcomes (Aylward, 2002b). The incidence of disability increases with decreasing birth weight for preterm infants with 9–57% disability for children born <800 g in the 1990s (Hack & Fanaroff, 1999). The findings from this study suggest that variations in birth weight and perinatal morbidity may affect functional performance, health, and motor outcomes differently.
The regression model clearly demonstrates the cumulative effect of neonatal illness severity, birth weight, and preschool health status and motor development on functional performance. The results illustrate how a child seen in a pediatric clinic with health and/or motor problems may also present with difficulties in self-care tasks or social interaction. The importance of functional performance assessment in follow-up is in how prematurity, low birth weight, and perinatal morbidity affect function in daily activities as the preterm child develops and how the interconnection of health status and motor problems informs the understanding of outcomes. When the conceptual definition of functional performance is applied to children, it is defined as an individual child’s ability to perform the tasks of daily living and fill expected social roles both physically and emotionally (Vohr & Msall, 1997).
We used the Hollingshead Four-Factor Index as the SES measure. In ANOVA, there were no significant differences between SES groups in self-care, F(4, 154) = 1.6, p = .16, mobility, F(1, 154) = 1.3, p = .25, and the total functional performance scores, F(4, 154) = 1.9, p = .10. However, for the cognition subscale, Group 4 (low SES) had lower cognitive scores than the groups with the highest SES (Groups 1 and 2), F(1, 154) = 3.9, p = .005. This subscale is composed of items in social communication that are components of traditional assessments of cognitive development. Thus, our results show that with the exception of cognition for age-appropriate function, functional performance does not differ by SES status.
In his theoretical writing, Bronfenbrenner has suggested that proximal processes (enduring forms of interaction in the immediate environment usually between child and parent, such as child care activities, reading, play, etc.) are more powerful effects than the environmental contexts in which they occur (Bronfenbrenner & Ceci, 1994; Bronfenbrenner & Morris, 1998). Proximal processes alone do not tell the whole story; their developmental effectiveness is a function of the environmental characteristics in which they occur. Parents and families are influenced by environmental contexts that influence the proximal processes. We specifically chose our SES measurement because it accounts for both mother and father education and occupation status in a weighted system and is widely used in child development research. Although distinct proximal process measures were beyond the scope of this study, the SES measure was robust and included key variables of education and occupation, both found to influence child development (Bradley et al., 1994; Magyary, Brandt, Hammond, & Barnard, 1992).
The mechanisms by which a child learns functional performance tasks are suggested in the bidirectional child-environment nature of Bronfenbrenner’s model. That is, the parents recognize innate functional abilities in their child and reinforce more complex functions over time. Because we report lower levels of functional performance for preterm children compared with full-term children, it may be that parents perceive their child’s readiness for learning functional tasks differently than parents of full-term children. With no functional performance differences due to SES, our regression models support neonatal, motor, and health status to be important considerations. The model shows that SES alone contributes 14% (12% [adjusted]) of the variance. However, we acknowledge that environmental effects can be more sensitively conceptualized and measured. Precise measurement of proximal processes and environmental variables would enable researchers to test child-context models of development such as the Bronfenbrenner model and provide further understanding of these processes.
The functional performance demands are many and complex for a preschool child who may not be able to gain placement in nursery school with same-aged peers due to delay in mastery of functional performance tasks. When functional performance is used as a criterion, we are able to ask questions such as how the preterm child uses motor skills to negotiate his or her environment within the developmental demands of preschool. For example, a child with asthma may miss school, resulting in poor academic grades. Asthma may also affect motor performance participation in active preschool play and social functioning such as peer group activities. Thus, there is an interrelationship between child health and our outcomes. Drotar (2004) calls for specification of the relationships among pediatric outcome domains and factors that might influence them. It is important to note that the relationships among our outcome variables at age 4 are reciprocal and may indicate different profiles for different children and conditions. For example, a child with fine motor difficulty may have normal health status but needs greater assistance in self-care functional tasks although he or she is age appropriate in functional communication and social behaviors, whereas the limited functional performance of another child may be attributed to activity limitations due to illness symptoms which, in turn, affect his or her confidence in social activities with peers.
Findings from this research can be related to clinical outpatient and inpatient nursing care by using a functional perspective, that is, assessing the degree of assets and challenges for each child. Clinically, instead of asking “Can your child dress himself?” we could ask more specific functional questions such as “Can your child dress without your help?” In the United States, as many as 1 in 3 children live at a psychosocial disadvantage due to poverty, having parent(s) who did not finish high school, or having parents with mental illness including substance abuse (Msall, 2005). This occurs in the context of rising numbers of premature births with no decline in morbidity. Given the prevalence of these environmental and biological risks and the importance of optimizing the outcomes of these at-risk children, incorporating functional assessment by community health nurses could direct family and/or school interventions and reveal concurrent health or motor problems. The WeeFIM has been successfully used to document change over time in the preschool years in a diverse cohort of children with motor, communicative, health, and developmental challenges (Ottenbacher et al., 2000). Advanced practice nurses may find that the WeeFIM is a helpful way to track progress in their young, vulnerable clients.
Explicitly measuring functional skills in conjunction with motor and health outcomes will enhance our understanding of preterm developmental competencies and pathways to risk and resiliencies. The cumulative impact has important implications on how parents view their child and react to their vulnerability and how pediatric health professionals prioritize ongoing interventions. Until preterm birth can be prevented, strategies to optimize health as well as developmental and functional status will be required.
Acknowledgments
This work was supported by NIH Grant NICHD RO3 37627.
The authors wish to thank Sharon Capuano, MS, RNP, Maribeth Walesko, MS, RNP; Christina Poore, MS, RNP; Melissa MacNeill, MA; and Katheleen Hawes, MS, RN, CS, for their assistance with the home visits and data collection.
References
- Aylward G. Methodological issues in outcomes studies of at-risk infants. Journal of Pediatric Psychology. 2002a;27:37–45. doi: 10.1093/jpepsy/27.1.37. [DOI] [PubMed] [Google Scholar]
- Aylward G. Cognitive and neuropsychological outcomes: More than IQ scores. Mental Retardation and Developmental Disabilities Research Reviews. 2002b;8:234–240. doi: 10.1002/mrdd.10043. [DOI] [PubMed] [Google Scholar]
- Aylward GP. Neurodevelopmental outcomes of infants born prematurely. Journal of Developmental and Behavioral Pediatrics. 2005;26:427–440. doi: 10.1097/00004703-200512000-00008. [DOI] [PubMed] [Google Scholar]
- Beery KE. The Beery–Buktenica developmental test of visual–motor integration. 4. Parsippany, NJ: Modern Curriculum Press; 1997. [Google Scholar]
- Botting N, Powls A, Cooke RWI, Marlow N. Attention deficit hyperactivity disorders and other psychiatric outcomes in very low birth weight children at 12 years. Journal of Child Psychology and Psychiatry. 1997;38:931–941. doi: 10.1111/j.1469-7610.1997.tb01612.x. [DOI] [PubMed] [Google Scholar]
- Bradley RH, Whiteside L, Mundfrom DJ, Casey PH, Caldwell BM, Barrett K. Impact of the infant health and development program (IHDP) on the home environments of infants born prematurely and with low birthweight. Journal of Educational Psychology. 1994;86:531–541. [Google Scholar]
- Bronfenbrenner U, Ceci SJ. Nature–nurture reconceptualized in developmental perspective: A bioecological model. Psychological Review. 1994;101:1–33. doi: 10.1037/0033-295x.101.4.568. [DOI] [PubMed] [Google Scholar]
- Bronfenbrenner U, Morris PA. The ecology of developmental processes. In: Damon W, editor. Handbook of child psychology. 5. New York: Wiley; 1998. pp. 993–1028. Theoretical models of human development. [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2. New Jersey: Erlbaum; 1988. [Google Scholar]
- Dammann O, Leviton A. Possible strategies to protect the preterm brain against the fetal inflammatory response. Developmental Medicine and Child Neurology Supplement. 2001;86:8–20. doi: 10.1111/j.1469-8749.2001.tb04141.x. [DOI] [PubMed] [Google Scholar]
- Drotar D. Validating measures of pediatric health status, functional status, and health-related quality of life: Key methodological challenges and strategies. Ambulatory Pediatrics. 2004;4:358–364. doi: 10.1367/A03-101R.1. [DOI] [PubMed] [Google Scholar]
- Hack M, Fanaroff AA. Outcomes of children of extremely low birthweight and gestational age in the 1990’s. Early Human Development. 1999;53:193–218. doi: 10.1016/s0378-3782(98)00052-8. [DOI] [PubMed] [Google Scholar]
- Hack M, Taylor HG, Klein N, Minich NM. Functional limitations and special health care needs of 10- to 14-year old children weighing less than 750 grams at birth. Pediatrics. 2000;106:554–560. doi: 10.1542/peds.106.3.554. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four factor index of social status. New Haven, CT: Yale University Press; 1975. [Google Scholar]
- Magyary D, Brandt P, Hammond M, Barnard K. School-age follow-up of the development of preterm infants: Infant and family predictors. In: Friedman SL, Sigman MD, editors. The psychological development of low-birthweight children. Norwood, NJ: Ablex; 1992. pp. 299–314. [Google Scholar]
- Marlow N, Roberts BL, Cooke RW. Outcome at 8 years for children with birthweights of 1250 grams or less. Archives of Disease in Childhood. 1993;68:286–290. doi: 10.1136/adc.68.3_spec_no.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe M, Granger C. Content validity of a pediatric functional independence measure. Applied Nursing Research. 1990;3:120–122. doi: 10.1016/s0897-1897(05)80128-4. [DOI] [PubMed] [Google Scholar]
- McCarthy D. McCarthy scales of children’s abilities. New York: The Psychological Corporation; 1972. [Google Scholar]
- Msall ME. Measuring functional skills in preschool children at risk for neurodevelopmental disabilities. Mental Retardation and Developmental Disabilities Research Reviews. 2005;11:263–273. doi: 10.1002/mrdd.20073. [DOI] [PubMed] [Google Scholar]
- Msall ME, DiGaudio K, Duffy LC, LaForest S, Braum S, Granger CV. WeeFIM: Normative sample of an instrument for tracking functional independence in children. Clinical Pediatrics. 1994;33:431–438. doi: 10.1177/000992289403300709. [DOI] [PubMed] [Google Scholar]
- Msall ME, DiGaudio K, Rogers BT, et al. The functional independence measure for children (WeeFIM) Clinical Pediatrics. 1994;33:421–430. doi: 10.1177/000992289403300708. [DOI] [PubMed] [Google Scholar]
- Msall ME, Phelps DL, Hardy RJ, Dobson V, Quinn GE, Summers CG, et al. Educational and social competencies at 8 years in children with threshold retinopathy of prematurity (ROP) in the CRYO-ROP multicenter study. Pediatrics. 2004;113:790–799. doi: 10.1542/peds.113.4.790. [DOI] [PubMed] [Google Scholar]
- Msall ME, Tremont MR. Functional outcomes in self-care, mobility, communication, and learning in extremely low birth weight infants. In: Vohr BR, editor. Clinics in perinatology. Philadelphia: W.B. Saunders; 2000. [DOI] [PubMed] [Google Scholar]
- Msall ME, Tremont MR, Ottenbacher KJ. Functional assessment of preschool children: Optimizing developmental and family supports in early intervention. Infants and Young Children. 2001;14:46–66. [Google Scholar]
- Niswander KR, Gordon M. The collaborative perinatal study: The women and their pregnancies. Philadelphia: W.B. Saunders; 1972. [Google Scholar]
- O’Shea TM. Cerebral palsy in very preterm infants: New epidemiological insights. Mental Retardation and Developmental Disabilities Research Reviews. 2002;8:135–145. doi: 10.1002/mrdd.10032. [DOI] [PubMed] [Google Scholar]
- Ottenbacher KJ, Msall ME, Lyon NR, Duffy LC, Granger CV, Braun S. Interrater agreement and stability on the Functional Independence Measure for Children (WeeFIM): Use in children with developmental disabilities. Archives of Physical Medicine and Rehabilitation. 1997;78:1309–1315. doi: 10.1016/s0003-9993(97)90302-6. [DOI] [PubMed] [Google Scholar]
- Ottenbacher KJ, Msall ME, Lyon NR, Duffy LC, Granger CV, Braun S. Measuring developmental and functional status in children with disabilities. Developmental Medicine and Child Neurology. 1999;41:186–194. doi: 10.1017/s0012162299000377. [DOI] [PubMed] [Google Scholar]
- Ottenbacher KJ, Msall ME, Lyon NR, Duffy LC, Granger CV, Braun S. The WeeFIM instrument: Its utility in detecting change in children with developmental disabilities. Archives of Physical Medicine and Rehabilitation. 2000;81:1317–1326. doi: 10.1053/apmr.2000.9387. [DOI] [PubMed] [Google Scholar]
- Palta M, Sadek-Badawi M, Evans M, Weinstein MR, McGuinness G. Functional assessment of a multicenter very low-birth-weight cohort at age 5 years. Newborn Lung Project. Archives of Pediatrics & Adolescent Medicine. 2000;154:23–30. [PubMed] [Google Scholar]
- Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evaluation of subependymal and intraventricular hemorrhage: A study of infants with birth weights less than 1,500 gm. Journal of Pediatrics. 1978;92:529–534. doi: 10.1016/s0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Vohr B, Cannistraci CJ, Dolberg S, Schneider KC, Katz CH. Regional brain volume abnormalities and long-term cognitive outcomes in preterm infants. Journal of the American Medical Association. 2000;15:1939–1947. doi: 10.1001/jama.284.15.1939. [DOI] [PubMed] [Google Scholar]
- Powls A, Botting N, Cooke RWI, Marlow N. Motor impairment in children 12 to 13 years old with a birthweight of less than 1250 grams. Archives of Disease in Childhood. 1995;72:F62–F66. doi: 10.1136/fn.73.2.f62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prechtel H, Beitema D. The neurological examination of the full-term newborn infant. London: Heineman; 1967. [Google Scholar]
- Richardson DK. SNAP-II scoring manual, version 1.1. Kaiser Permante, Beth Israel Deaconess Medical Center, University of British Columbia, Children’s & Women’s Health Centre of British Columbia; 1999. [Google Scholar]
- Saigal S, Rosenbaum PL, Stoskopf BL, Hoult L, Furlong W, Feeney D, et al. Comprehensive assessment of the health status of extremely low birth weight children at eight years of age. Journal of Pediatrics. 1994;125:411–417. doi: 10.1016/s0022-3476(05)83288-3. [DOI] [PubMed] [Google Scholar]
- Schraeder BD, Heverly MA, O’Brien C, McEvoy-Shields K. Finishing first grade: A study of school achievement in very-low-birth-weight children. Nursing Research. 1992;41:354–361. [PubMed] [Google Scholar]
- Taylor HG, Klein N, Schatschneider C, Hack M. Predictors of early school age outcomes in very low birth weight children. Journal of Developmental and Behavioral Pediatrics. 1998;19:235–243. doi: 10.1097/00004703-199808000-00001. [DOI] [PubMed] [Google Scholar]
- Vohr BR, Msall ME. Neuropsychological and functional outcomes of very low birth weight infants. Seminars in Perinatology. 1997;21:202–220. doi: 10.1016/s0146-0005(97)80064-x. [DOI] [PubMed] [Google Scholar]
- Volpe J. Perinatal brain injury: From pathogenesis to neuroprotection. Mental Retardation and Developmental Disabilities Research Reviews. 2001;7:56–64. doi: 10.1002/1098-2779(200102)7:1<56::AID-MRDD1008>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- WeeFIM system clinical guide: version 5. Buffalo, NY: University at Buffalo; [Google Scholar]