Abstract
Developmental Origins Theory has received little coverage in the nursing literature, even though it has received much attention in other sciences. The theory proposes that prenatal stress provokes adaptive changes in endocrine and metabolic processes that become permanently programmed and impact later adult health. This paper reviews the theory and describes the primary neuroendocrine mechanism of hypothalamic-pituitary-adrenal axis function. Supporting research evidence in preterm infant and adult samples is presented. Through knowledge of the theory and the long-term sequelae for preterm infants, nurses will have a different theoretical perspective and growing evidence to consider in their care for pregnant women and infants.
Keywords: cortisol, hypothalamic-pituitary-adrenal axis, prematurity, fetal origins hypothesis
Prematurity is a rising national public health problem and economic burden. Of the 4 million infants born in the United States in 2006, 12.5% were premature, an 18% increase since 1990, and 30% since 1981 (Martin et al., 2006). Hospital care of premature infants represent half of all infant charges—on average $77,000 compared to $1,700 for full-term infants in 2003 (March of Dimes, 2005). An estimated 50% to 70% of premature infants with birthweight less than 1,500 g have later dysfunctions, such as learning disabilities, attention problems, cognitive deficits, neuropsychological deficits, and behavioral problems (Aylward, 2003). Not only do these preterm infants suffer serious health consequences but also their families carry an enormous emotional burden as well (Singer et al., 1999; Vohr et al., 2000).
There is reason for renewed concern about the long-term outcomes of preterm infants. The Developmental Origins Theory, previously known as the Fetal Origins Hypothesis, proposes that prenatal stress provokes adaptive changes in endocrine and metabolic processes that become permanently programmed and impact later health, memory, learning, and executive function (Barker, 2007; Dalziel, Parag, Rodgers, & Harding, 2007; Hofman, Regan, & Cutfield, 2006). Retrospective epidemiological studies throughout the world have shown that early adverse events marked by premature birth are linked to adult disorders, such as hypertension, coronary heart disease, stroke, and type 2 diabetes (Barker, 2002; Ward, Syddall, Wood, Chrousos, & Phillips, 2004). Now, these researchers propose that neuroendocrine stress systems may be a mechanism underlying chronic illnesses in adulthood. The Developmental Origins Theory continues to command attention in the fields of obstetrics and gynecology. It appears, however, that it has had little coverage in the nursing literature, even though it has received much attention in other sciences. Since obstetric and neonatal nursing emphasizes health promotion for women and infants from pregnancy through birth, knowledge of this theory and its proposed neuroendocrine mechanisms has the potential to influence practice approaches that not only impact the stress of immediate neonatal care but also may influence long-term adult health.
In this paper, the authors review the Developmental Origins Theory and the primary neuroendocrine mechanism of hypothalamic-pituitary-adrenal (HPA) axis function. Supporting research evidence in preterm infant and adult samples will also be presented. Through knowledge of the theory and the long-term sequelae for preterm infants, nurses will have a different theoretical perspective and growing evidence to consider in their care for pregnant women and infants.
Fetal Origins of Adult Disease and HPA Axis Function
The Developmental Origins Theory was proposed to explain the observations linking early life events such as low birthweight and/or prematurity with later adult pathology (Barker, 2002, 2007). It has been hypothesized that persons exposed to an adverse fetal or neonatal environment develop compensatory physiological responses to survive that become permanent or programmed. This adaptation may subsequently alter the set points of physiological systems including those that sustain homeostasis. Such responses become mal-adaptive if the environment for which they are developed is not what is expected in the intrauterine environment. For instance, reduced fetal growth occurs in conditions of chronic intrauterine deprivation. With the birth of a 28-week gestation infant, the extrauterine Neonatal Intensive Care Unit (NICU) nursery environment is not the intrauterine environment expected by nature. Critically ill infants require prolonged NICU stays; sometimes as long as 5 months, yet the typical NICU environment is inherently stressful for infants and their families (Bremmer, Byers, & Kiehl, 2003; Byers, 2003; Shaw et al., 2006). This produces maladaptive physiological processes and predisposes the infant to later disease (Hofman et al., 2006; Welberg & Seckl, 2001).
Recent evidence suggests that the major systems involved in the compensatory response include the HPA axis and the autonomic nervous system. Intrauterine programming of the HPA axis is suggested as a central mechanism linking intrauterine growth with later adult disease. This is an intricate system of stimulation and feedback where, when stimulated, neurons in the paraventricular nucleus (PVN) of the hypothalamus produce corticotrophin-releasing hormone and vasopressin. Corticotrophin-releasing hormone induces adrenocortico synthesis and releases corticotroph cells from the anterior pituitary. Adrenocorticotropin initiates the production and release of glucocorticoids (cortisol) from the adrenal cortex. Glucocorticoids act at multiple loci within the body to maintain homeostasis and also act in the brain to modify behavior and learning (Kapoor, Dunn, Kostaki, Andrews, & Matthews, 2006). Elevated glucocorticoids provide the physiologic environment for an adaptive stress response, while immediately interacting with corticoid receptors to inhibit the stress response via negative feedback. Chronic exposure to stress and high levels of glucocorticoids can result in structural damage to the hippocampus. The primary roles of the hippocampus, located in the medial temporal lobe, are learning and memory as well as storing and processing spatial information. Glucocorticoid and mineral corticoid receptors in the limbic system and glucocorticoid receptors (GRs) in the PVN and anterior pituitary mediate negative HPA feedback (Talge, Neal, Glover, & The Early Stress Translational Research and Prevention Science Network: Fetal and Neonatal Experience on Child and Adolecent Mental Health, 2007).
The effects of prolonged glucocorticoid exposure appear to be highly dependent upon the timing of the stress (Kapoor et al., 2006). When a stimulus or stressor occurs at a critical period in fetal or neonatal development, it may result in permanent changes in structure and physiology. During the intrauterine period, stress can permanently alter later behavioral and/ or physiological reactivity to stressors (Welberg & Seckl, 2001)
There is compelling data from animal studies that the HPA axis is highly susceptible to programming during fetal and neonatal development (Matthews, 2002). When a pregnant animal is exposed to a dangerous environment that requires increased vigilance, a stress signal is transmitted to the fetus. This signal programs the fetal HPA axis and related behaviors to develop increased vigilance, a necessary adaptation for survival in a hostile environment. Accordingly, prenatal stress has long-lasting effects on the HPA axis of adult offspring, programming a persistently hyperactive system.
This programming of a persistently hyperactive system is evidenced by a number of diverse prenatal and post-natal manipulations that permanently modify HPA development and function in offspring of several species including rats, sheep, goats, guinea pigs, cattle, and primates. Prenatal manipulations include maternal stress (exposure to unpredictable light and noise), synthetic glucocorticoid exposure, and nutrient restriction. Intrauterine growth restriction (IUGR) induced by utero-placental insufficiency has been modeled in animal studies. The offspring had growth restriction and decreased glucose, insulin, amino acids, and progressive dysfunction of insulin secretion—the same profile found in human studies (Simmons, 2004).
Postnatal manipulations used in research studies include neonatal handling, exposure to synthetic glucocorticoid, altered maternal behavior, maternal deprivation, and infection (Kapoor et al., 2006; Matthews, 2002; Welberg & Seckl, 2001). Juveniles and adult offspring of species exposed to prenatal stress exhibit various alterations in HPA function, such as lower hippocampal GR binding, which has implications for hippocampal negative feedback and termination of the pituitary-adrenal response following activation. Additionally, handling of neonatal rats in the first 2 weeks of life raised hippocampal GR, increased glucocorticoid negative feedback, and reduced HPA activity when the rats reached adulthood. Neonatal handling also increased thyroid activity and increased serotonin turnover in the hippocampus. The data suggest that the ascending seratonergic system is involved in glucocorticoid-induced HPA programming prenatally. As such, prenatal stress has been shown to have long-term influence on the hippocampus, which may influence HPA function (see Kapoor et al.).
Prematurity, Postnatal Stress, and HPA Function
Findings in human infancy studies of the effects of stress on HPA function parallel those of animal studies. Cortisol is a widely accepted endocrine marker of HPA axis activity. Cortisol in saliva is a valid and reliable marker of the hormone in the blood (Hanrahan, McCarthy, Kleiber, Lutgendorf, & Tsalikian, 2006; John and Catherine MacArthur Research Network, 1999). There is a circadian rhythm to daily cortisol levels in infants from 3 months of age, with highest levels occurring 20 to 45 minutes after awakening and decreasing throughout the day with lowest levels in the late evening (Gunnar, 1992; Smyth et al., 1997). As a primary stress hormone, cortisol is rapidly responsive and indicative of physiological reactivity to an acute stressor.
Cortisol levels differ between full term, sick preterm, and healthy preterm infants suggesting differences in HPA dysregulation due to neonatal stress characterized by morbidity (Watterberg & Scott, 1995). Grunau and colleagues examined serum cortisol in 87 preterm infants at 32 weeks gestational age in the NICU. Cortisol levels were assessed after two common, but stressful, neonatal procedures over 2 days. The procedures were a series of tactile nursing procedures (diaper change, measuring abdominal girth, taking axillary temperature, cleaning the mouth with gauze and sterile water) and a clinical blood draw (warming with a foot pack, swabbing the heel, lancing the heel, and gently squeezing for a small amount of blood). Infants who were born less than or equal to 28 weeks gestational age had a lower cortisol stress response indicating a downregulation of the HPA axis which was not counteracted with morphine (Grunau et al., 2005; Holsti, Weinberg, Whitfield, & Grunau, 2007).
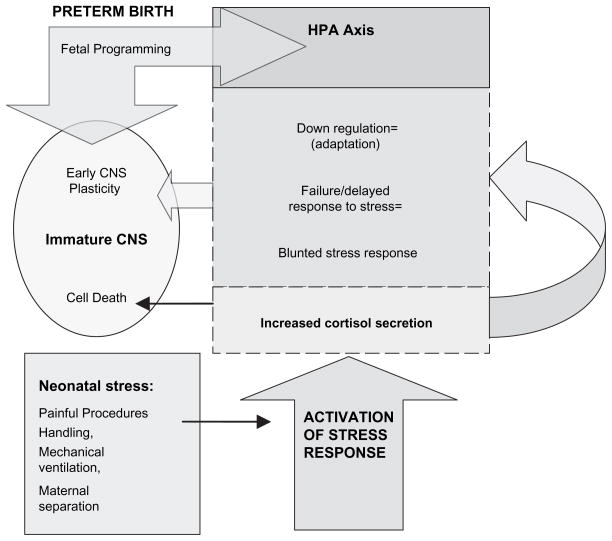
At 3 months corrected age, a subsample of 24 preterm infants had lower cortisol levels and smaller cortisol responses compared to 18 full-term infants (Haley, Weinberg, & Grunau, 2006). In a later follow-up to 18 months, basal cortisol in infants less than or equal to 28 weeks gestation shifted from significantly low levels at 8 months to high levels at 18 months suggesting a long-term “resetting” and “reprogramming ” of endocrine stress systems (Grunau et al., 2007). Figure 1 depicts the mechanisms of HPA axis activity and programming due to fetal and neonatal events including physiological immaturity at birth and stress related to common NICU procedures.
Figure 1.
Hypothalamic-pituitary-adrenal axis and response to stress.
Note. HPA = hypothalamic-pituitary-adrenal; CNS = central nervous system.
Not all adverse consequences can be attributed solely to fetal programming, premature birth, and stress physiology. Caregiving and social conditions in the growing infant’s environment may increase the sequelae of prematurity. Some behavioral and developmental delays reflect the fact that preterm infants are disproportionately more likely to come from disadvantaged environments, suggesting the theory of double jeopardy. In double jeopardy, prematurity places the infant at risk for later behavioral and developmental outcomes and this risk is increased when the child lives in poverty or other risk conditions (prematurity + environmental risk = double jeopardy; Parker, Greer, & Zuckerman, 1988). In stress reactivity studies, a blunting of the cortisol response was found in children exposed to high-risk environments (Gunnar & Vazquez, 2001). Postnatal handling has been found to reverse some effects of prenatal stress in animal studies but caution must be exercised in extrapolating to humans (Welberg & Seckl, 2001). Later, lifestyle choices such as smoking, exercise, and diet might moderate effects of early programming.
Linking HPA Dysfunction and Adult Outcomes
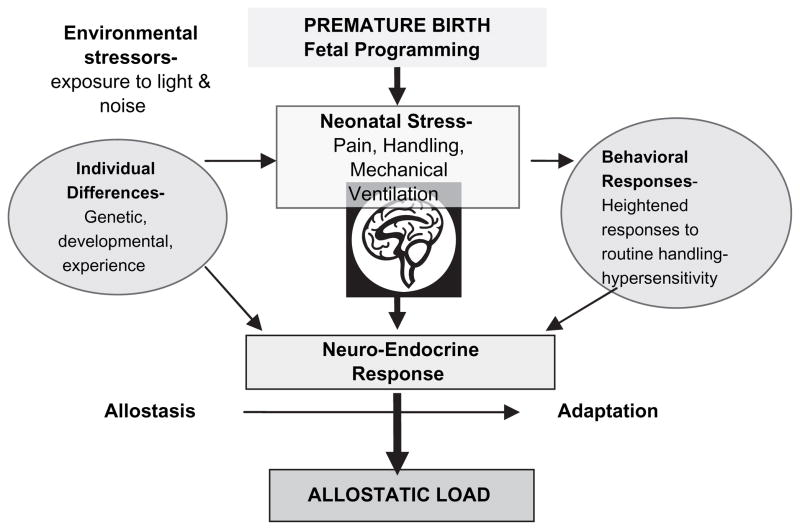
Repeated stress leads to high allostatic load defined as the cumulative negative costs of brain and body adaptation to repeated physiological and psychological challenge (McEwen, 1998). The HPA axis, the autonomic nervous, cardiovascular, metabolic, and immune systems protect the body by responding to stress. The cost for this stability is allostatic load. The term allostatic load, used in relation to HPA function, is a unifying concept because cumulative stress contributes to disease (Barker, 1995; Walker, Irving, Andrew, & Belton, 2002; Ward et al., 2004). In Figure 2, HPA function influenced by fetal/neonatal programming is further affected by allostatic load and individual differences in response to stress.
Figure 2.
Cumulative sources of allostatic load.
Prolonged high cortisol exposure is linked to poor health and behavior problems at adulthood. Higher cortisol levels have been found in formerly preterm infants into adulthood (Phillips, 2007). Raised fasting plasma cortisol was found in adults from three populations and was associated with their low birthweight, while higher plasma cortisol was associated with higher blood pressure in obese adults only (Phillips et al., 2000). There is significant evidence that the perinatal environment programs’ HPA function and hence, associated behavior throughout the lifespan (Phillips, 2007; Phillips et al., 2000). Chronic high cortisol may affect the hippocampal and prefrontal cortex (PFC) brain regions where preterm children demonstrated a suppressed reaction to novelty and “back off” novel stimuli (Whitfield, Grunau, & Holsti, 1997). The hippocampus is critical to learning and memory, and the PFC is critical to emotional regulation and executive function. Thus, these brain regions are needed for effective coping and self-regulation (Compas, 2006; Taylor, Repetti, & Seeman, 1997).
A recent meta-analysis indicated that chronic stress exposure in adults is associated with lower morning cortisol and higher concentrations of afternoon and evening cortisol levels, a flatter diurnal rhythm, and a higher daily volume of cortisol output. These findings suggest that there is a dysregulated pattern of hormone secretion associated with chronic stress (Miller, Chen, & Zhou, 2007). Gunnar and Vazquez (2001) found dampening of the early morning peak and lower basal cortisol levels in several studies of high-risk children. A flattening of the expected daily rhythm (a result from lower values near the early morning peak) was also found. Examining patterns of cortisol recovery after stressors is very significant to health outcomes because when recovery is prevented from occurring pathology results. A slow return to baseline may result in longer overall exposure to stress hormones indicating underlying HPA dysregulation (Dickerson & Kemeny, 2004).
Robust correlations have been found between cortisol concentrations, birthweight, and the development of hypertension and type 2 diabetes mellitus (Matthews, 2002). Specifically, it has been shown that low birthweight predicts increased cortisol concentrations in adults. Additionally, high cortisol concentrations correlate positively with higher blood pressure and coronary artery calcification in adulthood (Cohen et al., 2006; Phillips, 2002; Whitworth, Williamson, Mangos, & Kelly, 2005).
In addition to chronic health diseases, recent research has reported that young adults who were less than 1,500 g at birth had lower educational achievement, poorer physical abilities, higher blood pressure, and poorer respiratory function (Hack & Klein, 2006). Low birthweight has adverse consequences for health-related quality of life and functional outcomes, such as activities of daily living (Hack, Taylor, Klein, & Minich, 2000). Saigal, Burrows, Stoskopf, Rosenbaum, and Streiner (2000) found evidence for slightly lower health-related quality of life in their sample of extremely low-birthweight (ELBW; less than 1,000 g) children relative to normal-birthweight children. Cortisol, as a marker for HPA function, has not been prospectively studied in preterm samples to adulthood.
Summary
An overview of the Developmental Origins Theory has been presented which has been widely supported in large studies worldwide. Evidence from animal and human research studies suggests that prenatal intrauterine stress and postnatal prematurity activate the HPA axis as a stress response. Early findings were based on retrospective studies of adults who were born low birth-weight. More recently, prospective studies in adults have shown HPA dysfunction. Grunau and colleagues’ studies suggest that cortisol reprogramming in preterm infants may be one mechanism for later behavioral problems.
There has been criticism of the theory. Flaws in epidemiological approaches were cited in the initial publications; however, recent studies have reduced these concerns. One methodological flaw is the statistical issues of the “reversal paradox” (Tu, West, Ellison, & Gilthorpe, 2005; Weinberg, 2005). Inappropriate statistical adjustment may show artificial significant associations of birthweight and adult blood pressure that are not found without the adjustment. In the past 15 to 20 years, research showing associations of birthweight with adult disease have been replicated in studies across age, geography, race, ethnic group, and authors. Alternate mechanisms need investigation given the complex interplay of genetic, endocrine, metabolic, and vascular systems in the course of fetal and neonatal life (Gillman & Rich-Edwards, 2000). The preponderance of evidence supporting the theory has concerned the postnatal period. This may be because the infancy period is more accessible to research and possible interventions as compared to the fetal period. A commentary on the controversy called for combined interdisciplinary expertise to identify causal pathways and opportunities for intervention (McMillen, Owens, Coulter, & Robinson, 2001).
Other lines of research with formerly preterm infants illustrate the involvement of other body systems. Neuro-imaging studies have identified anatomical brain abnormalities, decreased white matter, and increased ventricular size in preterm children compared to full-term controls. These anatomical differences were associated with lower cognitive scores in formerly preterm infants at age 8 (Petersen et al., 2000).
Undoubtedly, other physiological mechanisms are also associated with later preterm outcomes. Birthweight is the common predictor in preterm follow-up studies where infants with lower birthweight are more likely to have developmental sequelae, with the smallest babies likely to have later problems (Aylward, 2002). However, not all ELBW children have impairment, while some low-birthweight children do have impairment, suggesting the role of perinatal morbidities in outcomes. The power of the infant’s environment both everyday (proximal environment) and remote (distal environment) demands consideration when estimating later outcomes. In bio-ecological theories, the interplay between the infant/ child and environment over time are the primary mechanisms that produce development.
Nurses provide continual intensive care to preterm infants and are the primary clinical professionals responsible to manage the neonatal environment in which the infants grow and develop. Nurses’ clinical roles are based on monitoring and control of infant physiological stability and collaborative decision making in regard to interventions. They are responsible for controlling the neonatal environment including light, noise levels, and appropriate touch (Turrill, 2003). Typically, the NICU nurse maintains the delicate balance of optimum oxygen levels, temperature for warmth, body positioning for skin integrity, skeletal and muscle development, and precisely administered medication to prevent and treat respiratory and heart ailments, infection or other problems. In addition to a preterm infants’ physical well-being, it is becoming more and more apparent that special attention to the infants’ neurodevelopment is paramount to long-term, successful outcomes (Ment et al., 2003; Vohr & Allen, 2005). Some of these studies have shown that when infant care is individualized and developmentally supportive to the infant, the infant demonstrates behaviors that are better coordinated in motor activity, sleep, and wakefulness and integrated with physiology of heart rate and oxygen levels (Als et al., 1994; Beal, 2005).
A recent Institute of Medicine report recommended long-term outcome studies into young adulthood for preterm infants to determine the extent of recovery, if any, and to monitor them for the onset of disease (Behrman & Stith Butler, 2007). There is little known about adult outcomes for preterm infants. In the only U.S. study, Hack and Klein (2006) reported that neurodevelopmental and growth sequelae persist to young adulthood in formerly very low-birthweight (VLBW; less than 1,500 g) preterm infants. Young adults who were VLBW had lower educational achievement, poorer physical abilities, higher blood pressure, and poorer respiratory function. More chronic health conditions, functional limitations, and neurosensory impairments were found in a Canadian cohort of ELBW infants (Saigal, Stoskopf, Pinelli, et al., 2006; Saigal, Stoskopf, Streiner, et al., 2006; Saigal et al., 2007).
Currently, there is a national emphasis on examining the physiological processes of behavior and health. However, integration and interpretation of biomarkers and behavioral data is challenging (Granger & Kivlighan, 2003). Salivary cortisol levels are widely recognized as reliable biomarkers of HPA function (Turner-Cobb, 2005). The collection of salivary cortisol is a noninvasive procedure that poses no risks to the subjects and reveals the underlying processes of health and behavioral outcomes (Kirschbaum, Pirke, & Hellhammer, 1993).
The ultimate reason to understand the etiology of long-term outcomes is identification of possibilities for preventative interventions. There is limited research for developing evidence-based intervention despite millions of preterm survivors. Formulating a science of prevention requires attention to complex interactions between persons and their contexts across periods of time (Coie et al., 1993). When developmental trajectories from premature birth through young adulthood, and the processes that affect them are understood, we will be better able to make early identification of those at risk to more accurately pinpoint the timing and content of intervention.
Acknowledgments
Supported by NIH RO1 NINR NR02263.
References
- Als H, Lawhorn G, Duffy FH, McAnulty GB, Gibes-Grossman R, Blickman JG. Individualized developmental care for the very low-birth-weight preterm infant. Journal of the American Medical Association. 1994;272:853–858. [PubMed] [Google Scholar]
- Aylward G. Methodological issues in outcomes studies of at-risk infants. Journal of Pediatric Psychology. 2002;27:37–45. doi: 10.1093/jpepsy/27.1.37. [DOI] [PubMed] [Google Scholar]
- Aylward G. Cognitive function in preterm infants: No simple answers. Journal of the American Medical Association. 2003;289:752–753. doi: 10.1001/jama.289.6.752. [DOI] [PubMed] [Google Scholar]
- Barker DJ. Fetal programming of coronary heart disease. Trends in Endocrinology Metabolism. 2002;13:364–368. doi: 10.1016/s1043-2760(02)00689-6. [DOI] [PubMed] [Google Scholar]
- Barker DJ. The origins of the developmental origins theory. Journal of Internal Medicine. 2007;261:412–417. doi: 10.1111/j.1365-2796.2007.01809.x. [DOI] [PubMed] [Google Scholar]
- Barker DJP. The fetal and infant origins of disease. European Journal of Clinical Investigations. 1995;25:457–463. doi: 10.1111/j.1365-2362.1995.tb01730.x. [DOI] [PubMed] [Google Scholar]
- Beal JA. Evidence for best practices in the neonatal period. American Journal of Maternal Child Nursing. 2005;30:397–403. doi: 10.1097/00005721-200511000-00008. [DOI] [PubMed] [Google Scholar]
- Behrman RE, Stith Butler A, editors. Preterm Birth: Causes, Consequences, and Prevention. Washington, DC: The National Academies Press; 2007. Committee on Understanding Premature Birth and Assuring Healthy Outcomes (2007) [PubMed] [Google Scholar]
- Bremmer P, Byers JF, Kiehl E. Noise and the premature infant: Physiological effects and practice implications. Journal of Obstetric, Gynecologic, and Neonatal Nursing. 2003;32:447–454. doi: 10.1177/0884217503255009. [DOI] [PubMed] [Google Scholar]
- Byers JF. Components of developmental care and the evidence for their use in the NICU. American Journal of Maternal Child Nursing. 2003;28:175–180. doi: 10.1097/00005721-200305000-00007. [DOI] [PubMed] [Google Scholar]
- Cohen S, Schwartz JE, Epel E, Kirschbaum C, Sidney S, Seeman T. Socioeconomic status, race, and diurnal cortisol decline in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Psychosomatic Medicine. 2006;68(1):41–50. doi: 10.1097/01.psy.0000195967.51768.ea. [DOI] [PubMed] [Google Scholar]
- Coie JD, Watt NF, West SG, Hawkins D, Asarnow JR, Markman HJ, et al. The science of prevention: A conceptual framework and some directions for a national research program. American Psychologist. 1993;48:1013–1022. doi: 10.1037//0003-066x.48.10.1013. [DOI] [PubMed] [Google Scholar]
- Compas BE. Psychobiological processes of stress and coping: Implications for resilience in children and adolescents–comments on the papers of Romeo & McEwen and Fisher et al. Annals of the New York Academy of Science. 2006;1094:226–234. doi: 10.1196/annals.1376.024. [DOI] [PubMed] [Google Scholar]
- Dalziel SR, Parag V, Rodgers A, Harding JE. Cardiovascular risk factors at age 30 following pre-term birth. International Journal of Epidemiology. 2007;36(4):907–15. doi: 10.1093/ije/dym067. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Gillman MW, Rich-Edwards JW. The fetal origin of adult disease: From sceptic to convert. Paediatric and Perinatal Epidemiology. 2000;14:192–193. doi: 10.1046/j.1365-3016.2000.00265.x. [DOI] [PubMed] [Google Scholar]
- Granger DA, Kivlighan KT. Integrating biological, behavioral, and social levels of analysis in early child development: Progress, problems, and prospects. Child Development. 2003;74:1058–1063. doi: 10.1111/1467-8624.00590. [DOI] [PubMed] [Google Scholar]
- Grunau RE, Haley DW, Whitfield MF, Weinberg J, Yu W, Thiessen P. Altered basal cortisol levels at 3, 6, 8 and 18 months in infants born at extremely low gestational age. Journal of Pediatrics. 2007;150:151–156. doi: 10.1016/j.jpeds.2006.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunau RE, Holsti L, Haley DW, Oberlander T, Weinberg J, Solimano A, et al. Neonatal procedural pain exposure predicts lower cortisol and behavioral reactivity in preterm infants in the NICU. Pain. 2005;113:293–300. doi: 10.1016/j.pain.2004.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR. Reactivity of the hypothalamic-pituitary-adrenocortical system to stressors in normal infants and children. Pediatrics. 1992;90(3 Pt 2):491–497. [PubMed] [Google Scholar]
- Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected daily rhythm: Potential indices of risk in human development. Development and Psychopathology. 2001;13:515–538. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- Hack M, Klein N. Young adult attainments of preterm infants. Journal of the American Medical Association. 2006;295:695–696. doi: 10.1001/jama.295.6.695. [DOI] [PubMed] [Google Scholar]
- Hack M, Taylor HG, Klein N, Minich NM. Functional limitations and special health care needs of 10- to 14- year old children weighing less than 750 grams at birth. Pediatrics. 2000;106:554–560. doi: 10.1542/peds.106.3.554. [DOI] [PubMed] [Google Scholar]
- Haley DW, Weinberg J, Grunau RE. Cortisol, contingency learning, and memory in preterm and full-term infants. Psychoneuroendocrinology. 2006;31:108–117. doi: 10.1016/j.psyneuen.2005.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanrahan K, McCarthy AM, Kleiber C, Lutgendorf S, Tsalikian E. Strategies for salivary cortisol collection and analysis in research with children. Applied Nursing Research. 2006;19:95–101. doi: 10.1016/j.apnr.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Hofman PL, Regan F, Cutfield WS. Prematurity –another example of perinatal metabolic programming? Hormone Research. 2006;66:33–39. doi: 10.1159/000093230. [DOI] [PubMed] [Google Scholar]
- Holsti L, Weinberg J, Whitfield MF, Grunau RE. Relationships between adrenocorticotropic hormone and cortisol are altered during clustered nursing care in preterm infants born at extremely low gestational age. Early Human Development. 2007;83:341–348. doi: 10.1016/j.earlhumdev.2006.08.005. [DOI] [PubMed] [Google Scholar]
- John D, Catherine T MacArthur Research Network. Salivary cortisol measurement. Socioeconomic Status and Health. 1999 Jun 9;2000 Retrieved April 1, 2007, from http://www.mac-sesucsf.edu/Research/Allostatic/notebook/salivarycort.html. [Google Scholar]
- Kapoor A, Dunn E, Kostaki A, Andrews MH, Matthews SG. Fetal programming of hypothalmo-pituitary-adrenal function: Prenatal stress and glucocorticoids. Journal of Physiology. 2006;572:31–44. doi: 10.1113/jphysiol.2006.105254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The Trier Stress Test: A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- March of Dimes. About prematurity. Premature Birth-Economic Costs. 2005 Retrieved March 15, 2007, from http://www.mar.chofdimes.com/prematurity/21198_10734.asp.
- Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Kirmeyer S. Births: Final data for 2004. National Vital Statistics Reports. 2006;55(1):1–26. [PubMed] [Google Scholar]
- Matthews SG. Early programming of the hypothalamo-pituitary-adrenal axis. Trends in Endocrinology & Metabolism. 2002;13:373–380. doi: 10.1016/s1043-2760(02)00690-2. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators. Seminars in Medicine of the Beth Israel Deaconess Medical Center. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- McMillen C, Owens JA, Coulter CL, Robinson JS. Introduction: Moving on from controversies to mechanisms. Clinical and Experimental Pharmacology and Physiology. 2001;28:930. doi: 10.1046/j.1440-1681.2001.03551.x. [DOI] [PubMed] [Google Scholar]
- Ment LR, Vohr B, Allan W, Katz KH, Schneider KC, Westerveld M, et al. Change in cognitive function over time in very low-birth-weight infants. Journal of the American Medical Association. 2003;289:705–711. doi: 10.1001/jama.289.6.705. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Parker S, Greer S, Zuckerman B. Double jeopardy: The impact of poverty on early child development. Pediatric Clinics of North America. 1988;35:1227–1240. doi: 10.1016/s0031-3955(16)36580-4. [DOI] [PubMed] [Google Scholar]
- Petersen BS, Vohr B, Cannistraci CJ, Dolberg S, Schneider KC, Katz CH, et al. Regional brain volume abnormalities and long-term cognitive outcomes in preterm infants. Journal of the American Medical Association. 2000;15:1939–1947. doi: 10.1001/jama.284.15.1939. [DOI] [PubMed] [Google Scholar]
- Phillips D. Endocrine programming and fetal origins of adult disease. Trends in Endocrinology and Metabolism. 2002;13:363. doi: 10.1016/s1043-2760(02)00696-3. [DOI] [PubMed] [Google Scholar]
- Phillips DI. Programming of the stress response: A fundamental mechanism underlying the long-term effects of the fetal environment? Journal of Internal Medicine. 2007;261:453–460. doi: 10.1111/j.1365-2796.2007.01801.x. [DOI] [PubMed] [Google Scholar]
- Phillips DI, Walker BR, Reynolds RM, Flanagan DE, Wood PJ, Osmond C, et al. Low birth weight predicts elevated plasma cortisol concentrations in adults from 3 populations. Hypertension. 2000;35:1301–1306. doi: 10.1161/01.hyp.35.6.1301. [DOI] [PubMed] [Google Scholar]
- Saigal S, Burrows E, Stoskopf BL, Rosenbaum PL, Streiner D. Impact of extreme prematurity on families of adolescent children. Journal of Pediatrics. 2000;137:701–706. doi: 10.1067/mpd.2000.109001. [DOI] [PubMed] [Google Scholar]
- Saigal S, Stoskopf B, Boyle M, Paneth N, Pinelli J, Streiner D, et al. Comparison of current health, functional limitations, and health care use of young adults who were born with extremely low birth weight and normal birth weight. Pediatrics. 2007;119:e562–e573. doi: 10.1542/peds.2006-2328. [DOI] [PubMed] [Google Scholar]
- Saigal S, Stoskopf B, Pinelli J, Streiner D, Hoult L, Paneth N, et al. Self-perceived health-related quality of life of former extremely low birth weight infants at young adulthood. Pediatrics. 2006;118:1140–1148. doi: 10.1542/peds.2006-0119. [DOI] [PubMed] [Google Scholar]
- Saigal S, Stoskopf B, Streiner D, Boyle M, Pinelli J, Paneth N, et al. Transition of extremely low-birth-weight infants from adolescence to young adulthood: Comparison with normal birth-weight controls. Journal of the American Medical Association. 2006;295:667–675. doi: 10.1001/jama.295.6.667. [DOI] [PubMed] [Google Scholar]
- Shaw RJ, Deblois T, Ikuta L, Ginzburg K, Fleisher B, Koopman C. Acute stress disorder among parents of infants in the neonatal intensive care nursery. Psychosomatics. 2006;47:206–212. doi: 10.1176/appi.psy.47.3.206. [DOI] [PubMed] [Google Scholar]
- Simmons R. Fetal origins of adult disease: Concepts and controversies. NeoReviews. 2004;5:e511–e515. [Google Scholar]
- Singer L, Salvator A, Guo S, Collin M, Lilien L, Baley J. Maternal psychological distress and parenting stress after the birth of a very low-birth-weight infant. Journal of the American Medical Association. 1999;281:799–805. doi: 10.1001/jama.281.9.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth JM, Ockenfels MC, Gorin AA, Catley D, Porter LS, Kirschenbaum C, et al. Individual differences in the diurnal cycle of cortisol. Psychoneuroendocrinology. 1997;22:89–105. doi: 10.1016/s0306-4530(96)00039-x. [DOI] [PubMed] [Google Scholar]
- Talge NM, Neal C, Glover Y The Early Stress Translational Research and Prevention Science Network: Fetal and Neonatal Experience on Child and Adolecent Mental Health. Antenatal maternal stress and long term effects on child neurodevelopment: How and why? Journal of Child Psychology and Psychiatry. 2007;48:245–261. doi: 10.1111/j.1469-7610.2006.01714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Repetti RL, Seeman T. Health psychology: What is an unhealthy environment and how does it get under the skin? Annual Review of Psychology. 1997;48:411–447. doi: 10.1146/annurev.psych.48.1.411. [DOI] [PubMed] [Google Scholar]
- Tu YK, West R, Ellison GT, Gilthorpe MS. Why evidence for the fetal origins of adult disease might be a statistical artifact: The “reversal paradox” for the relation between birth weight and blood pressure in later life. American Journal of Epidemiology. 2005;161:27–32. doi: 10.1093/aje/kwi002. [DOI] [PubMed] [Google Scholar]
- Turner-Cobb JM. Psychological and stress hormone correlates in early life: a key to HPA-axis dysregulation and normalisation. Stress. 2005;8(1):47–57. doi: 10.1080/10253890500095200. [DOI] [PubMed] [Google Scholar]
- Turrill S. A focus of care for neonatal nursing: The relationship between neonatal nursing practice and outcomes. Part 1. Paediatric Nursing. 2003;15:13–17. doi: 10.7748/paed.15.4.13.s19. [DOI] [PubMed] [Google Scholar]
- Vohr BR, Allen M. Extreme prematurity—The continuing dilemma. New England Journal of Medicine. 2005;352:71–72. doi: 10.1056/NEJMe048323. [DOI] [PubMed] [Google Scholar]
- Vohr BR, Wright LL, Dusick AM, Mele L, Verter J, Steichen JJ, et al. Neurodevelopmental and functional outcomes of extremely low birth weight infants in the National Institute of Child Health and Human Development Neonatal Research Network, 1993–1994. Pediatrics. 2000;105:1216–1226. doi: 10.1542/peds.105.6.1216. [DOI] [PubMed] [Google Scholar]
- Walker BR, Irving RJ, Andrew R, Belton NR. Contrasting effects of intrauterine growth retardation and premature delivery on adult cortisol secretion and metabolism in man. Clinical Endocrinology. 2002;57:351–355. doi: 10.1046/j.1365-2265.2002.01606.x. [DOI] [PubMed] [Google Scholar]
- Ward AMV, Syddall HE, Wood PJ, Chrousos GP, Phillips DIW. Fetal programming of the hypothalamic-pituitary-adrenal (HPA) axis: Low birth weight and central HPA regulation. Journal of Clinical Endocrinology & Metabolism. 2004;89:1227–1233. doi: 10.1210/jc.2003-030978. [DOI] [PubMed] [Google Scholar]
- Watterberg KL, Scott SM. Evidence of early adrenal insufficiency in babies who develop bronchopulmonary dysplasia. Pediatrics. 1995;95:120–125. [PubMed] [Google Scholar]
- Weinberg CR. Invited commentary: Barker meets Simpson. American Journal of Epidemiology. 2005;161:33–35. doi: 10.1093/aje/kwi003. [DOI] [PubMed] [Google Scholar]
- Welberg LAM, Seckl JR. Prenatal stress, glucocorticoids and the programming of the brain. Journal of Endocrinology. 2001;13:113–128. doi: 10.1046/j.1365-2826.2001.00601.x. [DOI] [PubMed] [Google Scholar]
- Whitfield MF, Grunau RV, Holsti L. Extremely premature (< or = 800 g) schoolchildren: Multiple areas of hidden disability. Archives of Disease in Childhood: Fetal & Neonatal Edition. 1997;77:F85–F90. doi: 10.1136/fn.77.2.f85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitworth JA, Williamson PM, Mangos G, Kelly JJ. Cardiovascular consequences of cortisol excess. Vascular Health Risk Management. 2005;1:291–299. doi: 10.2147/vhrm.2005.1.4.291. [DOI] [PMC free article] [PubMed] [Google Scholar]