Abstract
Glutamate antagonists decrease dyskinesia and augment the antiparkinsonian effects of levodopa in animal models of Parkinson’s disease (PD). In a randomized, double-blind, placebo-controlled clinical trial we investigated the acute effects of placebo and two doses of a NR2B subunit selective NMDA glutamate antagonist, CP-101,606, on the response to two-hour levodopa infusions in 12 PD subjects with motor fluctuations and dyskinesia. Both doses of CP-101,606 reduced the maximum severity of levodopa-induced dyskinesia approximately 30% but neither dose improved parkinsonism. CP-101,606 was associated with a dose-related dissociation and amnesia. These results support the hypothesis that glutamate antagonists may be useful antidyskinetic agents. However, future studies will have to determine if the benefits of dyskinesia suppression can be achieved without adverse cognitive effects.
Keywords: Parkinson’s disease; levodopa; dyskinesia; NR2B subunit selective glutamate antagonist; CP-101,606; amnesia; dissociation
Introduction
Alterations in glutamate neurotransmission are hypothesized to underlie the motor complications that detract from the clinical efficacy of levodopa in Parkinson’s disease (PD) 1. Glutamate actions are mediated through at least three inotropic receptors (N-methyl-D-aspartate (NMDA), alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and kainate receptors and a family of metabotropic receptors.2. NMDA receptors are thought to be particularly important for synaptic neurotransmission and long-lasting changes in synaptic efficacy as in long-term potentiation (LTP) 3. NMDA receptors contain four subunits, two NR1 subunits for which there are 7 isoforms, and two NR2 subunits with four, A –D, variants. NMDA receptors with different subunit compositions are distributed in the nervous system in regionally specific patterns. NMDA receptors with NR2-B subunits are in highest concentration in motor cortical regions, hippocampus and striatum3. Subunit selective NMDA antagonists may offer selective glutamate effects for therapeutic advantage without the cognitive and psychiatric adverse effects encountered with nonselective glutamate antagonists.
In monkeys with MPTP-induced parkinsonism and in people with PD, a variety of alterations in binding, expression, distribution and phosphorylation of NMDA receptor subtypes have been associated with parkinsonism, with levodopa treatment and with motor complications 4,5,6,7. Nonselective NMDA antagonists as well as NR2B selective antagonists have been reported to augment the effects of levodopa in animal models of PD 8,9,10,11,12,13,14. Dyskinesia may also be suppressed by nonselective atagonists 15 and selective NMDA antagonists 16 and development of dyskinesia reduced during long-term treatment with levodopa 17.
CP-101,606 is a NR2B selective NMDA antagonist 18. CP-101,606 penetrates into the CNS as evidenced by CSF concentrations that are 90% of plasma levels 19. CP-101,606 is metabolized by cytochrome P450 2D6 and has a half-life of approximately 3.5 hours in humans with the extensive metabolizer phenotype.20. The drug has been tested for neuroprotective effects in humans with traumatic brain injury and subarachnoid hemorrhage 21,22,19 In these clinical trials, no clinically significant effects on vital signs, cardiac arrhythmias or mental function were encountered. CP101,606 has anti-parkinsonian actions as well as anti-dyskinesia effects in some but not all rat and monkey models of PD 12,23,14.
Based on these data suggesting the importance of NMDA receptor mediated glutamate neurotransmission in basal ganglia circuitry, the alterations of NMDA receptor function with PD and long-term levodopa therapy and the effects of NMDA antagonists in rat and monkey models of PD, we have studied the acute interaction of CP-101,606 and levodopa in PD subjects with levodopa-induced dyskinesia and motor fluctuations. Our primary hypothesis was that CP-101,606 would suppress dyskinesia.
METHODS
The study was a randomized, double-blind, placebo-controlled, crossover trial that examined the effects of placebo and low and high doses of CP101,606 on the response to two-hour infusions of levodopa. The protocol was approved by the Oregon Health & Sciences University (OHSU) Institutional Review Board.
Subjects
Subjects had idiopathic PD by the UK Parkinson’s Disease Brain Bank criteria 24) and no historical, physical examination, or laboratory findings suggestive of a different diagnosis 25. Subjects were on levodopa, had motor fluctuations and dyskinesia, and had had a stable PD medication regimen for at least 4 weeks before randomization. The subjects had dyskinesia scores of 2 or greater in at least one body part and a 10% or greater increase in finger tapping in response to levodopa. MMSE scores were greater than 24. Subjects had had no neurosurgical procedures for PD. None were taking amantadine. Because CP-101,606 is metabolized by cytochrome P450 2D6, subjects were screened for the slow metabolism alleles of P450 2D6. Poor or intermediate metabolizers were excluded from participation. CP 101,606 prolongs the QT interval and therefore prolongation of the QTcF beyond 500 msec was an exclusion criterion. Clinically significantly laboratory abnormalities, medical conditions and substance abuse were additional exclusion criteria.
Protocol
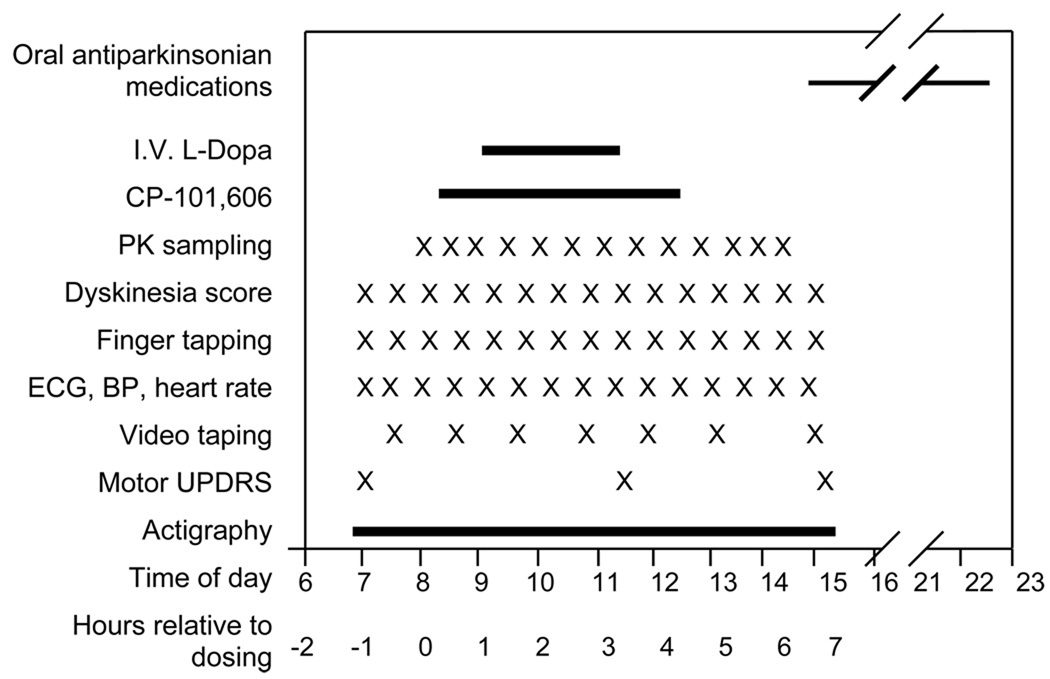
Subjects who met screening criteria were admitted to the OHSU General Clinical Research Center (GCRC) for four days. The day of admission, the subject practiced the various measures to be used in the study. The second to fourth day the subjects received in a balanced and randomized sequence, four-hour infusions of low dose CP-101,606, high dose CP-101,606 and placebo between 8AM (protocol time 0) and 12 noon (protocol time 4 hours). Doses were selected based on the pharmacokinetics of CP-101,606 observed in traumatic brain injury and pain programs with this compound 21,22,19. The “low” dose group targeted a plateau concentration of 60 ng/ml and the “high” dose targeted 200 ng/ml. In addition, on each study day the subject received a 1 mg/kg/hr infusion of levodopa between 9 and 11 AM (protocol hours 1 to 3). PD medications were held from 2200 the evening before each study day until 1500 the following day. The timing of various measurements is indicated in the study schematic [Figure 1].
Figure 1. Timing of protocol interventions and measurements.
The sample size for this pilot study was empirical, based on feasibility. The time points for primary and secondary efficacy endpoints were chosen based on two pharmacodynamic features of levodopa. First, dyskinesia and antiparkinsonian effects are not very dose sensitive 26 so that at peak levodopa concentrations (2.5 to 3.5 hours), any effects of CP-101,606 on dyskinesia and tapping would be less apparent. Second, duration of levodopa-induced antiparkinsonian effects is dose sensitive 26 and an interaction between CP 101,606 and levodopa would be most evident as an alteration of the duration (altered onset and offset) of the response to levodopa. The primary efficacy endpoint was the change in the Dyskinesia Rating Scale scores during the hour before CP-101,606 infusion was started (baseline) and the average of the dyskinesia scores 1.5 to 3 hours after the start of the CP-101,606 infusion to capture dyskinesia from onset to expected peak response. The secondary efficacy endpoint, tapping speed, an index of bradykinesia 27, was the change from baseline to the average of the tapping speeds at 3.5 and 4.0 hours after the start of the CP-101,606 infusion to capture lengthening of the antiparkinsonian actions of levodopa.
Measures
The finger tapping measures the number of times a subject can alternately tap with the index finger of their more affected arm, 2 counters 20 cm apart, in 1 minute 28. Dyskinesia was measured using the Dyskinesia Rating Scale (0–4), in 7 body parts (4 limbs, face, neck and trunk) 29. Tapping and dyskinesia were measured every 30 minutes from 0800 to 1400. Dyskinesia and bradykinesia (UPDRS finger and foot tapping tasks) scores were also derived from video tapes taken at approximately hourly intervals during and after the levodopa infusion and scored by a blinded rater post hoc. Actigraphs (accelerometers) were placed on both wrists to measure movements as an exploratory measure. Motor UPDRS was scored at 0800 before the CP-101,606 infusion was begun, at 1100 at peak plasma levodopa concentrations and at 1400 at the end of the study day measurements. Sitting blood pressure and heart rate were measured with an automated blood pressure machine. ECG monitoring was performed for 1 to 5 minutes each half hour. Blood samples for plasma CP-101,606 and levodopa concentrations were collected at 30 minute intervals through a separate intravenous line not used for infusion of either drug. Adverse events were collected from study nurse and investigators’ observations and patient reports.
Plasma concentrations of CP-101,606 and levodopa were measured by liquid chromatography combined with amperometric electrochemical detection by Bioanalytical Systems Inc.
Drugs
CP-101,606 was supplied as a liquid concentrate and prepared by the OHSU Investigational Pharmacy for infusion rates determined by subject weight. “ Low dose” CP 101,606 was administered at 0.25 mg/kg/hr for 2 hours followed by 0.12 mg/kg/hr for 2 hours, targeting a minimum plasma concentration of 60 ng/ml. “High dose” CP 101,606 was administered at 0.75 mg/kg/hr for 2 hours and then at 0.36 mg/kg/hr for 2 hours, targeting a minimum plasma concentration of 200 ng/ml 19. Matching placebo infusions were administered the control day. The study drug (CP-101,606 or placebo) were infused at a same infusion rates for each subject via a dedicated intravenous line.
Levodopa was prepared as a 1 mg/ml solution and delivered intravenously by a dedicated intravenous line at 1 mg/kg/hour for 2 hours each day, starting 1 hour after the CP-101,606 or placebo infusion was begun. Carbidopa, 25mg, was administered orally at study hours 0, 2 and 4.
Statistical Methods
The primary and secondary efficacy endpoints were analyzed using a linear mixed effects model with the following terms: sequence, subject, period, treatment, hour, treatment-by-hour, and residual error. The covariance structure for the within-subject residual error assumed compound symmetry. Since this was a repeated measures cross-over study, it had two time structures (3 periods and 15 half-hourly measurements) with a small number of subjects. The covariance structure for residual error was naturally rolled out into a single time structure as hours within each period instead of forming a Kronecker product of the two time profiles, in order to ensure that computational algorithms worked appropriately.
To evaluate safety, incidence and magnitude of ECG changes, adverse events, laboratory and vital sign abnormalities were measured. Standard safety analyses were performed using appropriate descriptive statistics.
RESULTS
Subjects
Eighteen patients recruited from OHSU outpatient clinics were screened, after giving written informed consent. Twelve patients met inclusion and exclusion criteria and participated in the inpatient portion of the study. The average age was 50 (range 51 to 75 years), eight were male and all were Caucasian. Average UPDRS motor score “off” was 27 (range 8 – 41) and MMSE was 28 (25–30).The subjects had had PD for 11 years (7 – 17), had received levodopa for 8 years (3 – 13). In addition to levodopa, nine subjects were using dopamine agonists, six entacapone, two anticholinergics and one selegiline. Body weights averaged 81 kg (range 61 to 115 kg) and BMIs averaged 27 (range 22 to 34).
Pharmacokinetics of CP 101,606 and levodopa
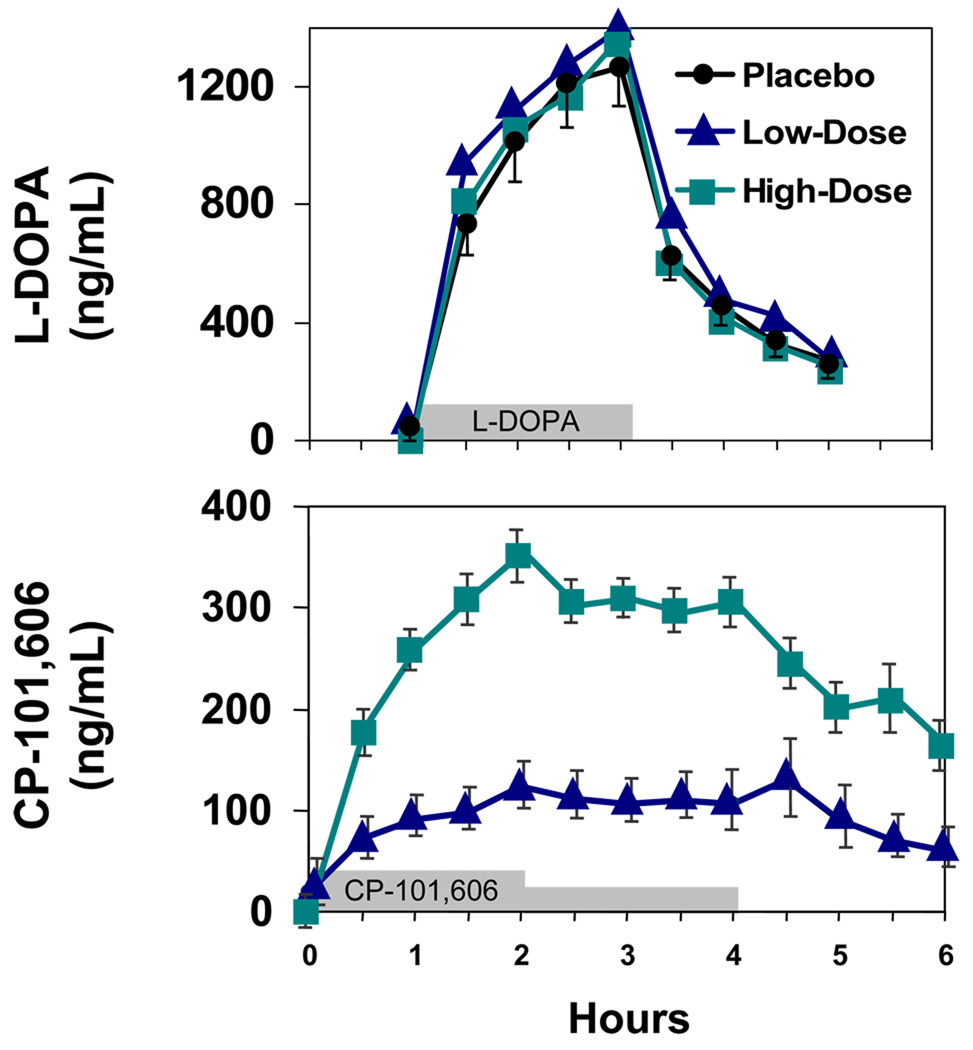
Plasma concentrations of CP-101,606 rose for the first two hours of infusion and were maintained at a relatively constant concentration for the subsequent two hours after the infusion rate was halved [Figure 2] The average plasma concentration for the second two hours of the infusions were 109 +/− SD ng/ml for the low infusion rate and 294 +/− SD ng/ml for the high infusion rate, consistent with the targeted concentrations.
Figure 2. Time course of plasma concentrations of levodopa and CP-101,606.
Mean and standard errors for plasma levodopa concentrations (upper panel) and CP-101,606 concentrations (lower panel). For clarity, standard errors for the levodopa concentrations are shown only for the day the concomitant infusion was placebo. The time of the drug infusions are represented by the bars on the abscissa. N = 8 tp 12 subjects at each time point.
Levodopa concentrations peaked at two hours at approximately 1300 ng/ml on all three days as intended with identical infusion rates all three days for each subject [Figure 2].
Effect of CP-101,606 on Dyskinesia
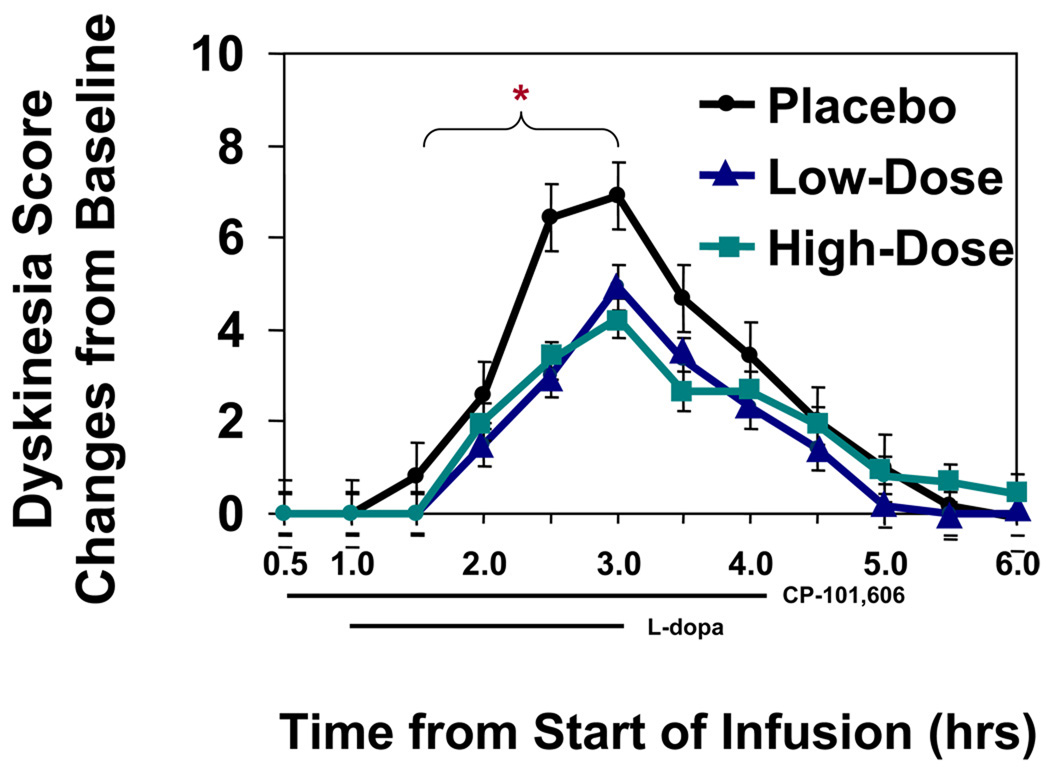
Low and high infusion rates of CP 101,606 suppressed the least squares mean of dyskinesia scores at 1.5 to 3 hours, the pre-specified primary endpoint, to 2.35 and 2.40 respectively from the least square mean of 4.19 for the placebo infusion. The mean and 95% confidence interval for treatment difference between the high dose group and placebo was −1.79[−2.57, −1.01], and the mean and 95% CI of the difference between the low-dose group and placebo was −1.83 [−2.62, −1.05]. Thus dyskinesia suppression by CP 101,606 was not dose dependent at the concentrations examined [Figure 3].
Figure 3. Time course of dyskinesia scores.
Least Squares Mean +/− SE of change in dyskinesia scores from baseline. The preplanned times for comparison of scores is indicated by the bracket. Placebo treatment differed from the two CP-101,606 treatments. N = 12
An exploratory measure of dyskinesia using the 0–4 scale for 7 body parts by a blinded rater was obtained from hourly videotapes between 10:30 AM and 12:30 PM of the subjects while sitting and performing a set routine of tasks from the motor UPDRS scale and undergoing a categorical naming exercise. Mean dyskinesia score dropped from 11.6 =+/− 3.5 (SD) with placebo to 8.5 +/− 4.3 and 6.5 +/− 3.8 with high and low dose CP101,606 respectively (p = 0.023)
Effect of CP-101,606 on Parkinsonism
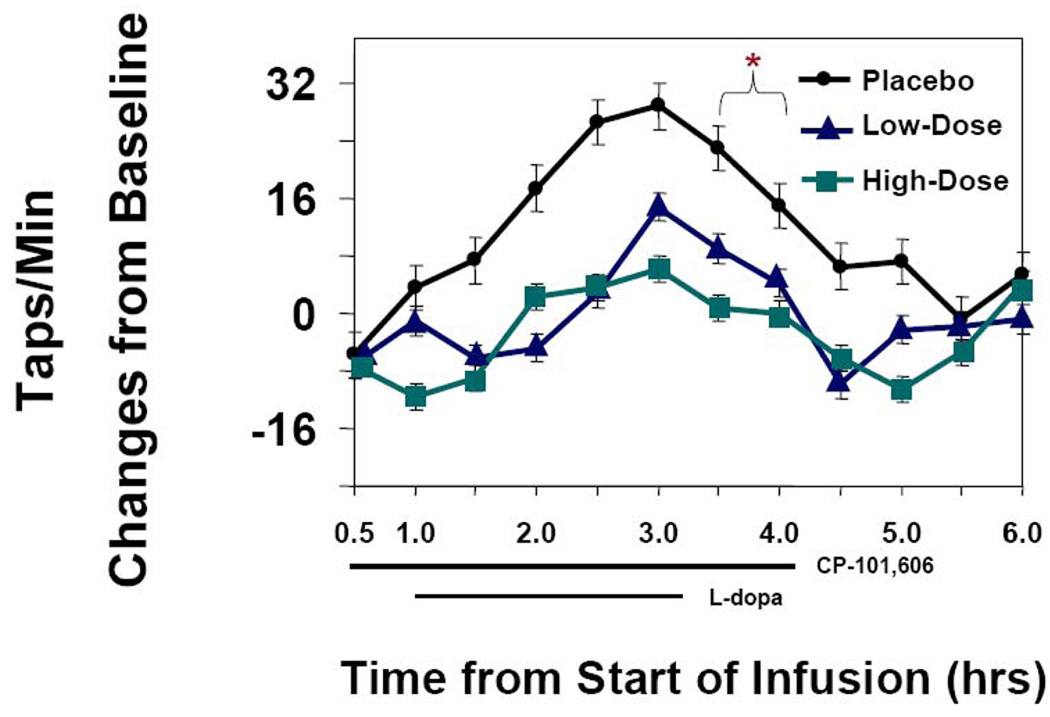
The levodopa-induced increase in speed of finger tapping from baseline (8 to 9 AM, study hours 0 to1) to study hours 3.5 to 4 was the endpoint for the effect of CP 101,606 on parkinsonism [Figure 4]. CP 101,606 reduced the increment from a least squares mean (SE) of 18.88 (4.61) for placebo to a LS mean (SE) of 6.58 (4.68) for the low-dose CP-101,606 and to a LS mean (SE) of 0.34 (4.68) for the high dose group The treatment difference and 95% confidence interval between the high dose group and placebo was −18.53 [−28.98, −8.08], and the difference and 95% CI between the low-dose group and placebo was −12.29 [−22.75, −1.84]. The difference between the low and high doses was not significant although there was the suggestion of a dose responsive effect of CP-101,606.
Figure 4. Time course of tapping speed.
Least Squares Mean +/− SE of change in tapping speed from baseline. The preplanned time of comparison of tapping scores is represented by the bracket. Placebo differed from the two CP-101,606 treatments. N = 12
Effects of CP 101,606 on motor UPDRS scores at peak response was a post hoc measure of effects of CP 101,606 on levodopa response. In contrast to the effects on tapping speed, the motor UPDRS at the end of the levodopa infusion, protocol hour 3, was improved by the levodopa infusion similarly on all three study days.(from a mean of 27 to 14)
A measure of bradykinesia from finger and foot tapping derived from the video of UPDRS tasks between 10:30 and 12:30 likewise did not demonstrate any difference between treatments. The mean of the UPDRS score for finger tapping and foot tapping (maximum score 16 for four limbs) was 10.1 +/− 2.0 (SD) for placebo, 10.5 +/− 2.5 for high dose CP-101,606 and 11.1 +/− 2.2 for low dose CP-101,606.
A final exploratory measure of movement was actigraphy which measured all movements of the more affected arm and could thus include voluntary and associated movements as well as tremor and dyskinesia. Actigraphy counts did not differ for the first hour of CP-101,606 infusion before the levodopa infusion started (8 to 9 AM) indicating that CP-101,606 by itself did not affect movements, as also indicated by tapping scores (Figure 4). However, between 10:30 and 11:30 at peak levodopa effects, the actigraphy counts were reduced from a mean of 18079 +/− 1659 (SE) for placebo treatment to 10882 +/− 1706 for high dose CP-101,606 and to 10725 +/− 1614 for low dose CP-101,606 (p < 0.0001), consistent with the tapping scores.
Adverse Events
Some combination of abnormal thinking, depersonalization and amnesia occurred in 10 subjects receiving high dose CP 101,606 and 5 subjects receiving low dose CP 101,606 [Table]. The abnormal thinking was often subtle, picked up by a visiting spouse or reported by the subject in retrospect when they found that they could not remember parts of the study day. Other adverse events were generally mild and related to study procedures rather than the experimental drug [Table 2].
Table.
Adverse Events
| Placebo | Low CP-101,606 | High CP-101,606 | |
|---|---|---|---|
| Abnormal Thinking | 0 | 5 | 10 |
| Phlebitis | 3 | 3 | 1 |
| Chest Pain | 1 | 0 | 0 |
| Abdominal Pain | 1 | 1 | 0 |
| Fall | 1 | 0 | 0 |
| Diarrhea | 0 | 0 | 1 |
Safety
CP 101,606 had no significant effects on blood pressure or on heart rate. No significant prolongation of the QT interval was detected with EKG. No significant changes in routine blood chemistries or in urine were noted during the study.
DISCUSSION
Pharmacokinetics
The CP-101,606 infusion rates produced plasma concentrations that were effective in preclinical studies and in studies on pain and depression in humans, ranging from 100 to 200 ng/ml (Unpublished results, Dr. Jaren Landen, Pfizer.Global Research and Development). The identical time-plasma levodopa concentration curves during the placebo and CP-101,606 infusions indicated that CP-101,606 had no effects on the peripheral pharmacokinetics of levodopa.
Dyskinesia
The NMDA NR2B subtype selective glutamate antagonist, CP-101,606, reduced dyskinesia as measured by clinical scoring during the study. This observation was confirmed by scoring of videotapes. These findings are consistent with several preclinical studies suggesting that NR2B glutamate antagonists could prevent development of dyskinesia or suppress dyskinesia 16,17,23. Importantly, the effects of CP 101,606 on dyskinesia were not dose related at the doses examined in our study, suggesting that lower doses of the drug might be equally efficacious in reducing dyskinesia.
Parkinsonism
The effects of CP-101,606 on the parkinsonism were more difficult to interpret. Tapping speed was reduced by CP-101,606 with a trend suggesting a dose response. Despite the fact that tapping speed generally correlates with measures of bradykinesia, 30,27 the improvement in the motor UPDRS at peak levodopa concentration was not reduced by CP-101,606. This observation suggests that the slowing of tapping speed might not be due to antagonism of the antiparkinsonian actions of levodopa but to the mental effects induced by CP-101,606. Video tape scoring of bradykinesia evaluated during tapping of fingers and toes as in the motor UPDRS tasks also did not find evidence of improvement or worsening of bradykinesia by CP-101,606. Actigraphy indicated less arm movement during the CP-101,606 treatments although this could represent a decrease in dyskinesia as well as in normal voluntary movements. The possible worsening of the parkinsonism and certainly, no improvement in the parkinsonism, is in contrast to numerous preclinical studies suggesting that NR2B subtype selective agents augmented the effects of levodopa 16,12,11,13,14. The differences in results from preclinical studies and this clinical study may be related to differences in the NMDA antagonists studied, the doses of the antagonists and levodopa employed in the trials and the differences between toxin-induced parkinsonism in animals and idiopathic parkinsonism in humans.
Cognitive Function
CP-101,606 was associated with dissociation, abnormal thinking and amnesia. Amnesia and dissociation are well recognized with ketamine, another glutamate antagonist used for anesthesia.31 Although it had been speculated that these side effects might be less with CP-101,60632,22, the high concentrations of NR2B receptors in the hippocampus probably explain these effects.3 The dissociation and amnesia with an NR2B antagonist in this study suggest that NR2B agonists might be useful in memory or post traumatic stress disorders.
Conclusion
Our study lends support to the hypothesis that NR2B glutamate antagonists may suppress dyskinesia but also indicates that the effects of NR2B glutamate antagonists on motor and cognitive function may limit the usefulness of this class of drug. However, the fact that the antidyskinetic effects were maximal with the low dose of CP-101,606 and the cognitive effects were dose responsive suggests that lower doses of CP-101,606 could yield an acceptable therapeutic ratio of benefit to adverse effects.
Acknowledgements
We thank the subjects for their voluntary participation in a demanding protocol. This project was funded by a grant from Pfizer Global Research and Development.
Footnotes
Trial registered at clinicaltrials.gov # NCT 00163085
Reference List
- 1.Chase TN, Bibbiani F, Oh JD. Striatal glutamatergic mechanisms and extrapyramidal movement disorders. Neurotox Res. 2003;5(1–2):139–146. doi: 10.1007/BF03033378. [DOI] [PubMed] [Google Scholar]
- 2.Meldrum BS. Glutamate as a neurotransmitter in the brain: review of physiology and pathology. J Nutr. 2000;130(4S Suppl):1007S–1015S. doi: 10.1093/jn/130.4.1007S. [DOI] [PubMed] [Google Scholar]
- 3.Loftis JM, Janowsky A. The N-methyl-D-aspartate receptor subunit NR2B: localization, functional properties, regulation, and clinical implications. Pharmacol Ther. 2003;97(1):55–85. doi: 10.1016/s0163-7258(02)00302-9. [DOI] [PubMed] [Google Scholar]
- 4.Dunah AW, Wang Y, Yasuda RP, Kameyama K, Huganir RL, Wolfe BB, et al. Alterations in subunit expression, composition, and phosphorylation of striatal N-methyl-D-aspartate glutamate receptors in a rat 6-hydroxydopamine model of Parkinson's disease. Mol Pharmacol. 2000;57(2):342–352. [PubMed] [Google Scholar]
- 5.Calon F, Rajput AH, Hornykiewicz O, Bedard PJ, Di Paolo T. Levodopa-induced motor complications are associated with alterations of glutamate receptors in Parkinson's disease. Neurobiol Dis. 2003;14(3):404–416. doi: 10.1016/j.nbd.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Hallett PJ, Dunah AW, Ravenscroft P, Zhou S, Bezard E, Crossman AR, et al. Alterations of striatal NMDA receptor subunits associated with the development of dyskinesia in the MPTP-lesioned primate model of Parkinson's disease. Neuropharmacology. 2005;48(4):503–516. doi: 10.1016/j.neuropharm.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Hurley MJ, Jackson MJ, Smith LA, Rose S, Jenner P. Immunoautoradiographic analysis of NMDA receptor subunits and associated postsynaptic density proteins in the brain of dyskinetic MPTP-treated common marmosets. Eur J Neurosci. 2005;21(12):3240–3250. doi: 10.1111/j.1460-9568.2005.04169.x. [DOI] [PubMed] [Google Scholar]
- 8.Klockgether T, Truski L. NMDA antagonists potentiate antiparkinsonian actions of L-dopa in monoamine-depleted rats. Ann Neurol. 1990;28:539–546. doi: 10.1002/ana.410280411. [DOI] [PubMed] [Google Scholar]
- 9.Greenamyre JT, Eller RV, Zhang Z, Ovadia A, Kurlan R, Gash DM. Antiparksonian effects of remacemide hydrochloride, a glutamate antagonist, in rodent and primate models of Parkinson's disease. Ann Neurol. 1994;35:655–661. doi: 10.1002/ana.410350605. [DOI] [PubMed] [Google Scholar]
- 10.Nash JE, Hill MP, Brotchie JM. Antiparkinsonian actions of blockade of NR2B-containing NMDA receptors in the reserpine-treated rat. Exp Neurol. 1999;155(1):42–48. doi: 10.1006/exnr.1998.6963. [DOI] [PubMed] [Google Scholar]
- 11.Nash JE, Fox SH, Henry B, Hill MP, Peggs D, McGuire S, et al. Antiparkinsonian actions of ifenprodil in the MPTP-lesioned marmoset model of Parkinson's disease. Exp Neurol. 2000;165(1):136–142. doi: 10.1006/exnr.2000.7444. [DOI] [PubMed] [Google Scholar]
- 12.Steece-Collier K, Chambers LK, Jaw-Tsai SS, Menniti FS, Greenamyre JT. Antiparkinsonian actions of CP-101,606, an antagonist of NR2B subunit-containing N-methyl-d-aspartate receptors. Exp Neurol. 2000;163(1):239–243. doi: 10.1006/exnr.2000.7374. [DOI] [PubMed] [Google Scholar]
- 13.Loschmann PA, De Groote C, Smith L, Wullner U, Fischer G, Kemp JA, et al. Antiparkinsonian activity of Ro 25–6981, a NR2B subunit specific NMDA receptor antagonist, in animal models of Parkinson's disease. Exp Neurol. 2004;187(1):86–93. doi: 10.1016/j.expneurol.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 14.Nash JE, Ravenscroft P, McGuire S, Crossman AR, Menniti FS, Brotchie JM. The NR2B-selective NMDA receptor antagonist CP-101,606 exacerbates L-DOPA-induced dyskinesia and provides mild potentiation of anti-parkinsonian effects of L-DOPA in the MPTP-lesioned marmoset model of Parkinson's disease. Exp Neurol. 2004;188(2):471–479. doi: 10.1016/j.expneurol.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Verhagen L, Del Dotto P, van den Mundkhof P, Fang J, Mouradian MM, Chase TN. Amantadine as treatment for dyskinesias and motor fluctuations in Parkinson's disease. Neurology. 1998;50:1323–1326. doi: 10.1212/wnl.50.5.1323. [DOI] [PubMed] [Google Scholar]
- 16.Blanchet PJ, Konitsiotis S, Whittemore ER, Zhou ZL, Woodward RM, Chase TN. Differing effects of N-methyl-D-aspartate receptor subtype selective antagonists on dyskinesias in levodopa-treated 1-methyl-4-phenyl-tetrahydropyridine monkeys. J Pharmacol Exp Ther. 1999;290(3):1034–1040. [PubMed] [Google Scholar]
- 17.Hadj TA, Gregoire L, Darre A, Belanger N, Meltzer L, Bedard PJ. Effect of a selective glutamate antagonist on L-dopa-induced dyskinesias in drug-naive parkinsonian monkeys. Neurobiol Dis. 2004;15(2):171–176. doi: 10.1016/j.nbd.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 18.Chenard BL, Bordner J, Butler TW, Chambers LK, Collins MA, De Costa DL, et al. (1S,2S)-1-(4-hydroxyphenyl)-2-(4-hydroxy-4-phenylpiperidino)-1-propanol: a potent new neuroprotectant which blocks N-methyl-D-aspartate responses. J Med Chem. 1995;38(16):3138–3145. doi: 10.1021/jm00016a017. [DOI] [PubMed] [Google Scholar]
- 19.Yurkewicz L, Weaver J, Bullock MR, Marshall LF. The effect of the selective NMDA receptor antagonist traxoprodil in the treatment of traumatic brain injury. J Neurotrauma. 2005;22(12):1428–1443. doi: 10.1089/neu.2005.22.1428. [DOI] [PubMed] [Google Scholar]
- 20.Pfizer Global research and Development. Investigator's Brochure: CP-101,606. 2004 Ref Type: Report. [Google Scholar]
- 21.Bullock MR, Merchant RE, Carmack CA, Doppenberg E, Shah AK, Wilner KD, et al. An open-label study of CP-101,606 in subjects with a severe traumatic head injury or spontaneous intracerebral hemorrhage. Ann N Y Acad Sci. 1999;890:51–58. doi: 10.1111/j.1749-6632.1999.tb07980.x. [DOI] [PubMed] [Google Scholar]
- 22.Merchant RE, Bullock MR, Carmack CA, Shah AK, Wilner KD, Ko G, et al. A double-blind, placebo-controlled study of the safety, tolerability and pharmacokinetics of CP-101,606 in patients with a mild or moderate traumatic brain injury. Ann N Y Acad Sci. 1999;890:42–50. doi: 10.1111/j.1749-6632.1999.tb07979.x. [DOI] [PubMed] [Google Scholar]
- 23.Wessell RH, Ahmed SM, Menniti FS, Dunbar GL, Chase TN, Oh JD. NR2B selective NMDA receptor antagonist CP-101,606 prevents levodopa-induced motor response alterations in hemi-parkinsonian rats. Neuropharmacology. 2004;47(2):184–194. doi: 10.1016/j.neuropharm.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 24.Gibb WRG, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson's disease. J Neurol Neurosurg Psychiatry. 1988;51:745–752. doi: 10.1136/jnnp.51.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gelb DJ, Oliver G, Gilman S. Diagnostic criteria for Parkinson's disease. Arch Neurol. 1999;56:33–39. doi: 10.1001/archneur.56.1.33. [DOI] [PubMed] [Google Scholar]
- 26.Metman LV, van den Mundkhof P, Klaassen AAG, Blanchet PJ, Mouradian MM, Chase TN. Effects of supra-threshold levodopa doses on dyskinesia in advanced Parkinson's disease. Neurology. 1997;49:711–713. doi: 10.1212/wnl.49.3.711. [DOI] [PubMed] [Google Scholar]
- 27.van Hilten JJ, Wagemans EAH, Ghafoerkhan SF, van Laar T. Movement characteristics in Parkinson's disease: determination of dopaminergic responsiveness and threshold. Clin Neuropharmacol. 1997;20:402–408. [PubMed] [Google Scholar]
- 28.Nutt JG, Woodward WR, Hammerstad JP, Carter JH, Anderson JL. The "On-off" phenomenon in Parkinson's disease: Relation to levodopa absorption and transport. N Engl J Med. 1984;310:483–488. doi: 10.1056/NEJM198402233100802. [DOI] [PubMed] [Google Scholar]
- 29.Nutt JG, Woodward WR, Hammerstad JP, Carter JH, Anderson JL. "On-off" phenomenon in Parkinson's disease. N Engl J Med. 1984;311:193–194. doi: 10.1056/NEJM198402233100802. [DOI] [PubMed] [Google Scholar]
- 30.van Laar T, Jansen EN, Essink AW, Neef C, Dosterloo S, Roos RA. A double-blind study of the efficacy of apomorphine and its assessment in 'off'-periods in Parkinson's disease. Clin Neurol Neurosurg. 1993;95:231–235. doi: 10.1016/0303-8467(93)90128-4. [DOI] [PubMed] [Google Scholar]
- 31.Newcomer JW, Krystal JH. NMDA receptor regulation of memory and behavior in humans. Hippocampus. 2001;11(5):529–542. doi: 10.1002/hipo.1069. [DOI] [PubMed] [Google Scholar]
- 32.Chazot PL. CP-101606 Pfizer Inc. Curr Opin Investig Drugs. 2000;1(3):370–374. [PubMed] [Google Scholar]