Abstract
Inflammation and damage promote monocyte adhesion to endothelium and cardiovascular disease (CVD). Elevated inflammation and increased monocyte-endothelial cell interactions represent the initial stages of vascular remodeling associated with a multitude of cardiovascular diseases. Cathepsins are proteases produced by both cell types that degrade elastin and collagen in arterial walls, and are upregulated in cardiovascular disease. We hypothesized that the inflammatory cytokine tumor necrosis factor alpha (TNFα) and monocyte binding would stimulate cathepsins K and V expression and activity in endothelial cells, that may be responsible for initiating local proteolysis during cardiovascular disease. Confluent human aortic endothelial cells (HAECs) were stimulated with TNFα or THP-1 monocyte co-cultures, and multiplex cathepsin zymography was used to detect changes in levels of active cathepsins K, L, S, and V. Direct monocyte-endothelial cell co-cultures stimulated with TNFα generated maximally observed cathepsin K and V activities compared to either cell type alone (n=3, p<0.05) by a c-Jun N-terminal kinase (JNK) dependent manner. Inhibition of JNK with SP6000125 blocked upregulation of cathepsin K activity by 49% and cathepsin V by 81% in endothelial cells. Together, these data show that inflammatory cues and monocyte-endothelial cell interactions upregulate cathepsin activity via JNK signaling axis and identify a new mechanism to target towards slowing the earliest stages of tissue remodeling in cardiovascular disease.
Keywords: cathepsins, atherosclerosis, monocytes, endothelial cells, inflammation
Introduction
In atherosclerosis and other cardiovascular disease, endothelial cells initiate vascular responses to inflammatory cytokines, such as tumor necrosis factor alpha (TNFα). Activation of the endothelium results in increased surface expression of cell adhesion molecules and secretion of powerful chemokines essential for the recruitment of circulating monocytes to the vascular wall [1]. Once adhered, physical, paracrine, and juxtacrine signaling between monocytes and endothelial cells arrest monocytes along the endothelium, and permit transmigration to the subendothelial space [2,3]. Ultimately, these inflammatory pathologies will cue extensive vascular remodeling and luminal narrowing as atherosclerotic plaques form.
Collagen and elastin are two important extracellular matrix proteins degraded by cathepsins during atherosclerotic lesion formation [4–6]. Cathepsins are a family of cysteine proteases that have been highly implicated in cardiovascular disease [7,5], and endothelial cells contribute to their production and this pathology [8,6]. Cathepsins K and V, in particular, have gained attention due to their potent proteolytic activity. Cathepsin K is both the most potent human collagenase identified, as well as an extremely powerful elastase [9], and has been shown to be highly expressed in atherosclerotic lesions [10,6]. Cathepsin V is the most powerful mammalian elastase yet identified, and is expressed in human monocyte-derived macrophages [11]. Studies have shown that the human cathepsin V homolog, murine cathepsin L [12,13], significantly contributes to cardiovascular disease in mouse models [14,15]. However, elucidating the contributions of specific cell types and their stimulation of each other is still an important goal to treat this disease.
We have previously shown that endothelial cells play an important role in proteolytic vascular remodeling via production of cathepsins in response to altered hemodynamics and disturbed flow [8,6], but the influence of inflammatory cytokines and communication between newly infiltrated monocytes and the resident vascular cells on proteolytic activity have not been explored. In this study, effects of TNFα stimulation and monocyte binding on cathepsin production and activity by human aortic endothelial cells was investigated to understand early stimuli that promote proteolytic remodeling of the arterial wall using a multiplex cathepsin zymography technique to simultaneously quantify cathepsins K, L, S, and V expression levels of active enzyme [16,17]. Furthermore, we investigated key phosphorylation of Akt, ERK 1/2, JNK, and c-jun to identify intracellular signaling cascades linking TNFα stimulation and monocyte binding to increased levels of cathepsins K and V activity to suggest a mechanism for pharmaceutical targeting.
Materials and Methods
Cell Culture
Human aortic endothelial cells (HAECs) (Lonza) were cultured in MCDB medium 131 (Mediatech) containing 10% fetal bovine serum (FBS), 1% L-glutamine, 1% penicillin/streptomycin, and 1% endothelial cell growth serum (ECGS). Human THP-1 acute monocytic leukemia cells (American Type Culture Collection [ATCC]) were cultured in RPMI medium 1640 (Mediatech) containing 10% FBS, 0.05% β-mercaptoethanol, 1% L-glutamine, and 1% penicillin/streptomycin. HAECs were transfected with cathepsin K overexpression plasmids on the pCMVSport6 background at 50–80% confluence with Lipofectin (Invitrogen) in OptiMEM according to manufacturer’s instructions.
Monocyte adhesion and endothelial cell co-cultures
HAECs were preconditioned in the presence or absence of 10 ng/mL recombinant human TNFα (Invitrogen) and cultured for 4 hours prior to adding 500,000 monocytes/ml. THP-1 monocytes were allowed to adhere for 45 minutes prior to washing with PBS, and co-cultures adhered to HAECs were maintained for an additional 20 hours. Indirect co-cultures were generated by suspending 500,000 monocytes/mL above pre-stimulated HAECs using a transwell insert with a 0.2μm pore size for 20 hours. For TNFα blocking studies, HAECs were stimulated with growth media containing 10 ng/mL TNFα combined with either 10 μg/mL anti-TNFα antibody (R&D Systems) or 10 μg/mL isotype control (R&D Systems) for 24 hours prior to collecting cell lysates. For JNK inhibition studies, HAECs were preconditioned with 10 μg/mL of SP6000125 (EMD Biosciences) for one hour prior to addition of THP-1s and co-culture.
Multiplex cathepsin zymography
Cathepsin zymography was performed as described previously [17]. Briefly, cell lysates were collected using zymography lysis buffer (20 nM Tris–HCl at pH 7.5, 5 mM EGTA, 150 mM NaCl, 20 mM β-glycerol-phosphate, 10 mM NaF, 1 mM sodium orthovanadate, 1% Triton X-100, 0.1% Tween-20) with 0.1 mM leupeptin freshly added to stabilize enzymes during electrophoresis and lysates were collected and cleared by centrifugation. Specific cathepsin species were resolved by 12.5% SDS–polyacrylamide gels containing 5mg/mL gelatin at 4 °C. Gels were removed and enzymes renatured in 65 mM Tris buffer, pH 7.4 with 20% glycerol, followed by incubation in activity buffer (0.1 M sodium phosphate buffer, pH 6.0, 1 mM EDTA, and 2 mM DTT freshly added). For determination of cathepsin V band, a 0.1 M sodium acetate buffer of pH 4 was used. The gels were rinsed once with deionized water and incubated for 1 h in Coomassie stain (10% acetic acid, 25% isopropanol, 4.5% Coomassie Blue) followed by destaining (10% isopropanol and 10% acetic acid). Gels were imaged using an ImageQuant LAS 4000, and cathepsins K, V, L, and S can be visualized, in that order of decreasing apparent molecular weight. Images were inverted in Adobe Photoshop and densitometry was performed using Scion Image.
In situ zymography
Co-cultures of HAECs and THP-1 monocytes were prepared as above; after the 20 hour incubation time, cultures were rinsed with PBS and incubated in zymography assay buffer (0.1M sodium phosphate buffer, 1mM EDTA, 2mM DTT, pH 6.0) containing 0.5mM Z-GPR-MβNA (Enzo) and 1mM 5-nitrosalicylic acid (Sigma). To isolate cathepsin K signal, serine proteases were inhibited with 1mM PMSF (Sigma), matrix metalloproteinases (MMPs) were inhibited with 10 mM EDTA (Sigma), and cathepsin B was inhibited with CA-074 (EMD Biosciences). 5μM of the broad-spectrum cathepsin inhibitor, E-64 (EMD Biosciences), was added for negative controls. Cultures were incubated for 8 hours, washed, and imaged using a Nikon Ti-E™ fluorescent microscope. Fluorescence was quantified by averaging pixel intensity across images of a given area using ImageJ.
Phosphorylated kinase analysis with Bioplex
HAEC or co-culture lysates were prepared according to Bioplex instructions (BioRad), and beads conjugated with antibodies for phosphorylated Akt, extracellular signal-regulated kinases 1 and 2 (ERK 1/2), c-Jun NH2-terminal kinase (JNK), and c-Jun (BioRad) were incubated overnight, followed by labeling with biotinylated secondary antibodies for 1 hour, then with avidin/streptavidin conjugated with phycoerythrin. Phosphorylated kinase levels were measured using a BioPlex 200 System (BioRad).
Statistical Analysis
Each experimental condition was repeated with a minimum of three biological replicates and each data point is presented as the mean value and standard error of the mean. Representative images are shown. Unpaired student t-tests were used to determine statistical significance (*p<0.05) between experimental groups.
Results
TNFα and monocyte adhesion differentially induce cathepsins K and V activity
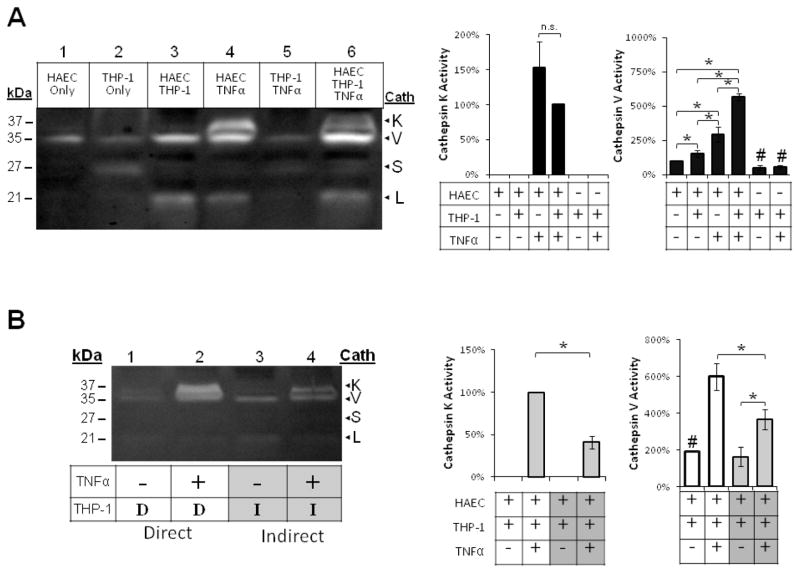
To determine how TNFα and monocyte interactions, individually and cooperatively, regulate cathepsin activity in large artery endothelial cells, we co-cultured human aortic endothelial cells (HAECs) and THP-1 monocytes, as described in the Materials and Methods. TNFα-stimulated mature cathepsin K expression and activity (37 kDa) in HAECs and HAEC/monocyte co-cultures, and also increased cathepsin V expression and activity (35 kDa) by two-fold (Fig 1A; n=3, p<0.05). THP-1 monocytes alone did not stimulate cathepsin K activity, but co-culture with endothelial cells stimulated a 50% increase in cathepsin V activity (Fig 1A lane 3). TNFα and co-culturing with THP-1 monocytes stimulated a 460% increase in cathepsin V active enzyme compared to HAEC controls (Fig 1A lane 6; n=3, p<0.05).
Fig 1.
TNFα and direct monocyte adhesion induced cathepsin K and V activities in endothelial cell-monocytes co-cultures. Endothelial cells, THP-1 monocytes, and co-cultures were conditioned with 10ng/mL TNFα. Monocytes were allowed to interact either (A) directly (indicated by “D”), or (B) indirectly, suspended above in a Transwell insert with a 0.2μm pore size (indicated by “I”). (A) Cell lysates were collected and loaded for cathepsin zymography. Cathepsin K active enzyme bands were quantified with densitometry and normalized to HAEC, THP-1, TNFα samples, and cathepsin V active enzyme bands were normalized to unstimulated endothelial cell controls (n=7, *p<0.05, # represents significant difference from EC control, SEM bars shown). (B) Lysates from Transwell cultures were also collected and loaded for zymography and active enzyme quantified with densitometry (n=3, *p<0.05, SEM bars shown).
In order to ascertain if the increased active cathepsin observed in the co-cultures was mediated by direct monocyte-endothelial cell contacts, paracrine factors, or some combination of both, we implemented a transwell culture system permitting exchange of soluble factors between the cell types, while being physically separated by a 0.22 μm pore size filter. Indirect communication between monocytes and endothelial cells failed to increase cathepsin V activity as high as direct contact cultures; additionally, there was no detectable cathepsin K activity without TNFα stimulation (Fig 1B).
TNFα is sufficient to turn on cathepsin K activity in endothelial cells
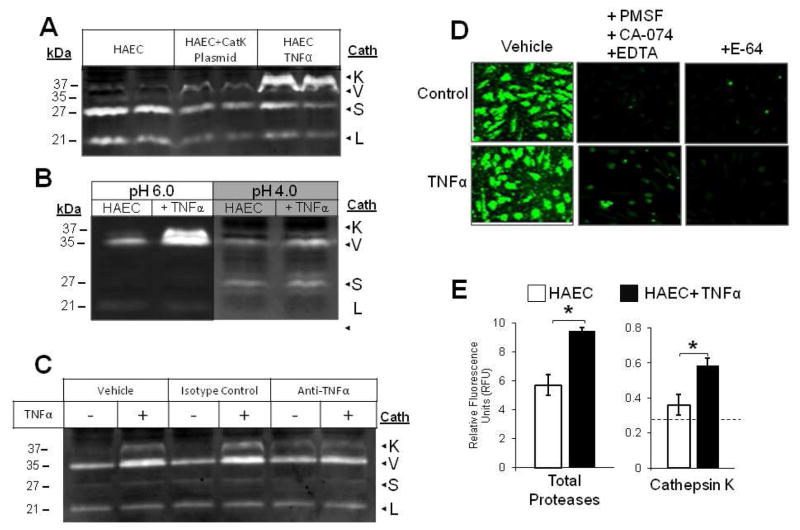
To confirm the identity of the apparent TNFα-dependent, 37kDa active band as cathepsin K, HAECs were transfected with CMVSport6 plasmid with cathepsin K gene to drive constitutive overexpression. We achieved 25% transfection efficiency as estimated from parallel transfections with GFP vector with same concentration and protocol (data not shown). Lysates from transfected HAECs were loaded for zymography in the same gel as lysates from HAECs stimulated with TNFα or vehicle, and results are shown in figure 2. Transfected HAECs displayed an active band at the same electrophoretic migration distance as that of HAECs stimulated with TNFα, and with greater intensity than control cells confirming the 37kDa band as cathepsin K (Fig 2A). Further confirmation was achieved with an exclusionary cathepsin zymography modification; we previously demonstrated that lowering the pH from 6 to 4 during overnight incubation selects for cathepsin V activity and reduces the cathepsin K signal [17]. When incubated at pH 4, the upper 37 kDa band intensity diminished in the TNFα-stimulated samples, but cathepsin V (35 kDa) signal remained detectable under both conditions (Fig 2B) confirming the upper band as cathepsin K.
Fig 2.
TNFα turns on cathepsin K activity in endothelial cells (A) ECs were transfected with cathepsin K gene on pCMVSport6 to drive overexpression. Cell lysates collected from HAECs treated with and without 10ng/mL TNFα, and ECs transfected with cathepsin K plasmid were lysed, prepared, and loaded for cathepsin zymography. (B) Cell lysates collected from HAECs treated with and without 10 ng/mL TNFα were incubated in assay buffer of pH 4 or 6 to observe the disappearance of the 37 kDa cathepsin K band at pH 4. The cathepsin K bands and TNFα stimulated bands appeared at the same molecular weight in the zymogram. (C) HAECs were stimulated with 10ng/mL TNFα combined with either 10μg/mL anti-TNFα antibody, or isotype controls. Cell lysates were collected and cathepsin activity was assessed via gelatin zymography. (D) For in situ zymography, endothelial cells treated with or without 10 ng/mL TNFα were incubated in zymography assay buffer containing 1mM 5-NSA and 0.5 mM Z-GPR-MβNA only or 10mM EDTA, 2mM DTT, 1mM PMSF, and 10μM CA-074 to select for cathepsin K activity, endothelial cells were also treated with 5μM E-64 to block all cathepsin activity. (E) Fluorescent images of cultures were taken and mean fluorescence intensity for total fluorescent signal and cathepsin K specific cathepsin activity was quantified
Next, we sought to confirm that cathepsin K activity in endothelial cells was dependent on TNFα stimulation. HAECs were conditioned for 24 hours in media containing 10 ng/mL TNFα and either 10 μg/mL anti-TNFα antibody or isotype control, and cell lysates were collected. As expected, TNFα stimulated robust cathepsin K activity, while co-incubation with the blocking antibody substantially reduced activity levels (Fig 2C). These data, taken with the observed responses in Figure 1, indicate that TNFα is sufficient and necessary to stimulate cathepsin K activity in these studies.
Quenched, fluorescent synthetic substrates are commonly used to quantify the activity of cathepsin family members in cells and in vitro studies [18–20], and we used this method to identify TNFα stimulated cathepsin K activity in situ as increased fluorescence captured by microscopy. After HAECs were stimulated with TNFα, culture media was replaced with zymography assay buffer containing the cathepsin K cleavable substrate Z-GPR-MβNA (5 μM), and fluorescent images were captured. To select for the cathepsin K activity among other proteases that can cleave this substrate, parallel cultures were inhibited with 5 μM E-64 to block all cathepsin activity or with a protease inhibitor cocktail (10 μM CA-074, 1 mM phenylmethanesulfonylfluoride (PMSF), and 10 mM EDTA to inhibit cathepsin B, serine proteases, and matrix metalloproteinases, respectively) thereby identifying the residual activity as cathepsin K. TNFα stimulation increased total fluorescent intensity, and more importantly, the fluorescence due to cathepsin K seen after incubation with the protease inhibitor cocktail (Fig 2D). E-64 incubation significantly reduced fluorescent intensity as expected, as shown in the picture and indicated by the dashed line on the graph (Fig 2D,E).
TNFα stimulation and monocyte interactions with endothelial cells increased JNK and Akt phosphorylation
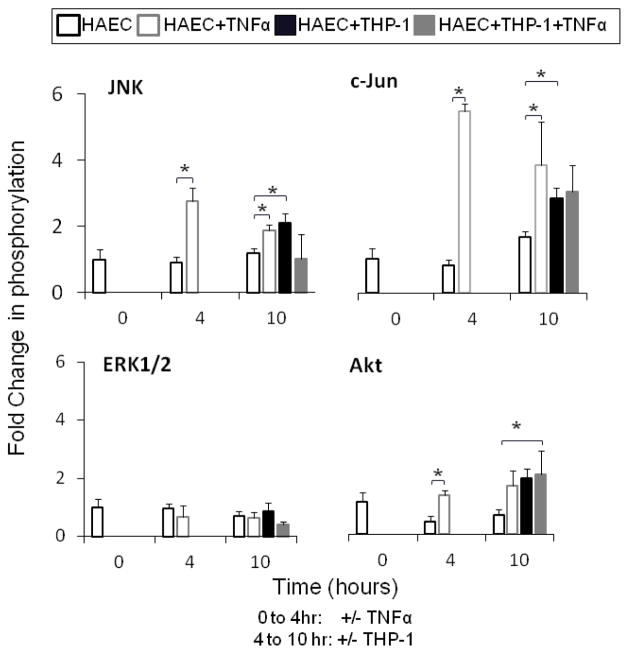
Next, the intracellular signal cascades initiated by TNFα and THP-1 monocyte adhesion, which appeared to have increased cathepsin K and V activities, were investigated at baseline (0 hours), after TNFα stimulation and monocyte binding (4 hours), and six hours of co-culture (10 hours). Co-cultures were maintained for 6 hours instead of 20, to shorten the length of time between stimulation and analysis to quantify the phosphorylated kinase signal before it was quiesced. Cell lysates were analyzed for phosphorylation of Akt, ERK1/2, JNK, and c-Jun using Bioplex technology, and results shown in figure 3. JNK and its downstream signaling protein substrate, c-Jun showed the greatest activation in response to TNFα stimulation by 2.8 and 5.3 fold, respectively (Fig 3, n=3, p<0.01). Akt phosphorylation was significantly increased by TNFα stimulation and monocyte binding (Fig 3, n=3, p<0.01). There were no changes in ERK 1/2 phosphorylation in any condition for all time points measured (Fig 3).
Fig 3.
TNFα and monocytes interactions increase JNK and Akt phosphorylation. Confluent HAECs and co-cultures were pre-conditioned with 10ng/mL TNFα prior to monocyte adhesion as described earlier. HAEC and co-culture cell lysates were collected for kinase analysis using the BioPlex 200™ machine that uses Luminex technology. Kinase lysates were collected prior TNFα stimulation (0 hour), 4 hours post stimulation (4 hours), and then after another 6 hours of co-culture with monocytes (10 hours). Levels of phosphorylated (A) ERK1/2, Akt, JNK, and c-Jun were measured and phosphorylated protein signal was normalized to unstimulated EC control (n=3, *p<0.05, SEM bars shown)
JNK inhibition significantly decreased TNFα and THP-1 monocyte induced cathepsin K and V activities
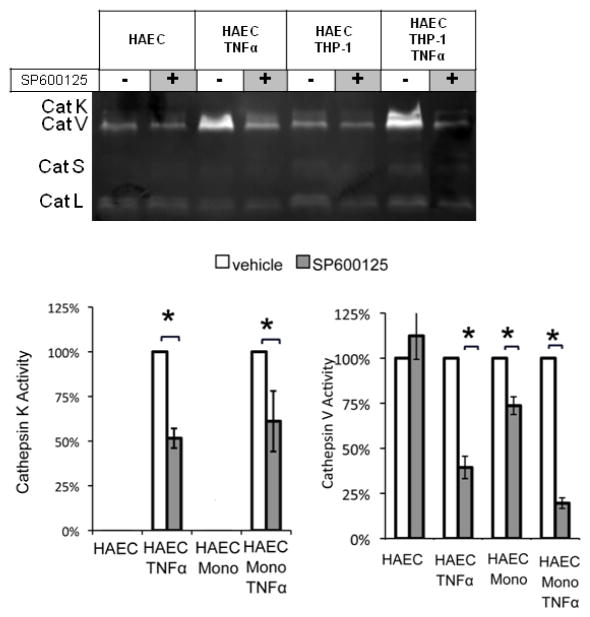
Since TNFα stimulation of HAECs increased cathepsin K and V activities, and JNK and c-Jun were highly activated in response, we next tested the hypothesis that inhibiting JNK pathway would reduce cathepsin K and V activity. Endothelial cells were incubated for 1 hour with the JNK inhibitor SP6000125 (10 μM), followed by stimulation with 10ng/mL TNFα or vehicle for 4 hours, and co-culture with THP-1 monocytes. Inhibition of JNK significantly reduced cathepsin K active enzyme by 49% in HAEC cultures stimulated with TNFα and by 39% in co-cultures stimulated with TNFα (Fig 4; n=3, p<0.05). In the absence of TNFα stimulation, there was no detectable cathepsin K activity. A similar effect was observed for cathepsin V; JNK inhibition reduced TNFα stimulated active cathepsin V by 60% (n=3, p<.005) in HAECs, by 27% in co-cultures (n=3, p<0.005), and by 81% in TNFα stimulated co-cultures (n=3, p<0.001) (Fig 4).
Fig 4.
Cathepsins K and V activities induced by THP-1 monocytes are significantly reduced by JNK inhibition with SP6000125. (A) HAECs were incubated with or without 10μM of SP6000125 for 1 hour, followed by conditioning with TNFα or vehicle for 4 hours. THP-1 monocytes were subsequently added, non-adhered cells were removed, and co-cultures were maintained for an additional 20 hours. Cell lysates were collected analyzed via cathepsin zymography. (B) Densitometric analysis quantified cathepsin K and cathepsin V activity (n=3, *p<0.05, SEM bars shown)
Discussion
Increased cathepsin activity has been linked to tissue destructive mechanisms in the cardiovascular system including atherosclerotic elastic lamina degradation [21,6,10], stent restenosis [22,23], abdominal aortic aneurysm formation [24], and heart valve remodeling under hypertensive conditions [8]. The identification of TNFα and monocyte adhesion as both separate and partnering mediators of cathepsins K and V activation in endothelial cells via JNK signaling provides new insight into the initiation of proteolytic remodeling in cardiovascular diseases. While the progression of arterial remodeling in atherosclerosis is well described, little is known of the initial degradation or breaks in elastic lamina that will later result in smooth muscle cell phenotypic switch and migration into neointimal space to initiate lesion formation. Here we propose that induction of cathepsin expression and mature, active cathepsins by monocyte binding to endothelial cells during the earliest steps participates in this initial elastin proteolysis.
Indirect contact between the two cell types increased cathepsin activity, but direct monocyte-endothelial cell contact induced even higher levels of active cathepsins K and V activity, even in the presence of TNFα, suggesting that juxtacrine communication is involved. Pro-TNFα present on monocyte plasma membranes is proteolytically cleaved to release the soluble cytokine [25]. Soluble TNFα then binds primarily to TNFR1 with low affinity for TNFR2, but membrane bound pro-TNFα has greater affinity for TNFR2 [26]. The direct contact between monocytes and endothelial cells may place the pro-TNFα on monocyte surfaces in close enough contact to ligate TNFR2 on endothelial cell surfaces, which may be a mechanism to explain the elevated induction of cathepsin activity with direct vs. indirect contact co-cultures (Fig 1B). Stimulation of either TNFR1 or TNFR2 pathway with soluble TNFα or pro-TNFα on monocyte surfaces may explain the differential regulation of cathepsins K and V in these results, but further studies are still needed.
The significant effect of JNK inhibition (Fig 4) on reducing cathepsin K and V activity in the co-cultures and after TNFα stimulation implicates JNK signaling cascade as a potentially successful target for therapeutic intervention. Although JNK inhibition has been shown to block ICAM-1 expression [27,28], our studies did not show a reduction in monocyte adhesion after culturing with the JNK inhibitor, SP6000125 (data not shown) but did reduce cathepsin activity in response. It was shown previously that the transcription factor AP-1, comprised of the subunits c-fos and c-Jun, a target of JNK, stimulates cathepsin K promoter activity in macrophages [29], so the link shown here between JNK activation downstream of TNFα stimulation and cathepsin K and V induction may involve AP-1 as well and further investigation of these pathways may be informative for reducing proteolysis during cardiovascular disease progression due to multiple cell types and their heterotypic interactions.
Acknowledgments
The authors of this study would like to thank Eric Kopfle, Alex Miller, and Sindhuja Surapeneni for assistance with data collection. This work was funded by Georgia Tech startup funds and NIH New Innovator grant #1DP2OD007433-01 (M.O.P.). The project described was supported by Award Number DP2OD007433 from the Office of the Director, National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Office of the Director, National Institutes of Health or the National Institutes of Health. P.M.K. was supported by an NSF graduate research fellowship.
References
- 1.Mestas J, Ley K. Monocyte-endothelial cell interactions in the development of atherosclerosis. Trends Cardiovasc Med. 2008;18(6):228–232. doi: 10.1016/j.tcm.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belcher J, Marker P, Weber J, Hebbel R. Activated monocytes in sickle cell disease: potential role in the activation of vascular endothelium …. Blood. 2000 [PubMed] [Google Scholar]
- 3.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117(1):195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li DY, Brooke B, Davis EC, Mecham RP, Sorensen LK, Boak BB, Eichwald E, Keating MT. Elastin is an essential determinant of arterial morphogenesis. Nature. 1998;393(6682):276–280. doi: 10.1038/30522. [DOI] [PubMed] [Google Scholar]
- 5.Lutgens E, Lutgens SPM, Faber BCG, Heeneman S, Gijbels MMJ, de Winther MPJ, Frederik P, van der Made I, Daugherty A, Sijbers AM, Fisher A, Long CJ, Saftig P, Black D, Daemen MJAP, Cleutjens KBJM. Disruption of the cathepsin K gene reduces atherosclerosis progression and induces plaque fibrosis but accelerates macrophage foam cell formation. Circulation. 2006;113(1):98–107. doi: 10.1161/CIRCULATIONAHA.105.561449. [DOI] [PubMed] [Google Scholar]
- 6.Platt MO, Ankeny RF, Shi G-P, Weiss D, Vega JD, Taylor WR, Jo H. Expression of cathepsin K is regulated by shear stress in cultured endothelial cells and is increased in endothelium in human atherosclerosis. Am J Physiol Heart Circ Physiol. 2007;292(3):H1479–1486. doi: 10.1152/ajpheart.00954.2006. [DOI] [PubMed] [Google Scholar]
- 7.Liu J, Sukhova GK, Sun J-S, Xu W-H, Libby P, Shi G-P. Lysosomal cysteine proteases in atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24(8):1359–1366. doi: 10.1161/01.ATV.0000134530.27208.41. [DOI] [PubMed] [Google Scholar]
- 8.Platt MO, Ankeny RF, Jo H. Laminar shear stress inhibits cathepsin L activity in endothelial cells. Arterioscler Thromb Vasc Biol. 2006;26(8):1784–1790. doi: 10.1161/01.ATV.0000227470.72109.2b. [DOI] [PubMed] [Google Scholar]
- 9.Kafienah W, Brömme D, Buttle DJ, Croucher LJ, Hollander AP. Human cathepsin K cleaves native type I and II collagens at the N-terminal end of the triple helix. Biochem J. 1998;331 ( Pt 3):727–732. doi: 10.1042/bj3310727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sukhova GKSG, Simon DI, Chapman HA, Libby P. Expression of the elastolytic cathepsins S and K in human atheroma and regulation of their production in smooth muscle cells. The Journal of Clinical Investigation. 1998;102:576–583. doi: 10.1172/JCI181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yasuda Y, Li Z, Greenbaum D, Bogyo M, Weber E, Brömme D. Cathepsin V, a novel and potent elastolytic activity expressed in activated macrophages. J Biol Chem. 2004;279(35):36761–36770. doi: 10.1074/jbc.M403986200. [DOI] [PubMed] [Google Scholar]
- 12.Brömme D, Li Z, Barnes M, Mehler E. Human cathepsin V functional expression, tissue distribution, electrostatic surface potential, enzymatic characterization, and chromosomal localization. Biochemistry. 1999;38(8):2377–2385. doi: 10.1021/bi982175f. [DOI] [PubMed] [Google Scholar]
- 13.Tolosa E, Li W, Yasuda Y, Wienhold W, Denzin LK, Lautwein A, Driessen C, Schnorrer P, Weber E, Stevanovic S, Kurek R, Melms A, Bromme D. Cathepsin V is involved in the degradation of invariant chain in human thymus and is overexpressed in myasthenia gravis. J Clin Invest. 2003;112(4):517–526. doi: 10.1172/JCI18028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang M, Zhang Y, Pan J, Sun J, Liu J, Libby P, Sukhova GK, Doria A, Katunuma N, Peroni OD, Guerre-Millo M, Kahn BB, Clement K, Shi G-P. Cathepsin L activity controls adipogenesis and glucose tolerance. Nat Cell Biol. 2007;9(8):970–977. doi: 10.1038/ncb1623. [DOI] [PubMed] [Google Scholar]
- 15.Kitamoto S, Sukhova GK, Sun J, Yang M, Libby P, Love V, Duramad P, Sun C, Zhang Y, Yang X, Peters C, Shi G-P. Cathepsin L deficiency reduces diet-induced atherosclerosis in low-density lipoprotein receptor-knockout mice. Circulation. 2007;115(15):2065–2075. doi: 10.1161/CIRCULATIONAHA.107.688523. [DOI] [PubMed] [Google Scholar]
- 16.Li WA, Barry ZT, Cohen JD, Wilder CL, Deeds RJ, Keegan PM, Platt MO. Detection of femtomole quantities of mature cathepsin K with zymography. Analytical biochemistry. 2010;401(1):91–98. doi: 10.1016/j.ab.2010.02.035. [DOI] [PubMed] [Google Scholar]
- 17.Wilder CL, Park K-Y, Keegan PM, Platt MO. Manipulating substrate and pH in zymography protocols selectively distinguishes cathepsins K, L, S, and V activity in cells and tissues. ARCHIVES OF BIOCHEMISTRY AND BIOPHYSICS. 2011 doi: 10.1016/j.abb.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lecaille F, Weidauer E, Juliano MA, Brömme D, Lalmanach G. Probing cathepsin K activity with a selective substrate spanning its active site. Biochem J. 2003;375(Pt 2):307–312. doi: 10.1042/BJ20030468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruettger A, Schueler S, Mollenhauer JA, Wiederanders B. Cathepsins B, K, and L are regulated by a defined collagen type II peptide via activation of classical protein kinase C and p38 MAP kinase in articular chondrocytes. J Biol Chem. 2008;283(2):1043–1051. doi: 10.1074/jbc.M704915200. [DOI] [PubMed] [Google Scholar]
- 20.Wilder CL, Platt MO. Manipulating substrate and pH in zymography protocols selectively identifies cathepsins K, L, S, and V activity in cells and tissues. Review. 2011 doi: 10.1016/j.abb.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gacko MGS. Expression of the elastolytic cathepsins S and K in human atheroma and regulation of their production in smooth muscle cells. Clinical Chemistry. 1998;36:449–452. doi: 10.1172/JCI181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burns-Kurtis CL, Olzinski AR, Needle S, Fox JH, Capper EA, Kelly FM, McQueney MS, Romanic AM. Cathepsin S expression is up-regulated following balloon angioplasty in the hypercholesterolemic rabbit. Cardiovasc Res. 2004;62(3):610–620. doi: 10.1016/j.cardiores.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Garcia-Touchard A, Henry TD, Sangiorgi G, Spagnoli LG, Mauriello A, Conover C, Schwartz RS. Extracellular proteases in atherosclerosis and restenosis. Arterioscler Thromb Vasc Biol. 2005;25(6):1119–1127. doi: 10.1161/01.ATV.0000164311.48592.da. [DOI] [PubMed] [Google Scholar]
- 24.Abdul-Hussien H, Soekhoe RG, Weber E, von der Thusen JH, Kleemann R, Mulder A, van Bockel JH, Hanemaaijer R, Lindeman JH. Collagen degradation in the abdominal aneurysm: a conspiracy of matrix metalloproteinase and cysteine collagenases. Am J Pathol. 2007;170(3):809–817. doi: 10.2353/ajpath.2007.060522. 170/3/809 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ha S-D, Martins A, Khazaie K, Han J, Chan BMC, Kim SO. Cathepsin B is involved in the trafficking of TNF-alpha-containing vesicles to the plasma membrane in macrophages. J Immunol. 2008;181 (1):690–697. doi: 10.4049/jimmunol.181.1.690. [DOI] [PubMed] [Google Scholar]
- 26.Thoma B, Grell M, Pfizenmaier K, Scheurich P. Identification of a 60-kD tumor necrosis factor (TNF) receptor as the major signal transducing component in TNF responses. J Exp Med. 1990;172 (4):1019–1023. doi: 10.1084/jem.172.4.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang Y-l, Chen C-l, Kuo C-L, Chen B-c, You J-s. Glycyrrhetinic acid inhibits ICAM-1 expression via blocking JNK and NF-kappaB pathways in TNF-alpha-activated endothelial cells. Acta Pharmacol Sin. 2010;31(5):546–553. doi: 10.1038/aps.2010.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hui C, Like W, Yan F, Tian X, Qiuyan W, Lifeng H. S-allyl-L-cysteine sulfoxide inhibits tumor necrosis factor-alpha induced monocyte adhesion and intercellular cell adhesion molecule-1 expression in human umbilical vein endothelial cells. Anat Rec (Hoboken) 2010;293(3):421–430. doi: 10.1002/ar.21070. [DOI] [PubMed] [Google Scholar]
- 29.Pang M, Martinez AF, Fernandez I, Balkan W, Troen BR. AP-1 stimulates the cathepsin K promoter in RAW 264.7 cells. Gene. 2007;403(1–2):151–158. doi: 10.1016/j.gene.2007.08.007. [DOI] [PubMed] [Google Scholar]