BACKGROUND
Myelomeningocele (MMC) is a congenital neural tube defect that occurs in approximately 1 in 2900 live births in the United States.1,2 This neurological anomaly arises from incomplete neural tube closure during early development, resulting in an open spinal canal with exposed neural elements in the form of a flat neural placode. The neural placode then undergoes further traumatic injury in utero. These events form the basis of the “two-hit” hypothesis of neurologic injury in MMC, with the first “hit” being the developmental defect itself and the second “hit” being the additional injury to the exposed neural elements of the spinal cord. In utero intervention to repair the spinal cord does not address the developmental defect, but it can ameliorate the subsequent trauma associated with the second hit.
The exact etiology of MMC is unknown, but its origin is likely multifactorial. Nutritional, environmental, and genetic factors have all been implicated in the pathogenesis of MMC. Folate deficiency in mothers is associated with an increased incidence of neural tube defects. Folate supplementation can decrease the risk of MMC in the pregnancy by as much as 50%, but it has not eradicated the anomaly.3 Environmental exposure to toxins and drugs has been implicated in the development of neural tube defects. Additionally, genetic abnormalities may have a role in some patients, as seen in patients with in PAX3 mutation in Waardenburg’s syndrome.
Patients with MMC can display a broad array of clinical findings depending on the severity of their neurological defect. Most infants survive the neonatal period without significant morbidity; however, up to 30% of patients die before adulthood due to respiratory, urinary, or central nervous system complications. Long-term morbidity and mortality is related both to neurological damage from the spinal cord lesion itself as well as to brain abnormalities from leakage of cerebrospinal fluid.4 Continued leakage of cerebrospinal fluid is thought to lead to an intercerebral pressure gradient, resulting in a hindbrain herniation known as Arnold-Chiari II malformation. Virtually all newborns with MMC have a Chiari malformation and over 90% will develop hydrocephalus, requiring ventriculo-peritoneal (VP) shunting. The spinal level of the defect determines the degree of motor and somatosensory deficit. In addition, these patients often have dysfunction of bladder and bowel control, as well as loss of sexual function that manifests in adolescence and early adulthood.5
RATIONALE FOR FETAL REPAIR OF MMC
The rationale for fetal repair of MMC targets the second hit of the two-hit hypothesis.6,7 By limiting in utero damage to the exposed spinal cord and preventing continued leakage of cerebrospinal fluid, in utero coverage of the spinal defect could potentially normalize the intercerebral gradient and lead to improved neurological outcomes. Several observations in both humans and animals support the two-hit hypothesis. First, early prenatal ultrasound examinations of human fetuses with MMC has demonstrated normal hind limb movement.8 This suggests a later loss of function attributable to in utero injury to the spinal cord. Second, postmortem analysis of stillborn and aborted fetuses with MMC has suggested recent in utero injury to the exposed neural placode.6 Third, patients with milder forms of neural tube defects in which the abnormal neural elements remain covered with skin or a membrane have more normal neural development than those patients with MMC.9 Finally, observations in mice with neural tube defects have demonstrated normal early anatomy and function which is then progressively lost in utero.10
ANIMAL MODELS
Animal models were developed to the study the natural history of MMC and potential fetal interventions before attempting fetal MMC repair in humans. Surgical models in rodent, rabbit, sheep, and non-human primates share similar a similar phenotype as found in human MMC: paraplegia, extremity deformity, urinary and bowel dysfunction, hydrocephalus, and the Chiari malformation. Prenatal closure of these surgically created defects has produced improvement in motor function, urinary function, and reversal of the Chiari malformation with normal or near-normal hindbrain development and morphology. Several animal models have demonstrated that the Chiari malformation occurs with surgically created myelomeningocele and, more importantly, that it can be reversed after in utero repair.11–15 Further animal studies have also demonstrated improved distal neurologic function after in utero repair of surgically created MMC. In a rabbit model of fetal MMC repair, neurologic function was improved after birth, as measured by somatosensory-evoked potentials.16 In studies examining anal sphincter development in the sheep model, histological examination of rectum and anal sphincter muscles revealed preservation of longitudinal muscles in the sphincter complex and the submucosal nerve plexus in lambs who had undergone fetal repair.17,18
EARLY HUMAN EXPERIENCE
Fetoscopic Repair
Prior to prenatal repair for MMC, human fetal surgery had been reserved for situations in which there would be expected perinatal mortality. This approach was adopted in order to maximize potential benefit in the fetus while minimizing risk and morbidity to the mother. Fetal diseases for which prenatal intervention was considered included severe obstructive uropathies, congenital diaphragmatic hernia, placental anomalies such as twin-twin transfusion syndrome, and non-immune hydrops secondary to an anatomical defect (e.g. sacrococcygeal teratoma, congenital cystic adenoid malformation). MMC was the first nonlethal fetal malformation treated with human in utero surgery.
In an attempt to minimize maternal morbidity, early human fetal MMC repairs were approached fetoscopically.19–21 This technique was independently attempted at the Vanderbilt University Medical Center and at the University of California, San Francisco (UCSF). The Vanderbilt group reported four fetoscopic repairs via maternal laparotomy and a three-port technique for in utero access. The amniotic fluid was replaced with carbon dioxide and the defects were covered with a maternal skin graft. Two of four fetuses survived to birth; both survivors required reoperation postnatally because no evidence of the skin graft was found at birth.
The UCSF group reported three attempts at fetoscopic MMC repair, only one of which was successfully closed fetoscopically. In this patient, the MMC defect was closed with a decellularized dermal matrix patch; however, at birth, the repair was incomplete and required an additional operation to close the defect. The two other attempts at fetoscopic repair were converted to open fetal repair due to technical difficulties. Fetoscopic repair would eventually be largely abandoned in favor of open fetal repair, which was technically easier to complete despite its increased maternal morbidity.
Open repair
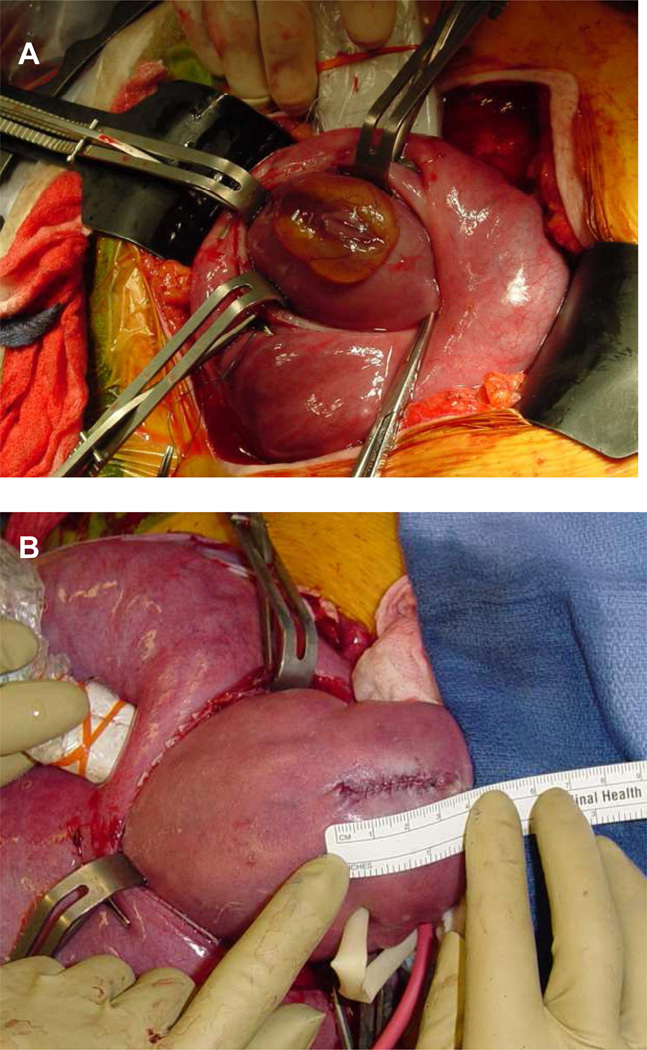
After these early attempts at minimally invasive repair, fetal surgeons began performing open fetal MMC repair (Figure 1). The Vanderbilt group was the first to report their results in 1999 and concluded that the open repair method was superior to the fetoscopic method. They also found that hindbrain herniation was improved in MMC patients who had undergone fetal surgical repair and that the need for VP shunting was significantly lower in those patients: 59% vs. 91% in historic controls.22 These data were limited by their small sample size and the lack of a standardized protocol for postnatal VP shunt placement. A follow-up study with subset analysis demonstrated that repair earlier in gestation, lower level lesions, and smaller ventricles prior to repair were all associated with a lower VP shunt rate.23,24
Figure 1.
Open fetal repair for myelomeningocele. a) Myelomeningocele defect before repair. b) Final skin closure of a myelomeningocele defect.
Concurrently, the group at the Children’s Hospital of Philadelphia (CHOP) confirmed the findings of reversal of hindbrain herniation and improvement in the Chiari malformation in patients who had undergone open fetal repair.25 These findings were corroborated with studies of head biometry in pre- and postnatal MRI scans.26 Decreased shunt rates and less ventriculomegaly were also reported, further reinforcing the findings at Vanderbilt.27
Neurologic function
At all three centers, immediate results did not reveal significant improvement in neurologic function or hind limb movement with fetal repair. Follow-up data was mixed. At UCSF, only two of nine survivors had functional improvement greater than 2 spinal levels above the MMC lesion. Neurologic function correlated well with the level of the spinal lesion in the remainder of patients.21 The Vanderbilt group initially compared the neurologic outcomes of 26 patients who had undergone fetal MMC repair to historical controls and found no improvement.28 They later compared their cohort to matched controls at the University of Alabama, Birmingham, and again they found no improvement in neurologic function.29 In contrast, CHOP examined 54 patients who had fetal MMC repair and found that 57% had neurologic function better than what was predicted from the level of their spinal defect.30 Their median follow up was 66 months and ranged from 36 to 133. Factors that were associated with a lower likelihood of independent ambulation included higher level lesions and presence of a clubfoot deformity.
Maternal morbidity
Open fetal surgery places the healthy mother at risk for significant operative and postoperative complications. Two early studies suggested that maternal morbidity was low and that fertility after fetal surgery was preserved.31,32 However, Vanderbilt’s data revealed more serious maternal morbidity: intraoperative placenta abruption, uterine dehiscence, and small bowel obstruction.33 Uterine dehiscence and rupture are particularly notable because these risks remain present for any subsequent pregnancies.
Prematurity
Two reports were published from the Vanderbilt group regarding prematurity in neonates who underwent fetal repair of MMC. The first report analyzed whether repair prior to 25 weeks gestation affected gestational age at birth and found that the degree of prematurity was independent of the gestational age at fetal repair.34 A follow-up study compared the complication rate of premature infants who had fetal repair of MMC with controls matched for gestational age, sex, birth weight, antenatal steroid use, and mode of delivery, and found no significant difference in the incidence of morbidity associated with prematurity.35
Urologic function
Studies of urologic outcome in infants who underwent prenatal closure of MMC have demonstrated no improvement in urologic function to date. The Vanderbilt group examined 16 patients with a mean age of 6.5 months and compared urodynamic studies with those from historic controls. They found similar results between the two groups.36 The UCSF group examined six patients who had fetal MMC repair and also showed no significant urologic improvement.37
THE MANAGEMENT OF MYELOMENINGOCELE STUDY (MOMS)
From these early reported case series, it was unclear whether prenatal repair of MMC was beneficial when compared to standard postnatal therapy, as no controlled comparisons had been made. To address this question, in 2003 CHOP, Vanderbilt, and UCSF began collaboration on a National Institutes of Health-sponsored prospective randomized controlled trial (Management of Myelomeningocele Study, or MOMS) comparing prenatal MMC repair to postnatal repair.38 Inclusion and exclusion criteria for the trial are listed in Box 1.
Box 1. Inclusion and exclusion criteria for the Management of Myelomeningocele Study.
Inclusion criteria:
Myelomeningocele at level T1 through S1 with hindbrain herniation.
Maternal age 18 years or older.
Gestational age at randomization of 190 to 256 weeks.
Normal karyotype.
Exclusion criteria:
Non-resident of the United States.
Non-singleton pregnancy.
Insulin-dependent pregestational diabetes.
Fetal anomaly not related to MMC.
Kyphosis in the fetus of 30 degrees or greater.
Current or planned cerclage or documented history of incompetent cervix.
Short Cervix (<20mm).
Placenta previa or placental abruption.
Body mass index 35 or greater.
Previous spontaneous delivery prior to 37 weeks’ gestation.
Maternal-fetal Rh isoimmunization, Kell sensitization or neonatal alloimmune thrombocytopenia
Maternal HIV or Hepatitis B status positive.
Known Hepatitis C positivity.
Uterine anomaly such as large or multiple fibroids or müllerian duct abnormality
Other maternal medical condition which is a contraindication to surgery or general anesthesia
Patient does not have a support person.
Inability to comply with travel and follow-up requirements.
Patient does not meet other psychosocial criteria to handle the implications of the trial.
Participation in another intervention study that influences maternal and fetal morbidity and mortality or participation in this trial in a previous pregnancy.
Maternal hypertension which would increase the risk of preeclampsia or preterm delivery.
There were two primary research questions in this trial. The first was whether fetal surgery for MMC improved outcomes as measured by death or the need for a VP shunt within the first year of life. The second question was whether prenatal repair of MMC improved neurologic function at 30 months of age as predicted by the spinal level of the lesion. Secondary research questions included whether the Chiari malformation was improved, whether neuromotor outcome was improved at 12 and 30 months of age, and the long-term psychological and reproductive consequences for the parents.
Outcomes
In 2011, enrollment in the MOMS trial was stopped early due to statistical evidence of benefit in the prenatal surgery group after the enrollment of 183 of a planned 200 patients. Initial analysis of the first 158 patients confirmed that fetal surgery for MMC had decreased the need for postnatal VP shunting in approximately one third of infants by 12 months [Table 1]. The proportion of infants who had no evidence of hindbrain herniation was significantly higher in the prenatal surgery group, and the proportion of infants who had moderate or severe hindbrain herniation was significantly reduced. The second primary outcome, neurological function as assessed by a score derived from mental and motor development at 30 months, was also significantly improved in the prenatal surgery group. In addition, several secondary outcomes were improved. Most notably, the percentage of patients able to independently ambulate at 30 months increased from 21% to 42% after prenatal repair compared to postnatal surgery, supporting the theory that distal neurological function can also be improved by in utero closure.
Table 1.
Summary of select outcomes from the Management of Myelomeningocele Study randomized controlled trial.
| Prenatal Surgery (N = 78) |
Postnatal Surgery (N = 80) |
Relative Risk (95% CI) |
P Value | |
|---|---|---|---|---|
| Primary outcomes | ||||
| Primary outcome at 12 months1 | 53 (68%) | 78 (98%) | 0.70 (0.58–0.84) | <0.001 |
| Primary outcome at 30 months2 | 148.6 ± 57.5 (SD) | 122.6 ± 57.2 (SD) | - | 0.007 |
| Secondary outcomes | ||||
| Hindbrain herniation (12 months) | 45/70 (64%) | 66/69 (96%) | 0.67 (0.56–0.81) | <0.001 |
| Shunt placement (12 months) | 31 (40%) | 66 (82%) | 0.48 (0.36–0.64) | <0.001 |
| Walking independently (30 months) | 26/62 (42%) | 14/67 (21%) | 2.01 (1.16–3.48) | 0.01 |
Based on incidence of fetal or neonatal death or need for shunt placement by 12 months.
Composite score based on Bayley Mental Developmental Index and the difference between observed and expected motor function at age 30 months. Higher values indicate better function.
There were no maternal deaths and two perinatal deaths in each group, suggesting that mortality was comparable between groups. Prenatal surgery was associated with higher rates of preterm birth, intraoperative complications, uterine-scar defects, and higher rates of maternal transfusion at delivery, all of which are known complications of open fetal surgery. Although enrollment into the MOMS trial has ended, long-term follow-up and analysis is ongoing. In addition, a new Management of Myelomeningocele Study (MOMS II) will follow-up the neurological outcome of these patients into later childhood.
NEW DIRECTIONS
To augment the still limited neurological function recovered by fetal MMC repair, novel regenerative medicine techniques are being actively studied in research laboratories. One study of murine neural stem cells applied to the MMC defect during fetal repair in sheep showed qualitative improvements in hind limb movement in the animals that received stem cells.39 The neural stem cells survived and engrafted, and they seemed to be more concentrated at areas of greatest damage, suggesting that they may “home in” on the most injured areas. On further histological analysis, the engrafted neural stem cells were found to remain in a largely undifferentiated state, possibly suggesting a neurotrophic secretory or chaperone-like role for these cells. Other groups are exploring the use of biomaterials such as gelatin microspheres and nanofibrous scaffolds as a means to maximize the unique benefits of prenatal MMC closure.40,41 This research is preliminary, but supports further study into the benefits of a multifaceted approach to MMC repair. Finally, due to the maternal morbidity of open fetal surgery, less invasive methods of fetal MMC treatment continue to be investigated.42,43 In addition, surgical experiments in fetal sheep have demonstrated the feasibility of robot-assisted closure of MMC defects.44 Given the push towards fetoscopic closure, it is likely that a less invasive fetal MMC technique will become accepted in the near future.
SUMMARY
Myelomeningocele is a devastating disability with significant morbidity and mortality within the first few decades of life. MMC was the first nonlethal disease to be considered and studied for fetal surgery. The recently completed MOMS trial has shown that fetal repair for MMC can improve hydrocephalus and hindbrain herniation associated with the Arnold-Chiari II malformation, can reduce the need for VP shunting, and may improve distal neurologic function in some patients. Potential improvements in outcome must be balanced with safety and well-being of the mother, in addition to that of the unborn patient. Further follow-up will determine the long-term benefit of fetal MMC repair.
Key Points.
-
*
Fetal surgery for myelomeningocele is the most common open fetal surgery currently performed and has been tested in a randomized controlled trial
-
*
Prenatal repair of myelomeningocele can improve hindbrain herniation and reduces postnatal shunt requirement
-
*
Distal neurological function may also be improved in some patients after prenatal repair
-
*
Fetoscopic approaches to myelomeningocele repair remain a challenge and are an active area of research
Acknowledgments
Diana L. Farmer is a principal investigator on the Management of Myelomeningocele Study (MOMS) and received NIH funding for this work (grant no. NICHD 3U10 HD041669).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement:
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
REFERENCES
- 1.Canfield MA, Honein MA, Yuskiv N, et al. National estimates and race/ethnic-specific variation of selected birth defects in the United States, 1999–2001. Birth Defects Res A Clin Mol Teratol. 2006;76(11):747–756. doi: 10.1002/bdra.20294. [DOI] [PubMed] [Google Scholar]
- 2.Boulet SL, Yang Q, Mai C, et al. Trends in the postfortification prevalence of spina bifida and anencephaly in the United States. Birth Defects Res A Clin Mol Teratol. 2008;82:527–532. doi: 10.1002/bdra.20468. [DOI] [PubMed] [Google Scholar]
- 3.De Wals P, Tairou F, Van Allen MI, et al. Reduction in neural-tube defects after folic acid fortification in Canada. NEJM. 2007;357(2):135–142. doi: 10.1056/NEJMoa067103. [DOI] [PubMed] [Google Scholar]
- 4.Steinbok P, Irvine B, Cochrane DD, Irwin BJ. Long-term outcome and complications of children born with meningomyelocle. Childs Nerv Syst. 1992;8(2):92–96. doi: 10.1007/BF00298448. [DOI] [PubMed] [Google Scholar]
- 5.Woodhouse CRJ. Myelomeningocele: neglected aspects. Pediatr Nephrol. 2008;23:1223–1231. doi: 10.1007/s00467-007-0663-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hutchins GM, Meuli M, Meuli-Simmen C, Jordan MA, Heffez DS, Blakemore KJ. Acquired spinal cord injury in human fetuses with myelomeningocele. Pediatr Pathol Lab Med. 1996;16(5):701–712. [PubMed] [Google Scholar]
- 7.Heffez DS, Aryanpur J, Hutchins GM, Freeman JM. The paralysis associated with myelomeningocele: clinical and experimental data implicating a preventable spinal cord injury. Neurosurgery. 1990;26(6):987–992. [PubMed] [Google Scholar]
- 8.Korenromp MJ, van Gool JD, Bruinese HW, Kriek R. Early fetal leg movements in myelomeningocele. Lancet. 1986;1(8486):917–918. doi: 10.1016/s0140-6736(86)91022-6. [DOI] [PubMed] [Google Scholar]
- 9.Oya N, Suzuki Y, Tanemura M, et al. Detection of skin over cysts with Spina bifida may be useful not only for preventing neurological damage during labor but also for predicting fetal prognosis. Fetal Diagn Ther. 2000;15(3):156–159. doi: 10.1159/000020996. [DOI] [PubMed] [Google Scholar]
- 10.Stiefel D, Copp AJ, Meuli M. Fetal spina bifida in a mouse model: loss of neural function in utero. J Neurosurg. 2007;106(Suppl 3):213–221. doi: 10.3171/ped.2007.106.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pedreira DA, Sanchez e Oliveira Rde C, Valente PR, Abou-Jamra RC, Araujo A, Saldiva PH. Validation of the ovine fetus as an experimental model for the human myelomeningocele defect. Acta Cir Bras. 2007;22(3):168–173. doi: 10.1590/s0102-86502007000300003. [DOI] [PubMed] [Google Scholar]
- 12.von Koch CS, Compagnone N, Hirose S, Yoder S, Harrison MR, Farmer DL. Myelomeningocele: characterization of a surgically induced sheep model and its central nervous system similarities and differences to the human disease. Am J Obstet Gynecol. 2005;193(4):1456–1462. doi: 10.1016/j.ajog.2005.02.110. [DOI] [PubMed] [Google Scholar]
- 13.Weber Guimaraes Barreto M, Ferro MM, Guimaraes Bittencourt D, Violin Pereira LA, Barini R, Sbragia L. Arnold-Chiari in a fetal rat model of dysraphism. Fetal Diagn Ther. 2005;20(5):437–441. doi: 10.1159/000086827. [DOI] [PubMed] [Google Scholar]
- 14.Paek BW, Farmer DL, Wilkinson CC, et al. Hindbrain herniation develops in surgically created myelomeningocele but is absent after repair in fetal lambs. Am J Obstet Gynecol. 2000;183(5):1119–1123. doi: 10.1067/mob.2000.108867. [DOI] [PubMed] [Google Scholar]
- 15.Galvan-Montano A, Cardenas-Lailson E, Hernandez-Godinez B, Ibanez-Contreras A, Martinez-Del Olmo A, Aragon-Inclan J. Development of an animal model of myelomeningocele and options for prenatal treatment in Macaca mulatta. Cir Cir. 2007;75(5):357–362. [PubMed] [Google Scholar]
- 16.Julia V, Sancho MA, Albert A, et al. Prenatal covering of the spinal cord decreases neurologic sequelae in a myelomeningocele model. J Pediatr Surg. 2006;41(6):1125–1129. doi: 10.1016/j.jpedsurg.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 17.Yoshizawa J, Sbragia L, Paek BW, et al. Fetal surgery for repair of myelomeningocele allows normal development of the rectum in sheep. Pediatr Surg Int. 2003;19(3):162–166. doi: 10.1007/s00383-002-0910-4. [DOI] [PubMed] [Google Scholar]
- 18.Yoshizawa J, Sbragia L, Paek BW, et al. Fetal surgery for repair of myelomeningocele allows normal development of anal sphincter muscles in sheep. Pediatr Surg Int. 2004;20(1):14–18. doi: 10.1007/s00383-003-1073-7. [DOI] [PubMed] [Google Scholar]
- 19.Bruner JP, Richards WO, Tulipan NB, Arney TL. Endoscopic coverage of fetal myelomeningocele in utero. Am J Obstet Gynecol. 1999;180(1 Pt 1):153–158. doi: 10.1016/s0002-9378(99)70167-5. [DOI] [PubMed] [Google Scholar]
- 20.Bruner JP, Tulipan NB, Richards WO, Walsh WF, Boehm FH, Vrabcak EK. In utero repair of myelomeningocele: a comparison of endoscopy and hysterotomy. Fetal Diagn Ther. 2000;15(2):83–88. doi: 10.1159/000020981. [DOI] [PubMed] [Google Scholar]
- 21.Farmer DL, von Koch CS, Peacock WJ, et al. In utero repair of myelomeningocele: experimental pathophysiology, initial clinical experience, and outcomes. Arch Surg. 2003;138(8):872–878. doi: 10.1001/archsurg.138.8.872. [DOI] [PubMed] [Google Scholar]
- 22.Bruner JP, Tulipan N, Paschall RL, et al. Fetal surgery for myelomeningocele and the incidence of shunt-dependent hydrocephalus. JAMA. 1999;282(19):1819–1825. doi: 10.1001/jama.282.19.1819. [DOI] [PubMed] [Google Scholar]
- 23.Tulipan N, Sutton LN, Bruner JP, Cohen BM, Johnson M, Adzick NS. The effect of intrauterine myelomeningocele repair on the incidence of shunt-dependent hydrocephalus. Pediatr Neurosurg. 2003;38(1):27–33. doi: 10.1159/000067560. [DOI] [PubMed] [Google Scholar]
- 24.Bruner JP, Tulipan N, Reed G, et al. Intrauterine repair of spina bifida: preoperative predictors of shunt-dependent hydrocephalus. Am J Obstet Gynecol. 2004;190(5):1305–1312. doi: 10.1016/j.ajog.2003.10.702. [DOI] [PubMed] [Google Scholar]
- 25.Sutton LN, Adzick NS, Bilaniuk LT, Johnson MP, Crombleholme TM, Flake AW. Improvement in hindbrain herniation demonstrated by serial fetal magnetic resonance imaging following fetal surgery for myelomeningocele. JAMA. 1999;282(19):1826–1831. doi: 10.1001/jama.282.19.1826. [DOI] [PubMed] [Google Scholar]
- 26.Danzer E, Johnson MP, Bebbington M, et al. Fetal head biometry assessed by fetal magnetic resonance imaging following in utero myelomeningocele repair. Fetal Diagn Ther. 2007;22(1):1–6. doi: 10.1159/000095833. [DOI] [PubMed] [Google Scholar]
- 27.Johnson MP, Sutton LN, Rintoul N, et al. Fetal myelomeningocele repair: short-term clinical outcomes. Am J Obstet Gynecol. 2003;189(2):482–487. doi: 10.1067/s0002-9378(03)00295-3. [DOI] [PubMed] [Google Scholar]
- 28.Tulipan N, Bruner JP, Hernanz-Schulman M, et al. Effect of intrauterine myelomeningocele repair on central nervous system structure and function. Pediatr Neurosurg. 1999;31(4):183–188. doi: 10.1159/000028859. [DOI] [PubMed] [Google Scholar]
- 29.Tubbs RS, Chambers MR, Smyth MD, et al. Late gestational intrauterine myelomeningocele repair does not improve lower extremity function. Pediatr Neurosurg. 2003;38(3):128–132. doi: 10.1159/000068818. [DOI] [PubMed] [Google Scholar]
- 30.Tulipan N, Bruner JP. Myelomeningocele repair in utero: a report of three cases. Pediatr Neurosurg. 1998;28(4):177–180. doi: 10.1159/000028645. [DOI] [PubMed] [Google Scholar]
- 31.Farrell JA, Albanese CT, Jennings RW, Kilpatrick SJ, Bratton BJ, Harrison MR. Maternal fertility is not affected by fetal surgery. Fetal Diagn Ther. 1999;14(3):190–192. doi: 10.1159/000020918. [DOI] [PubMed] [Google Scholar]
- 32.Danzer E, Gerdes M, Bebbington MW, et al. Lower extremity neuromotor function and short-term ambulatory potential following in utero myelomeningocele surgery. Fetal Diagn Ther 28. 2009;25(1):47–53. doi: 10.1159/000197359. [DOI] [PubMed] [Google Scholar]
- 33.Longaker MT, Golbus MS, Filly RA, Rosen MA, Chang SW, Harrison MR. Maternal outcome after open fetal surgery. A review of the first 17 human cases. JAMA. 1991;265(6):737–741. [PubMed] [Google Scholar]
- 34.Hamdan AH, Walsh W, Heddings A, Bruner JP, Tulipan N. Gestational age at intrauterine myelomeningocele repair does not influence the risk of prematurity. Fetal Diagn Ther. 2002;17(2):66–68. doi: 10.1159/000048010. [DOI] [PubMed] [Google Scholar]
- 35.Hamdan AH, Walsh W, Bruner JP, Tulipan N. Intrauterine myelomeningocele repair: effect on short-term complications of prematurity. Fetal Diagn Ther. 2004;19(1):83–86. doi: 10.1159/000074267. [DOI] [PubMed] [Google Scholar]
- 36.Holzbeierlein J, Pope JI, Adams MC, Bruner J, Tulipan N, Brock JW., 3rd The urodynamic profile of myelodysplasia in childhood with spinal closure during gestation. J Urol. 2000;164(4):1336–1339. [PubMed] [Google Scholar]
- 37.Holmes NM, Nguyen HT, Harrison MR, Farmer DL, Baskin LS. Fetal intervention for myelomeningocele: effect on postnatal bladder function. J Urol. 2001;166(6):2383–2386. doi: 10.1016/s0022-5347(05)65596-6. [DOI] [PubMed] [Google Scholar]
- 38.Adzick NS, Thom EA, Spong CY, et al. A randomized trial of prenatal versus postnatal repair of myelomeningocele. N Engl J Med. 2011;364(11):993–1004. doi: 10.1056/NEJMoa1014379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fauza DO, Jennings RW, Teng YD, Snyder EY. Neural stem cell delivery to the spinal cord in an ovine model of fetal surgery for spina bifida. Surgery. 2008 Sep;144(3):367–373. doi: 10.1016/j.surg.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 40.Saadai P, Nout YS, Encinas J, Wang A, Downing TL, Beattie MS, Bresnahan JC, Li S, Farmer DL. Prenatal repair of myelomeningocele with aligned nanofibrous scaffolds- a pilot study in sheep. J Pediatr Surg. 2011;46:2279–2283. doi: 10.1016/j.jpedsurg.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 41.Watanabe M, Li H, Roybal J, et al. A tissue engineering approach for prenatal closure of myelomeningocele: comparison of gelatin sponge and microsphere scaffolds and bioactive protein coatings. Tissue Eng Part A. 2011;17(7–8):1099–1110. doi: 10.1089/ten.TEA.2010.0390. [DOI] [PubMed] [Google Scholar]
- 42.Pedreira DA, Oliveira RC, Valente PR, Abou-Jamra RC, Araujo A, Saldiva PH. Gasless fetoscopy: a new approach to endoscopic closure of a lumbar skin defect in fetal sheep. Fetal Diagn Ther. 2008;23(4):293–298. doi: 10.1159/000123616. [DOI] [PubMed] [Google Scholar]
- 43.Kohl T, Hartlage MG, Kiehitz D, et al. Percutaneous fetoscopic patch coverage of experimental lumbosacral full-thickness skin lesions in sheep. Surg Endosc. 2003;17(8):1218–1223. doi: 10.1007/s00464-002-9184-0. [DOI] [PubMed] [Google Scholar]
- 44.Aaronson OS, Tulipan NB, Cywes R, et al. Robot-assisted endoscopic intrauterine myelomeningocele repair: a feasibility study. Pediatr Neurosurg. 2002;36(2):85–89. doi: 10.1159/000048358. [DOI] [PubMed] [Google Scholar]