Abstract
BACKGROUND
Low vitamin D has been associated with low levels of high-density lipoprotein (HDL) cholesterol, a marker of coronary risk. Whether atheroprotective HDL particle composition accounts for this association and whether fat affects this association is not known.
OBJECTIVE
To explore the association between HDL particle composition and 25-hydroxy vitamin D (25[OH]D) in post-menopausal women.
METHODS
Vitamin D levels and lipoprotein composition were assessed in fasting blood samples of apparently healthy women from a diverse Chicago community. Visceral (VAT) and subcutaneous (SAT) abdominal fat area were assessed using computed tomography. Total body fat mass was measured by dual-energy X-ray absorptiometry.
RESULTS
We enrolled 78 women (50% black; 50% white), age 48 to 64 years, all of whom were participants in a longitudinal study of fat patterning. They had a mean 25[OH]D of 31 ± 15 µg/L, HDL cholesterol 57 ± 11 mg/dL, and large HDL particle subclass 8.6 ± 3.4 µmol/L. In a multivariable-adjusted regression model, each 5 µg/L higher 25[OH]D predicted 0.57 µmol/L (95%CI 0.20–0.95) higher large HDL particles, independent of race, season, and total HDL particle concentration. This association was only partially confounded by total body fat mass (0.49, 95%CI 0.10–0.89), SAT (0.50, 95%CI 0.11–0.90), or VAT (0.37, 95%CI 0.01–0.74). Age did not significantly influence the strength of associations.
CONCLUSIONS
Higher 25[OH]D levels are associated with large HDL particles. This association is stronger than that of HDL cholesterol and only partially confounded by body fat. Theoretically, vitamin D may protect against cardiovascular risk by promoting formation of large HDL particles, affecting reverse cholesterol transport.
Higher levels of vitamin D,1 assessed by serum 25-hydroxyvitamin D concentration (25[OH]D), and high- density lipoprotein (HDL) cholesterol2 are both related to lower risk of coronary artery disease (CAD). Although several studies3–5 suggested an association between 25[OH]D and HDL cholesterol, there are no published reports exploring whether this relationship can be better explained by HDL particle composition pattern (particularly, large HDL particle subclass, or particle number and size). Because HDL particle composition may be instrumental in understanding the link between HDL cholesterol and CAD risk,6 determining the association between 25[OH]D and HDL particle composition can help elucidate any links between 25[OH]D and CAD risk.
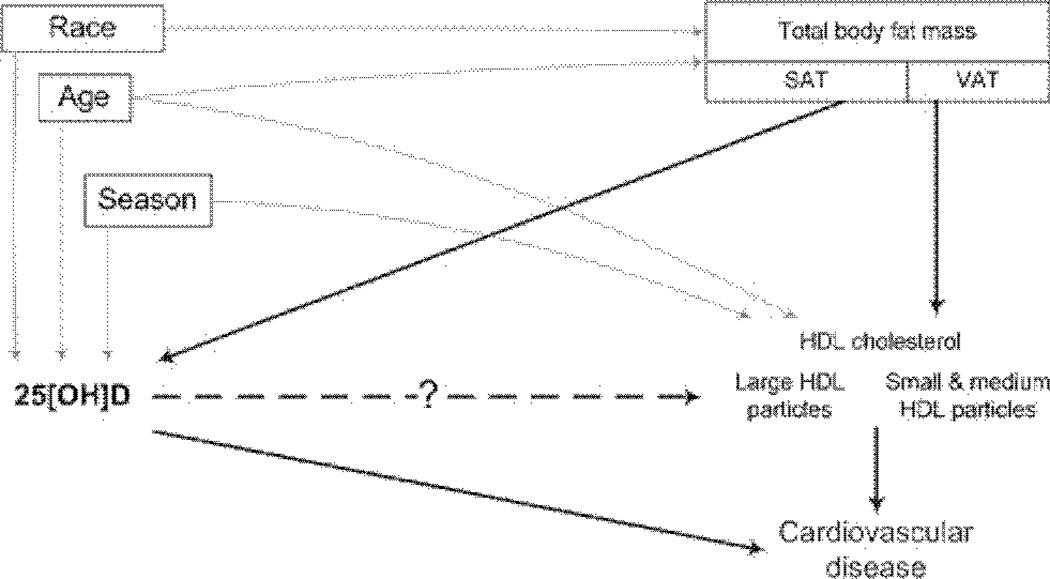
It is particularly meaningful to investigate the association between 25[OH]D and HDL particle composition in postmenopausal women because this period is a critical one of increasing cardiovascular risk.7 Adipose tissue may confound any relationship between vitamin D and HDL particle subclasses (Fig. 1). Because of fat-soluble 25[OH]D sequestration,8 vitamin D levels are lower in people with more total adipose tissue,9 subcutaneous adipose tissue (SAT),9,10 and visceral adipose tissue (VAT).10 VAT is related to HDL cholesterol and HDL particle composition11,12 and is associated with increased cardiovascular risk during menopause.13 Our focus was to explore whether (1) vitamin D is associated with atheroprotective HDL particle subclasses in women of postmenopausal age; and (2) these associations are independent of adipose tissue.
Figure 1.
The diagram of association between vitamin D and HDL variables. The square around box around the nodes represents conditioning for variable in multivariable analysis.
Methods
Subjects
Women included in this analysis were participants in a study of the impact of menopause on fat patterning.13 Women were randomly selected from a population census with excellent representativeness and were approximately equivalent within both races on indices of socioeconomic status. For this report, we randomly selected from the ongoing study 80 postmenopausal women, ages 48 to 64 years. Selected participants had no history or symptoms of CAD and no known chronic debilitating health conditions, no estrogen replacement, and no diabetes or impaired fasting glucose. Two women did not have measurements required for the current analysis. Thus, our final sample consisted of 78 women (39 white; 39 black). The study was approved by the institutional review board at the Rush University Medical Center, and all women provided a written informed consent.
Measurements
The blood pressure was taken with the use of standard techniques.14 High blood pressure was defined according to the National Cholesterol Education Program Adult Treatment Panel III guidelines as a blood pressure greater than 130/85 mm Hg or taking antihypertensive medications. All 78 women underwent a fasting blood collection. Cardiovascular risk factors were measured using standard self-report questionnaires.
High-density lipoprotein composition
Lipoprotein subclasses were determined by nuclear magnetic resonance (NMR) spectroscopy (LipoScience Inc, Raleigh, NC), as previously described.15 In brief, lipoprotein particles were quantified by their proton NMR signals, which differ according to particle diameter. The total plasma signal was deconvoluted into derived signal amplitudes for each lipoprotein subclass by the use of data from previously modeled reference subclasses. The quantity of each subclass is proportional to its signal amplitude, which is multiplied by a standard lipid amount to provide the cholesterol content carried in HDL particles (HDL cholesterol), or triglyceride content carried in very low-density lipoprotein (VLDL) and chylomicrones.
The NMR-derived measures for HDL cholesterol and triglycerides (but not for total and low-density lipoprotein cholesterol) correlate closely to the chemically measured values. The lipoprotein subclasses were categorized as large (60 to 200 nm), medium (35 to 60 nm), and small (27 to 35 nm) VLDL; large (21.3 to 23.0 nm), medium (19.8 to 21.2 nm), and small (18.3 to 19.7 nm) low-density lipoprotein (LDL); and large (8.2 to 13 nm) and small-to-medium (7.3 to 8.2 nm) HDL particles. Mean particle size was calculated for each lipid particle subclass (HDL, LDL, and VLDL) by weighting the concentration of each subclass by its standard reference diameter. The total HDL particle concentration was defined as the number of HDL particles per plasma volume, expressed in µmol/L.
Vitamin D
Serum vitamin D testing was performed by PPD Global Central Labs (Highland Heights, KY) with the use of a Chromosystems high-performance liquid chromatography kit for 25-OH vitamin D2 and 25-OH vtamin D3 and a Waters isocratic high-performance liquid chromatography pump system with UV detector (Milford, MA). Each assay detection limit was 5 µg/L; intraassay variability varied from 0.8% at 57.9 µg/L to 3.0% at 24.9 µg/L, and the interassay variation varied from 1.9% at 84.5 µg/L to 4.6% at 23.2 µg/L. We used total 25-OH vitamin D (25-OH vitamin D2 + 25-OH vitamin D3) concentration in the statistical analyses. Calcidiol (25-OH vitamin D3) is the predominant source of vitamin D in humans and reflects endogenous vitamin D production, whereas ergocalciferol (vitamin D2) is derived from plants and frequently used in vitamin D supplements. The 25[OH]D samples taken from December through May were categorized as winter/spring season, and those taken from June through November were categorized as summer/fall season.16
Abdominal adipose tissue distribution
The women underwent an abdominal computed tomography (CT) scan at the level of L4–5 intervertebral space to quantify VAT and SAT. The CT scan was performed in supine position with both arms stretched above the head.17 A single 6-mm slice was taken while the participant was in suspended respiration after normal expiration. Total abdominal adipose tissue area (in cm2) was measured delineating the body surface with a receiver operator instrument and then computing the adipose tissue area in attenuation range of −190 to −30 Hounsfield units.17 VAT area was quantified by delineating abdominal cavity at the internal aspect of the abdominal wall and the posterior aspect of the vertebral body with an receiver operator instrument.17 SAT was calculated by subtracting VAT from total abdominal adipose tissue area.
Total body fat mass
Total body fat mass (in kilograms) was assessed the same day as the CT scans with whole body dual-energy X-ray absorptiometry (DXA) scans using a General Electric Lunar Prodigy scanner (enCORE software, GE-Lunar, Madison, WI) with a participant in the supine position, arms by her side, wearing only a hospital gown. The system software first determines bone mineral content and soft tissue compartments. Then the software further separates soft tissue into fat-free soft-tissue mass and total body fat mass in kilograms. Because DXA-measured total body fat mass quantifies total body adipose tissue more precisely than body mass index or waist circumference, we used total body fat mass to explore the confounding relationship of adipose tissue between 25[OH]D and HDL.
Statistical analysis
The cohort was described with standard descriptive statistics. Subgroup comparisons of categorical variables were performed with chi-squared (χ2) analysis, and the subgroup means were compared by Student t test (Table 1). Linear correlations between the lipoproteins, 25[OH]D and adiposity variables were conducted using Pearson correlation coefficients (Table 2). We used logarithmic transformation to convert skewed variables (triglyceride concentration and VLDL/chylomicron concentration) into normally distributed variables.
Table 1.
Participant characteristics
| Comparison by race | ||||
|---|---|---|---|---|
| Characteristic | Total sample, n = 78 |
Black women, n = 39 (50%) |
White women, n= 39 (50%) |
P value |
| General characteristics | (χ2) | |||
| Mean age (range), years | 56 (48–64) | 57 (48–63) | 56 (51–63) | .99 |
| Hypertension, n (%) | 39 (50) | 29 (74) | 12 (30) | <.001 |
| Smoking, n (%) | 13 (17) | 7 (23) | 6 (16) | .51 |
| Vitamin D supplement use, n (%) | 8 (10) | 4 (10) | 4 (10) | 1.00 |
| Adiposity, mean ± SD | (t-test) | |||
| Body mass index, kg/m2 | 30 (± 6) | 32 (± 6) | 28 (± 5) | <.001 |
| Waist circumference, cm | 91 (± 12) | 96 (± 12) | 87 (± 11) | .004 |
| Total body fat mass, kg | 34 (± 12) | 38 (± 13) | 30 (± 11) | .007 |
| Visceral, adipose tissue area, cm2 | 102 (± 52) | 101 (± 46) | 102 (± 57) | .96 |
| Subcutaneous abdominal adipose tissue area, cm2 | 411 (± 168) | 460 (± 184) | 362 (± 136) | .01 |
| Lipids, mean ± SD | (t-test) | |||
| HDL cholesterol, mmol/L | 57 (± 11) | 55 (± 11) | 58 (± 11) | .25 |
| HDL particle size, nm | 9.1 (± 0.4) | 9.0 (± 0.4) | 9.1 (± 0.4) | .70 |
| HDL particle concentration, µmol/L : | 35 (± 5) | 35 (± 5) | 35 (± 5)) | .20 |
| Large HDL | 8.6 (± 3.4) | 8.4 (± 3.7) | 9.5 (± 3.2) | .77 |
| Small-to-medium HDL | 26 (± 6) | 25 (± 5) | 27 (± 7) | .38 |
| LDL particle concentration, mmol/L | 1.3 (± 0.4) | 1.2 (± 0.4) | 1.2 (± 0.4) | .72 |
| LDL particle size, nm | 21.4 (± 0.8) | 21.5 (± 0.8) | 21.4 (± 0.8) | .62 |
| Triglycerides, mg/dL | 79 (60–146)* | 75 (54–138)* | 81 (60–184)* | .11 |
| VLDL and chylomicron concentration, nmol/L | 40 (17–94)* | 31 (14–94)* | 45 (17–118)* | .13 |
| 25-hydroxy vitamin D, mean ± SD | (t-test) | |||
| Total 25[0H]D, µg/L | 31 (± 15) | 22 (± 12) | 39 (± 14) | <.001 |
| in summer/fall, µg/L | 35 (± 15) | 24 (± 11) | 41 (± 13) | <.001 |
| in winter/spring, µg/L | 25 (± 14)† | 22 (± 12) | 32 (± 16)† | <.001 |
| Calcidiol (25-hydroxy vitamin D3), µg/L | 30 (± 15) | 21 (± 11) | 38 (± 15) | <.001 |
Significant P values are indicated in bold print.
HDL, high-density lipoprotein; LDL, low-density lipoprotein; VLDL, very-low-density lipoprotein.
The variable, shown as median (10th–90th percentile), was logarithmically transformed for comparison between white and black women.
Mean total 25[0H]D was significantly lower in winter/spring than in summer/fall season (one-way t-test, P < .05) in white women.
Table 2.
Pearson correlations: lipid, vitamin D, and adiposity variables
| Characteristic | HDL cholesterol |
HDL particle size |
HDL particle concentration |
Total 25-0H vitamin D |
|
|---|---|---|---|---|---|
| Total | Large | ||||
| HDL variables | |||||
| HDL cholesterol, mg/dL | - | - | - | - | 0.33* |
| HDL particle size, nm | 0.77* | - | - | - | 0.18† |
| HDL particle concentration, µmol/L : | 0.47* | −0.16† | - | - | 0.20† |
| Large HDL particle subclass | 0.83* | −0.92* | −0.03 | - | 0.30* |
| Small-to-medium HDL particle subclass | −0.09 | 0.64* | −0.83* | 0.59* | 0.004 |
| Other lipid variables | |||||
| LDL particle concentration, mmol/L | −0.57* | −0.56* | −0.12 | −0.49* | 0.06 |
| LDL particle size, nm | 0.62* | 0.76* | −0.07 | 0.71* | 0.04 |
| Trigycerides, mg/dL | −0.23* | −0.41* | −0.22* | −0.39* | 0.12 |
| VLDL and chylomicrone particle concentration, nmol/L | −0.18† | −0.41* | 0.28* | −0.38* | 0.04 |
| VLDL particle size, nm | 0.03 | 0.08 | −0.08 | 0.05 | −0.11 |
| Adiposity and Hypertension variables | |||||
| Visceral adipose tissue area, cm2 | −0.41* | −0.38* | −0.02 | −0.45* | −0.16† |
| Subcutaneous abdominal adipose tissue area, cm2 | −0.26* | −0.11 | −0.18† | −0.21† | −0.38* |
| Total body fat mass, kg | −0.32* | −0.16† | −0.21† | −0.24* | −0.36* |
| High blood pressure (>140/90 mm Hg or treated hypertension) | −0.02 | −0.03 | −0.10 | −0.09 | −0.46* |
HDL, high-density lipoprotein; LDL, Low-density lipoprotein; VLDL, very-low-density Lipoprotein.
Numbers in bold indicate significance at P < .05.
P < .20.
Univariable linear regression models tested the association between 25[OH]D and each of the HDL outcome variables. Any adiposity measurements with Pearson's correlations which had a P value less than .2 were considered in the modeling. Multivariable-adjusted linear regression models tested the hypothesis that the relationship between 25[OH]D and HDL outcome variables was affected by total body fat mass or abdominal adipose tissue compartments (VAT and SAT). Figure 1 depicts a causal diagram18 exploring the relationships between 25[OH]D and HDL variables. In multivariable analysis we conditioned for confounding by indication (race) and for common causes affecting 25[OH]D and HDL levels—the season of sample collection (as both 25[OH]D and HDL cholesterol have seasonal variation9,19). We added to the multivariable models adjustment for age, which affects both HDL and vitamin D.9 We explored each of the models including quadratic and cubic transformation of 25[OH]D variable to assess for any nonlinear relation. Analyses were carried out using Stata™ 10.1 system for Windows (StataCorp, Inc., College Station, TX).
Results
Subject characteristics
Table 1 lists the characteristics of study participants. Because women with darker skin pigmentation tend to have lower 25[OH]D,20 we compared black and white women. Black and white women had similar characteristics; however, a greater proportion of black women had high blood pressure, larger body mass index, larger waist circumference, and greater total body adipose tissue and SAT area. VAT area was not different between racial groups (P = .96). The lipid parameters were similar in both races.
All but 8 (10%) women had undetectable levels of 25-hydroxy vitamin D2 and the proportion was similar in both races (P = .70), suggesting low rates of vitamin D supplement use. The 25-hydroxy vitamin D3 (calcidiol) accounted for most of the total 25-hydroxy vitamin D concentration. The 25[OH]D concentrations were significantly lower during winter/spring than summer/fall season in white, but not in black women (P < .03).
Correlations of high-density lipoprotein characteristics with 25[0H]D and adiposity
Table 2 shows correlations among lipids, adiposity, and 25[OH]D variables.
Correlations among HDL variables
We explored the relationships between HDL cholesterol (the standard method to assess high-density lipoproteins in clinical practice) and the HDL composition variables in our study sample. HDL cholesterol correlated best with the large HDL particle subclass (r = 0.83, P < .001) compared with all other HDL composition variables. Thus, cholesterol carried by the large HDL particle subclass accounted for the major share of HDL cholesterol concentration.
Correlations of 25[0H]D and lipid variables
The 25[OH]D positively correlated with HDL cholesterol (r = 0.33, P = .003) and with the large HDL particle subclass (r = 0.30, P < .009). We found marginal correlation of 25[OH]D with total HDL particle concentration (r = 0.20, P = .08) and with HDL particle size (r = 0.18, P = .11).
Triglyceride and VLDL variables correlated with the HDL variables but not with 25[OH]D. The LDL particle size and concentration correlated with HDL cholesterol, HDL particle size, and the large HDL particle subclass but not with 25[OH]D. These findings suggest that the triglyceride, VLDL, and LDL variables may not directly contribute to the relationship between 25[OH]D and HDL variables in our study sample.
Correlations of adipose tissue variables with HDL variables and 25[0H]D
VAT inversely correlated with the large HDL particle subclass (r = −0.44, P < .001) and HDL cholesterol (r = −0.41, P < .001), and marginally with 25[OH]D (r = −0.16, P = .17). SAT correlated inversely with 25[OH]D (r = −0.37, P < .001) and HDL cholesterol (r = −0.26, P < .02) but only marginally with large HDL particle subclass (r = −0.37, P = .06). Total body fat mass correlated inversely with 25[OH]D (r = −0.36, P = .001), HDL cholesterol (r = −0.32, P = .005), and large HDL particle subclass (r = −0.24, P = .04). The pattern of these correlations suggest that either total body fat mass, SAT, or VAT may confound the relationship between 25[OH]D and HDL cholesterol or large HDL particle subclass.
The multivariable-adjusted associations of vitamin D and HDL composition
Table 3 summarizes the contribution of 25[OH]D to HDL composition outcomes in multivariable-adjusted models. No important nonlinear associations of 25[OH]D and HDL variables were found. Therefore, we did not include quadratic and cubic 25[OH]D variable transformation in final models. The associations between 25[OH]D and HDL variables were similar in regression models substituting 25[OH]D by calcidiol. Including age as a confounding in all models did not significantly affect the associations of 25[OH]D and HDL variables, perhaps because of the narrow age range of the postmenopausal women in our study.
Table 3.
The association of 25-hydroxy vitamin D Levels with HDL cholesterol, HDL particle size, and Large HDL particle subclass, adjusted for confounders
| The effect of 25[0H]D in the model (for each 5 mcg/L 25[0H]D change): |
HDL cholesterol, mg/dL |
HDL Particle size, nm | Large HDL particle subclass, µmol/L |
|---|---|---|---|
| Adjusted for race and season† | 1.39 (0.13–2.64)* | 0.04 (−0.01 to 0.09) | 0.57 (0.20–0.95)* |
| Adjusted for race, season,† and total body fat mass | 1.08 (−0.28 to 2.28) | 0.03 (−0.01 to 0.08) | 0.49 (0.10–0.89)* |
| Adjusted for race, season,† SAT | 1.10 (−0.20 to 2.41) | 0.03 (−0.02 to 0.09) | 0.50 (0.11–0.90)* |
| Adjusted for race, season,† VAT | 0.74 (−0.48 to 1.96) | 0.02 (−0.03 to 0.07) | 0.37 (0.01–0.74)* |
HDL, high-density lipoprotein; LDL, low-density lipoprotein; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
P < .05.
In the models predicting HDL particle size and large HDL particle subclass, we also adjusted for total HDL particle concentration.
25[0H]D and HDL cholesterol
The HDL cholesterol is the most common clinical method of assessing high-density lipoproteins in clinical practice. A 5 µg/L greater 25[OH]D concentration was associated with 1.39 mg/dL (95% confidence intervall 0.13–2.64, P = .03) greater HDL cholesterol after adjustment for race and season. This relationship was confounded by total body fat mass, SAT and VAT. These findings indicate that adipose tissue is a significant confounder in association between 25[OH]D and HDL cholesterol.
25[0H]D and large HDL particle subclass
Large HDL particle subclass is an important marker of reverse cholesterol transport21 and, therefore, atheroprotective effect of HDL particles. A 5 µg/L greater 25 [OH]D concentration was associated with 0.58 µmol/L (95% confidence interval 0.16–0.95, P = .003) greater large HDL subclass particle concentration, independent of season, race, and total HDL particle concentration. This relationship remained significant after adjustment for total body fat mass (P = .02), SAT (P = .02), or VAT (P = .04), indicating that 25[OH]D and large HDL particles are significantly associated even after adjustment for confounding bias.
Discussion
The major finding of this report is that the association between HDL cholesterol and vitamin D appears to be accounted for by large HDL particle subclass in postmenopausal women. The positive association between HDL cholesterol and vitamin D has been reported previously in observational studies.5,22–26 In Rancho Bernardo study, the association between HDL cholesterol and vitamin D could not be demonstrated; however, participants had nearly 45% greater vitamin D levels than participants of other large observational studies,4,5 suggesting a threshold effect of vitamin D. None of the large epidemiological studies explored the association between HDL composition and vitamin D levels.
The association of large HDL subclass with 25[OH]D can be a key to understanding the cardioprotective effects of greater serum 25[OH]D concentrations. The large HDL particles are linked to cardiovascular protection in epidemiological studies, as suggested by EPIC-Norfolk data.6 Although we cannot assume causal relationship between 25[OH]D and large HDL particle subclass because of descriptive nature of our study, there may be a biologically plausible causal path. Large HDL particles carry cholesterol from atherosclerotic plaque in a process called reverse cholesterol transport, which is an important atheroprotective HDL particle function.21 The increase in large HDL particles is driven by cholesterol efflux from cholesterol-loaded macrophages. Furthermore, macrophage function is regulated by vitamin D.27 Thus, vitamin D can play a key role in regulating the reverse cholesterol transport.
Adipose tissue can be a confounder in the relationship between vitamin D and HDL cholesterol, which is an important mediator of association between VAT and cardiovascular disease (Fig. 1).28 Vitamin D is associated with HDL cholesterol and waist circumference.4,5 Analysis of Framingham Heart Study data showed a significant association of vitamin D with SAT and VAT10 but did not explore whether SAT or VAT confounds the relationship between vitamin D and HDL cholesterol. We find that the association between vitamin D and large HDL particle subclass is independent of total body fat mass, SAT, and is partially confounded by VAT. This finding has not been reported previously.
The strength of our study was the sample, which featured a wide range of 25[OH]D concentrations and fat distribution, sociodemographic characteristics that were balanced between the black and white women and an age range that is suitable for potential CAD risk intervention.
The limitation of our study is its cross-sectional design, preventing us from drawing conclusions about the causality in the relationship between large HDL particle subclass and 25[OH]D concentrations. If indeed 25[OH]D promotes large HDL particle formation and, therefore, reverse cholesterol transport, then 25[OH]D deficiency may be an attractive therapeutic target for a simple and cost-effective intervention improving HDL particle composition and thus reducing risk of cardiovascular events. The plausibility of this association is supported by synergistic effects of vitamin D and statins,29 which imply that vitamin D may be inhibiting HMG Co-A reductase,30 which is important in cholesterol synthesis.
A sample featuring both black and white women has a wider range of 25[OH]D levels and minimizes spectrum bias. However, the sample composition adds possible heterogeneity to the relation between 25(OH)D and HDL metabolism. It is well known, that black women tend to have lower vitamin D levels than white women as the result of darker skin pigmentation (decreased photosynthesis of vitamin D) and greater adiposity, and these racial groups appear to differ in HDL metabolism in general and HDL particle size in particular. Our data suggest this potential for heterogeneity on the basis of race (Table 1): there are body fat differences (total body fat mass and SAT but not VAT) between the white and black women, but these differences do not translate into differences in lipid parameters. Moreover, these racial differences in HDL metabolism may not relate to differences in risk of vascular disease. A large sample that would allow for assessment of effect modification by race would be a wonderful addition to the literature, but our study, is too small to address the heterogeneity.
The limitation of VAT measurements is small variability (approximately 2.8%) on repeated testing. It is possible that a strong confounding effect of VAT on the association between HDL measures and vitamin D would attenuate the association even more, if very high precision VAT measurement technique was available and even more residual confounding was eliminated.
Conclusion
The large HDL particle subclass is associated with vitamin D. Unlike HDL cholesterol, the relationship of large HDL particle subclass with vitamin D was independent of total body fat mass, and only partially confounded by association with visceral adipose tissue in post-menopausal black and white women. Theoretically, vitamin D can protect against cardiovascular risk by promoting formation of large HDL particles, affecting reverse cholesterol transport. Exploring the causal relationship of 25[OH]D on an atheroprotective HDL profile in an interventional trial, may clarify the value of vitamin D treatment on cardiovascular risk reduction.
Acknowledgments
Financial disclosures
This project was supported by a Pilot Study Grant from the Rush Translational Science Consortium awarded through the Scientific Leadership Council at Rush University Medical Center.
References
- 1.Wang TJ, Pencina MJ, Booth SL, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilson PW, Garrison RJ, Castelli WP, Feinleib M, McNamara PM, Kannel WB. Prevalence of coronary heart disease in the Framingham Offspring Study: role of lipoprotein cholesterols. Am J Cardiol. 1980;46:649–654. doi: 10.1016/0002-9149(80)90516-0. [DOI] [PubMed] [Google Scholar]
- 3.Dobnig H, Pilz S, Scharnagl H, et al. Independent association of low serum 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels with all-cause and cardiovascular mortality. Arch Intern Med. 2008;168:1340–1349. doi: 10.1001/archinte.168.12.1340. [DOI] [PubMed] [Google Scholar]
- 4.Reis JP, von Muhlen D, Miller ER, III, Michos ED, Appel LJ. Vitamin D status and cardiometabolic risk factors in the united states adolescent population. Pediatrics. 2009;124:e371–e379. doi: 10.1542/peds.2009-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shea MK, Benjamin EJ, Dupuis J, et al. Genetic and non-genetic correlates of vitamins K and D. Eur J Clin Nutr. 2007;63:458–464. doi: 10.1038/sj.ejcn.1602959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El Harchaoui K, Arsenault BJ, Franssen R, et al. High-density lipoprotein particle size and concentration and coronary risk. Ann Intern Med. 2009;150:84–93. doi: 10.7326/0003-4819-150-2-200901200-00006. [DOI] [PubMed] [Google Scholar]
- 7.Kannel WB, Hjortland MC, McNamara PM, Gordon T. Menopause and risk of cardiovascular disease: the Framingham study. Ann Intern Med. 1976;85:447–452. doi: 10.7326/0003-4819-85-4-447. [DOI] [PubMed] [Google Scholar]
- 8.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick ME. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72:690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 9.Young KA, Engelman CD, Langefeld CD, et al. Association of plasma vitamin D levels with adiposity in Hispanic and African Americans. J Clin Endocrinol Metab. 2009;94:3306–3313. doi: 10.1210/jc.2009-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng S, Massaro JM, Fox CS, et al. Adiposity, cardiometabolic risk, and vitamin D status: The Framingham Heart Study. Diabetes. 2010;59:242–248. doi: 10.2337/db09-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sam S, Haffner S, Davidson MH, et al. Relationship of abdominal visceral and subcutaneous adipose tissue with lipoprotein particle number and size in type 2 diabetes. Diabetes. 2008;57:2022–2027. doi: 10.2337/db08-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pascot A, Lemieux S, Lemieux I, et al. Age-related increase in visceral adipose tissue and body fat and the metabolic risk profile of premenopausal women. Diabetes Care. 1999;22:1471–1478. doi: 10.2337/diacare.22.9.1471. [DOI] [PubMed] [Google Scholar]
- 13.Janssen I, Powell LH, Kazlauskaite R, Dugan SA. Testosterone and visceral fat in midlife women: The Study of Women’s Health Across the Nation (SWAN) FatPatterning Study. Obesity. doi: 10.1038/oby.2009.251. [In Press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Health and Nutrition Examination Survey physician’s examination procedures manual. [Accessed February 4, 2010];1999 & 2000 Available at: http://www.cdc.gov/nchs/data/nhanes/pe.pdf.
- 15.Otvos J. Measurement of triglyceride-rich lipoproteins by nuclear magnetic resonance spectroscopy. Clin Cardiol. 1999;22(S2):II-21–II-27. doi: 10.1002/clc.4960221405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Engelman CD, Fingerlin TE, Langefeld CD, et al. Genetic and environmental determinants of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels in Hispanic and African Americans. J Clin Endocrinol Metab. 2008;93:3381–3388. doi: 10.1210/jc.2007-2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeNino WF, Tchernof A, Dionne IJ, et al. Contribution of abdominal adiposity to age-related differences in insulin sensitivity and plasma lipids in healthy nonobese women. Diabetes Care. 2001;24:925–932. doi: 10.2337/diacare.24.5.925. [DOI] [PubMed] [Google Scholar]
- 18.Hernan MA, Hernandez-Diaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004;15:615–625. doi: 10.1097/01.ede.0000135174.63482.43. [DOI] [PubMed] [Google Scholar]
- 19.Ockene IS, Chiriboga DE, Stanek EJ, III, et al. Seasonal variation in serum cholesterol levels: treatment implications and possible mechanisms. Arch Intern Med. 2004;164:863–870. doi: 10.1001/archinte.164.8.863. [DOI] [PubMed] [Google Scholar]
- 20.Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, ethnicity, and blood pressure in the Third National Health and Nutrition Examination Survey. Am J Hypertens. 2007;20:713–719. doi: 10.1016/j.amjhyper.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 21.Rye K-A, Bursill CA, Lambert G, Tabet F, Barter PJ. The metabolism and anti-atherogenic properties of HDL. J. Lipid Res. 2009;50(Suppl):S195–S200. doi: 10.1194/jlr.R800034-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reis JP, von Mühlen D, Kritz-Silverstein D, Wingard DL, Barrett-Connor E. Vitamin D, parathyroid hormone levels, and the prevalence of metabolic syndrome in community-dwelling older adults. Diabetes Care. 2007;30:1549–1555. doi: 10.2337/dc06-2438. [DOI] [PubMed] [Google Scholar]
- 23.Reis JP, von Muhlen D, Miller ER., III Relation of 25-hydroxyvitamin D and parathyroid hormone levels with metabolic syndrome among US adults. Eur J Endocrinol. 2008;159:41–48. doi: 10.1530/EJE-08-0072. [DOI] [PubMed] [Google Scholar]
- 24.Maki KC, Rubin MR, Wong LG, et al. Serum 25-hydroxyvitamin D is independently associated with high-density lipoprotein cholesterol and the metabolic syndrome in men and women. J Clin Lipidol. 2009;3:289–296. doi: 10.1016/j.jacl.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 25.Auwerx J, Bouillon R, Kesteloot H. Relation between 25-hydroxyvitamin D3, apolipoprotein A-I, and high density lipoprotein cholesterol. Arterioscler Thromb. 1992;12:671–674. doi: 10.1161/01.atv.12.6.671. [DOI] [PubMed] [Google Scholar]
- 26.Hypponen E, Power C. Vitamin D status and glucose homeostasis in the 1958 British birth cohort: the role of obesity. Diabetes Care. 2006;29:2244–2246. doi: 10.2337/dc06-0946. [DOI] [PubMed] [Google Scholar]
- 27.Matsuura F, Wang N, Chen W, Jiang XC, Tall AR. HDL from CETP-deficient subjects shows enhanced ability to promote cholesterol efflux from macrophages in an apoE- and ABCG1-dependent pathway. J Clin Invest. 2006;116:1435–1442. doi: 10.1172/JCI27602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mathieu P, Poirier P, Pibarot P, Lemieux I, Despres J-P. Visceral obesity: the link among inflammation, hypertension, and cardiovascular disease. Hypertension. 2009;53:577–584. doi: 10.1161/HYPERTENSIONAHA.108.110320. [DOI] [PubMed] [Google Scholar]
- 29.Grimes DS. Are statins analogues of vitamin D? Lancet. 2006;368:83–86. doi: 10.1016/S0140-6736(06)68971-X. [DOI] [PubMed] [Google Scholar]
- 30.Pérez-Castrillón JL, Vega G, Abad L, Sanz A, Chaves J, Hernandez G, Dueñas A. Effects of atorvastatin on vitamin D levels in patients with acute ischemic heart disease. Am J Cardiol. 2007;99:903–905. doi: 10.1016/j.amjcard.2006.11.036. [DOI] [PubMed] [Google Scholar]