Abstract
Background and Objectives
Poor homing efficiency is one of the major limitations of current stem cell therapy. Magnetic bionanoparticles (MPs) obtained from Magnetospirillum sp. AMB-1 have a lipid bilayer membrane and ferromagnetic properties. We evaluated a novel priming strategy using MPs to enhance the homing of transplanted progenitor cells to target tissue.
Materials and Methods
Effects of MP on proliferation, viability, and migration of late human endothelial progenitor cells (EPCs) were examined in vitro. Additionally, effects of MP on gene and protein expression related to survival and adhesion were evaluated. Homing and angiogenic efficiency of MP transferred late EPCs was evaluated in nude mouse hindlimb ischemia model.
Results
Below threshold concentration, MP transfer did not influence proliferation or survival of late EPCs, but enhanced migration and trans-endothelial migration of late EPCs toward magnet. Below threshold concentration, MP transfer did not influence gene and protein expression related to survival. In the mouse hindlimb ischemia model, late EPCs treated with high dose MP (5 ug/mL) showed enhanced homing of injected late EPCs in the ischemic limb by magnet, compared to low dose MP (1 ug/mL) treated late EPCs. In addition, high dose MP transferred EPC showed significantly better improvement of perfusion in ischemic limb compared to untreated EPC.
Conclusion
MP transfer with magnet application can be a promising novel strategy to enhance homing efficacy and outcomes of current stem cell therapy.
Keywords: Nanoparticles, Stem cells, Ischemia
Introduction
Stem cell therapy is a promising novel therapy for patients with ischemic cardiovascular diseases, however the efficacy of current stem cell therapy needs to be improved. In vivo and clinical studies have demonstrated that only a small portion of transplanted cells remained within the ischemic lesion even several hours after delivery.1),2) This has been regarded as one of the major causes of limited efficacy of stem cell therapy.
Magnetic particles have been used for tagging and labeling in magnetic resonance imaging where safety and feasibility for long term follow up has been demonstrated.3) Magnetic nanoparticles have the potential to capture moving cells in the target lesion by aid of magnetic forces. Previous studies reported the therapeutic potential of magnetic bionanoparticles (MPs) in both the artificial blood vessel model4) and myocardial infarction model.5) In this study we evaluated the therapeutic potential of MPs obtained from Magnetospirillum sp. AMB-1 with human endothelial progenitor cells (EPCs) in an in vivo model.
Materials and Methods
This study was approved by the institutional review board and Institutional Animal Care & Use Committee of the University Hospital.
Preparation of magnetic bionanoparticles
Magnetic bionanoparticles were obtained from Magnetospirillum sp. AMB-1. Preparation procedures of MPs were performed as previously described.4)
Endothelial progenitor cell culture
Peripheral blood (50 mL) was obtained from donors who signed informed consent. The mononuclear cells (MNCs) were fractionated from other components of peripheral blood by centrifugation on Histopaque 1077 (GE Healthcare, Waukesha, WI, USA) gradients according to the manufacturer's instructions. MNCs were isolated and washed 3 times with phosphate buffered saline (PBS) and resuspended in complete EGM-2MV™ medium (Lonza, Basel, Switzerland). Cells were seeded onto separate wells of a 6-well tissue culture plate, which was precoated with 1.5% Gelatin (Sigma, St. Louis, MO, USA), at 37℃ and 5% CO2, in a humidified incubator. Colonies of endothelial like cells appeared between 5 and 22 days of culture and were identified as well-circumscribed monolayers of cobblestone appearing cells.
Transmission electron microscopy
Endothelial progenitor cells were exposed to MPs for 3 days, washed with PBS and fixed with Karnovsky's electron microscopy fixative (2.5% glutaraldehyde and 2% paraformaldehyde in 80 mM phosphate buffer, pH 7.3-7.4). Secondary fixation was done in 1% osmium tetroxide with 1.5% potassium ferrocyanide in double distilled water for 1 hour at 4℃. Dehydration was performed through ascending concentrations of ethanol with three changes at 100%. Pure Epon-Araldite resin that did not contain methyl anhydride was added and infiltrated overnight at room temperature. All resin was removed the next day, and fresh resin was added to the appropriate depth. The sample was polymerized for 18 hours. Ultrathin sections of cells of interest were cut en face (parallel to the surface on which the cells were grown) using a JEM-100CX™ (JEOL, Tokyo, Japan) and then stained with uranyl acetate and lead citrate before viewing in a Philips CM120 electron microscope (FEI).
Cell viability and transfection efficacy
Magnetic bionanoparticles were mixed with EGM-2MV™ at concentrations of 50 ng/mL, 500 ng/mL, and 1000 ng/mL, and the media of EPCs was replaced with magnetite particles mixed medium. This step was repeated daily for 3 days. After transfection of MPs to EPC, cell viability for transfection was evaluated by tryphan blue exclusion assay.
The efficacy of transfection of MPs to EPC was evaluated by Prussian blue staining. For Prussian blue staining, which indicates the presence of iron, Cytospin slides were made by loading 3000 hEPCs in a total volume of 200 mL of EGM-2MV™ media per Cytofunnel™ (Thermo Electron, Pittsburgh, PA, USA) and centrifuging at 1350 rpm for 5 minutes in a Cytospin 3 cytocentrifuge (Thermo Electron Corp., Pittsburgh, PA, USA). Two slides were stained for analysis using Prussian blue and nuclear fast red staining. The cytospin slides were fixed with methyl alcohol, washed with distilled water, incubated for 30 minutes with 2% potassium ferrocyanide in 2% hydrochloric acid. Thereafter slides were washed again, and counterstained with nuclear fast red for 5 minutes. Proliferation capacity was assessed in subconfluent culture conditions and hypoxia-reoxygenation was done as previously described.6)
Scratch-wound assay
Endothelial progenitor cells were seeded into 6-well plates and transfected with MPs for 3 days. When the MP transfected EPCs had grown to subconfluence, EPC monolayers were scratched with a Cell lifter (Costar, Cambridge, MA, USA), creating a cell-free area ('scratch-wound') approximately 1 cm in width. 'Scratch-wounded' monolayers were washed twice with EGM-2MV™ to remove cell debris, and a defined area of the wound was photographed using phase-contrast microscopy. To remove the effect of cell proliferation, cells were pretreated with 1M thymidine (Sigma, St. Louis, MO, USA), which was added to the cell incubation medium. This treatment inhibits the proliferation of cells without killing them. In addition, a magnet was positioned in cell processing direction below the bottom of the plate. The migration was observed for 24 hours. For each observation the cell migration plate was placed on the stage of an inverted microscope (Olympus, Tokyo, Japan) equipped with a CCD camera. The image of the migration was analyzed by Image Pro Plus (Media Cybernetics, Bethesda, MD, USA). For magnet application, magnet was located in target position below culture plate.
Transendothelial migration assay
Human umbilical vein endothelial cell monolayers were grown to confluency on transwell inserts (3.0 µm pore size; BD Biosciences, Franklin Lakes, NJ, USA) that were subsequently placed in a fresh 24-well plate (BD Biosciences, Franklin Lakes, NJ, USA). To assess the effect of Stromal cell-derived factor (SDF)-1 on transendothelial migration in vitro, SDF-1 treated medium was added to the lower chamber, while the upper chamber contained Carboxyfluoroscein Diacetate Succinimidyl Ester (CFSE) labeled 2×104 EPCs in EGM-2MV™. For tropism of MP transfected cells, a magnet was positioned underneath the 24 well plate. Transmigrated cells were recovered from the lower chamber after 12 to 14 hours of incubation at 37℃/5% CO2. Enumeration of migrated cells was accomplished by counting CFSE labeled green cells. For magnet application, magnet was located in target position below culture plate.
Reverse transcript-polymerase chain reaction
Samples (1 µg) of each total RNA extract were used to perform first strand cDNA synthesis using a kit (Promega, Madison, WI, USA) in a total volume of 20 µL. One-tenth of each cDNA generated was used as a template for PCR using a Nova Taq polymerase. Primer sets for amplification of intercellular adhesion molecule-1, vascular cell adhesion molecule-1, SDF-1, KDR, Ve-Cadherin, Tie-2 and GAPDH were designed as previously described.7)
Western blot analysis
After transfection of MPs to EPCs, EPCs were exposed to hypoxia in a 1% O2 hypoxic system (GasPak Pouch Anaerobic System, BD Bioscience, Franklin Lakes, NJ, USA). Thereafter EPCs were washed in cold PBS and harvested by scraping in lysis buffer after the indicated procedures. The protein concentration of EPC whole cell extracts was determined using a BCA protein assay kit (Pierce, Rockford, IL, USA). The extracts (20 ug protein) of EPCs were separated on an SDS-PAGE gel, and were performed according to the procedures described previously with minor modification.8)
Anti-ILK was obtained from Upstate (Lake Placid, NY, USA), Anti-t-Akt, anti-NF-κB (p65), anti-Ve-cadherin, anti-Tie-2 and anti-t-GSK3b were from Santa Cruz Biotechnology (Santa Cruz, CA, USA), and anti-t-p44/42 (Erk1/2) MAP kinase, anti-p-p44/42 MAP kinase, anti-p-GSK3β (S9), and anti-t-b-catenin were from Cell Signaling (Berkely, MA, USA), while anti-HIF-1α was from BD Transduction Laboratories (Franklin Lakes, NJ, USA). Anti-a-tubulin was obtained from Calbiochem (Darmstadt, Germany). The secondary antibodies to each primary antibody utilized were as follows. Anti-mouse IgG HRP conjugated and anti-rabbit IgG HRP conjugated antibodies were obtained from Promega (Madison, WI, USA).
Anti-goat IgG HRP conjugated antibody was from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-mouse IgG Alexa flour 488 and anti-rabbit IgG Alexa flour 555 secondary antibody were purchased from Invitrogen (Carlsbad, CA, USA). 4',6-Diamidino-2-phenylindole dihydrochloride was obtained from Sigma-Aldrich. Co (St. Louis, MO, USA).
Mouse hindlimb ischemia model and endothelial progenitor cell transplantation
Six-week-old C57BL6/J mice (Biogenomics, Korea) were used for all animal experiments. Mice were anesthetized with 50 mg/kg intraperitoneal pentobarbital. To induce muscle ischemia, a unilateral femoral artery was removed as previously described.8) Daily observation and wound dressing were done to avoid significant infection of the wounds.
Six hours after unilateral femoral artery excision, mice were randomized into 6 groups: untreated EPCs, EPCs transfected with 1 ug/mL and 10 ug/mL of MP with and without magnet application. Approximately 5×105 cultivated EPCs in 100 uL of endothelial basal medium-2 medium without growth factors were administered systemically by cardiac puncture using an insulin syringe with a 27-gauge needle as previously reported.8) EPCs were labeled with a fluorescent dye, Vibrant-DiI (Invitrogen, USA), for tracking and quantification. A magnet was applied to the ischemic limb to enhance trapping of EPC for 1 hour after injection. Laser Doppler perfusion image analysis was done as previously described.8)
Tissue preparation and immunofluorescence
Mice were sacrificed at predetermined time points by administration of an overdose of sodium pentobarbital. The calf muscles were rinsed in PBS to remove excess blood, snap-frozen in liquid nitrogen, and stored at -80℃. Ten-micrometer-thick histological sections were prepared from snap-frozen tissue samples. Numbers of DiI labeled EPC were counted.
Statistical analysis
For cell culture experiments, results have been expressed as the mean±SD. P were calculated by Student t-test. For multiple comparisons, Bonferroni correction was done. Statistical Package for the Social Sciences (SPSS) version 19.0 program was used for analysis (SPSS Inc., Chicago, IL, USA).
Results
Transfection of magnetic bionanoparticles into endothelial progenitor cells
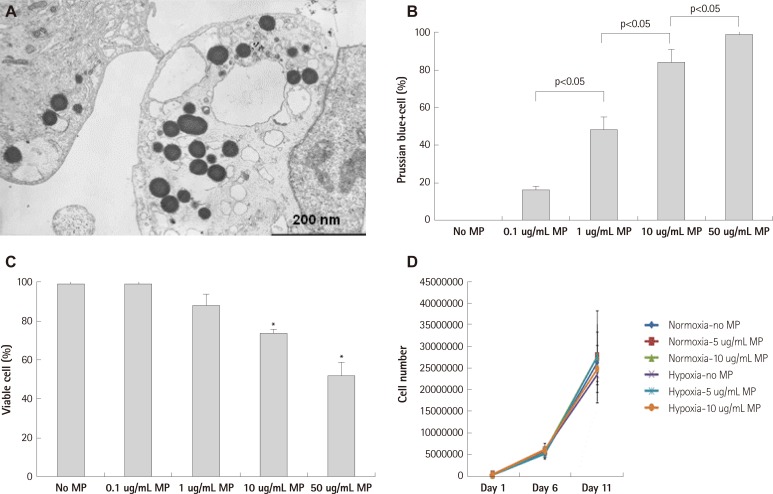
Magnetic bionanoparticles were transfected into EPCs in a dose dependent manner by simple co-incubation. Electron microscopic imaging showed round dense MPs in EPC cytoplasm (Fig. 1A). Transfection of MPs increased in a concentration-dependent manner up to 50 ug/mL (Fig. 1B). Below threshold concentration (10 ug/mL), repeated exposure to MPs for 3 days did not influence the viability of MP transfected EPCs. However, single day-exposure to MPs did not result in detrimental effects on EPC viability at 10 ug/mL of MP without measurable compromise of transfection efficacy (Fig. 1C). Proliferation capacity of EPC was not influenced by MP transfection during the observation period in normoxic and hypoxia-reoxygenation conditions (Fig. 1D).
Fig. 1.
Transfection of magnetic bionanoparticle. A: electron microscopic image of endothelial progenitor cell (EPC) which was transfected with magnetic bionanoparticles (MPs). B: transfection efficacy to EPCs was proportionally increased with concentration of MP, which was assessed by Prussian blue staining. C: viability of EPCs was significantly decreased at above 10 ug/mL of MPs, which was assessed by tryphan blue exclusion assay. D: proliferation of EPC was not influenced by MP transfection below threshold concentration (10 ug/mL) in normoxic and hypoxia-reoxygenation condition. There were no significant differences among groups (n=3 respectively). Error bars represented standard deviation. *p<0.05 compared with no MP group (n=3 respectively).
Influence of magnetic bionanoparticles on gene and protein expression related to angiogenesis and survival
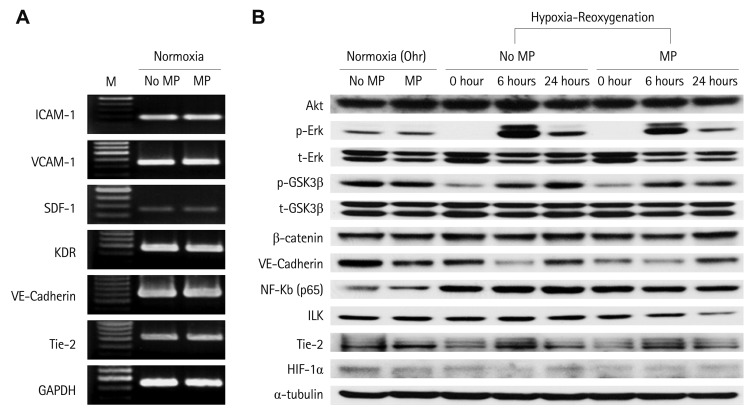
Up to 10 ug/mL of MP, transfection of MP into EPCs did not influence the expression of molecules related to cell adhesion, angiogenesis and survival. In hypoxia and hypoxia-reoxygenation conditions as well as normoxic conditions, these genes and protein expression were not significantly influenced by MP transfection (Fig. 2). Each set of experiments were repeated twice respectively and yielded the same results. This suggested that MP-transfected EPCs respond in same way to non MP-transfected EPCs and involve in angiogenesis.
Fig. 2.
Transfection of MP did not influence the gene and protein expression of key signaling and surface molecules related to survival and angiogenesis in normoxia and hypoxia-reoxygenation condition. A: reverse transcript-polymerase chain reaction. B: western blot (each experiments were repeated twice respectively and representative figures were presented). M: marker, MP: magnetic bionanoparticle, ICAM: intercellular adhesion mdecule, VCAM: vascular cell adhesion molecule, SDF: stromal cell-derived factor.
Magnetic bionanoparticle transfection enhanced directional-migration of endothelial progenitor cells under magnetic field
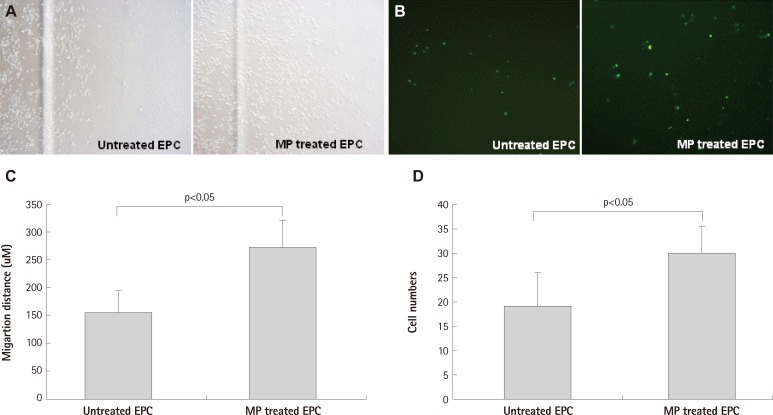
Magnetic bionanoparticle-transfected EPCs showed enhanced directional migration toward the magnet. In the scratch wound assay and the transendothelial migration assay, distance of EPC migration and numbers of penetrating EPCs were increased respectively (Fig. 3). There are potentials that MP transfection may influence migration capacity of EPCs and flexibility of EPC cytoplasm. Despite of potential concerns, transendothelial migration assay showed that MP transfection and magnet application enhanced directional migration.
Fig. 3.
Migration of EPC was enhanced by MP transfection with magnet apply. A and C: scratch wound assay: MP was transfected with concentration of 1 ug/mL and magnet was set in cell migrating direction (n=3 respectively). B and D: transendothelial migration assay: magnetic force enhanced MP transfected EPC transmigration through human umbilical vein endothelial cell monolayer: transmigrated cells at bottom of the porous membrane in the vehicle and MPs group (B). EPC were labeled CFSE before co-incubation (n=5 respectively). Error bars represented standard deviation. EPC: endothelial progenitor cell, MP: magnetic bionanoparticle, CFSE: Carboxyfluoroscein Diacetate Succinimidyl Ester.
Magnetic bionanoparticle transfection improved in vivo homing of endothelial progenitor cells and perfusion of ischemic limb
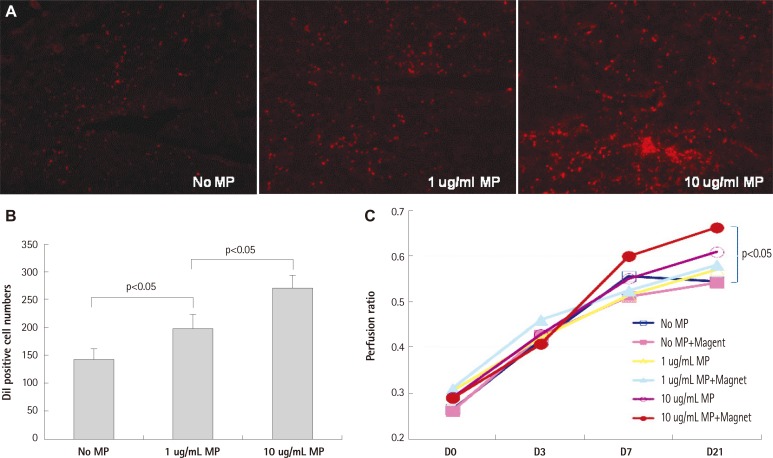
In mice hindlimb ischemia model, MP transfection with magnet significantly enhanced homing and trapping of systemically injected EPCs in ischemic limb compared to untreated EPCs. EPC transfected with higher dose (10 ug/mL) MP showed better perfusion recovery of ischemic limb only with magnet compared with EPC without MP transfection (Fig. 4). Although there were trends suggesting concentration-dependent enhancement of perfusion in ischemic limb, statistically significant differences were only observed between control and high dose MP+ magnet group.
Fig. 4.
Therapeutic efficacy of MP-transfected hEPCs. A and B: in an athymic mouse hindlimb ischemia model, EPCs transfected with MPs showed enhanced homing into ischemic limb in dose dependent manner (n=3 respectively). C: high dose MP (10 ug/mL) and magnet application significantly improved perfusion of ischemic limb compared to control group: MP untreated group without magnet application (n=5 respectively). Error bars represented standard deviation. MP: magnetic bionanoparticle, EPC: endothelial progenitor cell.
Discussion
Magnetic nanoparticles have been regarded as promising modalities for local delivery of drug or genes, additionally cell tracking or labeling modalities for MR imaging.9),10) In this study, we evaluated MPs obtained from magnetic bacteria, AMB-1. MPs have unique features compared with synthetic magnetic nanoparticles.11) Firstly, MPs have lipid-bilayered membrane, which can facilitate transfection of MPs into cells. And membrane can be utilized for drug or gene delivery. However it may cause immune or inflammatory reactions. Secondly, MPs has very uniform size and morphology. Thirdly, MPs have ferromagnetic magnetite (Fe3O4) core, which cause strong interaction with magnetic field.
In this study, we showed that MPs have potentials to be utilized for local cell delivery with systemic administration.12-14) MP can be efficiently transfected into EPCs and remained stable and relatively inert in vivo condition. Recently published study also reported that iron oxide labeling can facilitate targeting and retention of labeled EPC in rat myocardial ischemia/reperfusion model.5) However they could not show enhanced in vivo therapeutic efficacy of magnetically labeled EPC treatment over conventional cell infusion strategy.
Additionally MP itself did not influence proliferation and expression of gene and proteins related to survival and angiogenesis. Recent report also supports our data and showed superparamagnetic particle did not influence the stemness of transfected cells.15) However contamination of endotoxin during purification of MP is a potential problem, which can cause inflammatory responses. MP can enhance directional migration of EPC toward magnetic field in vitro and experimental blood vessel model. In spite of promising results from in vitro study, there have been concerns whether MP transfection could improve angiogenesis and perfusion in vivo. Firstly, depth and anatomical location of target lesion may limit trapping efficiency of MP-transfected cells with magnetic force. Secondly, clumping and trapping of cells may cause obstruction of blood vessel and blood flow, which can cause deterioration of ischemia. Thirdly, transfected MPs may limit flexibility and deformability of MP-transfected cells and inhibit in vivo function and migration of cells.
In this study we demonstrated the therapeutic efficacy of MP with magnet in vivo model. MP transfection and localization with magnet can improve trapping and homing of systemically administrated cells in ischemic lesion and trapped EPCs improved perfusion. With this promising novel strategy, we may enhance homing and trapping of cells into target lesion with aid of magnetic field.
Acknowledgments
This study was supported by grants from The Korean Society of Cardiology (2006) and the Innovative Research Institute for Cell Therapy, University Hospital (A062260), sponsored by the Ministry of Health, Welfare & Family, Republic of Korea.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Kang WJ, Kang HJ, Kim HS, Chung JK, Lee MC, Lee DS. Tissue distribution of 18F-FDG-labeled peripheral hematopoietic stem cells after intracoronary administration in patients with myocardial infarction. J Nucl Med. 2006;47:1295–1301. [PubMed] [Google Scholar]
- 2.Templin C, Kotlarz D, Marquart F, et al. Transcoronary delivery of bone marrow cells to the infarcted murine myocardium: feasibility, cellular kinetics, and improvement in cardiac function. Basic Res Cardiol. 2006;101:301–310. doi: 10.1007/s00395-006-0590-7. [DOI] [PubMed] [Google Scholar]
- 3.Agudelo CA, Tachibana Y, Noboru T, Iida H, Yamaoka T. Long-term in vivo magnetic resonance imaging tracking of endothelial progenitor cells transplanted in rat ischemic limbs and their angiogenic potential. Tissue Eng Part A. 2011;17:2079–2089. doi: 10.1089/ten.TEA.2010.0482. [DOI] [PubMed] [Google Scholar]
- 4.Kim JA, Lee HJ, Kang HJ, Park TH. The targeting of endothelial progenitor cells to a specific location within a microfluidic channel using mag-netic nanoparticles. Biomed Microdevices. 2009;11:287–296. doi: 10.1007/s10544-008-9235-y. [DOI] [PubMed] [Google Scholar]
- 5.Chaudeurge A, Wilhelm C, Chen-Tournoux A, et al. Can magnetic targeting of magnetically labeled circulating cells optimize intramyocardial cell retention? Cell Transplant. 2011 doi: 10.3727/096368911X612440. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 6.Kim HS, Skurk C, Maatz H, et al. Akt/FOXO3a signaling modulates the endothelial stress response through regulation of heat shock protein 70 expression. FASEB J. 2005;19:1042–1044. doi: 10.1096/fj.04-2841fje. [DOI] [PubMed] [Google Scholar]
- 7.Hur J, Yoon CH, Kim HS, et al. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol. 2004;24:288–293. doi: 10.1161/01.ATV.0000114236.77009.06. [DOI] [PubMed] [Google Scholar]
- 8.Yoon CH, Hur J, Park KW, et al. Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells: the role of angiogenic cytokines and miatrix metalloproteinases. Circulation. 2005;112:1618–1627. doi: 10.1161/CIRCULATIONAHA.104.503433. [DOI] [PubMed] [Google Scholar]
- 9.Hill JM, Dick AJ, Raman VK, et al. Serial cardiac magnetic resonance imaging of injected mesenchymal stem cells. Circulation. 2003;108:1009–1014. doi: 10.1161/01.CIR.0000084537.66419.7A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matuszewski L, Persigehl T, Wall A, et al. Cell tagging with clinically approved iron oxides: feasibility and effect of lipofection, particle size, and surface coating on labeling efficiency. Radiology. 2005;235:155–161. doi: 10.1148/radiol.2351040094. [DOI] [PubMed] [Google Scholar]
- 11.Komeili A. Molecular mechanisms of magnetosome formation. Annu Rev Biochem. 2007;76:351–366. doi: 10.1146/annurev.biochem.74.082803.133444. [DOI] [PubMed] [Google Scholar]
- 12.Gupta AK, Gupta M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials. 2005;26:3995–4021. doi: 10.1016/j.biomaterials.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 13.Lei Han, Li SY, Yong Yang, Zhao FM, Jie Huang, Jin Chang Research on the structure and performance of bacterial magnetic nanoparticles. J Biomater Appl. 2008;22:433–448. doi: 10.1177/0885328207079064. [DOI] [PubMed] [Google Scholar]
- 14.Kobayshi T, Ochi M, Yanada S, et al. Augmentation of degenerated human cartilage in vitro using magnetically labeled mesenchymal stem cells and an external magnetic device. Arthroscopy. 2009;25:1435–1441. doi: 10.1016/j.arthro.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 15.Balakumaran A, Pawelczyk E, Ren J, et al. Superparamagnetic iron oxide nanoparticles labeling of bone marrow stromal (mesenchymal) cells does not affect their "stemness". PLoS One. 2010;5:e11462. doi: 10.1371/journal.pone.0011462. [DOI] [PMC free article] [PubMed] [Google Scholar]