Abstract
Motivation to change is believed to be a key factor in therapeutic success in substance use disorders; however, the neurobiological mechanisms through which motivation to change impacts decreased substance use remain unclear. Existing research is conflicting, with some investigations supporting decreased and others reporting increased frontal activation to drug cues in individuals seeking treatment for substance use disorders. The present study investigated the relationship between motivation to change cocaine use and cue-elicited brain activity in cocaine-dependent individuals using two conceptualizations of “motivation to change:” 1) current treatment status (i.e., currently receiving vs. not receiving outpatient treatment for cocaine dependence) and 2) self-reported motivation to change substance use, using the Stages of Change Readiness and Treatment Eagerness Scale (SOCRATES; Miller and Tonigan, 1996). Thirty-eight cocaine-dependent individuals (14 currently in treatment) completed a diagnostic assessment and an fMRI cocaine cue-reactivity task. Whole-brain analyses demonstrated that both treatment-seeking and motivated participants had lower activation to cocaine cues in a wide variety of brain regions in the frontal, occipital, temporal, and cingulate cortices relative to non-treatment-seeking and less motivated participants. Future research is needed to explain the mechanism by which treatment and/or motivation impacts neural cue-reactivity, as such work could potentially aid in the development of more effective therapeutic techniques for substance-dependent patients.
Keywords: cocaine dependence, cue reactivity, fMRI, motivation to change, SOCRATES, treatment seeking
1. Introduction
Motivation to change is considered a necessary ingredient in therapeutic success for individuals with substance use disorders (Prochaska and DiClemente, 1982; Miller and Tonigan, 1996). However, the mechanisms by which motivation to change impacts drug seeking and using behaviors remain unclear. Identifying neurobiological differences between substance-dependent individuals who are motivated to change and those who are not could help to identify such mechanisms, and, in doing so, facilitate the development of more effective therapeutic techniques for individuals with substance use disorders. Brain activation to drug cues is a particularly important neurobiological parameter to examine in relation to change motivation, as chronic drug use is maintained in part by craving and drug-seeking behaviors that occur in response to drug cues that have been repeatedly paired with the reinforcing drug effects (Berridge and Robinson, 1998; Kalivas and Volkow, 2005).
To our knowledge, only two previous publications have explored the impact of motivation to change on brain activation to drug cues in individuals with substance use disorders. These studies both used “treatment-seeking status,” typically defined as whether participants responded to an advertisement for a treatment or a non-treatment study, as an indicator of motivation to change substance use. Wilson and colleagues (2004) reviewed 18 cue-elicited craving functional Magnetic Resonance Imaging (fMRI) studies and advanced the hypothesis that variability in cue-elicited dorsolateral prefrontal cortex (DLPFC) and orbitofrontal cortex (OFC) activations across studies may have been due to between-study differences in participants’ treatment-seeking status. Specifically, studies with “non-treatment-seeking” participants demonstrated reliable cue-elicited activation of DLPFC and OFC, whereas studies with “treatment-seeking” participants did not. The authors suggested that treatment-seeking participants may have not demonstrated frontal activation to drug cues due to efforts to inhibit cue-elicited craving during the scan in an attempt to maintain abstinence, whereas non-treatment-seeking participants may have indulged craving and planned for future use during the scan (Wilson et al., 2004). More recently, Claus and colleagues (2011) pooled cue-elicited fMRI data across two large studies of individuals with alcohol dependence to investigate the impact of a variety of factors, including treatment-seeking status, on brain activation to alcohol cues. In contrast to Wilson et al. (2004), Claus et al. (2011) found that treatment-seeking participants had increased left DLPFC, left amygdala, and nucleus accumbens activation relative to non-treatment-seeking participants. The authors suggested two possible explanations for these unexpected findings: 1) treatment-seeking participants may have activated more to alcohol cues in frontal regions because they were attempting to control their urge to use during the scan, or 2) treatment-seeking participants may have activated more to alcohol cues because they were in a later stage of alcohol dependence relative to non-treatment-seekers. Claus and colleagues (2011) findings are arguably more consistent with the latter explanation because, a) treatment-seeking participants in their study had significantly higher scores on the Alcohol Dependence Scale and the Alcohol Use Disorders Identification Test, had been drinking more heavily in the past 30 days, and had been drinking for more than twice as many years as non-treatment-seeking participants, and b) both frontal (DLPFC) and non-frontal (amygdala, nucleus accumbens) brain regions were more highly activated to alcohol cues in treatment-seeking participants relative to non-treatment-seeking participants. These non-frontal regions (amygdala, nucleus accumbens) are typically involved in cue-elicited craving, and are not typically implicated in frontal control of behavior (Kalivas and Volkow, 2005).
In sum, the relationship between motivation to change and cue-elicited brain activity in substance-dependent individuals remains unclear. The only empirical fMRI investigation of this association (Claus et al., 2011) cannot be clearly interpreted because treatment-seeking participants had substantially more severe alcohol dependence than non-treatment-seeking participants. To advance the literature, the present study investigated the relationship between motivation to change cocaine use and cue-elicited brain activity in cocaine-dependent individuals using two complementary conceptualizations of “motivation to change:” 1) current treatment status (i.e., currently receiving vs. not receiving outpatient treatment for cocaine dependence) and 2) self-reported motivation to change substance use, using the Stages of Change Readiness and Treatment Eagerness Scale (SOCRATES; Miller and Tonigan, 1996). In accordance with Wilson et al. (2004), we hypothesized that current treatment participation, as well as higher scores on the SOCRATES, would be associated with less activation to cocaine cues, particularly in frontal regions.
2. Methods
2.1. Participants
Cocaine-dependent men and women were recruited for a larger, ongoing investigation of D-cycloserine facilitation of cocaine-cue extinction through media advertisements and clinical referrals in the local Charleston, SC area. All study procedures were performed in accordance with Good Clinical Practice Guidelines and the Declaration of Helsinki, with approval from the Medical University of South Carolina Institutional Review Board.
All participants met DSM-IV criteria for Cocaine Dependence and indicated cocaine as their primary drug of choice. Participants were right-handed. Exclusionary criteria included medications for addiction (e.g., topirimate, naltrexone, suboxone, antabuse), major medical (e.g., diabetes, HIV) and psychiatric conditions (e.g., affective disorders, posttraumatic stress disorder), pregnancy or nursing, DSM-IV criteria for non-cocaine substance dependence (except caffeine, nicotine, marijuana, or alcohol) within the past 60 days, and ferrous metal implants or pacemakers.
2.2. Procedure
All procedures included in the present report were conducted prior to the application of any experimental manipulations of the parent study. Following a phone or in-person screening, participants were scheduled for a baseline diagnostic visit. During this visit participants completed a diagnostic interview for DSM-IV disorders along with a number of self-report psychometric measures (see “Measures” below). Once all inclusion and no exclusion criteria were met, participants were scheduled for a baseline fMRI visit within one-week. They were instructed to remain abstinent from all drugs of abuse (including cocaine) for at least 72 hours preceding their fMRI visit; participants with positive breath alcohol or urine drug screens were rescheduled. This abstinence requirement ensured that participants were not acutely under the influence of alcohol or drugs when they completed the fMRI scan.
2.3. Measures
Substance use disorders were assessed using the appropriate modules of the Structured Clinical Interview for DSM-IV (SCID; First et al., 1994). Axis I psychiatric disorders were assessed using the Mini International Neuropsychiatric Interview (MINI; Sheehan et al., 1998). Cocaine use in the three months preceding the first visit, as well as in between the first visit and the fMRI visit, was assessed using the Timeline Follow-back method (Sobell and Sobell, 1996). Demographics and treatment status variables (e.g., duration of current treatment) were assessed using an in-house questionnaire. Motivation to change cocaine use was assessed using the Stages of Change Readiness and Treatment Eagerness Scale (SOCRATES; Miller and Tonigan, 1996). The SOCRATES is composed of three scales derived from factor analytic research: 1) ambivalence, 2) recognition, and 3) taking steps. Individuals who score high on “ambivalence” wonder if they may have problems with cocaine; they are contemplating change. Individuals who score high on “recognition” acknowledge that they have problems with cocaine use and strongly desire to make changes. Finally, individuals who score high on “taking steps” are already doing things to make positive changes in their cocaine use; if they have stopped using, they are taking steps towards preventing relapse. All three scales of the SOCRATES are positively keyed, such that increased scores represent increased motivation to change.
2.4. Cocaine cue-exposure paradigm
The present investigation utilized a cocaine cue-exposure paradigm that was originally adapted from a well-studied alcohol-cue exposure paradigm (George et al., 2001; Myrick et al., 2008). The paradigm contains pictures of cocaine and related objects (e.g., crack pipe), neutral objects (e.g., furniture), and visual control images that match the cocaine pictures in color and hue but lack object recognition. Stimulus presentation occurs over six 90-second epochs. Each epoch contains three 24-second blocks (cocaine images, neutral objects, control images), containing five pictures displayed for 4.8 seconds each, and one 18 second rest block (i.e., cross-hair). The image blocks were all balanced with respect to luminosity (i.e., brightness). Blocks, and stimuli within blocks, are presented in pseudorandom order. During the task, participants were asked to rate their craving, from zero (“none”) to four (“severe”), after each block using a handpad. The present cocaine cue-exposure paradigm was previously developed for a placebo-controlled trial of N-acetylcysteine (NAC) for cocaine dependence (LaRowe et al., 2005; LaRowe et al., 2007).
2.5. Image acquisition
MRI scans were performed in a Siemens 3.0T Trio (Erlangen, Germany) MR scanner with a 12-channel head coil. Following localizer and anatomical scans, the cue exposure scan was acquired using an echo-planer gradient-echo pulse sequence (TR = 2200 ms, TE = 35 ms, flip angle = 90%). Images were acquired with approximate AC-PC alignment. Each brain volume consisted of 36 transverse slices (64 × 64 matrix, 3.0 mm thickness, no gap). Voxel size was 3.0 × 3.0 × 3.0 mm3.
2.6. Image analysis
fMRI analyses were conducted using Statistical Parametric Mapping software 8 (SPM8, The Wellcome Department of Cognitive Neurology, London). All volumes within the cue exposure scan were realigned to the first volume. Images were stereotactically normalized into a standard space, with a resolution of 2 × 2 × 2 mm voxels using a Montreal Neurological Institute (MNI) template. Data were smoothed with an isotropic 8 mm Gaussian kernel and were high-pass filtered with a cut off period of 240 s (i.e., twice the task cycle duration). Following preprocessing, fMRI data were analyzed within a general linear model (GLM) mixed effects framework. Within-task data from individual participants were analyzed using fixed-effects GLM, with cocaine-cue activity modeled as a box-car function convolved with the standard canonical hemodynamic response function; six movement parameters (3 rotation values in radian and 3 translation values in mm) were included as covariates to control for the influence of residual head motion. Autocorrelation was statistically controlled using an AR(1) model. Following these intra-individual GLM analyses, cocaine minus neutral image contrast maps were generated and entered into inter-individual random-effects analyses. To identify brain regions that activated significantly more to cocaine cues relative to neutral objects, we performed a one-sample t-test on participants’ cocaine minus neutral image contrast maps. To examine differences in cue-reactivity between participants currently in treatment and those not currently in treatment, we performed an independent group t-test on participants’ contrast maps. Finally, we used linear regression to determine the association between each SOCRATES scale (i.e., one scale at a time) and the cocaine minus neutral image contrast map. All group-level statistical maps were thresholded using Gaussian random field theory as implemented in cluster-level thresholding in SPM8. For the one-sample t-test, we used a voxelwise threshold of z = 3.09 (i.e., p < 0.01) and corrected cluster threshold of p < 0.05. For all other analyses, we used a voxelwise threshold of z = 2.33 (i.e., p < 0.05) and corrected cluster threshold of p < 0.05.
3. Results
3.1. Participant Characteristics
See Table 1 for participant characteristics organized by current treatment status. Thirty-eight cocaine-dependent participants (5 women and 33 men) completed the baseline assessment and fMRI scan. Twenty-four of the 38 participants reported that they were not presently undergoing any treatment for addiction. The remaining 14 participants reported that they were currently participating in outpatient psychosocial treatment for addiction. These 14 participants were either participating in an intensive outpatient addiction treatment program at the Charleston Veterans Affairs Medical Center, consisting of 2 hours of therapeutic groups daily (M-F) for four-weeks (n = 13), or an outpatient treatment program at a local state-run addiction center (n = 1). Participants currently in treatment reported an average treatment duration of 30 days (SD = 19.54). Treatment and non-treatment groups did not significantly differ from one another on demographics or cocaine use (i.e., years of use, age of onset, frequency and quantity of recent cocaine use) characteristics. Additionally, groups did not significantly differ regarding whether they had sought treatment for addiction in the past. Participants currently in treatment had significantly higher scores on the recognition and taking steps subscales of the SOCRATES, but were not significantly different on the ambivalence subscale, relative to participants not currently in treatment.
Table 1.
Baseline characteristics by treatment status (n = 38)
| TX + (n = 14) | TX − (n = 24) | p | |
|---|---|---|---|
| Demographics | |||
| Age, M (SD) | 47.86 (10.52) | 43.96 (9.60) | 0.25 |
| Gender, % male | 92.86 | 83.33 | 0.40 |
| Race, % African American | 85.71 | 75.00 | 0.44 |
| Marital status, % married | 28.57 | 13.00 | 0.24 |
| Education, % ≥ some college | 50.00 | 45.83 | 0.66 |
| Smoking status, % smokers | 92.86 | 70.83 | 0.11 |
| Treatment Motivation | |||
| Days in current treatment, M (SD) | 29.86 (19.54) | N/A | N/A |
| Past treatment for addiction, % yes | 64.29 | 50.00 | 0.39 |
| Ambivalence (SOCRATES), M (SD) | 3.71 (0.89) | 4.01 (0.81) | 0.29 |
| Recognition (SOCRATES), M (SD) | 4.82 (0.23) | 4.31 (0.63) | < 0.01 |
| Taking Steps (SOCRATES), M (SD) | 4.67 (0.37) | 3.88 (1.05) | < 0.01 |
| Cocaine Use | |||
| Total years of cocaine use, M (SD) | 18.11 (9.31) | 17.50 (5.79) | 0.83 |
| Age of CD onset, M (SD) | 30.21 (8.88) | 29.92 (9.82) | 0.93 |
| % cocaine use days (past 90), M (SD) | 40.45 (26.08) | 35.32 (22.64) | 0.53 |
| $ spent/day on cocaine (past 90), M (SD) | 19.54 (11.43) | 34.21 (53.20) | 0.32 |
Note. TX + = currently attending outpatient psychosocial treatment; TX − = not currently attending treatment; SOCRATES = Stages of Change Readiness and Treatment Eagerness Scale.
3.2. Cue activation across participants
Seven participants were excluded from imaging analyses due to excessive head motion (i.e., ≥ 2 mm/degrees in any direction), leaving an effective sample size of 31 (11 in the treatment group, 20 in the non-treatment group). Across all participants, cocaine cues, relative to neutral objects, were associated with a widespread pattern of activation including left occipital cortex, left superior frontal gyrus, right DLPFC, bilateral hippocampus, right amygdala, posterior cingulate cortex, and left OFC (Table 2, MFigure 1). Subjective craving was significantly higher following cocaine image blocks ( = 1.66, SD = 0.89) versus neutral object blocks (M = 0.58, SD = 0.61; p < 0.001).
Table 2.
Activation to cocaine versus neutral cues across participants
| Contrast | Cluster | Z Max | P | Voxel | MNI (x, y, z) | Anatomy |
|---|---|---|---|---|---|---|
| Cocaine > Neutral | 1 | 6.95 | < .001 | 6510 | −45, −52, −16 | L inferior temporal gyrus |
| 6.64 | −18, −94, −7 | L occipital pole | ||||
| 6.51 | −39, −64, −10 | L occipital fusiform gyrus | ||||
| 2 | 6.22 | < .001 | 3353 | −3, 29, 59 | L superior frontal gyrus (BA 6) | |
| 5.94 | −6, 59, 29 | L frontal pole | ||||
| 5.30 | −45, 5, 29 | L precentral gyrus | ||||
| 3 | 5.69 | .001 | 218 | −48, 35, 11 | L inferior frontal gyrus, pars triangularis | |
| 3.73 | −42, 29, 2 | L frontal operculum cortex | ||||
| 4 | 5.56 | < .001 | 1017 | 54, 41, 11 | R frontal pole (BA 46) | |
| 5.44 | 45, 11, 29 | R precentral gyrus | ||||
| 5.22 | 60, 20, 26 | R inferior frontal gyrus (BA 9) | ||||
| 5 | 4.87 | < .001 | 945 | 21, −28, −7 | R hippocampus | |
| 4.55 | −21, −28, −7 | L hippocampus | ||||
| 4.46 | 30, −1, −19 | R amygdala | ||||
| 6 | 4.76 | < .001 | 301 | 0, −34, 32 | Cingulate gyrus, posterior (BA 31) | |
| 4.69 | −3, −52, 26 | Cingulate gyrus, posterior | ||||
| 7 | 4.52 | .030 | 74 | −33, 32, −16 | L frontal orbital cortex (BA 47) | |
| 3.92 | −30, 35, −7 | L frontal orbital cortex |
Note: Analyses (one-sample t-test) completed using cluster thresholding (z > 3.09 and corrected cluster threshold of p < .05). Anatomy = most probable region identified by the Harvard-Oxford cortical and subcortical structural atlases; Brodmann areas (where available) identified by the Talairach-Tournoux Atlas. Z Max = local maximum z-value, MNI (x, y, z) = Montreal Neurological Institute coordinates for the local maximum, BA = Brodmann Area.
Figure 1.
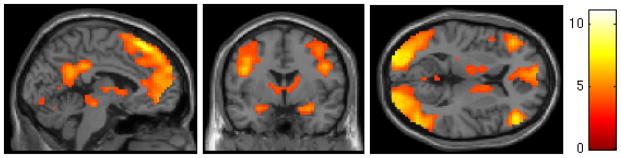
SPM map of blood oxygen level dependent (BOLD) response to cocaine cues minus neutral objects across all participants using a voxelwise threshold of z = 3.09 and a corrected cluster threshold of p < 0.05. Cocaine cues were associated with significant activation in left occipital cortex, left superior frontal gyrus, right DLPFC, bilateral hippocampus, right amygdala, posterior cingulate cortex, and left OFC.
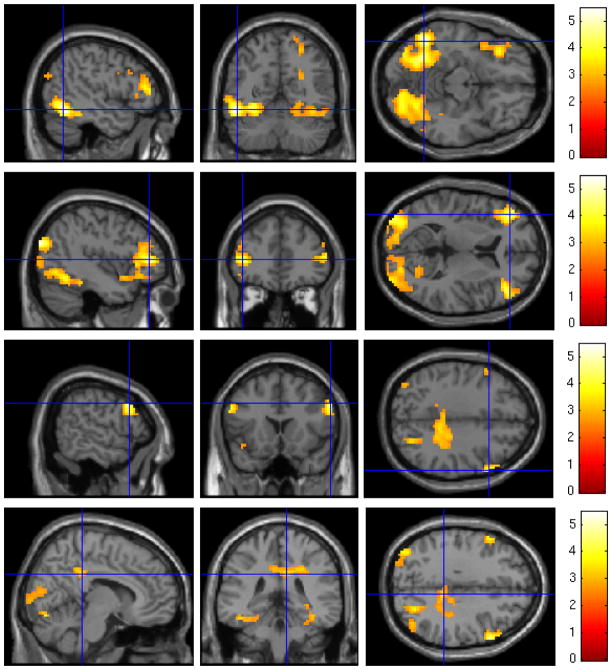
3.2. Cue activation in treated vs. untreated participants
Four distinct clusters of activation were identified that distinguished treated and non-treated participants’ brain reactivity to cocaine cues. Specifically, untreated participants had significantly higher activation to cocaine versus neutral images in the left occipital cortex, left DLPFC and OFC, right DLPFC, and posterior cingulate cortex (Table 3, MFigure 2). Non-treated participants reported marginally higher subjective craving than treated participants following both neutral object (non-treated: = 0.70, SD = 0.68; treated: M = 0.35, SD = 0.39; p = 0.08) and cocaine image (non-treated: M = 1.84, SD = 0.79; treated: (M = 1.32, SD = 0.98; p = 0.12) blocks. However, subjective craving to cocaine images minus subjective craving to neutral objects was not significantly different between treated (M = 0.97, SD = 1.00) and non-treated (M = 1.14, SD = 0.64) participants (p = 0.61).
Table 3.
Activation to cocaine versus neutral cues in treated versus untreated participants
| Contrast | Cluster | Z Max | P | Voxel | MNI (x, y, z) | Anatomy |
|---|---|---|---|---|---|---|
| Untreated > Treated | 1 | 4.49 | < .001 | 3303 | −48, −58, −13 | L inferior temporal gyrus (BA 37) |
| 4.35 | −42, −85, 26 | L lateral occipital cortex, superior | ||||
| 4.15 | 42, −73, 26 | R lateral occipital cortex, superior | ||||
| 2 | 4.15 | .002 | 448 | −42, 41, 8 | L frontal pole (BA 46) | |
| 3.60 | −54, 17, 29 | L inferior frontal gyrus (BA 9) | ||||
| 3.46 | −36, 29, −13 | L frontal orbital cortex | ||||
| 3 | 4.14 | .017 | 240 | 60, 17, 35 | R precentral gyrus (BA9) | |
| 3.71 | 45, 35, 8 | R frontal pole | ||||
| 3.45 | 54, 41, 11 | R frontal pole (BA 46) | ||||
| 4 | 3.10 | .005 | 368 | 9, −37, 32 | Cingulate gyrus, posterior | |
| 3.07 | 24, −34, 38 | R cerebral white matter | ||||
| 2.85 | −3, −34, 35 | Cingulate gyrus, posterior (BA 31) |
Note: Analyses (independent-samples t-test using the cocaine versus neutral cues contrast) completed using cluster thresholding (z > 2.33 and corrected cluster threshold of p < .05). Anatomy = most probable region identified by the Harvard-Oxford cortical and subcortical structural atlases; Brodmann areas (where available) identified by the Talairach-Tournoux Atlas. Z Max = local maximum z-value, P = cluster-level p value, MNI (x, y, z) = Montreal Neurological Institute coordinates for the local maximum, BA = Brodmann Area.
Figure 2.
SPM map of blood oxygen level dependent (BOLD) response to cocaine cues minus neutral objects in untreated versus treated participants using a voxelwise threshold of z = 2.33 and a corrected cluster threshold of p < 0.05. Untreated participants had higher activation to cocaine cues in the left occipital cortex (uppermost panel), left DLPFC and OFC (upper-middle panel), right DLPFC (lower-middle panel), and posterior cingulate cortex (lowermost panel).
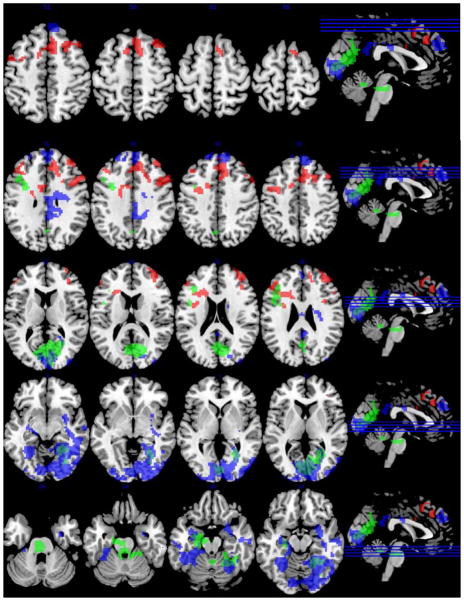
3.3. Associations between SOCRATES scales and cue activation
SOCRATES ambivalence and recognition scores were significantly correlated to one another (r = 0.41, p = 0.02); no other scales were significantly correlated. All three SOCRATES scales were significantly associated with activation to cocaine cues (Table 4, rFigure 3). Individuals who scored low on the recognition scale had higher activation in temporal and occipital regions, right superior frontal gyrus, right and left frontal poles, and anterior and posterior cingulate cortex. Individuals who scored low on the ambivalence scale had higher activation in left hippocampus, right intracalcarine cortex, and left middle frontal gyrus. Finally, individuals who scored low on the taking steps scale had higher activation in right DLPFC, right frontal pole, and right paracingulate gyrus. In sum, the three SOCRATES scales were each significantly associated with cue activation in different brain regions, such that lower scores on the SOCRATES were associated with increased cue-elicited activation. Subjective craving to cocaine cues was not significantly correlated with any of the SOCRATES scales (ambivalence: = 0.22, p = 0.23; recognition: r = −0.06, p = 0.75; taking steps: r = −0.06, p = 0.74).
Table 4.
Activation to cocaine versus neutral cues in participants with lower versus higher readiness to change their drug use
| Contrast | Cluster | Z Max | P | Voxel | MNI (x, y, z) | Anatomy |
|---|---|---|---|---|---|---|
| Lower > Higher Recognition | 1 | 4.75 | < .001 | 2301 | −6, −85, −10 | L lingual gyrus |
| 3.97 | 3, −88, −4 | R lingual gyrus (BA 18) | ||||
| 3.89 | 21, −91, −13 | R occipital pole | ||||
| 2 | 4.70 | .002 | 479 | −42, −43, −19 | L temporal fusiform cortex (BA 37) | |
| 4.11 | −30, −34, −22 | L temporal fusiform cortex, posterior | ||||
| 3.97 | −30, −55, −13 | L temporal occipital fusiform cortex | ||||
| 3 | 4.09 | .012 | 278 | 6, 53, 41 | R superior frontal gyrus (BA 8) | |
| 3.64 | 3, 59, 32 | R frontal pole (BA 9) | ||||
| 3.00 | −12, 56, 41 | L frontal pole | ||||
| 4 | 2.98 | .014 | 259 | 3, −13, 29 | Cingulate gyrus, anterior/posterior | |
| 2.97 | 18, −7, 32 | R cerebral white matter | ||||
| 2.90 | 27, −10, 29 | R cerebral white matter | ||||
| Lower > Higher Ambivalence | 1 | 4.31 | < .001 | 1254 | −24, −19, −16 | L hippocampus |
| 3.87 | 18, −70, 14 | R intracalcarine cortex (BA 30) | ||||
| 3.83 | 6, −67, 8 | R intracalcarine cortex (BA 30) | ||||
| 2 | 3.52 | .031 | 195 | −33, 8, 32 | L middle frontal gyrus | |
| 3.49 | −36, 32, 23 | L middle frontal gyrus | ||||
| 3.42 | −39, 23, 26 | L middle frontal gyrus | ||||
| Lower > Higher Taking Steps | 1 | 3.92 | .030 | 39, 50, 23 | R frontal pole (BA 9) | |
| 3.33 | 30, 62, 17 | R frontal pole (BA 10) | ||||
| 2.92 | 39, 41, 11 | R frontal pole | ||||
| 2 | 3.72 | < .001 | 18, 26, 32 | R cerebral white matter | ||
| 3.71 | 9, 26, 44 | R paracingulate gyrus | ||||
| 3.63 | 12, 38, 35 | R paracingulate gyrus |
Note: Analyses (multiple regression using the cocaine versus neutral cues contrast) completed using cluster thresholding (z > 2.33 and corrected cluster threshold of p < .05). Anatomy = most probable region identified by the Harvard-Oxford cortical and subcortical structural atlases; Brodmann areas (where available) identified by the Talairach-Tournoux Atlas. Z Max = local maximum z-value, MNI (x, y, z) = Montreal Neurological Institute coordinates for the local maximum, BA = Brodmann Area.
Figure 3.
Associations between blood oxygen level dependent (BOLD) response to cocaine cues minus neutral objects and scores on the SOCRATES using a voxelwise threshold of z = 2.33 and a corrected cluster threshold of p < 0.05. Low “recognition” scores were associated with higher activation in temporal and occipital regions, right superior frontal gyrus, right and left frontal poles, and anterior and posterior cingulate cortex (pictured in blue). Low “ambivalence” scores were associated with higher activation in left hippocampus, right intracalcarine cortex, and left middle frontal gyrus (pictured in green). Low “taking steps” scores were associated with higher activation in right DLPFC, right frontal pole, and right paracingulate gyrus (pictured in red).
4. Discussion
The present study investigated the relationship between motivation to change and brain activation to cocaine cues in cocaine-dependent individuals. Consistent with Wilson and colleagues (2004), participants currently in outpatient treatment had significantly lower activation to cocaine cues in the bilateral DLPFC and left OFC, and participants with higher scores on the SOCRATES “taking steps” subscale had significantly lower activation to cocaine cues in the right DLPFC. Expanding on Wilson and colleagues’ (2004) observations, participants currently in treatment also had significantly lower activation to cues in the left occipital and posterior cingulate cortices, and participants with higher scores on the three SOCRATES subscales had significantly lower activation to cues in a variety of frontal brain regions including bilateral frontal poles, right superior frontal gyrus, and left middle frontal gyrus, as well as a number of non-frontal brain regions including the occipital, temporal, and cingulate cortices. Interestingly, each of the three SOCRATES subscales was associated with cue activation in a different set of brain regions, suggesting that each facet of change motivation may be associated with different cognitive components of cue-reactivity. Together, these findings empirically substantiate and extend Wilson and colleagues’ (2004) report.
Conversely, our findings are seemingly inconsistent with those of Claus and colleagues’ (2011) examination of neurobiological phenotypes for alcohol use disorders. Specifically, their study found that treatment-seeking participants had increased activation to alcohol cues in DLPFC, amygdala, and nucleus accumbens relative to non-treatment-seeking participants, while the present study found that cocaine-dependent individuals currently in treatment or motivated to change their cocaine use had decreased activation to cocaine cues in DLPFC and OFC, along with a variety of other frontal, cingulate, and occipital regions as compared to untreated or less motivated individuals. The key difference between the present study and Claus et al. (2011) may be that treatment-seeking participants in Claus et al. (2011) had higher levels of alcohol dependence than non-treatment-seeking participants, whereas treated and non-treated participants in the present study had statistically equivalent levels of cocaine dependence. As a result, treatment-seeking participants in Claus et al. (2011) may have had higher activations to alcohol cues than non-treatment seeking participants because of their higher level of alcohol dependence. Research has consistently demonstrated that increased dependence severity is associated with increased neural cue-reactivity (Claus et al., 2011; Volkow et al., 2006). Of course, there could be other explanations for the differences in findings observed between these studies (e.g., the present study examined cocaine-dependent individuals, whereas Claus et al. examined alcohol-dependent individuals), and further research is needed to investigate these possibilities.
Wilson and colleagues (2004) originally suggested that only studies with non-treatment-seeking participants have demonstrated cue-elicited activations of DLPFC and OFC because non-treatment-seeking participants have anticipated using drugs following the scan whereas treatment-seeking participants have not. More specifically, they argued that cue-elicited activation of OFC and DLPFC reflects representation of drug use expectancy and the generation and maintenance of goals designed to obtain drug reward, respectively, and that neither region was therefore relevant to individuals who were not planning to use drugs in the near future. Although this interpretation is consistent with our findings, it is not sufficient to explain all of our results. Specifically, in the present investigation, treated and motivated participants demonstrated decreased cue-elicited activation relative to untreated and less motivated individuals throughout the brain, not only in the DLPFC and OFC. Furthermore, different facets of change motivation were associated with cue activation in largely non-overlapping sets of brain regions. In addition to their primary explanation described above, Wilson and colleagues (2004) also suggested that decreased cue-activation in treatment-seeking participants may alternatively be due to their attempts to inhibit craving during cue exposure. Our findings suggest that both of these proposed explanations, along with others, are plausible and most likely working in concert to produce decreased cue-elicited activation across various brain regions in treated and motivated cocaine-dependent individuals. However, further research is needed to determine why individuals who are in treatment demonstrate less brain activation to drug cues, as well as why different facets of change motivation are associated with cue activation in different brain regions. Better understanding of these intermediate mechanisms could aid in the development of more effective therapeutic techniques that optimally capitalize on the strategies that treated and motivated individuals use to enhance their success in reducing and/or stopping their dysfunctional substance use.
The findings from Wilson et al., 2004, Claus et al., 2011, and the present study are consistent with the broader literature on health behavior change. A recent secondary analysis of 11 large studies of health behaviors (i.e., smoking, diet, sun exposure) found four consistent predictors of long-term health behavior change: 1) treatment, 2) stage of change, 3) problem severity, and 4) effort (Blissmer et al., 2010). The findings from Wilson et al., 2004 and the present study demonstrate the relevance of the first two predictors of health behavior change (i.e., treatment and change motivation) to brain-reactivity to drug cues. We invoked the third predictor of change (i.e., problem severity) to explain the discrepancy in findings between Claus et al., 2011 and the present study. Finally, Wilson et al., 2004 suggested the fourth predictor of change (i.e., effort) as a possible explanation for why treated participants demonstrate less cue activation than non-treated participants. Blissmer and colleagues’ (2010) research on health behavior change thus provides a useful organizing framework for future investigations of predictors of brain-reactivity to drug cues in substance-dependent individuals.
As is often the case in addiction research, our subjective craving results did not closely mirror our fMRI results (Tiffany and Conklin, 2000; Myrick et al., 2008). Specifically, whereas treatment status and change motivation were associated with brain activation to drug cues, neither treatment status nor change motivation were significantly associated with subjective craving. Across participants, the cue-reactivity fMRI task did not appear to invoke a large amount of subjective craving. This may have created a floor effect that made it difficult to detect associations between treatment status or change motivation and subjective craving. Given the robust and consistent pattern of fMRI results in the present study, we do not believe that the lack of demonstrated associations between treatment status, change motivation, and subjective craving weakens the validity of our findings. Contemporary cognitive models of drug craving suggest that brain reactivity to drug cues and subjective craving may be controlled by different processes; unlike cue-elicited brain activity, subjective craving is posited to arise from a non-automatic process that serves to, at times, exert executive control over the automatic processes that proximally influence drug seeking and use (Tiffany and Conklin, 2000).
This discussion should be viewed in light of several limitations. Although our sample size was comparable to other fMRI investigations, replication with larger sample sizes, and other substances of abuse, would increase confidence in our findings. Replication with larger samples would also allow for the separation of unique vs. shared effects of treatment status and motivation on cue-reactivity via simultaneous statistical modeling. The majority of treated participants in the present study were Veterans, whereas the majority of non-treated participants were not. Although it is not clear how Veteran status (irrespective of substance use severity or the presence of comorbid psychiatric disorders) would impact between-group differences in brain activation to cocaine cues, and although our findings were supported in regression analyses using the SOCRATES across both Veteran and non-Veteran participants, replication of our findings in treated and non-treated participant groups that are balanced on Veteran status would increase confidence in our results.
In summary, the present study demonstrated associations between current treatment status, motivation to change, and brain activation to cocaine cues in cocaine-dependent individuals. Both treated and motivated participants had lower activation to cocaine cues in a wide variety of brain regions relative to non-treated and less motivated participants, respectively. Future research is needed to explain the mechanism by which treatment and/or motivation to change decreases neural cue-reactivity. Such research could potentially aid in the development of more effective therapeutic techniques for substance-dependent patients.
Acknowledgments
Funding for this study was provided by NIDA grants 5R01DA023188-04 and 3RO1DA023188-02S1; NIDA had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication. Dr. Prisciandaro was supported by 1F32DA032250-01.
Footnotes
Authors Contributions
Dr. Brady designed the study and wrote the protocol. Dr. McRae-Clark coordinated the study implementation. Dr. Myrick and Mr. Henderson designed the fMRI cue-exposure task and imaging protocol. Dr. Prisciandaro conducted the analyses and wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
References
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28:309–69. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Blissmer B, Prochaska JO, Velicer WF, Redding CA, Rossi JS, Greene GW, Paiva A, Robbins M. Common factors predicting long-term changes in multiple health behaviors. Journal of Health Psychology. 2010;15:205–14. doi: 10.1177/1359105309345555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus ED, Ewing SW, Filbey FM, Sabbineni A, Hutchison KE. Identifying neurobiological phenotypes associated with alcohol use disorder severity. Neuropsychopharmacology. 2011;36:2086–96. doi: 10.1038/npp.2011.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders, Research Version, Patient Edition. New York: New York State Psychiatric Institute; 1996. [Google Scholar]
- George MS, Anton RF, Bloomer C, Teneback C, Drobes DJ, Lorberbaum JP, Nahas Z, Vincent DJ. Activation of prefrontal cortex and anterior thalamus in alcoholic subjects on exposure to alcohol-specific cues. Arch Gen Psychiatry. 2001;58:345–52. doi: 10.1001/archpsyc.58.4.345. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–13. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- LaRowe SD, Myrick H, Hedden S, Mardikian P, Saladin M, McRae A, Brady K, Kalivas PW, Malcolm R. Is cocaine desire reduced by N-Acetylcysteine? Am J Psychiatry. 2007;164:1115–7. doi: 10.1176/ajp.2007.164.7.1115. [DOI] [PubMed] [Google Scholar]
- LaRowe SD, Myrick H, Henderson S, Kalivas PW, Malcolm R. Cue reactivity and neuroimaging in cocaine dependent subjects: a double-blind placebo-controlled pilot study involving N-Acetylcysteine. Poster presented at: the 35th annual meeting of the Society for Neuroscience; 2005; Washington, D. C. [Google Scholar]
- Miller WR, Tonigan JS. Assessing drinkers’ motivation for change: the Stages of Change Readiness and Treatment Eagerness Scale (SOCRATES) Psychol Addict Behav. 1996;10:81–9. [Google Scholar]
- Myrick H, Anton RF, Li X, Henderson S, Randall PK, Voronin K. Effect of naltrexone and ondansetron on alcohol cue-induced activation of the ventral striatum in alcohol-dependent people. Arch Gen Psychiatry. 2008;65:466–75. doi: 10.1001/archpsyc.65.4.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochaska JO, DiClemente CC. Transtheoretical therapy: toward a more integrative model of change. Psychol Psychother Theor Res Pract. 1982;19:276–88. [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavas J, Weiller E, Hergueta T, Baker R, Dunbar GC. The mini-international neuropsychiatric interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiat. 1998;59:22–33. [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline followback user’s guide: a calendar method for assessing alcohol and drug use. Toronto: Addiction Research Foundation; 1996. [Google Scholar]
- Tiffany ST, Conklin CA. A cognitive processing model of alcohol craving and compulsive alcohol use. Addiction. 2000;95 (Suppl 2):S145–S153. doi: 10.1080/09652140050111717. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006;14:6583–8. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA, Fiez JA. Prefrontal responses to drug cues: a neurocognitive analysis. Nat Neurosci. 2004;7:211–4. doi: 10.1038/nn1200. [DOI] [PMC free article] [PubMed] [Google Scholar]