Abstract
Running increases the formation of new neurons in the adult rodent hippocampus. However, the function of new neurons generated from running is currently unknown. One hypothesis is that new neurons from running contribute to enhanced cognitive function by increasing plasticity in the adult hippocampus. An alternative hypothesis is that new neurons generated from running incorporate into experience-specific hippocampal networks that only become active during running. The purpose of this experiment was to determine if new neurons generated from running are selectively activated by running, or can become recruited into granule cell activity occurring during performance on other behavioral tasks that engage the hippocampus. Therefore, the activation of new 5–6 week neurons was detected using BrdU, NeuN, and Zif268 triple-label immunohistochemistry in cohorts of female running and sedentary adult C57BL/6J mice following participation in one of three different tasks: the Morris water maze, novel environment exploration, or wheel running. Results showed that running and sedentary mice displayed a nearly equivalent proportion of new neurons that expressed Zif268 following each task. Since running approximately doubled the number of new neurons, the results demonstrated that running mice had a greater number of new neurons recruited into the Zif268 induction in the granule cell layer following each task than sedentary mice. The results suggest that new neurons incorporated into hippocampal circuitry from running are not just activated by wheel running itself, but rather become broadly recruited into granule cell layer activity during distinct behavioral experiences.
Keywords: Neurogenesis, Hippocampus, Mouse, Zif268, Exercise, Wheel running
Introduction
Regularly engaging in exercise can improve performance on tasks that involve the hippocampus in both humans and rodents (Cotman and Berchtold, 2002). Although the mechanisms are not known, data from rodent models suggest that cognitive enhancement could be due to the contribution of several changes occurring in the hippocampus from exercise including increased gliogenesis (Uda et al., 2006), vasculature (Clark et al., 2009; Van der Borght et al., 2009; van Praag et al., 2005), growth factors (Neeper et al., 1996), spine density (Eadie et al., 2005), and changes to dendrite structure (Redila and Christie, 2006). Another factor that may contribute to cognitive enhancement from exercise is increased formation of new hippocampal granule neurons (van Praag et al., 1999b). This possibility is intriguing because the hippocampus is one of two brain regions that unarguably develop new neurons throughout adulthood (Gould, 2007).
While the function of new neurons in the adult hippocampus remains unresolved, it has been hypothesized that neurogenesis from running provides additional plasticity that contributes to enhanced performance on hippocampus-involved tasks (Clark et al., 2008; van Praag et al., 1999a). However, an alternative hypothesis is that new neurons recruited into experience-specific networks only process information about the task that aided in their survival (Tashiro et al., 2007; Kee et al., 2007). Tasks that engage the rodent hippocampus, such as trace conditioning, Morris water maze, and enriched environment exploration enhance the survival of new neurons at a critical period of 7–14 days after labeling with the cell division marker 5-bromo-2-deoxyuridine (BrdU) (Ambrogini et al., 2000; Dobrossy et al., 2003; Gould et al., 1999; Leuner et al., 2004; Tashiro et al., 2007). Further, modest positive relationships are sometimes observed between the number of new neurons that survive and performance on hippocampal-dependent tasks (Ambrogini et al. 2000; Dalla et al., 2007; Dalla et al., 2009; Leuner et al., 2004), suggesting that the acquisition of a task may promote the survival of new neurons. Therefore, new neurons may preferentially incorporate into experience-specific networks, showing greater activity when an animal is re-engaged in the same task instead of contributing to the performance of other behavioral tasks (Tashiro et al., 2007; Kee et al., 2007). Similarly, running increases the survival of new neurons and induces the expression of neural activity markers in these neurons in a manner related to the distance traveled (Clark et al., 2011a; Clark et al., 2009; Rhodes et al., 2003a). Given that one hypothesized function of the hippocampus is to integrate sensory or motor information about voluntary movement (see Bland and Oddie, 2001), it is possible new neurons from running may become incorporated into experience-specific hippocampal circuitry that only becomes active during running activity (Bland and Oddie, 2001; Clark et al., 2011a). Whether new neurons generated from running, in addition, become recruited into neural activity during the performance of other tasks that engage the hippocampus is not known.
The goal of this study was to determine whether new neurons generated from running are recruited into experience-specific networks and thus only become activated during running, or whether distinct hippocampal-involved behavioral experiences can activate running-formed new neurons. One way to address whether new neurons from running may contribute to performance on other tasks is to provide evidence that these new neurons display an immediate early gene (IEG) response (Mello et al., 1992) while performing a distinct task. Therefore, the activation (as measured by Zif268 expression) of new 5–6 week old neurons were assessed in cohorts of running and sedentary C57BL/6J mice following participation in three different hippocampus engaging tasks: the Morris water maze, novel environment exploration, and wheel running.
By observing the proportion of new neurons that displayed Zif268 in response to each behavioral condition, we determined the extent to which new neurons were activated during distinct behavioral experiences (Tashiro et al., 2007). Running increases adult hippocampal neurogenesis by nearly doubling the survival of new neurons in C57BL/6J mice (Clark et al., 2011b; van Praag et al., 1999b). Therefore, we reasoned that if a greater number of new neurons displayed Zif268 in running compared to sedentary mice across each task, this would provide evidence that new neurons from running are broadly recruited into neuronal activation induced by experiences beyond the task that aided in their survival. Alternatively, if a similar number of new neurons display Zif268 in running and sedentary mice following water maze and novel environment exposure, but running mice have a greater number of Zif268 expressing new neurons than sedentary mice following access to running wheels, this would provide evidence new neurons incorporate into experience-specific circuitry that only becomes active during movement or running itself.
Materials and methods
Subjects
Upon arrival, five-week old female C57BL/6J mice (obtained from The Jackson Laboratory, Bar Harbor, ME) were group housed (4 per cage) in standard laboratory cages and left undisturbed for 4 weeks before the experiment. Females were chosen because they run farther and form more new neurons than male mice (Clark et al., 2011b). This provides a greater probability of finding new neurons that display Zif268 following task performance. Rooms were controlled for temperature (21 ± 1°C) and photo-period (12:12 L:D; lights on at 9am and off at 9pm) for the entire study.
Experimental Design
When mice were 9 weeks old, they were individually housed in cages with (running, n=50) or without (sedentary, n=50) access to running wheels. We used 9 inch (22.9 cm) diameter Mini Mitter wheels mounted in the cage top (Respironics, Bend, OR). Individual wheel running distances were obtained via magnetic switches interfaced to a computer running VitalView software (Respironics, Bend, OR). The first 10 days, all mice received a daily injection of BrdU (50 mg/kg) to label dividing cells. After day 30, running and sedentary mice performed different behavioral tasks (see below and Fig. 1). On day 42, mice were euthanized 2h following final performance on each behavioral task. Two hours was chosen because Zif268 expression peaks approximately 2h following a stimulus (Richardson et al., 1992; Zangenehpour and Chaudhuri, 2002). We chose to measure Zif268 expression in 5–6 week-old neurons for 2 reasons. First, BrdU-labeled neuron survival is measured several weeks after injections of BrdU. Thus the transient increase in cell proliferation and immature neuron number that occurs during proestrous of the estrous cycle should not have an effect on new neuron estimates (Tanapat et al., 1999). Second, studies using electrophysiological recordings or immediate early gene markers of neural activation suggest that 4–6 week old new neurons are more likely to become activated in response to a stimulus as compared to neurons not labeled with BrdU (Clark et al., 2011a; Clark et al., 2009; Ge et al., 2007; Kee et al., 2007; Stone et al., 2011). Running mice received access to running wheels for 30 days and then were subsequently moved to standard laboratory cages without running wheels for the final 12 days because wheel running stimulates Zif268 expression (Clark et al., 2009). Hence, mice cannot have access to running wheels in proximity to behavioral testing, as it would be difficult to distinguish whether Zif268 induction resulted from wheel running or behavioral performance on the other tasks. Note that mice were deliberately not housed in cages with locked wheels because mice climb in locked wheels and we wanted to keep physical activity to a minimum in the sedentary conditions (Koteja et al., 1999; Rhodes et al., 2000). Horizontal distance traveled while performing each task was recorded by video cameras mounted on the ceiling interfaced to a computer running TopScan software (CleverSys Inc., Vienna, VA) in order to assess the potential contributions of physical activity on Zif268 expression.
Figure 1.
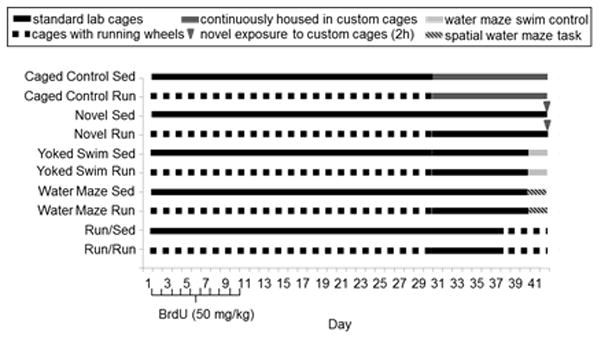
Experimental design. Mice were housed with or without running wheels for 30 days. The first 10 days the mice received daily injections of 50 mg/kg BrdU to label dividing cells. Behavioral tasks began during the final 12 days, days 31–42. All mice were housed in cages without access to running wheels on days 31–42 and throughout behavioral testing, except the Run/Sed and Run/Run groups (see below). Mice in the caged control group were continuously housed in custom cages (for video tracking) on days 31–42. The novel group received a single 2h exposure to the custom cages on day 42. The water maze group received 3 days (2 trials/day for 2 days, 3 trials on the 3rd day) of hidden platform water maze training. The yoked swim group received exposure to the water maze without a platform and served as a swim control for the water maze group. The run groups (Run/Sed and Run/Run) were continuously housed in cages with access to running wheels on days 37–42. All mice were euthanized on day 42, two hours following behavioral testing to measure Zif268 induction.
Behavioral tasks (Fig. 1)
1) Novel environment exploration
Running and sedentary mice were individually placed into novel custom-made cages (conducive for video tracking) for 2h and then perfused (see Zombeck et al., 2010 for custom cage details). These groups will hereafter be referred to as novel running (n=8), and novel sedentary (n=8) mice. Additional running and sedentary mice were individually housed in the same type of cages continuously for 12 days preceding euthanasia to serve as habituated controls. These mice will hereafter be referred to as caged control running (n=8) and caged control sedentary (n=8) mice. Caged control mice were briefly removed and placed back into their respective cages by the experimenter 2h before euthanasia to control for any potential Zif268 induction related to handling. Horizontal distance traveled was continuously monitored using video tracking. All mice were euthanized, two hours after peak activity (4 hours after lights shut off).
2) Water maze
Dimensions and parameters followed Clark et al. (2008). Running and sedentary mice were trained on the Morris water maze for 3 days. These mice will hereafter be referred to as water maze running (n=10) and water maze sedentary (n=10) mice. The first 2 days, mice received 2 trials per day from different start locations with 30s inter-trial intervals. A trial lasted either 60s or until the mouse reached the platform and remained on the platform for 10s. If a mouse did not reach the platform in 60s, it was gently guided there by hand. On the final day, mice were given 3 trials to assure maximal learning without causing physical fatigue from additional trials. Mice were trained on the water maze for only 3 days rather than additional days, because previous experiments from our lab using the same maze parameters have demonstrated that mice reach asymptotic performance by day 3 and we wanted to sample mice when most of the learning occurs (Clark et al., 2008). An additional group of running and sedentary mice swam in the water maze without a platform and served as yoked controls for induction of Zif268 from physical activity or non-goal oriented spatial learning. These groups will hereafter be referred to as yoked swim running (n=10) and yoked swim sedentary (n=10) mice. Each mouse in yoked groups swam in the water for a duration equivalent to a mouse trained with a hidden platform. Trials began each day, 2 hours after lights shut off. Mice were placed into their respective home cages following the last trial. All mice were euthanized 2h after the last trial.
3) Wheel running
Running and sedentary mice were placed into cages with running wheels for 5 days (days 38–42) before euthanasia. These groups will hereafter be referred to as Run/Run (n=6) and Run/Sed (n=6). Running mice were removed from wheels for 7 days before being placed back in cages with wheels to be consistent with the other groups. All mice were euthanized 2h after peak running (3h after lights shut off).
Immunohistochemistry
Following Clark et al., (2011a) mice were anesthetized with 150 mg/kg sodium pentobarbital (ip) and then transcardially perfused with 4% paraformaldehyde in a phosphate buffer solution (PBS). Brains were post-fixed overnight and then transferred to 30% sucrose in PBS. Brains were sectioned using a cryostat into 40 micron coronal sections and stored in tissue cryoprotectant at −20°C. Separate 1-in-6 series of free floating sections (i.e. series of sections throughout the rostral-caudal extent of the brain with 240 micron increments separating each section) were stained using Diaminobenzidine (DAB) or fluorescent immunohistochemistry. The following primary antibodies were used: rat anti-BrdU (1:100; AbD Serotec, Raleigh, NC) to measure the survival of cells that had divided 32–42 days before the animal was euthanized, mouse anti-NeuN (1:50; Millipore, Billerica, MA) to detect mature neurons, and rabbit-anti Zif268 to detect neuronal activation (1:12,000 for DAB or 1:3000 for fluorescent; Santa Cruz Biotechnology, Santa Cruz, CA). For DAB visualization, secondary antibodies were obtained from Vector (1:200; Burlingame, CA). Fluorescent secondary antibodies were obtained from Jackson ImmunoResearch (1:250; West Grove, PA). DAB-stained sections were lightly counter stained with methylene blue to highlight cell bodies in the dentate gyrus.
Image analysis
1) BrdU and Zif268-DAB
Following Clark et al. (2011a), the entire bilateral anterior and posterior granule layer in a 1-in-6 series was photographed by systematically advancing the field of view via a camera interfaced to computer under 100× total magnification. The field and condenser diaphragm on the microscope were set so that DAB+ nuclei could be easily observed through the entire z-plane of the tissue. Positively labeled cells in photographs were counted by eye to generate estimates of total number of labeled cells (BrdU or Zif268). Positively labeled nuclei that were predicted to be in the top plane of the sections were not included in cell counts to generate unbiased estimates. Only nuclei completely filled and stained darkly with DAB were counted for these analyses. In addition, volume of the granule layer represented in the series was also measured to express Zif268 as a density per cubic mm. Volume was estimated by outlining the granule cell layer to obtain area using ImageJ (http://rsbweb.nih.gov/ij/) and multiplying the total granule layer area by the space between sections. The number of Zif268 cells is presented as a total count and a density (per cubic mm) because the granule cell layer increases in both size and total number of granule cells as a result of running (Rhodes et al., 2003b). Hence, we wanted to determine whether the differences in Zif268 counts between groups was due to greater proportional activation of the granule cell layer as opposed to merely a consequence of different total numbers of granule neurons between running and sedentary groups.
2) Fluorescence
Following Clark et al. (2011a), images taken from a confocal microscope (400× total magnification) were used to determine the proportion of BrdU cells (BrdU+) that differentiated into neurons (NeuN+) and to determine the proportion of neurons (BrdU+/NeuN+, or BrdU−/NeuN+) displaying Zif268. Each image captured a single plane of granule cell layer (in the z-plane) that was non-overlapping with previous image, by systematically advancing the field of view (in the x & y-plane) via a camera interfaced to computer running Leica confocal software. Imaging was done until the entire medial to lateral extent of each bi-lateral granule layer was captured. The entire bi-lateral granule cell layer was imaged in 3 hippocampal sections (1 section chosen randomly from the anterior 3rd, medial 3rd, and posterior 3rd) of 3 running and 6 sedentary mice under each experimental condition, with the exception of the caged control condition where 3 running and 3 sedentary mice were analyzed. All the new (BrdU+/NeuN+) and pre-existing (BrdU−/NeuN+) granular neurons, as well as the number of neurons co-labeled with Zif268 (BrdU−/NeuN+/Zif268+, or BrdU+/NeuN+/Zif268+) in that plane were counted for each image. Only brightly and completely, or near completely labeled BrdU nuclei were counted. Further, all BrdU+/NeuN+/Zif268+ neurons observed during live imaging were zoomed in and scanned through the z-axis to confirm co-labeling. Only neurons that displayed co-labeling throughout the z-axis were included for analysis.
Zif268-expressing new neurons (BrdU+/NeuN+/Zif268+) were also analyzed for the location within the granule cell layer. The number of Zif268-expressing new neurons that shared a border directly adjacent to the hilus was compared with the proportion of Zif268-expressing new neurons that had migrated into the granule cell layer away from the hilus (as defined by not sharing a border with the hilus, or completely surrounded by granule cells). This analysis was conducted to determine whether the probability of Zif268 expression in new neurons differed depending on location in the granule layer.
Statistical Analysis
Data were analyzed using SAS (http://www.sas.com) and R (http://www.r-project.org). P<0.05 was considered statistically significant. Density of Zif268 cells (per mm3) were compared using separate 2-way ANOVAS with exercise treatment and behavioral condition as factors. The following behavioral conditions were analyzed: caged control vs. novel cage, caged control vs. running, yoked swim vs. water maze. Separate 2-way ANOVAs were conducted rather than one large omnibus ANOVA because each condition has a unique and carefully designed control condition to compare Zif268 induction. Moreover, not all of the behavioral conditions were matched for length of exposure to the Zif268-inducing stimuli and thus are not directly comparable. The total number of BrdU cells and BrdU cells co-labeled with NeuN (BrdU+/NeuN+) in the granule layer were compared between running and sedentary mice using unpaired T-tests. The proportion of BrdU cells co-labeled with NeuN were compared between sedentary and running mice by logistic regression. The proportion of BrdU−/NeuN+ and BrdU+/NeuN+ neurons expressing Zif268 were compared between groups using logistic regression. For logistic regression analyses, the deviance is reported in place of the F statistic.
Results
Zif268 induction in the granule layer from each task (Fig. 2A, B)
Figure 2.
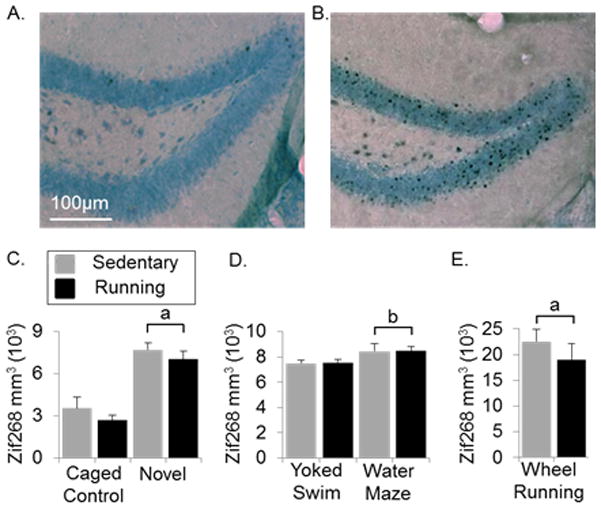
Zif268 induction in the granule cell layer from each task. A) Representative coronal section of a mouse from the caged control group containing the granule cell layer stained for Zif268 using DAB as the chromogen with a light Nissl stain to highlight the dentate gyrus. B) same as A) except containing representative section of a mouse that was running 2hr before euthanasia. C) Average number of Zif268 labeled cells (± SE) per cubic mm in the granule layer of sedentary and running mice in caged control and novel environment groups. D) same as C) except containing the yoked swim and water maze groups. E) Same as C) except containing the wheel running group. aP<0.0001 from caged control. bP<0.05 from yoked swim.
1) Novel environment
Mice in the novel group displayed a 2-fold increase in number of Zif268 positive cells over mice in the caged control group (Fig. 2C) [F(1,28)=54.8, P<0.0001]. For individual mice in the novel group, Pearson’s r for the correlation between Zif268 density and total distance traveled over the 2h exposure was 0.63 (P=0.009). In caged control mice, no relationship between Zif268 density and total distance traveled over 2 or 4h before euthanasia was observed.
2) Water maze
Both running and sedentary water maze mice learned the task as determined by decreased distance to reach the hidden platform over the three days of training [F(2,116)=36.9, P<0.0001]. A disproportionately larger number of sedentary mice discovered the platform, by chance, on the very first trial than running mice. This lead to an uneven starting point for both running and sedentary mice (an over 2 meter difference), making it impossible to address whether or not exercise had any benefits in performance on this task. In the running group, 1 out of 10 mice found the platform in less than 60s on the first trial, whereas in the sedentary group, 5 out of the 10 mice found the platform in less than 60s on the first trial. The average distance swam in the maze over the last 3 trials did not differ between the running or sedentary water maze and yoked swim groups.
The water maze group displayed a modest, yet significant, increase in number of Zif268-positive cells over the yoked swim group (Fig. 2D) [F(1,36)=5.6, P=0.02]. No significant correlation was observed between Zif268 expression and average distance to reach the platform on the 3rd day of swimming for either the yoked swim or water maze groups.
3) Wheel running
Mice in the run group displayed a 4-fold increase in numbers of Zif268-positive cells over caged control mice (Fig. 2C, E) [F(1,28)=94.7, P<0.0001]. Zif268 induction did not differ between Sed/Run and Run/Run groups (Fig. 2E). A significant correlation (r=0.77, P=0.008) was observed between Zif268 density and total distance traveled (km) on wheels 2 hours before euthanasia. The correlation was stronger (r=0.94, P<0.0001) for running distance 4 hours before euthanasia.
Zif268 induction in new granule neurons following behavioral performance (Fig. 3A)
Figure 3.
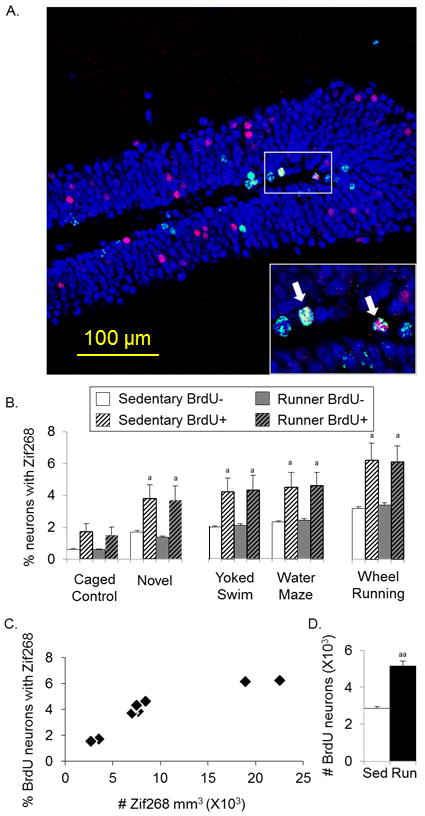
New neurons from running are broadly recruited into Zif268 induction during different behavioral tasks. A) Representative coronal section of the dentate gyrus stained for BrdU (green), NeuN (blue), and Zif268 (red) containing a region in the white box with arrows pointing at two zoomed in triple-labeled neurons. B) Percentage of BrdU−/NeuN+ and BrdU+/NeuN+ neurons that displayed Zif268 (± SE) in running and sedentary mice during performance on each behavioral task. C) Average density of Zif268 expression in the granule cell layer for each behavioral task plotted against the percentage of new neurons that displayed Zif268. Note that each point represents the mean values of multiple mice for each behavioral task, plotted separately for running and sedentary mice. D) Estimated number of BrdU+/NeuN+ cells (± SE) in running and sedentary mice aP<0.01 from BrdU− in each group, aaP<0.0001 from sedentary
The proportion of new neurons (BrdU+/NeuN+) and neurons not labeled with BrdU (BrdU−/NeuN+) expressing Zif268 was similar between sedentary and running mice for each task (Fig. 3B). For both sedentary and running mice, the percentage of neurons expressing Zif268 was approximately 2-fold greater in BrdU+ neurons than BrdU− neurons for each task (Fig. 3B) [Deviance=88.7, P<0.0001]. Collapsed across sedentary and running groups, the proportion of new neurons that expressed Zif268 differed depending on location within the granule layer [Deviance=27.3, P<0.0001]. Post-hoc analysis revealed that new neurons located away from the hilus in the granule layer were approximately twice more likely to display Zif268 than new neurons located adjacent to the hilus in yoked swim, water maze, and running conditions (each P<0.05). Sedentary and running mice exposed to the novel environment displayed a non-significant trend towards greater Zif268 expression in new neurons located away from the hilus than adjacent to the hilus. Due to the overall low numbers of Zif268-positive cells in caged control mice, an insufficient number of BrdU+ cells expressing Zif268 were observed to estimate differential proportions of Zif268 expression depending on location in the granule layer.
A strong and significant correlation (r=0.88, P<0.0001) was observed between the degree of Zif268 expression in the granule layer and proportion of new neurons displaying Zif268 for each task (Fig. 3C).
New neuron survival
The estimated absolute number of BrdU labeled cells in the granule layer of running mice was 5,706 (± 270) and in sedentary mice was 3,556 (± 108) [T(14)=7.4, P<0.0001]. The percentage of BrdU cells double labeled with NeuN was 90% (±1.2) in running and 80% (± 1.8) in sedentary mice [Deviance=21.2, P<0.001]. Thus, wheel running increased the number of new neurons (BrdU+/NeuN+) detected in the granule layer by approximately 2-fold relative to sedentary mice (Fig. 3D) [T(14)=8.9, P<0.0001].
Discussion
Our results demonstrate that new neurons generated from running express Zif268 following behavioral performance on distinct tasks that engage the hippocampus. The proportion of new neurons that expressed Zif268 was nearly equivalent in sedentary and running mice within each task (Fig. 3B). Since running mice had approximately twice the number of new neurons as sedentary mice (Fig. 3D), approximately twice as many new neurons were recruited into the Zif268 induction in the granule cell layer of running compared to sedentary mice following each task. These results suggest that new neurons generated from running can become activated during the performance of other hippocampus-involved behavioral tasks besides running. Moreover, the degree of Zif268 induction in the granule layer from each task was strongly correlated with the proportion of new neurons that expressed Zif268, independent of whether or not the mice had previous access to running wheels (Fig. 3C), demonstrating that a more active granule cell layer recruits a greater proportion of available new neurons. Taken together, these results suggest that new neurons generated basally or from running can become incorporated into granule cell activity during distinct behavioral experiences.
The result that new neurons generated from running are broadly recruited rather than preferentially engaged by the task that aided in their survival is different from what has been suggested by two earlier studies reporting that new neurons rescued during a specific behavioral-task were selectively activated during the same task (Kee et al., 2007; Tashiro et al., 2007). The reason for the difference is not entirely clear. However, it is possible that the functional integration of new neurons may differ depending on whether neurons become incorporated into the dentate gyrus by cognitive (e.g. learning) or physical processes (e.g. running). In the Kee et al., (2007) and Tashiro et al., (2007) studies, new neurons were integrated into the granule cell layer in association with hippocampus-engaged learning, namely as a result of water maze learning and the exploration of a relatively novel enriched environment. Therefore, neurons generated during hippocampal learning may favor direct recruitment into specific circuitry to support stable memories of that event. In contrast, new neurons that are integrated into the granule cell layer from running appear absent of hippocampal learning at the time they become incorporated. The increased survival of new neurons from running is, in part, a byproduct of peripheral spikes in growth factors that occur during strenuous physical activity (Aberg et al., 2000; Fabel et al., 2003; Wagner et al., 1999; Wahl et al., 2011), and may lack circuit specificity upon initial recruitment. Therefore, neurons rescued during running may represent highly plastic units that can later become incorporated into hippocampus-engaging cognitive processes.
Literature directly assessing the contribution of exercise-induced neurogenesis to cognitive performance is scarce (Winocur et al., 2011; Wojtowicz et al., 2008; Wong-Goodrich et al., 2010). Our lab recently found that performance benefits from running on the water maze are eliminated when neurogenesis is reduced by irradiation (Clark et al., 2008). The current study cannot directly assess whether new neurons from running contribute to improved hippocampus functioning. However, previous work has suggested that new 1–6 week old neurons have a lower threshold for long term potentiation (LTP) than older neurons which may play a crucial role in experience-dependent hippocampal plasticity and learning (Ge et al., 2007; Schmidt-Hieber et al., 2004). Therefore, results are consistent with the hypothesis that the new neurons contribute to enhanced behavioral performance because running mice had more new neurons recruited into the Zif268 induction than sedentary mice following each task (Fig. 3B). Taken together with observations from recent literature, results of this study provide an important foundation for the hypothesis that new neurons contribute to enhanced performance, because if more new neurons were not recruited into the Zif268 expression from the behavioral tasks in running mice, then it would seem unclear how running-enhanced neurogenesis could contribute to enhanced cognitive performance (van Praag et al., 1999a).
In the current study, new 5–6 week old neurons (in both sedentary and running mice) were more likely to display Zif268 over primarily pre-existing neurons following water maze, novel environment exposure, and wheel running (Fig. 3B). It makes sense that new neurons would be more likely to display IEGs over pre-existing neurons for two reasons. First, studies suggest that new neurons display unique electrophysiological properties that may result in an increased likelihood of firing an action potential over pre-existing neurons in response to an environmental stimulus (Ge et al., 2007; Schmidt-Hieber et al., 2004; Snyder et al., 2001; Wang et al., 2000). Secondly, while IEGs are widely used as markers for neural activity, they are commonly studied for transcriptional regulation of proteins involved in cellular plasticity (Dragunow, 1996). Thus, new neurons may be more likely to display Zif268 following a stimulus because they are more plastic during cellular development while competing for incorporation into the granule cell circuitry with thousands of already integrated neurons. On the other hand, a recent study used three different thymidine-analogue markers of cell division to directly compare a population of older labeled neurons to a distinct population of younger labeled neurons and found a similar proportion of c-Fos induction from a hippocampal task in each cohort of neurons (Stone et al., 2011). Given the recent Stone et al. (2011) findings, it is difficult to strongly conclude that new neurons preferentially display Zif268 over pre-existing neurons during hippocampal-task performance. However, we can conclude that a similar proportion of new neurons in sedentary and running mice are recruited into the Zif268 response during each of the behavioral tasks.
In conclusion, the current study provides novel evidence suggesting that new neurons integrated into granule cell circuitry from running can become recruited into granule cell activity during behavioral-experiences distinct from wheel running. While the precise contribution of new neurons from running to animal behavior remains a hotly debated topic, our data indicate that both basally and running-generated neurons are activated by a variety of hippocampus-engaging behavioral tasks. Future research will continue to elucidate whether having a greater number of activated new neurons causally contributes to cognitive advantages associated with exercise.
Acknowledgments
Special thanks to Beckman Institute Animal Care facility. This work was supported by grants from National Institutes of Health, MH083807 and DA027487.
References
- Aberg MA, Aberg ND, Hedbacker H, Oscarsson J, Eriksson PS. Peripheral infusion of IGF-I selectively induces neurogenesis in the adult rat hippocampus. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20(8):2896–903. doi: 10.1523/JNEUROSCI.20-08-02896.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrogini P, Cuppini R, Cuppini C, Ciaroni S, Cecchini T, Ferri P, Sartini S, Del Grande P. Spatial learning affects immature granule cell survival in adult rat dentate gyrus. Neurosci Lett. 2000;286(1):21–4. doi: 10.1016/s0304-3940(00)01074-0. [DOI] [PubMed] [Google Scholar]
- Bland BH, Oddie SD. Theta band oscillation and synchrony in the hippocampal formation and associated structures: the case for its role in sensorimotor integration. Behavioural brain research. 2001;127(1–2):119–36. doi: 10.1016/s0166-4328(01)00358-8. [DOI] [PubMed] [Google Scholar]
- Clark PJ, Bhattacharya TK, Miller DS, Rhodes JS. Induction of c-Fos, Zif268, and Arc from acute bouts of voluntary wheel running in new and pre-existing adult mouse hippocampal granule neurons. Neuroscience. 2011a;(184):16–27. doi: 10.1016/j.neuroscience.2011.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark PJ, Brzezinska WJ, Puchalski EK, Krone DA, Rhodes JS. Functional analysis of neurovascular adaptations to exercise in the dentate gyrus of young adult mice associated with cognitive gain. Hippocampus. 2009;19(10):937–50. doi: 10.1002/hipo.20543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark PJ, Brzezinska WJ, Thomas MW, Ryzhenko NA, Toshkov SA, Rhodes JS. Intact neurogenesis is required for benefits of exercise on spatial memory but not motor performance or contextual fear conditioning in C57BL/6J mice. Neuroscience. 2008;155(4):1048–58. doi: 10.1016/j.neuroscience.2008.06.051. [DOI] [PubMed] [Google Scholar]
- Clark PJ, Kohman RA, Miller DS, Bhattacharya TK, Brzezinska WJ, Rhodes JS. Genetic influences on exercise-induced adult hippocampal neurogenesis across 12 divergent mouse strains. Genes Brain Behav. 2011b;10(3):345–53. doi: 10.1111/j.1601-183X.2010.00674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25(6):295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- Dalla C, Bangasser DA, Edgecomb C, Shors TJ. Neurogenesis and learning: acquisition and asymptotic performance predict how many new cells survive in the hippocampus. Neurobiol Learn Mem. 2007;88(1):143–8. doi: 10.1016/j.nlm.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla C, Papachristos EB, Whetstone AS, Shors TJ. Female rats learn trace memories better than male rats and consequently retain a greater proportion of new neurons in their hippocampi. Proc Natl Acad Sci U S A. 2009;106(8):2927–32. doi: 10.1073/pnas.0809650106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrossy MD, Drapeau E, Aurousseau C, Le Moal M, Piazza PV, Abrous DN. Differential effects of learning on neurogenesis: learning increases or decreases the number of newly born cells depending on their birth date. Mol Psychiatry. 2003;8(12):974–82. doi: 10.1038/sj.mp.4001419. [DOI] [PubMed] [Google Scholar]
- Dragunow M. A role for immediate-early transcription factors in learning and memory. Behav Genet. 1996;26(3):293–9. doi: 10.1007/BF02359385. [DOI] [PubMed] [Google Scholar]
- Eadie BD, Redila VA, Christie BR. Voluntary exercise alters the cytoarchitecture of the adult dentate gyrus by increasing cellular proliferation, dendritic complexity, and spine density. The Journal of comparative neurology. 2005;486(1):39–47. doi: 10.1002/cne.20493. [DOI] [PubMed] [Google Scholar]
- Fabel K, Tam B, Kaufer D, Baiker A, Simmons N, Kuo CJ, Palmer TD. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. The European journal of neuroscience. 2003;18(10):2803–12. doi: 10.1111/j.1460-9568.2003.03041.x. [DOI] [PubMed] [Google Scholar]
- Ge S, Yang CH, Hsu KS, Ming GL, Song H. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron. 2007;54(4):559–66. doi: 10.1016/j.neuron.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E. How widespread is adult neurogenesis in mammals? Nat Rev Neurosci. 2007;8(6):481–8. doi: 10.1038/nrn2147. [DOI] [PubMed] [Google Scholar]
- Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999;2(3):260–5. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- Kee N, Teixeira CM, Wang AH, Frankland PW. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat Neurosci. 2007;10(3):355–62. doi: 10.1038/nn1847. [DOI] [PubMed] [Google Scholar]
- Koteja P, Garland T, Sax JK, Swallow JG, Carter PA. Behaviour of house mice artificially selected for high levels of voluntary wheel running. Animal Behaviour. 1999;58:1307–1318. doi: 10.1006/anbe.1999.1270. [DOI] [PubMed] [Google Scholar]
- Leuner B, Mendolia-Loffredo S, Kozorovitskiy Y, Samburg D, Gould E, Shors TJ. Learning enhances the survival of new neurons beyond the time when the hippocampus is required for memory. J Neurosci. 2004;24(34):7477–81. doi: 10.1523/JNEUROSCI.0204-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello CV, Vicario DS, Clayton DF. Song presentation induces gene expression in the songbird forebrain. Proc Natl Acad Sci U S A. 1992;89(15):6818–22. doi: 10.1073/pnas.89.15.6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeper SA, Gomez-Pinilla F, Choi J, Cotman CW. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res. 1996;726(1–2):49–56. [PubMed] [Google Scholar]
- Redila VA, Christie BR. Exercise-induced changes in dendritic structure and complexity in the adult hippocampal dentate gyrus. Neuroscience. 2006;137(4):1299–307. doi: 10.1016/j.neuroscience.2005.10.050. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Garland T, Jr, Gammie SC. Patterns of brain activity associated with variation in voluntary wheel-running behavior. Behav Neurosci. 2003a;117(6):1243–56. doi: 10.1037/0735-7044.117.6.1243. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Koteja P, Swallow JG, Carter PA, Garland T. Body temperatures of house mice artificially selected for high voluntary wheel-running behavior: repeatability and effect of genetic selection. Journal of Thermal Biology. 2000;25(5):391–400. doi: 10.1016/s0306-4565(99)00112-6. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, van Praag H, Jeffrey S, Girard I, Mitchell GS, Garland T, Jr, Gage FH. Exercise increases hippocampal neurogenesis to high levels but does not improve spatial learning in mice bred for increased voluntary wheel running. Behav Neurosci. 2003b;117(5):1006–16. doi: 10.1037/0735-7044.117.5.1006. [DOI] [PubMed] [Google Scholar]
- Richardson CL, Tate WP, Mason SE, Lawlor PA, Dragunow M, Abraham WC. Correlation between the induction of an immediate early gene, zif/268, and long-term potentiation in the dentate gyrus. Brain Res. 1992;580(1–2):147–54. doi: 10.1016/0006-8993(92)90938-6. [DOI] [PubMed] [Google Scholar]
- Schmidt-Hieber C, Jonas P, Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429(6988):184–7. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Kee N, Wojtowicz JM. Effects of adult neurogenesis on synaptic plasticity in the rat dentate gyrus. J Neurophysiol. 2001;85(6):2423–31. doi: 10.1152/jn.2001.85.6.2423. [DOI] [PubMed] [Google Scholar]
- Stone SS, Teixeira CM, Zaslavsky K, Wheeler AL, Martinez-Canabal A, Wang AH, Sakaguchi M, Lozano AM, Frankland PW. Functional convergence of developmentally and adult-generated granule cells in dentate gyrus circuits supporting hippocampus-dependent memory. Hippocampus. 2011 doi: 10.1002/hipo.20845. [DOI] [PubMed] [Google Scholar]
- Tanapat P, Hastings NB, Reeves AJ, Gould E. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19(14):5792–801. doi: 10.1523/JNEUROSCI.19-14-05792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro A, Makino H, Gage FH. Experience-specific functional modification of the dentate gyrus through adult neurogenesis: a critical period during an immature stage. J Neurosci. 2007;27(12):3252–9. doi: 10.1523/JNEUROSCI.4941-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uda M, Ishido M, Kami K, Masuhara M. Effects of chronic treadmill running on neurogenesis in the dentate gyrus of the hippocampus of adult rat. Brain Res. 2006;1104(1):64–72. doi: 10.1016/j.brainres.2006.05.066. [DOI] [PubMed] [Google Scholar]
- Van der Borght K, Kobor-Nyakas DE, Klauke K, Eggen BJ, Nyakas C, Van der Zee EA, Meerlo P. Physical exercise leads to rapid adaptations in hippocampal vasculature: temporal dynamics and relationship to cell proliferation and neurogenesis. Hippocampus. 2009;19(10):928–36. doi: 10.1002/hipo.20545. [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999a;96(23):13427–31. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999b;2(3):266–70. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25(38):8680–5. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner JP, Black IB, DiCicco-Bloom E. Stimulation of neonatal and adult brain neurogenesis by subcutaneous injection of basic fibroblast growth factor. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19(14):6006–16. doi: 10.1523/JNEUROSCI.19-14-06006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl P, Zinner C, Achtzehn S, Behringer M, Bloch W, Mester J. Effects of acid-base balance and high or low intensity exercise on VEGF and bFGF. European journal of applied physiology. 2011;111(7):1405–13. doi: 10.1007/s00421-010-1767-1. [DOI] [PubMed] [Google Scholar]
- Wang S, Scott BW, Wojtowicz JM. Heterogenous properties of dentate granule neurons in the adult rat. J Neurobiol. 2000;42(2):248–57. [PubMed] [Google Scholar]
- Winocur G, Becker S, Luu P, Rosenzweig S, Wojtowicz JM. Adult hippocampal neurogenesis and memory interference. Behavioural brain research. 2011 doi: 10.1016/j.bbr.2011.05.032. [DOI] [PubMed] [Google Scholar]
- Wojtowicz JM, Askew ML, Winocur G. The effects of running and of inhibiting adult neurogenesis on learning and memory in rats. Eur J Neurosci. 2008;27(6):1494–502. doi: 10.1111/j.1460-9568.2008.06128.x. [DOI] [PubMed] [Google Scholar]
- Wong-Goodrich SJ, Pfau ML, Flores CT, Fraser JA, Williams CL, Jones LW. Voluntary running prevents progressive memory decline and increases adult hippocampal neurogenesis and growth factor expression after whole-brain irradiation. Cancer research. 2010;70(22):9329–38. doi: 10.1158/0008-5472.CAN-10-1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zangenehpour S, Chaudhuri A. Differential induction and decay curves of c-fos and zif268 revealed through dual activity maps. Brain Res Mol Brain Res. 2002;109(1–2):221–5. doi: 10.1016/s0169-328x(02)00556-9. [DOI] [PubMed] [Google Scholar]
- Zombeck JA, Lewicki AD, Patel K, Gupta T, Rhodes JS. Patterns of neural activity associated with differential acute locomotor stimulation to cocaine and methamphetamine in adolescent versus adult male C57BL/6J mice. Neuroscience. 2010;165(4):1087–99. doi: 10.1016/j.neuroscience.2009.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]


