Abstract
It is uncertain whether late mortality rates after hematopoietic cell transplantation for severe combined immunodeficiency (SCID), non-SCID primary immunodeficiency diseases (non-SCID PIDD) and inborn error of metabolism (IEM) return to rates observed in the general population, matched for age, sex and nationality. We studied patients with SCID (n=201), non-SCID PIDD (n=405) and IEM (n=348) who survived for at least two years after transplantation with normal T-cell function (SCID) or >95% donor chimerism (non-SCID PIDD and IEM). Importantly, mortality rates were significantly higher than for the general population for several years after transplantation. This decreases towards normal rates in patients with SCID and non-SCID PIDD beyond 6 years after transplantation but not for IEM. Active chronic graft-versus-host disease at 2-years was associated with higher risks of late mortality for all diseases (HR 1.87, p=0.05). Additionally, for non-SCID PIDD, late mortality was higher in recipients of T-cell depleted grafts (HR 4.16, p=0.007) and for IEM, after unrelated donor (HR 2.72, p=0.03) and mismatched related donor (HR 3.76, p=0.01) transplants. The higher mortality rates in these long-term survivors for many years after transplantation confirm the need for long-term surveillance.
Keywords: Late mortality, primary immunodeficiency, inborn error of metabolism
INTRODUCTION
Severe combine immunodeficiency (SCID), non-SCID primary immunodeficiency diseases (non-SCID PIDD) and inborn errors of metabolism (IEM) are fatal disorders, and for many years, allogeneic hematopoietic cell transplantation was the only known definitive treatment available.1 In recent years, enzyme replacement therapy and gene therapy have been used successfully for some but not all of the primary immunodeficiency and metabolic diseases. Therefore, to-date, transplantation remains the standard of care for the majority of children with this diseases.2–8
SCID patients have been studied by several groups with reports detailing immune reconstitution, complications and survival after matched or mismatched related donor transplantation.2,6,7,8 However, most patients in these reports received allografts from related donors and did not receive transplant conditioning chemotherapy. Neven and colleagues7 report 2-year survival rates of 63% in 149 patients with SCID who received allografts from related donors except for two patients who received their allograft from unrelated donors and only half of these patients received transplant conditioning. There were an additional 8 deaths which occurred beyond two-years from poor immune reconstitution, chronic graft-versus-host disease (GVHD) and related complications, endocrine, autoimmune and inflammatory complications7. In the report by Railey and colleagues8, where all patients received their allografts from a related donor and none received transplant conditioning, the timing of transplantation predicted outcome. Transplantation within the first 3.5 months of life led to higher long-term survival, better nutritional status and fewer subsequent cellular infusions to “boost” engraftment.
Antoine and colleagues2 reported long-term survival after transplantation for SCID and non-SCID PIDD and identified better long-term survival when the donor was a matched sibling. Over 90% of SCID patients and 80% of non-SCID PIDD patients in that report received their allograft from matched or mismatched related donors.2 In a more recent report on long-term outcome after transplantation for Wiskott Aldrich syndrome, the 7-year event-free survival rates was 75% in 96 patients who survived for at least two years after transplantation.3 Sixty-six percent of patients in that report received allografts from matched or mismatched donors and late complications included GVHD, autoimmunity and infections.
Data describing long-term survival after transplantation for IEM and non-SCID PIDD, and in particular, long-term survival after unrelated donor transplantation is lacking. Further, published reports2, 3, 6–8 have not addressed the question of whether long-term survivors with immune deficiency diseases (SCID or non-SCID PIDD) and IEM have mortality rates similar to the general population. In contrast, late mortality rates in long-term survivors with severe aplastic anemia and hematological malignancies are well documented and their mortality rates are elevated for some years post transplantation.9–11 Socié and colleagues9 reported that the risk of death by the sixth year after transplantation for severe aplastic anemia and by the ninth year for acute myeloid leukemia did not differ significantly from that of a normal population. These data differ from the report by Bhatia and colleagues,10 in which late mortality risks remained significantly higher than that of a normal population in long-term survivors after transplantation. Similarly, a more recent report from the CIBMTR for patients with hematologic malignancies and severe aplastic anemia also confirmed lower life expectancy amongst long-term survivors of transplantation compared to the general population.11 While the study population in the report by Socié included fewer than 5% of unrelated donor transplant recipients the more recent reports have included higher proportions of unrelated donor transplant recipients which may explain the observed differences between the earlier and later reports.
We report on late mortality in patients with SCID, non-SCID PIDD and IEM who survived at least two-years after their transplantation with normal T-cell function (SCID) and >95% donor chimerism (non-SCID PIDD and IEM). Including only long-term survivors allowed us to determine mortality rates in these patients relative to mortality rates in an age, sex- and nationality matched general population and, to identify risk factors for late mortality in patients who are considered cured of their disease.
METHODS
Data Collection
Data were collected by the Center for International Blood and Marrow Transplant Research (CIBMTR), a voluntary organization of over 450 transplant centers worldwide that report consecutive transplantations facilitated at their center to a statistical center at the Medical College of Wisconsin. The CIBMTR database includes detailed patient, disease and transplant characteristics and their outcome data on approximately 60% of transplantations. All patients are followed longitudinally annually and compliance monitored by on-site audits. The Institutional Review Boards of the Medical College of Wisconsin and the National Marrow Donor Program approved the study.
Eligibility criteria
Eligibility for participation was limited to transplant centers that provided extended follow-up data on more than 85% of their surviving patients with SCID, non-SCID PIDD and IEM. All patients had to be alive for longer than two years after their transplantation and demonstrate sustained immunologic recovery or donor chimerism. Patients with SCID had normal T-cell function and those with non-SCID PIDD and IEM, >95% donor chimerism at study entry (i.e. two-years after transplantation). It is expected that deaths due to the early complications of transplantation would occur prior to the two-year period. Nine hundred and fifty-four patients were eligible and data were reported by 114 transplant centers. An additional 847 patients (SCID n=180; non-SCID PIDD n=327; IEM n=340) were transplanted at participating centers but died within two years after transplantation or had autologous recovery or failed to attain normal T-cell function within two years after transplantation making them ineligible for this analysis.
Statistical Methods
SCID, non-SCID PIDD and IEM disease groups were analyzed separately because of differences in biologic features and transplant strategies. Late deaths were defined as any death occurring beyond two years after transplantation. Reported causes of death were reviewed and categorized. Patients who died with active chronic GVHD were considered to have died of this complication. Deaths due to infection included only those patients without active GVHD.
The probability of long-term survival was calculated using the Kaplan-Meier estimator and the 95% confidence interval (CI) estimated using Greenwood’s formula.12 Death from any cause was considered an event and surviving patients censored at last follow up. Cox proportional hazards regression was used to identify risk factors associated with late deaths for each disease group.13 Multivariate models were built with the use of stepwise forward selection, with a p-value of 0.05 or less to indicate statistical significance; all variables met the proportional-hazards assumption. The variables considered in multivariate analysis were: age at transplantation, patient race, sex, donor type, graft type, conditioning regimen, use of anti-thymocyte globulin, recipient cytomegalovirus serostatus, graft-versus-host disease prophylaxis, donor-recipient sex match, history of grade 2–4 GVHD, chronic GVHD (none vs. resolved by 2-years after transplantation vs. active chronic at 2-years after transplantation) and year of transplantation (Table 1).
Table 1.
Patient, disease and transplant characteristics
| Characteristics | SCID* N (%) |
non-SCID PIID† N (%) |
IEM‡ N (%) |
|---|---|---|---|
| Number of patients | 201 | 405 | 348 |
| Age at transplant, years | |||
| <1 | 159 (79) | 108 (27) | 81 (23) |
| 1–2 | 36 (18) | 144 (36) | 142 (41) |
| 3–5 | 3 ( 1) | 79 (19) | 54 (15) |
| 6–9 | 3 ( 1) | 34 ( 8) | 38 (11) |
| 10–15 | 1 (<1) | 29 ( 7) | 16 ( 5) |
| >15 | 0 | 11 ( 3) | 17 ( 5) |
| Male | 118 (59) | 296 (73) | 201 (58) |
| Donor type | |||
| HLA-matched sibling | 51 (25) | 137 (34) | 108 (31) |
| Mismatched related | 93 (46) | 50 (12) | 41 (12) |
| Unrelated | 57 (28) | 218 (54) | 199 (57) |
| Graft type | |||
| Bone marrow | 161 (80) | 324 (80) | 260 (75) |
| Peripheral blood progenitor cells | 18 ( 9) | 23 ( 6) | 10 ( 3) |
| Umbilical cord blood | 22 (11) | 58 (14) | 78 (22) |
| Conditioning regimen | |||
| Total body irradiation + cyclophospahmide ± other | 5 ( 2) | 42 (11) | 85 (25) |
| Busulfan + cyclophosphamide ± other | 92 (46) | 333 (82) | 252 (72) |
| Busulfan + other | 10 ( 5) | 7 ( 2) | 4 ( 1) |
| Busulfan alone | 7 ( 3) | 1 (<1) | __ |
| Cyclophosphamide + other | 21 (10) | 10 ( 2) | 2 (<1) |
| Cyclophosphamide alone | 20 (10) | 4 ( 1) | 1 (<1) |
| Fludarabine + other | 5 ( 2) | 7 ( 2) | 4 ( 1) |
| ATG alone | 11 ( 5) | 1 (<1) | __ |
| None | 27 (13) | __ | __ |
| GVHD Prophylaxis | |||
| T-cell depletion | 79 (39) | 61 (15) | 77 (22) |
| Cyclosporine + methotrexate | 56 (28) | 211 (52) | 127 (36) |
| Cyclopsorine ± other agents | 55 (27) | 114 (28) | 128 (36) |
| Tacrolimus ± other agents | 2 (<1) | 8 ( 1) | 5 ( 1) |
| Methotrexate ± other agents | 7 ( 3) | 8 ( 2) | 11 ( 3) |
| Other agents | 2 ( 1) | 3 ( 1) | 0 |
| Year of transplant | |||
| 1980–1989 | 32 (15) | 33 ( 8) | 53 (15) |
| 1990–1000 | 112 (56) | 226 (56) | 172 (49) |
| 2001–2003 | 57 (28) | 146 (36) | 123 (36) |
| History of grade 2–4 acute GVHD | |||
| None | 125 (62) | 239 (59) | 207 (59) |
| Yes | 76 (38) | 166 (41) | 141 (41) |
| Acute GVHD grade | |||
| 0 | 105 (52) | 193 (48) | 153 (44) |
| 1 | 20 (10) | 46 (11) | 54 (15) |
| 2 | 46 (23) | 85 (21) | 75 (22) |
| 3 | 27 (13) | 72 (18) | 54 (15) |
| 4 | 3 ( 1) | 9 ( 2) | 12 ( 4) |
| Chronic GVHD | |||
| None | 163 (81) | 310 (77) | 272 (78) |
| Chronic GVHD resolved by 2 yrs after transplantation | 11 ( 5) | 36 ( 9) | 28 ( 8) |
| Chronic GVHD active for at least 2 yr after transplantation | 23 (11) | 54 (13) | 42 (12) |
| Chronic GVHD resolved by 2 years – not reported | 4 ( 2) | 5 ( 1) | 6 ( 2) |
| Age at last contact, years | |||
| 2–5 | 67 (33) | 79 (20) | 63 (18) |
| 6–9 | 61 (30) | 143 (35) | 101 (29) |
| 10–15 | 57 (28) | 111 (27) | 101 (29) |
| 16–0 | 15 ( 7) | 46 (11) | 48 (14) |
| 21–25 | 1 (<1) | 14 ( 3) | 18 ( 5) |
| >25 | 0 | 13 ( 3) | 17 ( 5) |
| Median follow-up of survivors, months§ | 93 (29–244) | 75 (25–284) | 90 (25–269) |
SCID: ADA deficiency n=9 (4%); T− B− ± NK activity n=76 (38%); T− B+ ± NK activity n=87 (43%); T+ B− ± NK activity n=20 (10%); T+ B+ ± NK activity n=6 (3%); Unknown n=3 (2%)
Non-SCID PIDD: Wiskott Aldrich syndrome n=144 (35%); hemophagocytic lymphohistiocytosis n=91 (25%); Langerhan cell histiocytosis n=12 (3%); Chediak-Higashi syndrome n=21 (4%); chronic granulomatous disease n=17 (4%); Kostmann agranulocytosis n=18 (4%); leukocyte adhesion deficiency n=15 (4%); X-linked lymphoproliferative disease n=12 (3%); other non-SCID PIDD n=75 (18%)
IEM: Osteopetrosis n=59 (17%); Hurler syndrome n=111 (32%); other mucopolysaccharidosis n=48 (12%); adrenoleukodystrophy n=36 (10%); metachromatic leukodystrophy n=33 (9%); globoid cell leukodystrophy n=20 (6%); Gaucher’s disease n=13 (4%); other IEM n=33 (10%)
N=645 patients were followed for at least 5 years, n= 440 were followed for at least 7 years and n=235 were followed for at least 10 years.
Estimates of relative mortality (excess risk of mortality) were calculated as described by Andersen and Vaeth taking into account differences among patients with regard to age, sex and nationality.14 Relative mortality is the relative risk of dying at a given time after transplantation as compared with a person of similar age, sex and nationality in the general population. We used age, sex and nationality specific rates for all countries from which transplantations were reported to calculate the relative mortality. The ratio of observed to expected cancers and the absolute excess risk (AER) were estimated. AER is the number of observed cases minus the number of expected cases per 10,000 person-years at risk. The number of person years at risk was calculated from the date of transplantation to date of diagnosis of solid cancer, date of death or date of last contact whichever occurred first. Analyses were performed using SAS (version 9.2), Cary, NC.
RESULTS
Patients
The characteristics of the study population are summarized in Table 1. Two hundred and one patients had SCID, 405, non-SCID PIDD and 348, IEM. Transplantations occurred between 1980 and 2003 and the median follow-up of surviving patients was 83 months (range 25–283). The median ages at last contact were 7, 9 and 10 years for patients with SCID, non-SCID PIDD and IEM, respectively. Age at transplantation varied with the disease for which transplantation was performed; SCID patients were younger compared to patients with non-SCID PIDD and IEM. Ninety-seven percent of patients with SCID received their transplant before two years of age compared to 65% of patients with non-SCID PIDD and IEM. Seventy percent of SCID patients received allografts from an HLA-matched sibling or mismatched relative. Transplantation of allografts from an unrelated donor was more common for non-SCID PIDD and IEM and accounted for 55% of transplants. Bone marrow was the predominant stem cell source. All but 27 patients with SCID received transplant-conditioning and 84% received conditioning regimens without total body irradiation. Most patients with non-SCID PIDD (86%) and IEM (90%) received myeloablative transplant conditioning regimens. Acute and chronic GVHD were assessed by standard criteria.15,16 Grade 2–4 acute GVHD developed in 383 of 954 (40%) of patients and most patients with GVHD had grade two or three acute GVHD. Chronic GVHD developed in 209 (22%) patients and 119 of these patients reported active chronic GVHD two-years after transplantation. One hundred and forty-eight of 209 (70%) patients with chronic GVHD also reported grade 2–4 acute GVHD prior to onset of chronic GVHD. Chronic GVHD was limited in 118 patients and extensive in 88 patients.
Long-term survival and late mortality
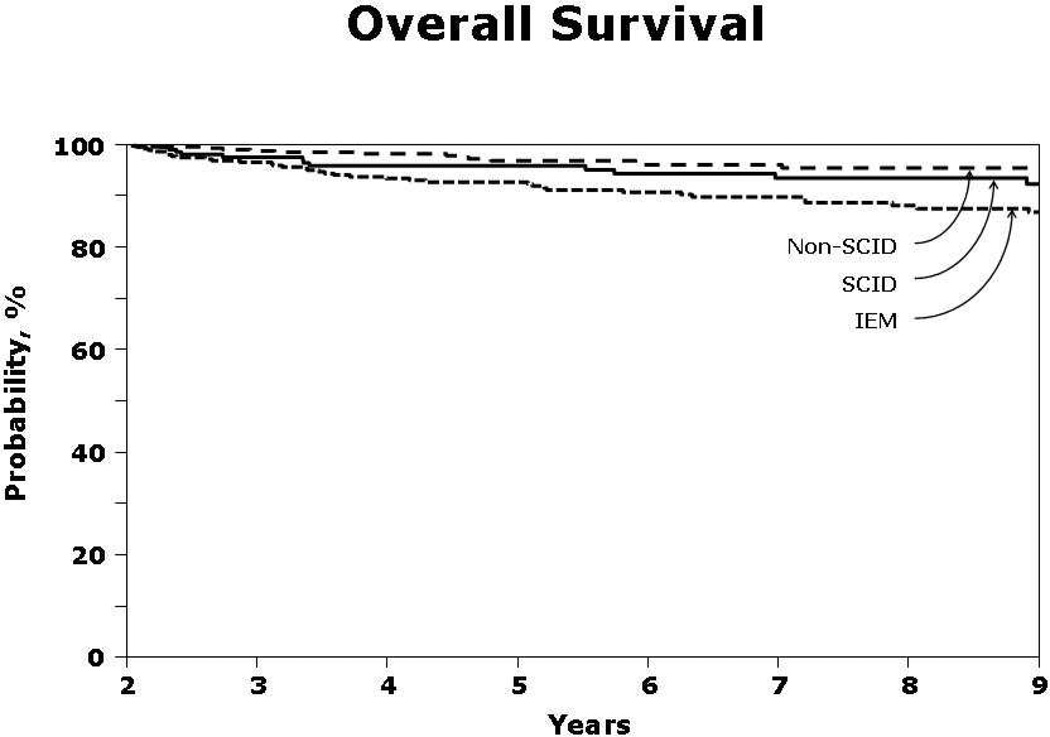
Fourteen patients (7%) with SCID, 15 patients (4%) with non-SCID PIDD and 37 patients (11%) with IEM died beyond two years after transplantation. The 7-year probabilities of overall survival were 93% (95% CI 89 – 97), 96% (95% CI 94 – 98) and 90% (95% CI 86 – 93) for SCID, non-SCID PIDD and IEM, respectively (Figure 1). Active chronic GVHD was associated with higher mortality risks for all patients, (HR 1.87, 95% CI 0.99 – 3.53, p=0.05). Other risk factors associated with late mortality differed by disease type. Late mortality was higher in recipients of T-cell depleted allografts (HR 4.16, 95% CI 1.49 – 11.76, p=0.007), for patients with non-SCID PIDD and for IEM, after transplantation of allografts from unrelated (HR 2.72, 95% CI 1.11 – 6.71, p=0.03) and mismatched related donors (HR 3.76, 95% CI 1.34 – 10.52, p=0.01) compared to HLA-matched sibling donors. Late mortality risk was not different in patients with B(−) SCID compared to B(+) SCID (HR 1.60, 95% CI 0.31 – 8.33, p=0.57). The primary causes of late mortality are shown in Tables 2A and 2B. There were 66 late deaths, 49 deaths occurring 2 – 6 years after transplantation and 17 deaths after 6 years. The most common causes of late death were chronic GVHD, infections not associated with chronic GVHD and organ failure.
Figure 1.
The 7-year probability of overall survival for patients who survived longer than two years after transplantation with normal T-cell function (SCID patients) and >95% donor chimerism (non-SCID PIDD and IEM patients) were 93% for SCID patients, 96% for non-SCID PIDD patients and 90% for IEM patients. The 9-year probabilities overall survival were 92% for SCID patients, 96% for non-SCID PIDD patients and 88% for IEM patients.
Table 2.
| A. Primary cause of death between 2 – 6 years | ||||
|---|---|---|---|---|
| Causes of death | SCID N=10 |
Non-SCID N=12 |
IEM N=27 |
Total N=49 |
| Chronic GVHD | 2 | 5 | 7 | 14 |
| Infection without GVHD* | 3 | 2 | 6 | 11 |
| Organ failure** | 3 | — | 10 | 13 |
| PTLD – EBV positive | 1 | 1 | — | 2 |
| Acute myeloid leukemia | — | 2 | — | 2 |
| Graft failure | — | 1 | — | 1 |
| Seizure | — | — | 1 | 1 |
| Accidental death | — | 1 | 1 | 2 |
| Acute abdomen | — | — | 1 | 1 |
| Not reported | 1 | — | 1 | 2 |
| B. Primary cause of death beyond 6 years | ||||
|---|---|---|---|---|
| Causes of death | SCID N=4 |
Non-SCID N=3 |
IEM N=10 |
Total N=17 |
| Chronic GVHD | — | — | 2 | 2 |
| Infection without GVHD* | 2 | 1 | 2 | 5 |
| Organ failure** | 1 | — | 2 | 3 |
| Brain stem glioma | — | 1 | — | 1 |
| Not reported | 1 | 1 | 4 | 6 |
Abbreviations: GVHD = graft-versus-host disease; PTLD-EBV = post-transplant lymphoproliferative disease – Epstein-Barr virus associated
Infection without GVHD: bacterial (n=8), viral (n=1) and no isolate (n=2)
Organ failure: multi-organ failure (n=1 IEM), cardiac (n=2 SCID; n=3 IEM) and pulmonary (n=1 SCID; n=5 IEM)
Abbreviations: GVHD = graft-versus-host disease
Infection without GVHD: bacterial infection (n=3), viral (n=1) and no isolate (n=1)
Organ failure: cardiac failure (n=1 SCID), pulmonary failure (n=1 IEM) and progressive neurological deterioration, Gaucher disease (n=1)
Malignancy accounted for 5% of late deaths (n=3). Eight malignancies were reported to have occurred two years after transplantation, excluding two patients with non-melanoma skin cancer and two patients with post-transplant lymphoproliferative disorder positive for Epstein Barr virus (PTLD-EBV), which occurred at 36 months and 39 months after transplantation. Two patients developed acute myeloid leukemia, 1 patient myelodysplastic syndrome and five patients, solid tumor (Hodgkin lymphoma n=1, kidney tumor n=1, brain stem glioma n=1, melanoma n=1 and mucoepidermoid carcinoma n=1) (Table 3). The median time to develop leukemia, myelodysplastic syndrome or solid tumor was 72 months (range 45 – 240) and occurred in patients with non-SCID PIDD. Acute myeloid leukemia and myelodysplastic syndrome developed in patients who had received total body irradiation (dose >1000 cGy). All malignancies occurred in patients aged less than 10 years at transplantation except for one patient.
Table 3.
Ratio of observed to expected cases and absolute excess risk of new malignancy occurring two-years after transplantation
| Primary disease |
New malignancy | Obs * |
Exp* | ObsExp (95% CI) | p-value | AER* |
|---|---|---|---|---|---|---|
| CGD | Melanoma of skin | 1 | 0.0027 | 370.33 (9.37, 2063) | 0.005 | 1.40 |
| WAS | Kidney | 1 | 0.0656 | 15.23 (0.39, 84.85) | 0.127 | 1.31 |
| HLH | Brain stem glioma | 1 | 0.0212 | 46.98 (1.19, 262) | 0.042 | 1.37 |
| HLH | Hodgkin lymphoma | 1 | 0.0727 | 13.75 (0.35, 76.63) | 0.140 | 1.30 |
| WAS | AML | 2 | 0.0533 | 37.55 (4.55, 135.66) | 0.003 | 2.72 |
| CHS | MDS | 1 | 0.0516 | 19.38 (0.49, 108) | 0.101 | 1.33 |
| LCH | Mucoepidermoid cancer | 1 | 0.2043 | 4.89 (0.12, 27.27) | 0.369 | 1.11 |
Abbreviations: 95% CI = 95% confidence interval; CGD=chronic granulomatous diseases; WAS=Wiskott Aldrich syndrome; HLH=hemophagocytic lymphohistiocytosis; SCID=T (−) B(+) severe combined immunodeficiency; CHS=Chediak Higashi syndrome; LCH=Langerhan cell histiocytosis;
AML=acute myeloid leukemia; MDS=myelodysplastic syndrome
Obs denotes observed cases and
Exp denotes expected cases
AER denotes absolute excess risk; number of observed cases minus number of expected cases per 10,000 person-years at risk
The relative mortality rate for each disease category was calculated to compare mortality in transplant recipients with that of an age-, sex- and nationality matched general population (Table 4). The risk of mortality 2 – 6 years after transplantation was in excess of that for the general population for all patients. Beyond 6 years, mortality risks were not different to that for the general population for patients with SCID and non-SCID PIDD. However, mortality risks remained in excess of that for the general population even beyond 6 years after transplantation for patients with IEM.
Table 4.
Estimated excess deaths per 1000 compared to an age−, sex−, and nationality matched general population
| Excess deaths per 1000 (95% confidence interval) | |||
|---|---|---|---|
| SCID | Non-SCID | IEM | |
| 2 – 6 years after HSCT | 54 (28, 79)* | 38 (25, 51)* | 90 (77, 103)* |
| 6 – 10 years after HSCT | 25 (−2, 51) | 16 (−6, 39) | 33 (15, 50)* |
denotes significant difference in excess deaths compared to an age and sex-matched general population
DISCUSSION
The risk of dying from a transplant-related complication or recurrence of primary disease is high within the first two years after transplantation. Thereafter, the risk is substantially lower such that the survival curves reach a plateau. However, the risk for late mortality exists for several years after transplantation and is linked to several factors including pre-transplant treatment, transplant conditioning regimen, chronic GVHD, infections and autoimmunity.2–11 We describe late mortality in a large group of patients with SCID, non-SCID PIDD and IEM who survived longer than two years after transplantation with normal T-cell function (SCID) and >95% donor chimerism (non-SCID PIDD and IEM). There are important differences in the characteristics of patients in the current analysis and those included in published reports.2–8 In this analysis: 1) patients had to live longer than two years after transplantation with sustained immunologic recovery or donor chimerism; 2) the majority of SCID patients received chemotherapy for transplant conditioning and a third received allografts from unrelated donors; and 3) over half of all patients with non-SCID PIDD and IEM received allografts from unrelated donors.
We found that most patients in the current analysis are long-term survivors with 7-year survival rates of 93%, 96% and 90% after transplantation for SCID, non-SCID PIDD and IEM, respectively. Overall, 7% of surviving patients died of transplant-related complications more than two-years after the procedure confirming that the risk of late mortality after transplantation is not negligible. High relative mortality in transplant recipients compared to an age, sex and nationality matched general population was observed in all patients as long as 6 years after transplantation. Excess mortality was seen only for IEM beyond six years. The observed excess mortality risk among patients with IEM may reflect failure of the transplantation procedure to prevent progression of end organ damage or fully correct organ dysfunction that may have occurred as a consequence of the disease process prior to transplantation or soon thereafter.4
Active chronic GVHD led to higher mortality in all disease groups. Others have also identified active chronic GVHD as a significant contributor to long-term morbidity and mortality after transplantation for malignant diseases, aplastic anemia, SCID and Wiskott Aldrich syndrome.3,6,7,9,10,11,17 In our analysis late mortality risks were not associated with donor source for transplantation for SCID and non-SCID PIDD. This differs from that reported by others.2 However, donor source was associated with late mortality for patients with IEM; mortality risks were higher after mismatched related and unrelated donor transplants compared to HLA-matched sibling transplants. Transplant strategies including donor selection (donor-recipient HLA matching at the allele-level at HLA-A, -B, -C, DRB1) and improvements in supportive care in recent years have resulted in fewer early deaths from transplant-related complications and may result in comparable improvement in late outcomes after alternative donor transplantations in the current era. Another hypothesis being the current analysis is limited to those who survived for two years or longer after transplantation and for diseases such as SCID and non-SCID PIDD, donor type may not be associated with survival beyond two years from transplantation.
Consistent with other reports, the most frequently reported causes of death were chronic GVHD, infection without chronic GVHD and organ failure.3,7,9,–11 Deaths occurring in patients with chronic GVHD may occur as a direct complication of GVHD such as bronchiolitis obliterans or as a consequence of the immunodeficiency associated with chronic GVHD which in turn increases susceptibility to infections and death.17,18 Though not the focus of this analysis and not feasible when utilizing data collected by a registry, information on the burden of morbidity in patients with chronic GVHD is not available.19
Death from infection in the absence of chronic GVHD was also frequent. Approximately, 75% of late infections were bacterial and indicate long-lasting immunodeficiency after transplantation.20 Organ failure was common and occurred more frequently after transplantation for IEM and SCID. Cardiac and pulmonary failures were the most frequently cited causes of organ failure. Death from organ failure may be related to the transplantation procedure and organ toxicity from radiation or chemotherapeutic agents used for transplant conditioning or the underlying disease. Patients with immunodeficiency are subject to frequent broncho-pulmonary infections prior to transplantation and patients with IEM may have cardio-pulmonary end organ dysfunction at time of transplantation. Ninety percent of IEM patients in this analysis received myeloablative conditioning regimens and it remains to be seen whether recent strategies aimed at lowering transplant-related complications such as substitution of treosulfan for busulfan,21 results in lower risks of fatal organ toxicity in long-term survivors. Organ failure can also be autoimmune mediated and autoimmunity without chronic graft-versus-host disease in long-term survivors with SCID and non-SCID PIDD is documented.3,6,7
Malignancy excluding EBV-PTLD accounted for 5% of late deaths; malignancy occurred only in patients with non-SCID PIDD. Increase in risk of malignancy compared to the general population matched for age, sex and nationality was confined to melanoma, brain stem glioma and acute myeloid leukemia. The study population is relatively small and the cancers noted to occur in excess limited by one or two events. Solid cancer after allogeneic transplantation is well documented and its incidence increases with longer follow-up.22–24 Post-transplant malignancy can be explained by the altered immune system/function after transplantation, very young age at transplantation, irradiation-containing transplant conditioning regimen and transplantation of T-cell depleted allografts all of which are known risk factors.22–24
Transplantation strategies have evolved and in recent years aggressive measures are instituted with respect to defining a suitably matched related or unrelated donor, graft-versus-host disease prevention, and, surveillance and treatment of infections. So, several of the late complications that resulted in death in this report may no longer be as relevant for patients receiving transplantation now. Only 25% of surviving patients in the current analysis have over 10 years of follow-up and complications occurring beyond this period are under reported. Curtis and colleagues22 have reported a sharp increase in new solid malignancies at 15 years after bone marrow transplantation for hematological malignancies and Baker and colleagues,25 an increase in diabetes, hypertension and cardiovascular events after transplantation in very long-term survivors.
Though the probability of long-term survival after transplantation for SCID, non-SCID PIDD and IEM is high, the risk of mortality is substantially higher than that expected for a normal general population for several years after transplantation. Allogeneic transplantation is unlikely to reverse end organ damage that occurred prior to transplantation and for some of the IEM diseases, progressive end organ damage continues despite adequate engraftment. Further, this treatment procedure may not fully correct the immunologic or metabolic defect resulting in continued interplay between inflammatory process and poorly regulated cellular repair. Consequently late mortality could be attributed to the primary disease as well as transplantation procedure. We recommend life-long surveillance to prevent and treat life-threatening late complications and better define the long-term risks of transplantation for SCID, non-SCID PIDD and IEM. Surveillance should include assessments for chronic GVHD, pulmonary function, cardiovascular risk including echocardiogram, lipid profile, diabetes, obesity and renal function tests in addition to the other recommended tests.26 Others have reported that survivors experience considerable difficulty in holding jobs and obtaining health insurance10 and this will likely hinder life-long surveillance of survivors of transplantation in countries without national health insurance.
ACKNOWLEDGEMENTS
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA76518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U01HL069294 from NHLBI and NCI; a contract HHSH234200637015C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-06-1-0704 and N00014-08-1-0058 from the Office of Naval Research; and grants from Allos, Inc.; Amgen, Inc.; Angioblast; Anonymous donation to the Medical College of Wisconsin; Ariad; Be the Match Foundation; Blue Cross and Blue Shield Association; Buchanan Family Foundation; CaridianBCT; Celgene Corporation; CellGenix, GmbH; Children’s Leukemia Research Association; Fresenius-Biotech North America, Inc.; Gamida Cell Teva Joint Venture Ltd.; Genentech, Inc.; Genzyme Corporation; GlaxoSmithKline; Kiadis Pharma; The Leukemia & Lymphoma Society; The Medical College of Wisconsin; Millennium Pharmaceuticals, Inc.; Milliman USA, Inc.; Miltenyi Biotec, Inc.; National Marrow Donor Program; Optum Healthcare Solutions, Inc.; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; RemedyMD; Seattle Genetics; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; Swedish Orphan Biovitrum; Tarix Pharmaceuticals; Teva Neuroscience, Inc.; THERAKOS, Inc.; and Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, or any other agency of the U.S. Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: We declare we have no conflict of interest.
Contributors: M Eapen, KW Ahn, SM Davies, P Veys and KS Baker designed the study. KW Ahn and A Hassebroek prepared and analyzed data. M Eapen and KS Baker had primary responsibility for drafting the manuscript. All co-authors contributed equally to interpretation of data and approval of final report.
REFERENCES
- 1.Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354:1813–1826. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- 2.Antione C, Muller S, Cant AJ, et al. European Group for Blood and Marrow Transplantation and European Society for Immunodeficiency. Long-term survival and transplantation of hematopoietic stem cells for immunodeficiencies: a report of the European experience 1968-99. Lancet. 2003;361:553–560. doi: 10.1016/s0140-6736(03)12513-5. [DOI] [PubMed] [Google Scholar]
- 3.Ozsahin H, Cavazzana-Calvo M, Notorangelo LD, et al. Long-term outcome after hematopoietic stem cell transplantation for Wiskott-Aldrich Syndrome: European Society for Immunodeficiency and the European Group for Blood and Marrow Transplantation. Blood. 2008;111:439–445. doi: 10.1182/blood-2007-03-076679. [DOI] [PubMed] [Google Scholar]
- 4.Prasad VK, Kurtzberg J. Transplant outcomes in mucopolysaccharidoses. Seminars in Hematology. 2010;47:59–69. doi: 10.1053/j.seminhematol.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 5.Costello DJ, Eichler AF, Eichler FS. Leukodystrophies: classification, diagnosis and treatment. Neurologist. 2009;15:319–328. doi: 10.1097/NRL.0b013e3181b287c8. [DOI] [PubMed] [Google Scholar]
- 6.Mazzolari E, Forino C, Guerci S, et al. Long-term immune reconstitution and clinical outcome after stem cell transplantation for severe T-cell immunodeficiency. Basic and Clinical Immunology. 2007;120:892–899. doi: 10.1016/j.jaci.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 7.Neven B, Leroy S, Decaluwe H, et al. Patients with adenosine deaminase deficiency surviving hematopoietic stem cell transplantation are at high risk of CNS complications. Blood. 2009;113:4114–4124. doi: 10.1182/blood-2006-07-034678. [DOI] [PubMed] [Google Scholar]
- 8.Railey MD, Lokhnygina Y, Buckley RH. Long-term clinical outcome of patients with severe combined immunodeficiency who received related donor bone marrow transplants without pretransplant chemotherapy or post-transplant GVHD prophylaxis. J Pediatr. 2009;155:834–840. doi: 10.1016/j.jpeds.2009.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Socie G, Veum-Stone J, Wingard JR, et al. Long-term survival and late deaths after allogeneic bone marrow transplantation. N Engl J Med. 1999;341:14–21. doi: 10.1056/NEJM199907013410103. [DOI] [PubMed] [Google Scholar]
- 10.Bhatia S, Francisco L, Carter A, et al. Late mortality after allogeneic hematopoietic cell transplantation and functional status of long-term survivors: report from the Bone Marrow Transplant Survivor Study. Blood. 2007;110:3784–3792. doi: 10.1182/blood-2007-03-082933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wingard JR, Majhail NS, Brazauskas R, et al. Long-term survival and late death after allogeneic hematopoietic cell transplantation. J Clin Oncol. 2011;29:2230–2239. doi: 10.1200/JCO.2010.33.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klein JP, Moeschberger ML. Survival Analysis: Techniques of Censored and Truncated Data, ed 2. New York, NY: Springer-Verlag; 2003. [Google Scholar]
- 13.Cox DR. Regression models and life tables. J R Stat Soc B. 1972;34:187–200. [Google Scholar]
- 14.Andersen PK, Vaeth M. Simple parametric and nonparametric models for excess and relative mortality. Biometrics. 1989;45:523–535. [PubMed] [Google Scholar]
- 15.Przepiorka D, Weisdorf DJ, Martin P, et al. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 16.Flowers ME, Kansu E, Sullivan KM. Pathophysiology and treatment of graft-versus-host disease. Hematol Oncol Clin North Am. 1999;13:1091–1112. doi: 10.1016/s0889-8588(05)70111-8. [DOI] [PubMed] [Google Scholar]
- 17.Lee SJ, Klein JP, Barrett AJ, et al. Severity of chronic graft-versus-host disease: association with treatment-related mortality and relapse. Blood. 2002;100:406–414. doi: 10.1182/blood.v100.2.406. [DOI] [PubMed] [Google Scholar]
- 18.Pavletic SZ, Vogelsang GB. Chronic graft-versus-host disease: Clinical manifestations and therapy. In: Appelbaum FR, Forman SJ, Negrin RS, Blume KG, editors. Thomas’s hematopoietic Cell Transplantation 4ed. Cambridge Mass: Wiley-Blackwell; 2009. pp. 1304–1324. [Google Scholar]
- 19.Fraser CJ, Bhatia S, Ness K, et al. Impact of chronic graft-versus-host disease on the health status of hematopoietic cell transplantation survivors: a report from the Bone Marrow Transplant Survivor Study. Blood. 2006;108:2867–2873. doi: 10.1182/blood-2006-02-003954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parkman R, Weinberg KI. Immune reconstitution following hematopoietic cell transplantation. In: Appelbaum FR, Forman SJ, Negrin RS, Blume KG, editors. Thomas’s hematopoietic Cell Transplantation 4ed. Cambridge Mass: Wiley-Blackwell; 2009. pp. 222–231. [Google Scholar]
- 21.Slatter MA, Rao K, Amrolia P, et al. Treosulfan-based conditioning regimens for hematopoietic stem cell transplantation in children with primary immunodeficiency: United Kingdom experience. Blood. 2011;117:4367–4375. doi: 10.1182/blood-2010-10-312082. [DOI] [PubMed] [Google Scholar]
- 22.Curtis RE, Rowlings PA, Deeg J, et al. Solid cancers after bone marrow transplantation. N Engl J Med. 1997;336:897–904. doi: 10.1056/NEJM199703273361301. [DOI] [PubMed] [Google Scholar]
- 23.Baker KS, DeFor TE, Burns LJ, et al. New malignancies after blood or marrow stem-cell transplantation in children and adults: Incidence and risk factors. J Clin Oncol. 2003;21:1352–1358. doi: 10.1200/JCO.2003.05.108. [DOI] [PubMed] [Google Scholar]
- 24.Rizzo JD, Curtis RE, Socie G, et al. Solid cancers after allogeneic hematopoietic cell transplantation. Blood. 2009;113:1175–1183. doi: 10.1182/blood-2008-05-158782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baker KS, Ness KK, Steinberger J, et al. Diabetes, hypertension, and cardiovascular events in survivors of hematopoietic cell transplantation: a report from the bone marrow transplantation survivor study. Blood. 2007;109:1765–1772. doi: 10.1182/blood-2006-05-022335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pulsipher MA, Skinner R, McDonald GB, et al. National cancer Institute, National heart Lung and Blood Institute, Pediatric Blood and Marrow Transplant Consortium, first international consensus conference on late effects after pediatric hematopoietic cell transplantation: the need for pediatric specific long-term follow up guidelines. Biol Blood and Marrow Transplant. 2012;18:334–347. doi: 10.1016/j.bbmt.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]