Abstract
Atrophy of brain white matter (WM) often is considered a signature injury of alcohol use disorders (AUDs). However, investigations into AUD-related changes in WM volume have yielded complex findings that are difficult to synthesize in a narrative review. The objective of this study was to obtain an averaged effect size (ES) for WM volume reduction associated with AUD diagnosis and to test potential moderators of ES. Study inclusion criteria were: 1) English language; 2) peer-reviewed; 3) published before December 2011; 4) use of MRI; 5) human participants; 6) inclusion of AUD group; 7) inclusion of non-AUD comparison group; 8) reporting or testing of total or cerebral WM volume. Moderators included study design, MRI methodology, and AUD characteristics. Nineteen studies with a total of 1,302 participants (70% male) were included, and calculated ES were confirmed by the corresponding author for 12 studies. The magnitude of the averaged ES adjusted for small sample bias (Hedges’ g) for WM reduction in AUDs was .304 (standard error = .134, range = −.57–1.21). Hierarchical linear modeling indicated that the overall ES differed significantly from 0, t(18) = 2.257, p = .037, and that the distribution of the 19 ES showed significant heterogeneity beyond sampling error, χ2(18) = 52.400, p < .001. Treatment-seeking status and length of abstinence were significant moderators of ES distribution. These results are suggestive of WM recovery with sustained abstinence and point to the need for further investigation of factors related to treatment-seeking status.
Keywords: meta-analysis, alcoholism, brain atrophy, white matter, magnetic resonance imaging
Introduction
Neuroscientific models of alcohol use disorders (AUDs) posit dysfunction of networks involved in self-regulation, motivation, and reward, leading to impaired insight and loss of control over drinking behavior (Koob and Volkow, 2010). Inquiry into the neural substrates of AUDs has been facilitated by rapid development of in vivo neuroimaging technology, particularly magnetic resonance imaging (MRI). White matter (WM) networks, which form the connective structure enabling communication among neurons, are a critical element in neuroscientific conceptualization of AUDs. WM is highly involved in cognition and emotion in general (Filley, 2010), and WM health has been linked to memory and visuospatial functioning in AUDs (Müller-Oehring et al., 2009; Sullivan et al., 2000). Experts have reached consensus that WM atrophy is a hallmark injury of AUDs (Kril and Halliday, 1999; Oscar-Berman and Marinković, 2007; Sullivan, 2000; Sullivan and Pfefferbaum, 2005), but the nature, magnitude, and moderators of WM reduction are complex and poorly understood. Given the broad functional significance of WM, characterizing the extent of atrophy is key to understanding the pathophysiology of AUDs. By combining effect sizes (ES) from MRI studies comparing AUD and control groups, this meta-analysis sought to provide a reliable estimate of WM volume reduction associated with AUD diagnosis. Factors that might systematically influence outcomes, such as methodological and design characteristics, were examined as moderators of ES distribution. The two main categories of moderators were those of broad importance to research and those specific to the study of AUDs. The former included year of publication, sample size, funding source, affiliation with Veterans Affairs (VA), and MRI methods. AUD-specific moderators were participant age, duration of AUD, lifetime consumption of alcohol, length of abstinence, and treatment-seeking status. In addition, group differences in education were considered as a proxy for socioeconomic differences. Rationale for selection of these variables is briefly discussed, followed by the methods and results of the meta-analysis. Longitudinal studies of WM volume were too few in number for meta-analysis but are reviewed in Supplementary Information.
Sample Characteristics
Age and duration of AUD
Because WM volume changes dynamically across the lifespan, age of participants may be a critical factor when studying effects of alcohol in the brain. Across the lifespan, WM volume exhibits a quadratic pattern, peaking in the 40’s and then decreasing (Ge et al., 2002; Walhovd et al., 2005). In clinical AUD samples, aging and AUD duration may interact to produce a synergistic effect on WM, making it difficult to isolate the effects of alcohol abuse (Pfefferbaum et al., 2006; Sullivan, 2000). Similarly, the effects of alcohol abuse on the adolescent brain are only beginning to be understood, and the meta-analysis included adolescent studies in the interest of providing a complete picture of WM changes from a developmental perspective.
Gender
Biological differences in alcohol metabolism have been thought to contribute to a “telescoping effect” whereby women experience more severe consequences within a shorter timeframe (Brady and Randall, 1999; Randall et al., 1999). Studies have found that alcoholic cirrhosis develops after shorter drinking duration, accompanies lower levels of consumption, and progresses more quickly in women than men (Saunders et al., 1981). Yet controversy exists over a telescoping effect specific to alcohol-related brain damage (Hommer, 2003). Jacobson (1986) reported that alcohol-dependent women with shorter duration of dependence and lower estimated intake than their male counterparts had comparable brain atrophy on CT scan, a finding that persisted even in subsets matched on age and duration of dependence. A recent CT study also demonstrated a similar extent of brain atrophy in alcohol-dependent women and men, even though average duration of dependence in women was about half that reported by men (Mann et al., 2005). This study closely replicated the results of an earlier CT study on an independent sample (Mann et al., 1992). However, other studies have found lesser damage in women even when controlling for or matching gender groups on intake and duration (Pfefferbaum et al., 2006; Sullivan et al., 2010).
Treatment-seeking status
Only 15% of individuals with AUDs ever receive treatment of any kind, and evidence indicates that this subset differs substantially from the AUD population as a whole on a number of relevant demographic, physiological, and psychological variables (Hasin et al., 2007). Epidemiological data show that factors associated with treatment-seeking include older age, male sex, and greater lifetime incidence of mood, personality, and other substance use disorders (Cohen et al., 2007). Fein and Landman (2005) compared alcohol use trajectories in treatment-naïve individuals and abstinent, treated individuals matched on age of alcohol dependence onset. Although groups did not differ in the time from initiation of drinking to onset of heavy drinking, the treated group had a significantly higher average intake and peak intake than the treatment-naive group (Fein and Landman, 2005).
Few neuroimaging studies have directly compared treatment-seeking and non-treatment-seeking AUD individuals. Gazdzinski et al. (2008) identified higher cerebrospinal fluid (CSF) volume, smaller gray matter (GM) volumes in cortical lobes and thalamus, and lower concentrations of metabolites reflecting neuronal viability in treatment-seeking versus treatmentnaïve AUD individuals. Moreover, few differences were found between the treatment-naïve AUD group and healthy controls. Therefore, convenience samples drawn from treatment-seeking populations may overestimate the magnitude of brain abnormality in the general population of AUD individuals.
Lifetime consumption and length of abstinence
Evidence from animal models of alcohol dependence suggests that alcohol-related neurodegeneration occurs primarily during intoxication, not during withdrawal as previously believed (Crews and Nixon, 2009; Obernier et al., 2002). In the oxidative stress model proposed by Crews and Nixon (2009), volume losses in AUDs are attributed to stimulation of proinflammatory cascades leading to cell dysfunction or death and inhibition of neurogenesis in adult neural stem cells located in the olfactory bulb and hippocampus. The observation that alcohol-related brain damage in humans appears to be more closely related to recency of drinking than duration or quantity of drinking lends support to this model (Crews and Nixon, 2009). Evaluating the relative importance of cumulative exposure, measured in duration of AUD or in lifetime consumption, versus recency of exposure is critical to understanding the neurobiology of alcohol-related WM damage and repair.
MRI Methodology
Procedures for collecting, segmenting, and analyzing images have evolved in parallel with rapid technological advances in MRI. Variables relevant to all structural MRI studies of AUDs and selected for analysis as moderators were field strength of the instrument, segmentation method, and adjustment for intracranial volume (ICV). Better resolution at higher field strengths increases the signal-to-noise ratio of MRI data, potentially increasing the ability to detect group differences (Moseley et al., 2009). Development of automated algorithms for classifying tissue on MRI images into WM, GM, and CSF has obviated the need for manual tracing of tissue compartments and may increase the precision of volume measures (Zhang et al., 2001). Adjusting tissue volumes for ICV has become standard practice in cross-sectional studies in recent years to reduce the influence of factors not associated with the mechanism of interest, including height (Friedman et al., 1997; Matsumae et al., 1996) and parental history of AUD (Gilman et al., 2007).
Materials and Methods
Literature Search
Keyword searches were conducted in Pubmed, ISI Web of Knowledge, and Google Scholar using the terms “alcoholism,” “alcohol abuse,” “alcohol,” “brain,” “atrophy,” “volume,” “white matter,” “adolescent,” and “magnetic resonance imaging.” Abstracts were examined for references to brain volumes in AUDs, and the full text of the study was retrieved if it appeared relevant. Reviews on structural brain changes in AUDs were examined for additional citations [e.g., (Bühler and Mann, 2011; Crews and Nixon, 2009; Sullivan and Pfefferbaum, 2005)].
Inclusion and Exclusion Criteria
Inclusion criteria were as follows: 1) English language; 2) peer-reviewed (i.e., dissertation and poster abstracts not eligible); 3) published before December 2011; 4) use of MRI for quantification of volume; 5) human participants; 6) inclusion of an AUD group; 7) inclusion of a non-AUD comparison group; 8) reporting or testing of total or cerebral WM volume. No minimum sample size was required.
Exclusion criteria were as follows: 1) study of primary drug of abuse other than alcohol; 2) no AUD group (i.e., study of alcohol effects in the population or moderate drinkers); 3) use of region-of-interest, diffusion tensor imaging (DTI), deformation-based morphometry (DBM), or voxel-based morphometry (VBM) methodology to study WM without reporting or testing of overall or cerebral WM volume. Practically speaking, most studies that used a region-of-interest approach also reported overall WM volume either in the same study or in a separate report on the same sample. The same was not necessarily true of DBM or VBM studies, yet their outcome measures (focal areas of difference reported as coordinates and Z-scores of significant clusters) diverge from global WM volume measures to the extent that combining these types of statistics would be of questionable meaning (see Ashburner and Friston, 2000). See Table 1 for examples of excluded studies.
Table 1.
Examples of excluded studies
| Criterion | Excluded study | Reason |
|---|---|---|
| Use of MRI for quantification of volume | Mann et al. (1992) | CT study |
| Mann et al. (2005) | CT study | |
| Inclusion of AUD group | Anstey et al. (2006) | Population-based sample |
| Sasaki et al. (2009) | Moderate drinking sample | |
| Alcohol as primary substance of abuse | Thompson et al. (2004) | Primary amphetamine dependence |
| Medina et al. (2007) | Primary marijuana use | |
| Reporting or testing of overall or cerebral WM volume | Chanraud et al. (2007) | VBM; no WM measure |
| Bartsch et al. (2007) | Global volume change; no WM measure | |
| Fein et al. (2010) | WM signal hyperintensities only | |
| Laakso et al. (2002) | Frontal lobe WM volume only |
In one case where data for a portion of the sample had been published previously [i.e., males in Pfefferbaum et al. (2001)], the ES was calculated from the report that included the largest number of participants.
Application of automated segmentation algorithms in MRI studies means that WM volumes are calculated incidentally by the majority of studies appearing in the literature. By itself, mention of tissue segmentation into GM, WM, and CSF was not sufficient for inclusion in the meta-analysis; reports were required to investigate WM volume as a variable of interest in AUDs. This criterion was met when the study either reported means and standards deviations for WM volume or performed a test of group differences in WM volume. In 3 cases (Gazdzinski et al., 2010; Gazdzinski et al., 2005; Lee et al., 2007) where WM volume differences had been tested but sufficient data to calculate the ES were absent (e.g., test statistics reported only as non-significant), the corresponding author was contacted for more information.
Coding
Moderators were coded as follows: affiliation with VA: 0 = no, 1 = yes; funding by National Institute on Alcohol Abuse and Alcoholism (NIAAA) and/or other National Institutes of Health (NIH): 0 = no, 1 = yes; tissue segmentation method: semi-automated (requiring some human input, rating, or judgment) = 0, fully automated (performed entirely by computerized algorithm) = 1; volume adjustment for ICV: 0 = no, 1 = yes; treatment-seeking status: non-treatment-seeking = 0; treatment-seeking = 1. Continuous data were recorded for year of publication, total sample size, MRI field strength in Tesla (T), average age of the total sample, percentage of males in the total sample, difference in years of education between control and AUD groups, duration of heavy drinking or AUD in the AUD group, lifetime consumption of alcohol in the AUD group, and number of days abstinent at MRI scan.
Statistics
Hedges’ g was chosen as the ES because it adjusts obtained ES by sample size (Hedges, 1981). ES and their variances were entered into hierarchical linear modeling [HLM (Raudenbush and Bryk, 2002)] using the HLM 6.04 software package (Raudenbush et al., 2000). ES were inversely weighted by their sampling variances so that ES with larger samples and lower sampling error received greater weight than smaller samples with greater sampling error. Using full maximum likelihood estimation, the random intercept model was first fitted to calculate the overall mean ES, test whether it was significantly different from 0, and assess the heterogeneity of the ES distribution. Following significant heterogeneity, moderators were set to fixed effects and assessed by the t-statistic in level 2 analyses (least squares estimates of fixed effects, with robust standard errors). Moderator analyses for difference in years of education, duration of AUD, lifetime consumption, number of days abstinent, and treatment-seeking status were performed within the subsets of studies reporting those variables. In addition, mean ES were calculated for male and female samples for each study reporting data from men and women separately. Moderator analyses were not performed within male and female subsets due to power limitations.
Because number of days abstinent showed positive skew, a square root transformation was applied prior to moderator analyses. In a study where mean number of days abstinent was not reported (Mechtcheriakov et al., 2007), the minimum required of participants at the time of scanning was substituted.
Author Confirmation
Following calculation of the cross-sectional ES, the corresponding author from each study was contacted via email, with two exceptions. In Cardenas et al. (2005), the obtained ES was simply compared to the ES published in the original report and found to be very similar. For Mechtcheriakov et al. (2007), a valid email address for the corresponding author could not be found. When contacting authors, the ES was presented along with a request for the author to evaluate it in light of his or her expertise on the study. The 12 authors who responded to this request indicated the acceptability of the calculated ES. In addition, authors were contacted to confirm the independence of samples in cases requiring clarification.
Interrater Reliability
The primary coder (MAM) trained an independent rater (RAY) on the coding scheme developed for this study in order to assess interrater reliability. Because ES for 12 of 19 studies were confirmed by a corresponding author, the independent rater coded 3 studies for which author confirmation was not obtained, chosen at random from within that subsample. For calculation of the overall ES, interrater reliability was excellent, Pearson’s r = .981, intraclass correlation coefficient (ICC) = .970, 95% confidence interval = .607–.999. Complete agreement for continuous measures was obtained for year of publication, MRI field strength, difference in education, percentage male, duration of AUD, and number of days abstinent. Reliability was acceptable to high for sample size, ICC = .678, and age, ICC = .998. For categorical measures, all kappa coefficients = 1, indicating complete agreement.
Results
From 127 articles retrieved on the basis of their abstracts, a total of 19 studies met criteria for inclusion in the meta-analysis. Table 2 summarizes design and sample characteristics for each study as well as the obtained ES and variance. For all ES, a positive sign indicates greater WM volume in the control group, and a negative sign indicates greater WM volume in the AUD group.
Table 2.
Design and sample characteristics and obtained effect sizes for all studies (N = 19).
| Study | Total N | % male in total sample |
HC group | HC age mean |
AUD group | AUD age mean |
AUD TS status |
WM measure | ES for both genders (variance) |
ES for men (variance) |
ES for women (variance) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cardenas et al. (2005) | 98 | 82 | 49 individuals (40 m/9 f) | 41.6 | 49 individuals (40 m/9 f) | 41.4 | NTS, with AUDa | Volume adjusted for ICV | .13 (.04) | .17 (.05) | .15 (.22) |
| Chen et al. (2011) | 235 | 65 | 111 individuals (73 m/38 f) | 34.0 | 124 individuals (79 m/45 f) | 40.0 | TS | Volume adjusted for ICV | 1.12 (.02) | 1.00 (.03) | 1.38 (.06) |
| De Bellis et al. (2000) | 36 | 42 | 24 individuals (10 m/14 f) | 17.0 | 12 individuals (5 m/12 f) | 17.2 | Unknown | Raw volume | 0.31 (.13) | — | — |
| Demirakca et al. (2011) | 116 | 53 | 66 individuals (34 m/32 f) | 45.0 | 50 individuals (27 m/23 f) | 46.6 | TS | Volume adjusted for ICV | .56 (.04) | .65 (.07) | .96 (.08) |
| Di Sclafani et al. (1995)b | 20 | 100 | 9 males | 63.0 | 11 males | 59.7 | TS | Volume adjusted for ICV | — | .25 (.20) | — |
| Fein et al. (2002) | 41 | 100 | 17 males | 30.0 | 24 males | 38.7 | NTS, with AUDc | Volume adjusted for ICV | — | .15d (.10) | — |
| Fein et al. (2009) | 97 | 52 | 46 individuals (23 m/23 f) | 45.5 | 51 individuals (27 m/24 f) | 46.7 | NTS, in long-term remission | Volume adjusted for ICV | −.20 (.04) | −.19 (.08) | −.32 (.09) |
| Gazdzinski et al. (2005) | 67 | 100 | 30 males | 45.3 | 37 males | 49.5 | TS | Volume adjusted for ICV | — | .58 (.06) | — |
| Gazdzinski et al. (2010) | 58e | 92 | 22 individuals (20 m/2 f) | 48.3 | 36 individuals (33 m/3 f) | 49.4 | TS | Volume adjusted for ICV | — | .16 (.07) | — |
| Jang et al. (2007) | 40 | 100 | 20 males | 44.5 | 20 males | 43.5 | TS | Volume adjusted for ICV | — | 1.21 (.12) | — |
| Lee et al. (2007) | 31 | 100 | 18 males | 32.9 | 13 males | 33.8 | TS | Raw volume | — | −.36 (.13) | — |
| Makris et al. (2008) | 42 | 100 | 21 males | 54.0 | 21 males | 50.7 | TS | Raw volume | — | −.57 (.10) | — |
| Mechtcheriakov et al. (2007) | 44 | 64 | 22 individuals (14 m/8 f) | 53.7 | 22 individuals (14 m/8 f) | 53.6 | TS | Raw volume | .52 (.09) | — | — |
| Nagel et al. (2005) | 30 | 60 | 17 individuals (10 m/7 f) | 16.5 | 13 individuals (8 m/5 f) | 62 | NTS, with AUD | Volume adjusted for ICV | −.43 (.14) | ||
| O’Neill et al. (2001) | 50 | 82 | 21 individualsf (18 m/3 f) | 38.0 | 29 individuals (23 m/6 f) | 43.4 | TS | Volume adjusted for ICV | .84 (.09) | — | — |
| Pfefferbaum et al. (1992) | 92 | 100 | 43 males | 45.5 | 49 males | 45.0 | TS | Raw volumes | — | .45 (.04) | — |
| Pfefferbaum et al. (2001) | 79g | 0 | 37 females | 42.9 | 42 females | 41.7 | TS | Raw volumes | — | — | −.12 (.05) |
| Rohlfing et al. (2006) | 23 | 0 | 16 females | 51.2 | 7 females | 47.8 | TS | Percent difference relative to HC | — | — | 1.05 (.23) |
| Symonds et al. (1999) | 115 | 70 | 63 individualsh (29 m/34 f) | 65.2 | 52 males | 48.4 | TS | Z-score relative to HC | .37 (.04) | — | — |
Abbreviations: AUD = alcohol use disorder; ES = effect size; HC = healthy control; ICV = intracranial volume; NTS = non-treatment-seeking; TS = treatment-seeking; WM = white matter.
Two individuals in this group did not meet DSM-IV criteria for current AUD, but may have met criteria for lifetime AUD. This study was included because the proportion of possibly non-AUD individuals in the AUD group was negligible.
Demographics were reported for the total sample (N = 25), only 20 of whom had WM volume data from which the ES was calculated.
All individuals met DSM-IV criteria for lifetime alcohol dependence.
The original report stated only that the difference between AUD and HC was non-significant. This effect size was estimated with the help of input from the corresponding author (D. J. Meyerhoff, personal communication, December 16, 2010).
Per the corresponding author (S. Gazdzinski, personal communication, December 9, 2010), data for 4 participants in the HC group and 8 participants in the AUD group were published previously. Removing these 12 duplicate cases brought the grand total for the meta-analysis to 1,302.
This group comprised both HC participants and participants with cocaine dependence without comorbid AUD.
Only the female subset was used, as data on males had been published as part of a larger sample in Pfefferbaum et al. (1992).
The HC group was an older, “normal aging” group.
Descriptive Characteristics
Publication dates ranged from 1992–2011, with 2005 being the median. VA affiliation was documented for 11 studies (58%), and 14 studies (74%) reported NIAAA/NIH funding.
MRI field strength was 1.5 T in 16 studies, 2.0 T in 1 study, and 3.0 T in 2 studies. Tissue segmentation on MR images was semi-automated in 9 studies and fully automated in 10 studies. WM volume was adjusted for ICV in 11 studies, with the remaining 8 studies reporting either raw volume or Z-score or percent difference with respect to the comparison group.
The grand total of participants was 1,302, of whom 70% were male. Average sample size was 69 participants (34 control, 35 AUD), with a median of 50. Seven investigations studied men exclusively. An additional study (Gazdzinski et al., 2010) was included in the male-only mean because males comprised > 90% of the sample. One study (Symonds et al., 1999) compared an all-male AUD group to a comparison group that was 54% female and was not included in the male-only mean. For one study that presented data for both genders, the male subsample had been published previously in a separate report and was not included twice in the meta-analysis (Pfefferbaum et al., 2001). Thus, 2 investigations presented novel data for females only, and 9 studies reported on both men and women. Fourteen studies (74%) recruited treatment-seeking participants. The 4 non-treatment-seeking samples were heterogeneous and included treatment-naïve, current heavy drinkers (Cardenas et al., 2005; Fein et al., 2002), AUD individuals with long-term abstinence (Fein et al., 2009), and teenagers with AUDs recruited from high schools (Nagel et al., 2005). Treatment-seeking status could not be determined for 1 study (De Bellis et al., 2000). The non-AUD comparison group was a healthy control sample for every study except O’Neill et al. (2001), which compared AUD participants with and without cocaine dependence to a combined group of healthy controls and individuals with cocaine dependence only. Descriptive characteristics for demographic and clinical characteristics of samples are presented in Table 3 to provide a snapshot of the “average” participant and to convey study heterogeneity.
Table 3.
Demographic and clinical characteristics of samples (N = 19).
| Characteristic | Number of studies reporting |
Mean (unweighted) |
Median | Range |
|---|---|---|---|---|
| Average age for all participants | 19 | 43.0 | 45.2 | 16.6 – 61.2 |
| Difference in years of education (control – AUD) | 14 | 1.7 | 1.8 | −.4 – 3.5 |
| Number of days abstinent at MRI scan | 14 | 390.3 | 26.1 | 4.5 – 2229.0 |
| Duration of AUD or heavy drinking in years | 15 | 15.1 | 12.7 | 1.4 – 27.4 |
| Lifetime consumption of pure ethanol in kg | 9 | 776.8 | 644.4 | 103.2 – 1361.0 |
| Percentage of AUD group who were current smokers | 8 | 73.6 | 77.5 | 43.0 – 100 |
Effect Sizes
Overall ES
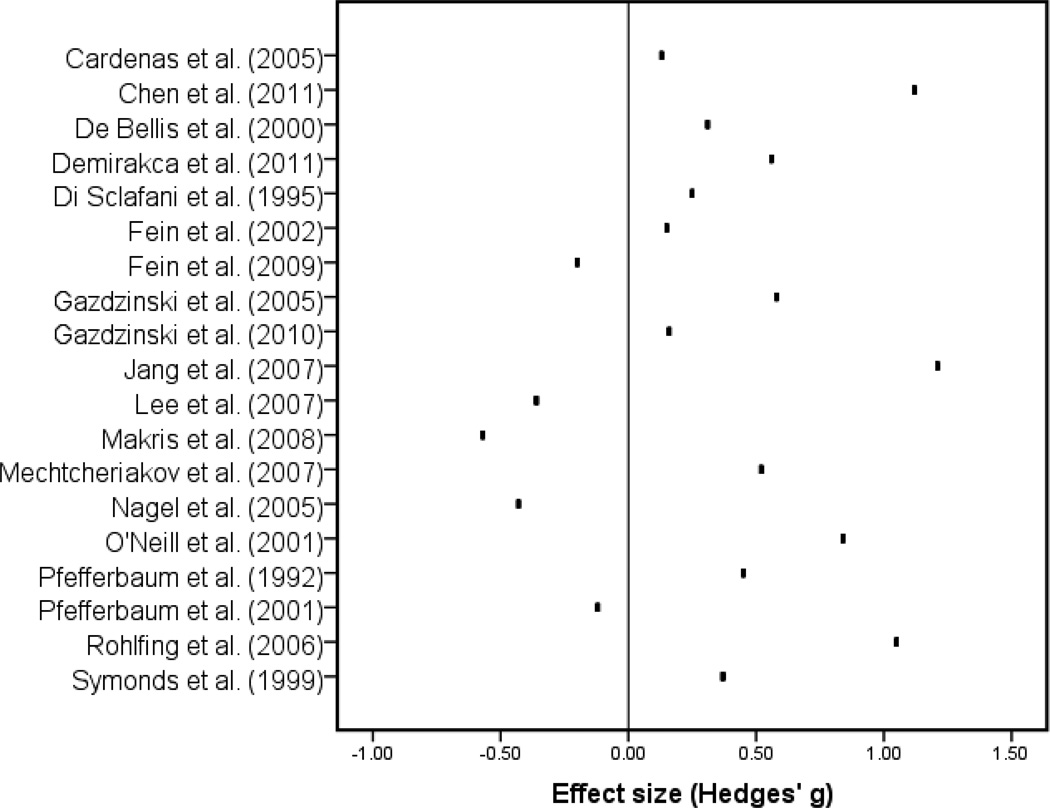
The averaged ES for all studies was g = .304 [standard error (SE) = .134; standard deviation (SD) = .371]. This ES was significantly different from 0, t(18) = 2.257, p = .037. The test for heterogeneity of ES was also significant, χ2(18) = 52.400, p < .001. These findings indicate that non-AUD participants had significantly greater WM volume relative to AUD participants and that the magnitude of this advantage varied significantly across studies (see Figure 1).
Figure 1.
Effect size by study.
ES for males and females
The mean ES for male samples (n = 12) was g = .239 (SE = .157, SD = .406). This ES was not significantly different from 0, t(11) = 1.525, p = .155. The mean ES for female samples (n = 6) was g = .538 (SE = .241, SD = .513), which showed a trend toward significance, t(5) = 2.233, p = .074. ES for men and women were not significantly different from each other, t(16) = .752, p = .463. Thus, the effect of AUD diagnosis on WM volume was not significantly different for men and women.
Moderators
Table 4 shows statistics for tests of moderators. VA affiliation, NIAAA/NIH funding, sample size, MRI field strength, segmentation method, adjustment for ICV, year of publication, age, percentage male, group difference in education, duration of AUD, and lifetime consumption were not significant moderators of ES.
Table 4.
Test statistics for moderators of effect size (N = 19).
| Moderator | Description | b | Standard error |
Degrees of freedom |
t-value | p-value |
|---|---|---|---|---|---|---|
| Publication date | Continuous | .002 | .017 | 17 | .137 | ns |
| MRI field strength | Continuous | −.006 | .411 | 17 | −.014 | ns |
| Sample size | Continuous | .003 | .002 | 17 | 1.656 | ns |
| Age | Continuous | .009 | .008 | 17 | 1.082 | ns |
| Percentage male | Continuous | −.001 | .004 | 17 | −.370 | ns |
| Education difference (n = 14) | Continuous | .081 | .125 | 12 | .652 | ns |
| Duration of AUD (n = 15) | Continuous | .004 | .012 | 13 | .325 | ns |
| Lifetime consumption (n = 9) | Continuous | < .001 | < .001 | 7 | −.760 | ns |
| Number of days abstinent (n = 14) | Continuous | −.013 | .006 | 12 | −2.247 | .044 |
| VA affiliation | No | .526 | .196 | 17 | 2.687 | .016 |
| Yes | .165 | .230 | 17 | −1.576 | ns | |
| NIAAA/NIH funding | No | .460 | .224 | 17 | 2.052 | .056 |
| Yes | .266 | .260 | 17 | −.747 | ns | |
| Segmentation method | Semi-automated | .112 | .137 | 17 | .820 | ns |
| Fully automated | .501 | .211 | 17 | 1.846 | .082 | |
| ICV adjustment | No | .206 | .174 | 17 | 1.186 | ns |
| Yes | .397 | .229 | 17 | .834 | ns | |
| Treatment-seeking status (n = 18) | Non-tx-seeking | −.088 | .121 | 16 | −0.724 | ns |
| Tx-seeking | .433 | .183 | 16 | 2.839 | .012 |
Treatment-seeking status was a significant moderator. Treatment-seeking and non-treatment-seeking samples had significantly different ES, t(16) = 2.839, p = .012. The ES for studies with non-treatment-seeking samples was b (unstandardized) = −.088 (SE = .121), whereas the ES for studies with treatment-seeking samples was positive, b = .433 (SE = .183). In short, the negligible difference between non-treatment-seeking samples and control groups stood in contrast to a medium-sized effect for WM reduction in treatment-seeking samples compared to control groups.
Number of days abstinent was also a significant moderator, b = −.013 (SE = .006), p = .044. The negative coefficient for this moderator signified that the magnitude of WM volume difference between AUD and healthy controls groups decreased as length of abstinence increased.
Discussion
The main finding of this meta-analysis was a small-to-medium ES (g = .304) for AUD diagnosis, indicating a significant WM volume deficit in AUD groups relative to healthy comparison groups. Of 14 contextual, methodological, and sample characteristics tested as moderators, only treatment-seeking status and length of abstinence at scanning significantly moderated ES distribution.
Because the population of studies that recruited non-treatment-seeking AUD groups was small and included both treatment-naive individuals and long-term abstinent individuals, generalizations about the importance of treatment-seeking status as a significant moderator of the ES distribution should be considered speculative. Yet this finding supports the assertion that treatment-seeking status marks an important distinction within the AUD population (Fein and Landman, 2005; Gazdzinski et al., 2008). A mere 15% of individuals with AUDs ever receive treatment (Hasin et al., 2007), raising questions about the generalizability of findings of neurobiological abnormality to the AUD population as a whole. Although treatment-seeking individuals with AUDs typically manifest impairment in attention, executive function, memory, and visuospatial abilities, a recent investigation of cognitive functioning in actively drinking, treatment-naïve individuals with alcohol dependence found no evidence of impairment on an extensive neuropsychological battery sensitive to the effects of alcohol abuse (Smith and Fein, 2010). Our finding of negligible WM effects in non-treatment-seeking samples, in contrast to substantial WM atrophy in treatment-seeking samples, is consistent with these differences in cognitive functioning. Because healthy WM is critical to normal attention, executive function, memory, and spatial orienting (Filley, 2001), it is likely that WM atrophy accounts in part for the impairment observed in treatment-seeking samples. Further, the harmful effects of alcohol abuse on WM and cognition may be especially problematic in the context of psychosocial treatments for AUDs, which typically require effortful information processing and reevaluation of reward. Better understanding of the neurobiological risk factors associated with greater cognitive impairment and WM atrophy in treatment-seeking individuals is an important next step in research on AUDs.
This meta-analysis found that length of abstinence was significantly, inversely associated with magnitude of atrophy in AUDs. On the basis of cross-sectional studies alone, this association constitutes weak evidence for recovery of WM tissue with abstinence. However, the longitudinal studies reviewed in Supplementary Information provide converging evidence of WM recovery beginning in early abstinence and continuing for several months. If length of abstinence, rather than how much or for how long the individual drank, is a major determinant of brain recovery, this finding would be highly encouraging in the context of AUD treatment. Simply knowing that brain injury can begin to reverse itself within days of drinking cessation and that present abstinence is more important than past drinking may strengthen the resolve of many individuals seeking to change their drinking behavior.
The significance of number of days abstinent but not lifetime consumption or duration of AUD as a moderator of ES distribution could be interpreted as support for a model in which alcohol-related brain damage occurs primarily during intoxication, not withdrawal (Crews and Nixon, 2009). The ability of the brain to heal itself after a sizable but time-limited dose of alcohol is supported by an animal model of binge drinking showing complete normalization of ventricular dilation after 7 days of recovery (Zahr et al., 2010). An alternative explanation is that duration of abstinence possessed greater predictive power than lifetime consumption or AUD duration because it was quantified with greater precision, thereby introducing less random error into analyses. Although the reliability and validity of lifetime consumption and AUD duration have proven adequate in most cases (Jacob et al., 2006; Koenig et al., 2009), these measures necessarily entail estimation and interpolation in the participant’s self-report. Many study participants resided in controlled environments with supervised abstinence prior to data collection, a factor likely to increase the accuracy of the abstinence variable.
Whether tested as a categorical variable or as a percentage, gender was not a significant moderator of ES distribution in this meta-analysis, and our study was not designed to directly address whether women manifest a telescoping effect in terms of health consequences. Nonetheless, a qualitative difference between ES for men and women arose, as the averaged ES in men (g = .239) was smaller than that for women (g = .538). Firm conclusions about relative vulnerability to alcohol-related WM damage cannot be made on the basis of the available evidence, especially given the small population of studies contributing ES for women, but a cautious interpretation is that women seem to be at least as severely affected as men.
Heightened vulnerability to alcohol-related brain damage with increasing age observed in some samples (e.g., Pfefferbaum et al., 2006) was not instantiated in this population of cross-sectional studies, as age was not a significant moderator. The average age range included adolescents as well as mature adults, suggesting that individuals with AUDs sustain a comparable extent of damage across most of the lifespan. However, the absence of studies focusing on adults in their 60’s and older limits conclusions and may account for the lack of an age effect.
The functional significance of WM reductions in AUDs, their reversal with sustained abstinence, and their ability to account for variance in AUD outcomes are issues of pressing importance. In a study of participants included in 2 studies above (Pfefferbaum et al., 1992; Pfefferbaum et al., 1995), increase in posterior WM volume was significantly correlated with recovery of memory function after several months of abstinence (Sullivan et al., 2000). A naturalistic longitudinal study found that processing speed, which relies heavily on intact WM (Filley, 2001), and a metabolic marker of neuronal integrity in frontal lobe WM were significant predictors of drinking outcomes following AUD treatment (Durazzo et al., 2008). In a DBM study of an overlapping sample, volumes of bilateral orbitofrontal cortex and surrounding WM at baseline were significantly smaller in those who relapsed than those who abstained during the year following treatment (Cardenas et al., 2011). In a DTI study, frontal WM integrity at baseline differed significantly between those who resumed heavy drinking and those who sustained treatment gains at 6-month follow-up (Sorg et al., 2011). These findings suggest that baseline differences in WM health, particularly in frontal lobes, constitute an important individual difference with potential treatment implications.
Due to demonstrated effects on neurophysiology, nicotine dependence (Durazzo et al., 2007), anxiety (van Tol et al., 2010), depression (Drevets et al., 2008; Peterson and Weissman, 2011), family history of AUD (Gilman et al., 2007), presence of Wernicke-Korsakoff syndrome (Kril et al., 1997), liver disease (Pfefferbaum et al., 2004), and comorbid drug use disorders (Berman et al., 2008; Lorenzetti et al., 2010; O'Neill et al., 2001) merit further consideration as factors influencing WM atrophy in AUDs. In particular, brain abnormality in AUDs overlaps considerably with changes found in mood, anxiety, and other substance use disorders. Because epidemiological data have linked AUD treatment-seeking to higher lifetime incidence of mood, personality, and other substance use disorders (Cohen et al., 2007), it is plausible that an overall greater burden of psychopathology accounts in part for the significantly larger ES observed in treatment-seeking samples in the present study. One limitation of the present study is that these variables could not be coded reliably due to inconsistent reporting in the original studies. Future studies systematically investigating the influence of comorbid disorders will assist in identifying shared versus unique effects on neurobiology.
In conclusion, the present meta-analysis found an ES of .304 for WM volume reduction in AUDs. This effect was robust with regard to potential confounds such as sample size, age, and MRI methodology. Treatment-seeking status and duration of abstinence were significant moderators of the ES distribution, with group differences maximized in treatment-seeking populations and in early abstinence. Because women and non-treatment-seeking individuals have been underrepresented in neuroimaging research in AUDs, conclusions about the effect of AUDs on WM in these populations remain tenuous. Future studies would profit from examining moderators and mediators of the effects of treatment-seeking status and length of abstinence in larger, more representative samples and with methods such as DTI, which is sensitive to changes in WM integrity.
Supplementary Material
Acknowledgments
The authors would like to thank the researchers who graciously responded to requests for ES confirmation and additional information. Support for this project was provided by an NIAAA training grant (1T32AA01818-01A) to the Center on Alcoholism, Substance Abuse, and Addictions (CASAA), on which Ms. Monnig is a predoctoral fellow.
Footnotes
The authors report no potential conflicts of interest.
Author Contributions
MAM originated the research question and developed the study design in collaboration with JST, RAY, BSM, and RJT. MAM performed literature review, literature search, coding of studies, data entry, and manuscript preparation. RAY acted as second coder on a subset of studies. JST supplied statistical and methodical consultation and performed the statistical analyses. All authors edited and revised the manuscript.
References
- Agartz I, Brag S, Franck J, Hammarberg A, Okugawa G, Svinhufvud K, Bergman H. MR volumetry during acute alcohol withdrawal and abstinence: a descriptive study. Alcohol Alcohol. 2003;38:71–78. doi: 10.1093/alcalc/agg020. [DOI] [PubMed] [Google Scholar]
- Anstey KJ, Jorm AF, Réglade-Meslin C, Maller J, Kumar R, von Sanden C, Windsor TD, Rodgers B, Wen W, Sachdev P. Weekly alcohol consumption, brain atrophy, and white matter hyperintensities in a community-based sample aged 60 to 64 years. Psychosom Med. 2006;68:778–785. doi: 10.1097/01.psy.0000237779.56500.af. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry—the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Bartsch AJ, Homola G, Biller A, Smith SM, Weijers HG, Wiesbeck GA, Jenkinson M, De Stefano N, Solymosi L, Bendszus M. Manifestations of early brain recovery associated with abstinence from alcoholism. Brain. 2007;130:36–47. doi: 10.1093/brain/awl303. [DOI] [PubMed] [Google Scholar]
- Berman S, O'Neill J, Fears S, Bartzokis G, London ED. Abuse of amphetamines and structural abnormalities in the brain. Ann N Y Acad Sci. 2008;1141:195–220. doi: 10.1196/annals.1441.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady KT, Randall CL. Gender differences in substance use disorders. Psychiatr Clin North Am. 1999;22:241–252. doi: 10.1016/s0193-953x(05)70074-5. [DOI] [PubMed] [Google Scholar]
- Bühler M, Mann K. Alcohol and the human brain: a systematic review of different neuroimaging methods. Alcohol Clin Exp Res. 2011;35:1771–1793. doi: 10.1111/j.1530-0277.2011.01540.x. [DOI] [PubMed] [Google Scholar]
- Cardenas VA, Durazzo TC, Gazdzinski S, Mon A, Studholme C, Meyerhoff DJ. Brain morphology at entry into treatment for alcohol dependence is related to relapse propensity. Biol Psychiatry. 2011;70:561–567. doi: 10.1016/j.biopsych.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Cardenas VA, Studholme C, Meyerhoff DJ, Song E, Weiner MW. Chronic active heavy drinking and family history of problem drinking modulate regional brain tissue volumes. Psychiatry Res. 2005;138:115–130. doi: 10.1016/j.pscychresns.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Chanraud S, Martelli C, Delain F, Kostogianni N, Douaud G, Aubin HJ, Reynaud M, Martinot JL. Brain morphometry and cognitive performance in detoxified alcohol-dependents with preserved psychosocial functioning. Neuropsychopharmacology. 2007;32:429–438. doi: 10.1038/sj.npp.1301219. [DOI] [PubMed] [Google Scholar]
- *.Chen CH, Walker J, Momenan R, Rawlings R, Heilig M, Hommer DW. Relationship Between Liver Function and Brain Shrinkage in Patients with Alcohol Dependence. Alcohol Clin Exp Res. 2011 doi: 10.1111/j.1530-0277.2011.01662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen E, Feinn R, Arias A, Kranzler HR. Alcohol treatment utilization: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug Alcohol Depend. 2007;86:214–221. doi: 10.1016/j.drugalcdep.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Crews FT, Nixon K. Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol Alcohol. 2009;44:115–127. doi: 10.1093/alcalc/agn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.De Bellis MD, Clark DB, Beers SR, Soloff PH, Boring AM, Hall J, Kersh A, Keshavan MS. Hippocampal volume in adolescent-onset alcohol use disorders. Am J Psychiatry. 2000;157:737–744. doi: 10.1176/appi.ajp.157.5.737. [DOI] [PubMed] [Google Scholar]
- *.Demirakca T, Ende G, Kämmerer N, Welzel-Marquez H, Hermann D, Heinz A, Mann K. Effects of alcoholism and continued abstinence on brain volumes in both genders. Alcohol Clin Exp Res. 2011;35:1678–1685. doi: 10.1111/j.1530-0277.2011.01514.x. [DOI] [PubMed] [Google Scholar]
- *.Di Sclafani V, Ezekiel F, Meyerhoff DJ, MacKay S, Dillon WP, Weiner MW, Fein G. Brain atrophy and cognitive function in older abstinent alcoholic men. Alcohol Clin Exp Res. 1995;19:1121–1126. doi: 10.1111/j.1530-0277.1995.tb01589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Cardenas VA, Studholme C, Weiner MW, Meyerhoff DJ. Non-treatment-seeking heavy drinkers: effects of chronic cigarette smoking on brain structure. Drug Alcohol Depend. 2007;87:76–82. doi: 10.1016/j.drugalcdep.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Gazdzinski S, Yeh PH, Meyerhoff DJ. Combined neuroimaging, neurocognitive and psychiatric factors to predict alcohol consumption following treatment for alcohol dependence. Alcohol Alcohol. 2008;43:683–691. doi: 10.1093/alcalc/agn078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Fein G, Di Sclafani V, Cardenas VA, Goldmann H, Tolou-Shams M, Meyerhoff DJ. Cortical gray matter loss in treatment-naive alcohol dependent individuals. Alcohol Clin Exp Res. 2002;26:558–564. [PMC free article] [PubMed] [Google Scholar]
- Fein G, Landman B. Treated and treatment-naive alcoholics come from different populations. Alcohol. 2005;36:19–26. [PMC free article] [PubMed] [Google Scholar]
- Fein G, Shimotsu R, Barakos J. Age-related gray matter shrinkage in a treatment naïve actively drinking alcohol-dependent sample. Alcohol Clin Exp Res. 2010;34:175–182. doi: 10.1111/j.1530-0277.2009.01079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Fein G, Shimotsu R, Di Sclafani V, Barakos J, Harper C. Increased white matter signal hyperintensities in long-term abstinent alcoholics compared with nonalcoholic controls. Alcohol Clin Exp Res. 2009;33:70–78. doi: 10.1111/j.1530-0277.2008.00812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filley C. The Behavioral Neurology of White Matter. New York: Oxford University Press; 2001. [Google Scholar]
- Filley CM. White matter: organization and functional relevance. Neuropsychol Rev. 2010;20:158–173. doi: 10.1007/s11065-010-9127-9. [DOI] [PubMed] [Google Scholar]
- Friedman L, Cerny C, Wiechers I, Jellema L, Latimer B, Schulz S, Buckley P. Prediction of intracranial volume from post-mortem head, stature and body frame measurements: Influences of gender, race, and implications for schizophrenia research. Schizophrenia Research. 1997;24:145. [Google Scholar]
- *.Gazdzinski S, Durazzo TC, Mon A, Yeh PH, Meyerhoff DJ. Cerebral white matter recovery in abstinent alcoholics--a multimodality magnetic resonance study. Brain. 2010;133:1043–1053. doi: 10.1093/brain/awp343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Gazdzinski S, Durazzo TC, Studholme C, Song E, Banys P, Meyerhoff DJ. Quantitative brain MRI in alcohol dependence: preliminary evidence for effects of concurrent chronic cigarette smoking on regional brain volumes. Alcohol Clin Exp Res. 2005;29:1484–1495. doi: 10.1097/01.alc.0000175018.72488.61. [DOI] [PubMed] [Google Scholar]
- Gazdzinski S, Durazzo TC, Weiner MW, Meyerhoff DJ. Are treated alcoholics representative of the entire population with alcohol use disorders? A magnetic resonance study of brain injury. Alcohol. 2008;42:67–76. doi: 10.1016/j.alcohol.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Y, Grossman RI, Babb JS, Rabin ML, Mannon LJ, Kolson DL. Age-related total gray matter and white matter changes in normal adult brain. Part I: volumetric MR imaging analysis. AJNR Am J Neuroradiol. 2002;23:1327–1333. [PMC free article] [PubMed] [Google Scholar]
- Gilman JM, Bjork JM, Hommer DW. Parental alcohol use and brain volumes in early- and late-onset alcoholics. Biol Psychiatry. 2007;62:607–615. doi: 10.1016/j.biopsych.2006.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007;64:830–842. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- Hedges L. Distribution theory for Glass's estimator of effect size and related estimators. Journal of Educational Statistics. 1981;6:107–128. [Google Scholar]
- Hommer D. Male and female sensitivity to alcohol-induced brain damage. Alcohol Res Health. 2003;27:181–185. [PMC free article] [PubMed] [Google Scholar]
- Jacob T, Seilhamer RA, Bargeil K, Howell DN. Reliability of Lifetime Drinking History among alcohol dependent men. Psychol Addict Behav. 2006;20:333–337. doi: 10.1037/0893-164X.20.3.333. [DOI] [PubMed] [Google Scholar]
- Jacobson R. The contributions of sex and drinking history to the CT brain scan changes in alcoholics. Psychol Med. 1986;16:547–559. doi: 10.1017/s003329170001031x. [DOI] [PubMed] [Google Scholar]
- *.Jang DP, Namkoong K, Kim JJ, Park S, Kim IY, Kim SI, Kim YB, Cho ZH, Lee E. The relationship between brain morphometry and neuropsychological performance in alcohol dependence. Neurosci Lett. 2007;428:21–26. doi: 10.1016/j.neulet.2007.09.047. [DOI] [PubMed] [Google Scholar]
- Koenig LB, Jacob T, Haber JR. Validity of the lifetime drinking history: a comparison of retrospective and prospective quantity-frequency measures. J Stud Alcohol Drugs. 2009;70:296–303. doi: 10.15288/jsad.2009.70.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kril JJ, Halliday GM. Brain shrinkage in alcoholics: a decade on and what have we learned? Prog Neurobiol. 1999;58:381–387. doi: 10.1016/s0301-0082(98)00091-4. [DOI] [PubMed] [Google Scholar]
- Kril JJ, Halliday GM, Svoboda MD, Cartwright H. The cerebral cortex is damaged in chronic alcoholics. Neuroscience. 1997;79:983–998. doi: 10.1016/s0306-4522(97)00083-3. [DOI] [PubMed] [Google Scholar]
- Laakso MP, Gunning-Dixon F, Vaurio O, Repo-Tiihonen E, Soininen H, Tiihonen J. Prefrontal volumes in habitually violent subjects with antisocial personality disorder and type 2 alcoholism. Psychiatry Res. 2002;114:95–102. doi: 10.1016/s0925-4927(02)00005-7. [DOI] [PubMed] [Google Scholar]
- *.Lee E, Jang DP, Kim JJ, An SK, Park S, Kim IY, Kim SI, Yoon KJ, Namkoong K. Alteration of brain metabolites in young alcoholics without structural changes. Neuroreport. 2007;18:1511–1514. doi: 10.1097/WNR.0b013e3282ef7625. [DOI] [PubMed] [Google Scholar]
- Lorenzetti V, Lubman DI, Whittle S, Solowij N, Yücel M. Structural MRI findings in long-term cannabis users: what do we know? Subst Use Misuse. 2010;45:1787–1808. doi: 10.3109/10826084.2010.482443. [DOI] [PubMed] [Google Scholar]
- *.Makris N, Oscar-Berman M, Jaffin SK, Hodge SM, Kennedy DN, Caviness VS, Marinkovic K, Breiter HC, Gasic GP, Harris GJ. Decreased volume of the brain reward system in alcoholism. Biol Psychiatry. 2008;64:192–202. doi: 10.1016/j.biopsych.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann K, Ackermann K, Croissant B, Mundle G, Nakovics H, Diehl A. Neuroimaging of gender differences in alcohol dependence: are women more vulnerable? Alcohol Clin Exp Res. 2005;29:896–901. doi: 10.1097/01.alc.0000164376.69978.6b. [DOI] [PubMed] [Google Scholar]
- Mann K, Batra A, Günthner A, Schroth G. Do women develop alcoholic brain damage more readily than men? Alcohol Clin Exp Res. 1992;16:1052–1056. doi: 10.1111/j.1530-0277.1992.tb00698.x. [DOI] [PubMed] [Google Scholar]
- Matsumae M, Kikinis R, Mórocz IA, Lorenzo AV, Sándor T, Albert MS, Black PM, Jolesz FA. Age-related changes in intracranial compartment volumes in normal adults assessed by magnetic resonance imaging. J Neurosurg. 1996;84:982–991. doi: 10.3171/jns.1996.84.6.0982. [DOI] [PubMed] [Google Scholar]
- *.Mechtcheriakov S, Brenneis C, Egger K, Koppelstaetter F, Schocke M, Marksteiner J. A widespread distinct pattern of cerebral atrophy in patients with alcohol addiction revealed by voxel-based morphometry. J Neurol Neurosurg Psychiatry. 2007;78:610–614. doi: 10.1136/jnnp.2006.095869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Nagel BJ, Park A, McQueeny T, Tapert SF. Depressive symptoms in adolescents: associations with white matter volume and marijuana use. J Child Psychol Psychiatry. 2007;48:592–600. doi: 10.1111/j.1469-7610.2007.01728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley ME, Liu C, Rodriguez S, Brosnan T. Advances in Magnetic Resonance Neuroimaging. Neurol Clin. 2009;27:1–xiii. doi: 10.1016/j.ncl.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Oehring EM, Schulte T, Fama R, Pfefferbaum A, Sullivan EV. Global-local interference is related to callosal compromise in alcoholism: a behavior-DTI association study. Alcohol Clin Exp Res. 2009;33:477–489. doi: 10.1111/j.1530-0277.2008.00858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Nagel BJ, Schweinsburg AD, Phan V, Tapert SF. Reduced hippocampal volume among adolescents with alcohol use disorders without psychiatric comorbidity. Psychiatry Res. 2005;139:181–190. doi: 10.1016/j.pscychresns.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.O'Neill J, Cardenas VA, Meyerhoff DJ. Separate and interactive effects of cocaine and alcohol dependence on brain structures and metabolites: quantitative MRI and proton MR spectroscopic imaging. Addict Biol. 2001;6:347–361. doi: 10.1080/13556210020077073. [DOI] [PubMed] [Google Scholar]
- Obernier JA, Bouldin TW, Crews FT. Binge ethanol exposure in adult rats causes necrotic cell death. Alcohol Clin Exp Res. 2002;26:547–557. [PubMed] [Google Scholar]
- Oscar-Berman M, Marinkovic K. Alcohol: effects on neurobehavioral functions and the brain. Neuropsychol Rev. 2007;17:239–257. doi: 10.1007/s11065-007-9038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BS, Weissman MM. A brain-based endophenotype for major depressive disorder. Annual Review of Medicine. 2011;62 doi: 10.1146/annurev-med-010510-095632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV. Dysmorphology and microstructural degradation of the corpus callosum: Interaction of age and alcoholism. Neurobiol Aging. 2006;27:994–1009. doi: 10.1016/j.neurobiolaging.2005.05.007. [DOI] [PubMed] [Google Scholar]
- *.Pfefferbaum A, Lim KO, Zipursky RB, Mathalon DH, Rosenbloom MJ, Lane B, Ha CN, Sullivan EV. Brain gray and white matter volume loss accelerates with aging in chronic alcoholics: a quantitative MRI study. Alcohol Clin Exp Res. 1992;16:1078–1089. doi: 10.1111/j.1530-0277.1992.tb00702.x. [DOI] [PubMed] [Google Scholar]
- *.Pfefferbaum A, Rosenbloom M, Deshmukh A, Sullivan E. Sex differences in the effects of alcohol on brain structure. Am J Psychiatry. 2001;158:188–197. doi: 10.1176/appi.ajp.158.2.188. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom MJ, Serventi KL, Sullivan EV. Brain volumes, RBC status, and hepatic function in alcoholics after 1 and 4 weeks of sobriety: predictors of outcome. Am J Psychiatry. 2004;161:1190–1196. doi: 10.1176/appi.ajp.161.7.1190. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, Shear PK, Rosenbloom MJ, Lim KO. Longitudinal changes in magnetic resonance imaging brain volumes in abstinent and relapsed alcoholics. Alcohol Clin Exp Res. 1995;19:1177–1191. doi: 10.1111/j.1530-0277.1995.tb01598.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Rosenbloom MJ, Mathalon DH, Lim KO. A controlled study of cortical gray matter and ventricular changes in alcoholic men over a 5-year interval. Arch Gen Psychiatry. 1998;55:905–912. doi: 10.1001/archpsyc.55.10.905. [DOI] [PubMed] [Google Scholar]
- Randall CL, Roberts JS, Del Boca FK, Carroll KM, Connors GJ, Mattson ME. Telescoping of landmark events associated with drinking: A gender comparison. Journal of Studies on Alcohol. 1999;60:252–260. doi: 10.15288/jsa.1999.60.252. [DOI] [PubMed] [Google Scholar]
- Raudenbush S, Bryk A. Hierarchical Linear Models: Applications and Data Analysis Methods. 2nd ed. Thousand Oaks, CA: Sage Publications; 2002. [Google Scholar]
- Raudenbush S, Bryk T, Congdon R. HLM 6 Hierarchical Linear and Nonlinear Modeling. Scientific Software International, Inc.; 2000. [Google Scholar]
- *.Rohlfing T, Sullivan EV, Pfefferbaum A. Deformation-based brain morphometry to track the course of alcoholism: differences between intra-subject and inter-subject analysis. Psychiatry Res. 2006;146:157–170. doi: 10.1016/j.pscychresns.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Sasaki H, Abe O, Yamasue H, Fukuda R, Yamada H, Takei K, Suga M, Takao H, Kasai K, Aoki S, Ohtomo K. Structural and diffusional brain abnormality related to relatively low level alcohol consumption. Neuroimage. 2009;46:505–510. doi: 10.1016/j.neuroimage.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Saunders JB, Davis M, Williams R. Do women develop alcoholic liver disease more readily than men? Br Med J (Clin Res Ed) 1981;282:1140–1143. doi: 10.1136/bmj.282.6270.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shear PK, Jernigan TL, Butters N. Volumetric magnetic resonance imaging quantification of longitudinal brain changes in abstinent alcoholics. Alcohol Clin Exp Res. 1994;18:172–176. doi: 10.1111/j.1530-0277.1994.tb00899.x. [DOI] [PubMed] [Google Scholar]
- Smith S, Fein G. Cognitive performance in treatment-naïve active alcoholics. Alcohol Clin Exp Res. 2010;34:2097–2105. doi: 10.1111/j.1530-0277.2010.01306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorg SF, Taylor MJ, Alhassoon OM, Gongvatana A, Theilmann RJ, Frank LR, Grant I. Frontal White Matter Integrity Predictors of Adult Alcohol Treatment Outcome. Biol Psychiatry. 2011 doi: 10.1016/j.biopsych.2011.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV. Services DoHaH. Bethesda, MD: 2000. Human brain vulnerability to alcoholism: Evidence from neuroimaging studies; pp. 473–508. [Google Scholar]
- Sullivan EV, Pfefferbaum A. Neurocircuitry in alcoholism: a substrate of disruption and repair. Psychopharmacology (Berl) 2005;180:583–594. doi: 10.1007/s00213-005-2267-6. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rohlfing T, Pfefferbaum A. Pontocerebellar volume deficits and ataxia in alcoholic men and women: no evidence for “telescoping”. Psychopharmacology (Berl) 2010;208:279–290. doi: 10.1007/s00213-009-1729-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Lim KO, Pfefferbaum A. Longitudinal changes in cognition, gait, and balance in abstinent and relapsed alcoholic men: relationships to changes in brain structure. Neuropsychology. 2000;14:178–188. [PubMed] [Google Scholar]
- *.Symonds LL, Archibald SL, Grant I, Zisook S, Jernigan TL. Does an increase in sulcal or ventricular fluid predict where brain tissue is lost? J Neuroimaging. 1999;9:201–209. doi: 10.1111/jon199994201. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, Simon SL, Geaga JA, Hong MS, Sui Y, Lee JY, Toga AW, Ling W, London ED. Structural abnormalities in the brains of human subjects who use methamphetamine. J Neurosci. 2004;24:6028–6036. doi: 10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Tol MJ, van der Wee NJ, van den Heuvel OA, Nielen MM, Demenescu LR, Aleman A, Renken R, van Buchem MA, Zitman FG, Veltman DJ. Regional brain volume in depression and anxiety disorders. Arch Gen Psychiatry. 2010;67:1002–1011. doi: 10.1001/archgenpsychiatry.2010.121. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Reinvang I, Lundervold A, Dale AM, Eilertsen DE, Quinn BT, Salat D, Makris N, Fischl B. Effects of age on volumes of cortex, white matter and subcortical structures. Neurobiol Aging. 2005;26:1261–1270. doi: 10.1016/j.neurobiolaging.2005.05.020. discussion 1275-1268. [DOI] [PubMed] [Google Scholar]
- Zahr NM, Mayer D, Rohlfing T, Hasak MP, Hsu O, Vinco S, Orduna J, Luong R, Sullivan EV, Pfefferbaum A. Brain injury and recovery following binge ethanol: evidence from in vivo magnetic resonance spectroscopy. Biol Psychiatry. 2010;67:846–854. doi: 10.1016/j.biopsych.2009.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001;20:45–57. doi: 10.1109/42.906424. *Study included in meta-analysis.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.