Abstract
Background
Both sensory and cognitive deficits have been associated with prenatal exposure to alcohol; however, very few studies have focused on sensory deficits in preschool aged children. Since sensory skills develop early, characterization of sensory deficits using novel imaging methods may reveal important neural markers of prenatal alcohol exposure.
Materials and Methods
Participants in this study were 10 children with a fetal alcohol spectrum disorder (FASD) and 15 healthy control children aged 3-6 years. All participants had normal hearing as determined by clinical screens. We measured their neurophysiological responses to auditory stimuli (1000 Hz, 72 dB tone) using magnetoencephalography (MEG). We used a multi-dipole spatio-temporal modeling technique (CSST – Ranken et al. 2002) to identify the location and timecourse of cortical activity in response to the auditory tones. The timing and amplitude of the left and right superior temporal gyrus sources associated with activation of left and right primary/secondary auditory cortices were compared across groups.
Results
There was a significant delay in M100 and M200 latencies for the FASD children relative to the HC children (p = 0.01), when including age as a covariate. The within-subjects effect of hemisphere was not significant. A comparable delay in M100 and M200 latencies was observed in children across the FASD subtypes.
Discussion
Auditory delay revealed by MEG in children with FASD may prove to be a useful neural marker of information processing difficulties in young children with prenatal alcohol exposure. The fact that delayed auditory responses were observed across the FASD spectrum suggests that it may be a sensitive measure of alcohol-induced brain damage. Therefore, this measure in conjunction with other clinical tools may prove useful for early identification of alcohol affected children, particularly those without dysmorphia.
Introduction
The teratogenic effects of alcohol on the developing fetus were first described in the literature approximately four decades ago (Lemoine et al., 1968). Jones and Smith (1973) coined the term fetal alcohol syndrome (FAS) to denote a pattern of birth defects and developmental delays observed in children born to alcoholic mothers. It is now recognized that alcohol-exposed children without physical dysmorphia also exhibit cognitive deficits related to prenatal alcohol exposure (Mattson et al., 1997) and only a small proportion of children show the dysmorphic features of FAS. Although not a diagnostic term, fetal alcohol spectrum disorder (FASD) is now used to describe this broader spectrum of physical and behavioral outcomes. Streissguth and colleagues (2004) further determined that these functional disabilities persist into adulthood, yet children who received early intervention had better life outcomes than those who did not. Due to co-morbid risk factors (e.g. poverty, malnutrition, abuse) also negatively impacting development (Farah et al., 2006), identification of a unique pattern of cognitive and behavioral problems is challenging. Therefore, the development and validation of tools for early identification of children affected by prenatal alcohol exposure without dysmorphia at a young age is of considerable clinical significance.
Over the last two decades there has been increased use of structural and functional methods to characterize alcohol-induced brain damage. Wide-ranging changes in brain structure associated with alcohol-induced cognitive deficits and behavioral difficulties have been identified through both animal and human research [for review see (Lebel et al., 2011; Schneider et al., 2011)]. Animal studies have confirmed both gross microcephaly and more subtle changes, such as decreases in cell count and abnormal connectivity in sensory cortex and other cortical and subcortical regions, resulting from a wide range of alcohol exposures [e.g. (Goodlett et al., 1990; Miller, 2006; Parnell et al., 2006; Oladehin et al., 2007)]. While microcephaly has been recognized as one of the characteristics of children with FAS (Jones and Smith, 1973), deficits in specific brain areas have also been identified in children and adults with FASD without gross microcephaly [e.g. hippocampus, cerebellum, corpus callosum (Riley et al., 1995; Sowell et al., 2001; Bookstein et al., 2006)].
Furthermore, functional deficits have been identified both in the presence and absence of anatomical differences [e.g. (Pettigrew and Hutchinson, 1984; Mattson et al., 1997)]. To search for functional biomarkers of FASD at the youngest age, one must turn to pre-linguistic functions, such as sensory-motor skills, since such functions rapidly develop in the first few years of life. While it is known that sensory deficits extend into adulthood in individuals with FASD (Streissguth et al., 1998) relatively little is known about the nature and extent of these problems in young children. A limited body of animal and human literature shows that basic sensory functions, including deficits in the auditory pathway, are affected by prenatal alcohol exposure (Pettigrew and Hutchinson, 1984; Church, 1987; Church and Gerkin, 1988; Rossig et al., 1994; Kaneko et al., 1996; Church et al., 1997; Slawecki et al., 2004; Medina et al., 2005; Oladehin et al., 2007). However, the human work has focused either on infants who have not yet been identified as having an FASD or on older children who may present different symptomatology than young children (Mattson et al, 1998). In addition to these physiological studies, Franklin et al. (2008) found that children with FASD were rated by their caregivers as having increased sensory and behavioral problems. However, no systematic study of sensory processing using objective measures in preschool aged children with FASD has been reported.
One noninvasive functional neuroimaging method suitable for studying young children is magnetoencephalography (MEG), which has not previously been used to assess neural functioning in children with FASD. The merits of MEG include excellent temporal resolution with millisecond precision (similar to EEG) and good spatial resolution without any distortion of spatial information from the skull. EEG signals, on the other hand, are distorted by the skull (Flemming et al., 2005). Therefore, one can more precisely infer the location and timing of possible electrophysiological deficits associated with FASD while employing simpler head models during MEG source modeling. Conversely, functional MRI measures changes in deoxyhemoglobin associated with changes in blood flow in response to neuronal activity while PET tracks radiotracers carried by the blood to monitor glucose uptake in response to neuronal activity. However, because blood flow changes are sluggish, the temporal resolution is orders of magnitude slower for fMRI and PET (1-10s sec) compared to MEG/EEG (~1 ms) and limits the ability of these techniques to identify subtle deficits in cortical processing speed.
The goal of this study was to characterize auditory responses in preschool aged children with FASD. Based on the results described above showing delays in auditory processing in infants and children prenatally exposed to alcohol, we hypothesized that children with FASD would exhibit delayed auditory responses and/or reduced amplitude of these responses relative to typically developing children.
Methods
Participants
We recruited parents/guardians of 19 healthy control (HC) children and 12 children identified as meeting criteria for fetal alcohol spectrum disorder (FASD) within the age range of 31-69 months. While the overall success rate was 96% for the MEG task, instrument error resulted in poor data in some children who succeeded in the task. Therefore, we describe only the good quality MEG data, collected from 15 HC and 10 children with FASD. This study was approved by the University of New Mexico Health Sciences Center Institutional Review Board and is in full compliance with the Declaration of Helsinki. The parent was fully informed of the study procedures prior to full participant recruitment and consented to the study prior to research procedures.
The children identified with FASD were recruited through the Fetal Alcohol Diagnostic Clinic at the Center for Development and Disability of the University of New Mexico Health Sciences Center. Children were diagnosed by a team of clinicians including a developmental pediatrician, clinical neuropsychologist, and a child clinical psychologist with extensive experience in the diagnosis and evaluation of children with FASD. Participants were classified as having fetal alcohol syndrome, partial fetal alcohol syndrome, or alcohol related neurodevelopmental disorder (ARND) using the Institute of Medicine Criteria (Stratton et al., 1996). While all participants in the FASD group had confirmed prenatal alcohol exposure, three of them met criteria for fetal alcohol syndrome, four for partial fetal alcohol syndrome, and three for ARND. Maternal alcohol consumption was confirmed directly (maternal interview) or through 1. Multiple eyewitness reports of maternal drinking during pregnancy or 2. Legal records confirming alcohol consumption during pregnancy (e.g., DWI arrest). While none of the participants had hearing problems as determined by state-mandated newborn screening (otoacoustic emissions test) and follow-up screens based on any concerns with hearing, three of them had their vision corrected with glasses. Four of the children with FASD were taking medication unrelated to the FASD diagnosis (2 Albuterol, 1 Keppra for previous seizures, and 1 on Prevacid/Zyrtec).
The healthy control group was recruited from the community by word of mouth, through posted flyers/recruitment brochures or from previous studies, when the parents had agreed to be contacted for future studies. The HC group was screened using a brief questionnaire and did not have developmental or neurological disorders or prenatal exposure to alcohol or other substances.
MEG Data collection
The children were scheduled for up to two MEG sessions and up to three MRI scan sessions, depending on success of each session. The MEG session was scheduled during the day at a time when the child was expected to be alert and well rested. The general MEG procedures are similar to those reported previously (Stephen et al., 2002; Stephen et al., 2006a; Hansen et al., 2010). Bipolar electrocardiogram (ECG) and electrooculogram (EOG) recordings were obtained in conjunction with the MEG to allow offline artifact removal. The MEG head position indicator coils (HPI) were taped to a child-sized cloth cap that could be form-fitted to each child’s head. This reduced the need to place tape in the hair of the children and eased participant preparation. The cloth cap was taped to the available skin surfaces to ensure that the cap did not move relative to the child’s head.
HPI coils were registered relative to standard fiducial points (left and right preauricular and nasion) using the Polhemus 3D position tracking device. Additional head shape points were collected to facilitate MEG/MRI coregistration.
MEG data collection was performed using the Elekta Neuromag 306-channel whole-head biomagneter. Once preparation was complete, the child laid in a supine position on the Elekta/Neuromag MEG system bed. The MEG system is located within a two-layer magnetically shielded room (Vacuumschmelze, Germany). The child’s head position within the helmet was optimized, and padding was used to reduce the potential for major head movements. Care was taken to ensure that the placement of the cloth cap did not move relative to the child’s head. During the sensory task, the children were allowed to watch a silent children’s movie. This was projected onto a rear-projection screen that was positioned above the child to allow for viewing from the supine position. An investigator (and a parent, if needed) remained in the shielded room with the child during data collection to ensure that the child was comfortable and complied with the task. Everyone who remained in the shielded room during data collection was required to remove all metal and electronic devices to reduce magnetic noise. Metal-free clothes were provided as needed (hospital scrubs for adults/child-size t-shirt for children). The adult(s) sat quietly during the measurement and provided words of encouragement to the child, if needed. The participants were not sedated or restrained in any way.
Stimuli
The auditory stimuli were presented as part of a larger sensory paradigm. Due to the complexity of performing source analysis on child data, we report herein the results obtained for the frequent tones presented during a P300-style auditory task. The frequent, 1000 Hz tones were presented at 72 dB measured at the helmet. Rare tones (1200 Hz; 72dB) were presented at a frequency of 16%. The duration of the stimuli was 50 ms (5 ms ramp at beginning and end) and the interstimulus interval was 1 ± 0.2 s. The tones were presented with speakers located in the room positioned to the left and right of the child. All auditory stimuli were presented binaurally and simultaneously from the left and right speakers. Approximately 600 trials were collected for the frequent tone condition. Intermixed with the auditory stimuli were tactile and simultaneous auditory/tactile conditions. The task took approximately 15 minutes to complete.
After the child was positioned within the helmet, the HPI coils were activated to determine the child’s head position relative to the sensor array. The MEG data were collected using the Elekta Neuromag 306-channel biomagnetometer. The continuous HPI option was employed to monitor head movement during the scan. The Elekta software then corrects for head movement offline. Wehner and colleagues (2008) demonstrated that the head movement correction is effective in child studies.
MRI Data collection
We obtained structural MRIs using a 3T Siemens Triotim MRI system to allow us to map the MEG activity onto the anatomical locations provided by the T1-weighted MRI sequence. The MRI and MEG data were collected during separate visits. Most children were requested to return for a night scan to allow MRI scanning to occur during sleep and minimize movement during the scan. Six children who had difficulty sleeping and were compliant during the MEG were successfully scanned while awake. These children were allowed to watch a movie during the MRI scanning procedures. The room attendant and MRI technician monitored motion to re-run scan sequences as needed. The child was accompanied by an adult at all times, and a trained room attendant was positioned next to the child during the MRI scan. The child was provided with double ear protection (MRI headphones and ear inserts) to reduce the risk of hearing loss and waking the child. Sagittal T1-weighted anatomical images were obtained with a multi-echo 3D MPRAGE sequence [TR/TE/TI = 2530/1.64, 3.5, 5.36, 7.22, 9.08/1200 ms, flip angle = 7°, field of view (FOV) = 256 × 256 mm, matrix = 256 × 256, 1 mm thick slice, 192 slices, GRAPPA acceleration factor = 2].
Data analysis
The MEG data were preprocessed using the temporal, signal space separation method available in the Elekta Maxfilter software (Taulu and Kajola, 2005) to correct head position and reduce noise from external sources. Data quality was assessed by viewing the continuous data offline. Heartbeat and auditory speaker artifact were projected out of the data using the Elekta signal space projection software. The stimuli were averaged by condition and source analysis was performed for the frequent auditory condition (described below).
The MEG data were registered to each participant’s MRI in most cases. In the event that we were not successful in obtaining an MRI, we used another child’s MRI based on matched head size and age. This is common for source analysis in studies where MRIs were not obtained (Dalal et al., 2011). An automatic fitting routine in MRIVIEW was used to co-register the MEG and MRI data after identification of the fiducial points (right and left preauricular and nasion) on the MRI (Ranken et al., 2002). The MRI was then segmented and the cortical volume identified using automated algorithms in MRIVIEW. A pixilated set of points based on the cortical volume was identified to provide starting locations for the dipole fitting procedure. Local, sensor-fitted multiple spheres (Huang et al., 1999) were used as the head model for calculating the forward solution. We used the cortical-start spatio-temporal (CSST) analysis approach for performing the source modeling of the MEG data. This approach has a number of advantages. First, while the initial starting locations originate from the cortical volume, the locations during the search procedure are not constrained. Second, the procedure allows for multi-dipole spatio-temporal modeling of the data. This is preferred to fitting single dipoles since under-modeling can impact the source timing obtained from the model (Supek and Aine, 1993). Third, there are no a priori assumptions about where the source activity originates. The starting locations for the minimization procedure are chosen randomly from the entire cortical volume. This minimizes bias introduced by using a priori assumptions about source locations for a particular task. Fourth, the minimization procedure is performed thousands of times with different random starting locations for each fit. This helps to ensure that a global minimum is reached. When consistent locations arise across the thousands of fits and across participants using this approach, one can more confidently assert the accuracy of the source locations for the task.
The CSST source modeling approach has been employed by our group and others over the last ten years (e.g. (Susac et al.; Stephen et al., 2003b; Stephen et al., 2007; Stephen et al., 2010)) and the methods have been described in detail previously. Briefly, random starting locations are chosen from the pixilated set of points identified within the cortical volume. A preprocessing step helps simplify the number of parameters to be resolved by identifying dipolar activity using a MUSIC approach for a subset of the sources (for details see Ranken et al. 2002). This reduced complexity data set is submitted to a fully unconstrained Nelder-Mead minimization procedure. The Nelder-Mead procedure has been shown to reduce the likelihood that the minimization procedure will be caught in local minima. Once this procedure is performed for the specified number of fits (2500-5000 fits depending on the number of dipoles being modeled), the best fits (based on a reduced-chi square fitting criterion and 90th percentile in the chi-square ranking or greater) are then subjected to a finer-grained Nelder-Mead minimization procedure. This two-step process reduces the overall analysis time by focusing the fine-grain search on the best fits. Once this fine grain search is complete, the best ten solutions are displayed. As is necessary with dipole modeling, multiple dipole models are tested for each condition for each participant. The best dipole model is chosen based on the criteria identified by Supek and Aine (2003).
Once the best dipole model was identified for the frequent auditory condition, the sources common across subjects were identified. The timecourses obtained from the dipole model were compiled within source across subjects and statistical analysis was performed on the latency and amplitude of the prominent peak(s) identified in the source timecourses. Timecourses for sources located in primary auditory cortex are reported in this manuscript (see Fig. 1)
Fig. 1.

Example results of the CSST analysis for a 60 month old HC child. The example source locations are shown in both the 3-dimensional view as well as the coronal slice (radiologic convention) showing good registration to left and right superior temporal gyrus consistent with primary/secondary auditory cortex. The timecourses (‘Dipole’ plot) of the left and right STG sources are shown in blue and red, respectively. The original waveforms correspond to the ‘Measured’ plot and the waveforms generated by the best model for this condition and subject correspond to the ‘Forward’ plot. The ‘Error’ plot is the difference between the ‘Measured’ and ‘Forward’ and shows that the model accounted for the visible response.
Signal to noise ratio (SNR) was obtained from the averaged auditory waveforms for each participant. The reported signal to noise ratio is the maximum SNR across channels obtained by identifying the maximum signal in the response time window (20-350 ms) and dividing by the standard deviation of the baseline (−100 – 0 ms) noise for each channel.
Statistical Analysis
Differences in latencies and amplitudes were tested using repeated measures ANOVA, each with age as a covariate, peak and hemisphere as the within subjects factors and diagnosis as the between subjects factor. A repeated measures ANOVA was performed to test whether the FASD diagnostic groups differed on the variables that were found significant in FASD vs. control comparisons.
Results
The demographic data for the participants are shown in Table 1. Although the FASD children appeared to be younger than the HC group on average, there was no significant difference in age between the groups. However, despite this lack of statistical significance, age was included in the statistical model due to the strong negative correlation (r = −0.35, p = 0.08) between age and auditory latencies at this young age. We were able to bring back 22/25 children for the MRI procedure. Of this group of 22, the MRI success rate was 92% for HC (12/13) and 78% for the FASD (7/9) children.
Table 1.
Participant Demographics of analyzed group
| HC (N = 15) | FASD (N = 10) | |
|---|---|---|
| Age | 52 (11.4) months | 48 (10.7) months |
| Gender | 9 girls/6 boys (60% girls) | 6 girls/4 boys (60% girls) |
| Race/Ethnicity | 13 Caucasian (6 Hispanic origin) 1 Asian / 1 Native American |
6 Caucasian (3 Hispanic origin) 3 Native American 1 Mixed race |
| FASD Subcategories | 3 – ARND/PAE, 4 pFAS, 3 – FAS |
Based on head position tracking, we found that most children were able to maintain a consistent head position in the supine position. In particular, we consistently identified brief intervals of movement (position adjustments) with a close return to the original position. We did not identify any relationship between age or diagnosis and the child’s ability to remain still during the task. Examples of head movement across the 15-minute data collection are presented in supplementary Fig. 1.
The SNR and the number of trials for the two groups were equivalent, indicating that the reported results are not due to differences in data quality across the groups. The maximum SNR was 39 ± 4 (mean ± SEM) for HC and 33 ± 4 for children with an FASD with no significant difference between groups (t (22) = 1.0, p = 0.2). The number of trials was statistically equivalent between groups (t (22) = 0.33, p = 0.4) with an average of 603 ± 32 trials for HC and 588 ± 30 trials for children with an FASD. In addition, we consistently identified two prominent peaks in the auditory response (M100 and M200) in the source timecourses across subjects and groups (see Fig. 2). Although it is well known that peak latencies decrease with increasing age (Paetau et al., 1995), the changes from 3-6 years of age are modest enough to allow one to reliably identify the same peak across the age range.
Fig. 2.
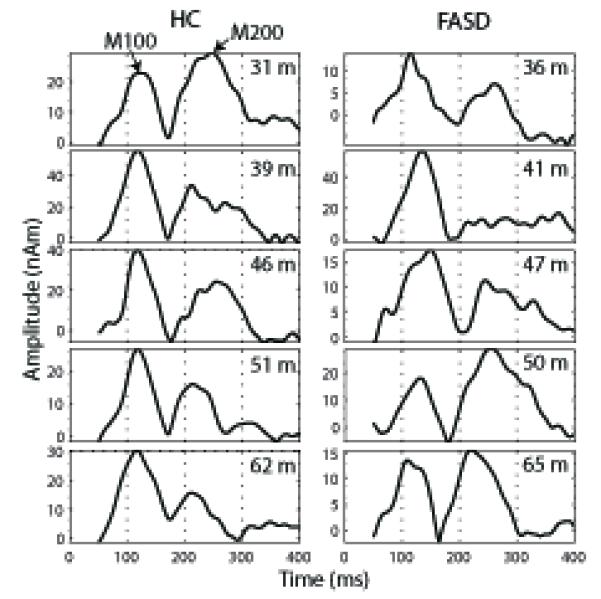
Example STG timecourses are shown across age (in months) for HC children and children with FASD. The M100 and M200 peaks are labeled (see arrows) in one example timecourse and are clearly visible across timecourses. The duration of the timecourses corresponds to the time window chosen for source analysis (50-400 ms).
The 2 × 2 × 2 RM-ANOVA for latency with peak and hemisphere as within subjects factors and diagnosis as the between subjects factor showed a significant between-subjects effect of diagnosis, with M100 and M200 latencies delayed for the FASD children relative to the HC (Table 2; F (1,19) = 8.1, p = 0.01). As expected, there was a significant within-subjects’ effect of peak latency between the M100 and M200 peaks (Table 2; F (1,19) = 53.4 p < 0.001). There were no significant interactions between peak, diagnosis or hemisphere. The average timecourses from the right superior temporal gyrus (STG) source are shown in Fig. 3 (left STG showed a similar pattern). The individual M100 STG peak latencies are shown in Fig. 4. The RM ANOVA comparing FASD subcategories showed no significant difference in latency and no trend of increasing latency with increasing disease severity (e.g. mean M100 latency ARND – 124 ms, pFAS – 129 ms, FAS – 123 ms) based on subcategory (F (2,5) = 0.69, p = 0.54). There were also no significant effects of medication on peak latencies within the FASD group (p > 0.05). There were no significant amplitude differences for either the M100 or M200 peak.
Table 2.
Mean (SEM) latencies and amplitudes for the M100 and M200 peaks
| M100 Latency | M200 Latency | M100 Amplitude | M200 Amplitude | |
|---|---|---|---|---|
| HC (N = 15) | 114.4 (2.8) | 234.9 (4.3) | 35.1 (6.6) | 29.9 (6.7) |
| FASD (N = 10) | 125.0 (3.4) | 247.8 (4.9) | 30.1 (7.9) | 17.5 (7.6) |
Fig. 3.
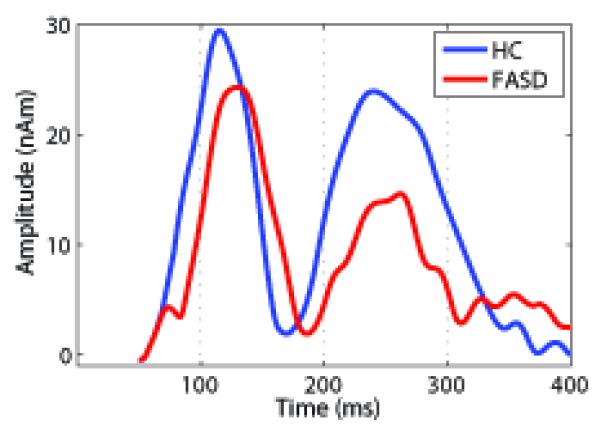
The group-averaged timecourses for the right STG source are shown to demonstrate the difference in latency observed for the M100 and M200 peaks. There were no significant differences in amplitude for the M100 or M200 peak. One can see a clear M100 and M200 peak for both groups as well as the delay in the peak latencies for the FASD relative to the HC children.
Fig. 4.
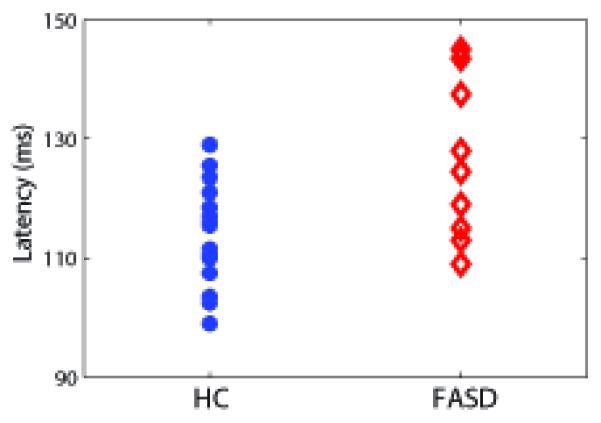
The M100 latencies averaged across left and right STG sources (no within-subjects effect of hemisphere). This plot denotes the difference in average STG latencies between HC and FASD children with an average 11 ms delay for the FASD group relative to HC.
Discussion
Consistent with our hypothesis, there was a significant main effect of diagnosis with auditory M100 and M200 latencies delayed in the FASD group relative to the HC group. However, no group differences in amplitude were observed. This study is consistent with animal literature reporting widespread auditory deficits with prenatal ethanol exposure. This study also extends the previous results in humans by performing source analysis to determine that this latency delay is present at the level of the auditory cortex and by identifying these latency changes in very young children across the FASD spectrum.
Our hypothesis of delayed auditory processing was largely based on previous animal studies, in which prenatal alcohol exposure was carefully controlled. The animal studies have identified alterations in auditory functioning in both the peripheral and central nervous system. For example, Church et al. (1987) demonstrated delays in all four brainstem auditory evoked potential (BAEP) peak latencies across development in rats. In addition, they found the largest latency delays at the youngest ages (<17 days of age) with smaller differences in latency in rats 17 – 70 days of age (human age equivalent of ~18 months – 6 years). Kaneko et al. (1993) also identified latency delays in auditory evoked potentials measured in hippocampus in alcohol-exposed rats measured at 20-24 weeks of age. With a whole brainstem in vitro preparation, Pettigrew and Hutchinson (1984) identified poorly synchronized and delayed responses to auditory nerve stimulation in chick brainstem after a single exposure to alcohol early in incubation. The variability in responses in the alcohol-exposed chicks appeared similar to responses in younger control chicks, but the delays were larger in alcohol-exposed chicks; therefore, the central auditory processing deficits may be a result of both delayed and abnormal development following prenatal alcohol exposure. Histological studies on these brainstems confirmed atypical synaptic formation in the auditory brainstem nuclei. The results of Pettigrew and Subramanian (1985) suggest alcohol exposure causes a delay in Ca dependent mechanisms in the brainstem, which impacts brainstem auditory response amplitudes at younger ages. Our results are consistent with these animal studies and suggest that the cortical latency delay likely encompasses both brainstem and higher-order cortical processing deficits. One important distinction is that the current results do not represent a simple propagation of delays originating in brainstem; latency delays in BAEPs in rats are ~1ms compared to the ~10ms cortical delays reported in the current study.
Three studies in infants/children have also identified atypical BAEP (Pettigrew and Hutchinson, 1984; Rossig et al., 1994; Church et al., 1997). The incidence of abnormal BAEPs ranged from 50-79% across these studies showing clear agreement with the previous animal studies. Pettigrew and Hutchinson specifically reported that the most common abnormality was difficult in identifying peak V of the human BAEP, consistent with synchronicity problems identified in the animal component of their study. However, the clinical report of “abnormal” BAEP reported in the remaining two studies does not distinguish whether the abnormality is based on the amplitude or latency criterion. Therefore, further comparisons are difficult.
To the best of our knowledge, only one previous study reported cortical auditory deficits in children with FAS (Kaneko et al., 1996). Using auditory evoked potentials, Kaneko et al. (1996) contrasted auditory responses in 4-15 year old children with FAS, Down syndrome, and healthy controls using an oddball paradigm. The primary finding was a delayed parietal P300 response in the FAS group relative to Down syndrome and HCs in response to the rare stimulus. A secondary finding was a significant group effect for N100 latency to the rare tone, with a trend for delayed latencies in the FAS relative to the Down syndrome and HC children. However, their post-hoc tests did not reach significance for distinguishing the HC and FAS groups. At the same time, they did not identify any latency or amplitude differences to the frequent tones. The difference between the Kaneko study and the current results may be explained, in part, by the difference in age range across the two studies. The lack of significance in latency in the Kaneko study may be related to the broader age range and the continued development of the auditory system across this age range (Gage et al., 2003). Based on their rat studies, Church (1987) suggested that sensory deficits normalize with increasing age [also suggested by (Spohr and Steinhausen, 2008)] such that sensory deficits are less pronounced with increasing age. In a follow-up study, Church et al. (2011) confirmed the normalization of the auditory brainstem response latencies in rats prenatally exposed to alcohol when comparing very young post-weaned rats with young adult rats. The Kaneko study did not include age as a covariate, despite the larger age range. Therefore, the difference between the 14-15 year old children may have been small enough to reduce group latency differences below the level of significance. Furthermore, our study included children across the FASD spectrum. This perhaps unexpected consistency in the latency delay of the M100 with the previous report of children with FAS (where the delay was not found to be significant) is notable. Our results are consistent with the general pattern of early sensory deficits with prenatal alcohol exposure.
Hearing deficits in FAS children have been consistently reported (Church, 1987; Church and Gerkin, 1988; Rossig et al., 1994; Church et al., 1997). These deficits can include sensorineural hearing loss with an estimated incidence of up to 33% in FAS children compared to 2-3% of the general population, as well as conductive hearing loss due to recurrent ear infections which can have a prevalence of up to 92% in FAS children compared to <20% in the general population [for review see (Church and Kaltenbach, 1997)]. It is well recognized that peripheral hearing loss directly impacts language acquisition (Church and Kaltenbach, 1997). Based on the nature of the current study (to investigate cortical differences in auditory perception), we specifically recruited children without hearing loss. Kaneko et al. (1996) also excluded children with hearing loss from their study. Despite normal hearing, there was still a significant central auditory delay without documented peripheral hearing deficits. Interestingly, Church et al. (1997) reported deficits in dichotic listening and word recognition in noise in 100% of the children tested despite only 50% of these children having abnormal BAEPs. Their conclusion was that BAEPs only identify some of the deficits related to language deficits in FAS children. Our current results of latency delays in the absence of peripheral hearing loss may represent an additional deficit in the auditory pathway that leads to the difficulties with language acquisition in FAS and FASD children. The lack of latency differences between diagnostic subtype is also notable and may suggest that source analysis with MEG may provide a more sensitive measure of delays in auditory processing.
Despite the clear latency delays identified in the FASD children relative to the HC children, the current study has some limitations. First, the sample size was small, requiring a replication of the current study for full generalizability of the results. It is possible that the small number of subjects in the FASD subgroups led to a non-significant difference in auditory latency between the sub-categories. However, there was no trend for increased latency with increasing FASD severity (if one assumes the severity progression is from ARND, pFAS to FAS). Alternatively, the cortical auditory response latency may be related to atypical brain development at a later gestational age (GA) than the time window related to dysmorphia (8-13 weeks gestational age). Second, a more comprehensive developmental battery in FASD and HC children in future studies will allow one to better understand the broader cognitive consequences of these basic sensory deficits. Previous studies have confirmed language delays in FAS children with sensorineural hearing loss (Church and Kaltenbach, 1997). Third, a comprehensive hearing test for both HC and FASD children would provide additional evidence of the underlying deficits associated with prenatal alcohol exposure. Fourth, the latencies obtained from the primary auditory cortex may be the result of delays at multiple levels of auditory processing as the signal travels from the cochlea through the brainstem to auditory cortex. This summary latency may still provide a convenient marker, but without brainstem auditory potential measurements the source (peripheral versus central nervous system) cannot be determined. Finally, unlike animal studies, the human FASD group is not controlled for the amount or the pattern of alcohol exposure during pregnancy. Further prospective studies will be important for identifying if alcohol exposure at a particular gestational age is directly related to these delays in auditory processing.
Despite the above limitations, the current study is the first to document delayed auditory latencies in preschool aged children with FASD. This MEG study extends the FASD literature to identify neurophysiological changes in young children across the FASD spectrum and better describes where and when auditory deficits related to prenatal alcohol exposure occur. The fact that the significant group difference was obtained with a small sample and that the effect was noted in less severely affected children, suggests that delayed auditory signals may be a sensitive marker of prenatal alcohol exposure. This may prove to be a useful diagnostic tool, given the limited number of neurocognitive tests available for diagnosing young children with FASD who do not have physical dysmorphia. Since young children show greater plasticity than adults, the opportunity for early interventions is critically important for prevention of secondary disabilities in FASD. Furthermore, Church et al. (2011) identified a resurgence of auditory deficits in middle age rats suggesting that sensory deficits at a young age may predict early age-related hearing decline consistent with the Barker hypothesis of aging. These results provide evidence that early identification of sensory deficits may be important for intervention purposes across the age-spectrum.
Supplementary Material
Supplementary Fig. 1. Head movement monitoring during the task. The displayed figures provide a representation of the head motion during the auditory task for four children. The age (in months) of each child is noted. The orange line (dr) denotes the change in position (in cm) from the initial head position at the beginning of the data collection. The listed dr corresponds to the change in head position from the initial position at the black vertical line. The time of the black vertical line was chosen based on the approximately largest change in position for that child. For the examples shown here, the change in head position varied from 0.12 - 0.82 cm. In addition, it is clear that the head was stable for long periods with some position adjustments, with often a quick return to the child’s default head position. In general, the head motion was minimal with < 1 cm of movement across the 15-minute data collection.
Acknowledgements
We thank the parents and children who graciously offered their time to participate in this study. We thank Daniel Savage, Director of the New Mexico Alcohol Research Center, for his support of this study. This project was supported in part by NIH P20 AA017068, 5P20 RR021938, and an MRN Internal Award funded by DOE DE FG02 08ER64581.
Support: NIH P20 AA017068, 5P20 RR021938, DE FG02 08ER64581MRN Internal Award
References
- Bookstein FL, Streissguth AP, Connor PD, Sampson PD. Damage to the human cerebellum from prenatal alcohol exposure: the anatomy of a simple biometrical explanation. Anat Rec B New Anat. 2006;289:195–209. doi: 10.1002/ar.b.20114. [DOI] [PubMed] [Google Scholar]
- Church MW. Chronic in utero alcohol exposure affects auditory function in rats and in humans. Alcohol. 1987;4:231–239. doi: 10.1016/0741-8329(87)90017-6. [DOI] [PubMed] [Google Scholar]
- Church MW, Gerkin KP. Hearing disorders in children with fetal alcohol syndrome: findings from case reports. Pediatrics. 1988;82:147–154. [PubMed] [Google Scholar]
- Church MW, Kaltenbach JA. Hearing, speech, language, and vestibular disorders in the fetal alcohol syndrome: a literature review. Alcohol Clin Exp Res. 1997;21:495–512. doi: 10.1111/j.1530-0277.1997.tb03796.x. [DOI] [PubMed] [Google Scholar]
- Church MW, Eldis F, Blakley BW, Bawle EV. Hearing, language, speech, vestibular, and dentofacial disorders in fetal alcohol syndrome. Alcohol Clin Exp Res. 1997;21:227–237. [PubMed] [Google Scholar]
- Church MW, Hotra JW, Holmes PA, Anumba JI, Jackson DA, Adams BR. Auditory Brainstem Response (ABR) Abnormalities Across the Life Span of Rats Prenatally Exposed to Alcohol. Alcohol Clin Exp Res. 2011 doi: 10.1111/j.1530-0277.2011.01594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalal SS, Zumer JM, Guggisberg AG, Trumpis M, Wong DD, Sekihara K, Nagarajan SS. MEG/EEG source reconstruction, statistical evaluation, and visualization with NUTMEG. Comput Intell Neurosci. 2011;2011:758973. doi: 10.1155/2011/758973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farah MJ, Shera DM, Savage JH, Betancourt L, Giannetta JM, Brodsky NL, Malmud EK, Hurt H. Childhood poverty: specific associations with neurocognitive development. Brain Res. 2006;1110:166–174. doi: 10.1016/j.brainres.2006.06.072. [DOI] [PubMed] [Google Scholar]
- Flemming L, Wang Y, Caprihan A, Eiselt M, Haueisen J, Okada Y. Evaluation of the distortion of EEG signals caused by a hole in the skull mimicking the fontanel in the skull of human neonates. Clin Neurophysiol. 2005;116:1141–1152. doi: 10.1016/j.clinph.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Franklin L, Deitz J, Jirikowic T, Astley S. Children with fetal alcohol spectrum disorders: problem behaviors and sensory processing. The American journal of occupational therapy: official publication of the American Occupational Therapy Association. 2008;62:265–273. doi: 10.5014/ajot.62.3.265. [DOI] [PubMed] [Google Scholar]
- Gage NM, Siegel B, Roberts TP. Cortical auditory system maturational abnormalities in children with autism disorder: an MEG investigation. Brain Res Dev Brain Res. 2003;144:201–209. doi: 10.1016/s0165-3806(03)00172-x. [DOI] [PubMed] [Google Scholar]
- Goodlett CR, Marcussen BL, West JR. A single day of alcohol exposure during the brain growth spurt induces brain weight restriction and cerebellar Purkinje cell loss. Alcohol. 1990;7:107–114. doi: 10.1016/0741-8329(90)90070-s. [DOI] [PubMed] [Google Scholar]
- Hansen PC, Kringelbach ML, Salmelin R, editors. MEG: An introduction to methods. University Press; Oxford: 2010. [Google Scholar]
- Huang MX, Mosher JC, Leahy RM. A sensor-weighted overlapping-sphere head model and exhaustive head model comparison for MEG. Phys Med Biol. 1999;44:423–440. doi: 10.1088/0031-9155/44/2/010. [DOI] [PubMed] [Google Scholar]
- Jones KL, Smith DW. Recognition of the fetal alcohol syndrome in early infancy. Lancet. 1973;302:999–1001. doi: 10.1016/s0140-6736(73)91092-1. [DOI] [PubMed] [Google Scholar]
- Kaneko WM, Riley EP, Ehlers CL. Electrophysiological and behavioral findings in rats prenatally exposed to alcohol. Alcohol. 1993;10:169–178. doi: 10.1016/0741-8329(93)90099-a. [DOI] [PubMed] [Google Scholar]
- Kaneko WM, Ehlers CL, Philips EL, Riley EP. Auditory event-related potentials in fetal alcohol syndrome and Down’s syndrome children. Alcohol Clin Exp Res. 1996;20:35–42. doi: 10.1111/j.1530-0277.1996.tb01040.x. [DOI] [PubMed] [Google Scholar]
- Lebel C, Roussotte F, Sowell ER. Imaging the impact of prenatal alcohol exposure on the structure of the developing human brain. Neuropsychol Rev. 2011;21:102–118. doi: 10.1007/s11065-011-9163-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemoine P, Harouseau B, Borteryu JT, Menuet JC. Les enfants des parents alcooliques: Anomalies observees apropos de 127 cas. Ouest Medical. 1968;21:476–482. [Google Scholar]
- Mattson SN, Riley EP, Gramling L, Delis DC, Jones KL. Heavy prenatal alcohol exposure with or without physical features of fetal alcohol syndrome leads to IQ deficits. J Pediatr. 1997;131:718–721. doi: 10.1016/s0022-3476(97)70099-4. [DOI] [PubMed] [Google Scholar]
- Medina AE, Krahe TE, Ramoa AS. Early alcohol exposure induces persistent alteration of cortical columnar organization and reduced orientation selectivity in the visual cortex. J Neurophysiol. 2005;93:1317–1325. doi: 10.1152/jn.00714.2004. [DOI] [PubMed] [Google Scholar]
- Miller MW. Effect of prenatal exposure to ethanol on glutamate and GABA immunoreactivity in macaque somatosensory and motor cortices: critical timing of exposure. Neuroscience. 2006;138:97–107. doi: 10.1016/j.neuroscience.2005.10.060. [DOI] [PubMed] [Google Scholar]
- Oladehin A, Margret CP, Maier SE, Li CX, Jan TA, Chappell TD, Waters RS. Early postnatal alcohol exposure reduced the size of vibrissal barrel field in rat somatosensory cortex (SI) but did not disrupt barrel field organization. Alcohol. 2007;41:253–261. doi: 10.1016/j.alcohol.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paetau R, Ahonen A, Salonen O, Sams M. Auditory evoked magnetic fields to tones and pseudowords in healthy children and adults. J Clin Neurophysiol. 1995;12:177–185. doi: 10.1097/00004691-199503000-00008. [DOI] [PubMed] [Google Scholar]
- Parnell SE, Dehart DB, Wills TA, Chen SY, Hodge CW, Besheer J, Waage-Baudet HG, Charness ME, Sulik KK. Maternal oral intake mouse model for fetal alcohol spectrum disorders: ocular defects as a measure of effect. Alcohol Clin Exp Res. 2006;30:1791–1798. doi: 10.1111/j.1530-0277.2006.00212.x. [DOI] [PubMed] [Google Scholar]
- Pettigrew AG, Hutchinson I. Effects of alcohol on functional development of the auditory pathway in the brainstem of infants and chick embryos. Ciba Found Symp. 1984;105:26–46. doi: 10.1002/9780470720868.ch3. [DOI] [PubMed] [Google Scholar]
- Pettigrew AG, Subramanian S. Calcium dependence of neural transmission during development of auditory nuclei in the brainstsem of normal and alcohol exposed chick embryos. Neurosci Lett. 1985;19:S90. [Google Scholar]
- Ranken D, Best E, Stephen J, Schmidt D, George J, Wood C, Huang M. In: Nowak H, Haueisen J, Gießler F, Huonker R, editors. MEG/EEG forward and inverse modeling using MRIVIEW; Proceedings of the 13th International Conference on Biomagnetism; 2002.pp. 785–787. [Google Scholar]
- Riley EP, Mattson SN, Sowell ER, Jernigan TL, Sobel DF, Jones KL. Abnormalities of the corpus callosum in children prenatally exposed to alcohol. Alcohol Clin Exp Res. 1995;19:1198–1202. doi: 10.1111/j.1530-0277.1995.tb01600.x. [DOI] [PubMed] [Google Scholar]
- Rossig C, Wasser S, Oppermann P. Audiologic manifestations in fetal alcohol syndrome assessed by brainstem auditory-evoked potentials. Neuropediatrics. 1994;25:245–249. doi: 10.1055/s-2008-1073029. [DOI] [PubMed] [Google Scholar]
- Schneider ML, Moore CF, Adkins MM. The effects of prenatal alcohol exposure on behavior: rodent and primate studies. Neuropsychol Rev. 2011;21:186–203. doi: 10.1007/s11065-011-9168-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slawecki CJ, Thomas JD, Riley EP, Ehlers CL. Neurophysiologic consequences of neonatal ethanol exposure in the rat. Alcohol. 2004;34:187–196. doi: 10.1016/j.alcohol.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Mattson SN, Tessner KD, Jernigan TL, Riley EP, Toga AW. Voxel-based morphometric analyses of the brain in children and adolescents prenatally exposed to alcohol. Neuroreport. 2001;12:515–523. doi: 10.1097/00001756-200103050-00018. [DOI] [PubMed] [Google Scholar]
- Spohr HL, Steinhausen HC. Fetal alcohol spectrum disorders and their persisting sequelae in adult life. Dtsch Arztebl Int. 2008;105:693–698. doi: 10.3238/arztebl.2008.0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephen J, Romero L, Zhang T, Okada Y. In: Cheyne D, Ross B, Stroink G, Weinberg H, editors. Auditory and Somatosensory Integration in Infants; International Congress Series: New Frontiers in Biomagnetism: Proc 15th Intl Conf Biomagnetism; Vancouver, BC Canada. 2007. [Google Scholar]
- Stephen JM, Knoefel JE, Adair J, Hart B, Aine CJ. Aging-related changes in auditory and visual integration measured with MEG. Neurosci Lett. 2010;484:76–80. doi: 10.1016/j.neulet.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephen JM, Aine CJ, Christner RF, Ranken D, Huang M, Best E. Central versus peripheral visual field stimulation results in timing differences in dorsal stream sources as measured with MEG. Vision Res. 2002;42:3059–3074. doi: 10.1016/s0042-6989(02)00415-7. [DOI] [PubMed] [Google Scholar]
- Stephen JM, Davis LE, Aine CJ, Ranken D, Herman M, Hudson D, Huang M, Poole J. Investigation of the normal proximal somatomotor system using magnetoencephalography. Clin Neurophysiol. 2003b;114:1781–1792. doi: 10.1016/s1388-2457(03)00150-0. [DOI] [PubMed] [Google Scholar]
- Stephen JM, Ranken D, Best E, Adair J, Knoefel J, Kovacevic S, Padilla D, Hart B, Aine CJ. Aging changes and gender differences in response to median nerve stimulation measured with MEG. Clin Neurophysiol. 2006a;117:131–143. doi: 10.1016/j.clinph.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine . In: Fetal alcohol syndrome: Diagnosis, epidemiology, prevention, and treatment. Stratton K, Howe C, Battaglia FP, editors. National Academy Press; Washington, D.C.: 1996. [Google Scholar]
- Streissguth AP, Bookstein FL, Barr HM, Press S, Sampson PD. A fetal alcohol behavior scale. Alcohol Clin Exp Res. 1998;22:325–333. doi: 10.1111/j.1530-0277.1998.tb03656.x. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Bookstein FL, Barr HM, Sampson PD, O’Malley K, Young JK. Risk factors for adverse life outcomes in fetal alcohol syndrome and fetal alcohol effects. J Dev Behav Pediatr. 2004;25:228–238. doi: 10.1097/00004703-200408000-00002. [DOI] [PubMed] [Google Scholar]
- Supek S, Aine CJ. Simulation studies of multiple dipole neuromagnetic source localization: Model order and limits of source resolution. IEEE Trans Biomed Eng. 1993;40:529–539. doi: 10.1109/10.237672. [DOI] [PubMed] [Google Scholar]
- Susac A, Ilmoniemi RJ, Ranken D, Supek S. Face activated neurodynamic cortical networks. Med Biol Eng Comput. 49:531–543. doi: 10.1007/s11517-011-0740-4. [DOI] [PubMed] [Google Scholar]
- Taulu S, Kajola M. Presentation of electromagnetic multichannel data: The signal space separation method. Journal of Applied Physics. 2005;97 [Google Scholar]
- Wehner DT, Hamalainen MS, Mody M, Ahlfors SP. Head movements of children in MEG: quantification, effects on source estimation, and compensation. Neuroimage. 2008;40:541–550. doi: 10.1016/j.neuroimage.2007.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1. Head movement monitoring during the task. The displayed figures provide a representation of the head motion during the auditory task for four children. The age (in months) of each child is noted. The orange line (dr) denotes the change in position (in cm) from the initial head position at the beginning of the data collection. The listed dr corresponds to the change in head position from the initial position at the black vertical line. The time of the black vertical line was chosen based on the approximately largest change in position for that child. For the examples shown here, the change in head position varied from 0.12 - 0.82 cm. In addition, it is clear that the head was stable for long periods with some position adjustments, with often a quick return to the child’s default head position. In general, the head motion was minimal with < 1 cm of movement across the 15-minute data collection.


