Abstract
Activated glucocorticoid receptor (GR) acts via two different mechanisms: transcriptional regulation that requires DNA-binding, and protein–protein interaction between GR and other transcription factors, such as nuclear factor kappa B (NF-κB) or activator protein 1 (AP-1). It has been postulated that many important effects of glucocorticoids, including their anti-inflammatory properties, depend on GR’s transrepressive effects on NF-κB and AP-1. In the present study, we have employed a TPA-induced model of skin inflammation and epidermal hyperplasia to determine whether partial activation of the glucocorticoid receptor by Compound A (CpdA) is sufficient to reverse the effect of TPA treatment. CpdA is a nonsteroidal GR modulator with high binding affinity, is capable of partial activation of GR. Topical application of TPA twice per week for two weeks results in inflammation and epidermal hyperplasia. TPA treatment also elevates levels of c-jun (AP-1 component), cyclooxygenase-2 (COX-2), p50 (NF-κB component), interleukin-6 (IL-6) and tumor necrosis factor (TNF) in the skin. Fluocinolone acetonide (FA) (a full GR agonist) was able to completely reverse the above effects of TPA. When applied alone, CpdA increased the epidermal thickness and keratinocyte proliferation as well as levels of c jun, COX-2, IL-6 and IFN-γ. However CpdA treatment resulted in a decrease in the number of p50 positive cells induced by TPA, suggesting its role in inhibition of NF-κB. The level of metallothionein-1 mRNA, regulated by GR was also significantly decreased in skin samples treated with CpdA. Our results suggest that CpdA is able to inhibit GR transactivation and activate only some transrepression properties of GR.
Keywords: Glucocorticoid receptor, dissociated glucocorticoids, SENCAR mice
Introduction
Topically applied glucocorticoid hormones are potent inhibitors of tumor promoter-mediated epidermal proliferation and mouse skin tumorigenesis [1]. The control of cellular functions by glucocorticoids (GCs) is mediated via the glucocorticoid receptor (GR), a well-known transcription factor. Unliganded GR resides in the cytosol associated with a heat-shock protein complex. Following hormone binding, GR dissociates from the complex and migrates as a homodimer to the nucleus, where it binds to glucocorticoid-response elements (GRE) in gene promoters. GR acts via two different mechanisms: transcriptional regulation that requires DNA-binding (transactivation) and direct protein–protein inhibitory interactions between GR and certain inflammatory and oncogenic transcription factors (transrepression), such as NF-κB or AP-1 [2,3]. Experiments with transgenic mice harboring mutated GR, which cannot activate GRE containing promoters, clearly indicate that many important effects of glucocorticoids, including their anti-inflammatory effect and blockage of tumor promoter 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced response in skin, depend on GR’s transrepressive effects on NF-κB and AP-1 [4]. However, skin papillomas and carcinomas become resistant to growth inhibition by glucocorticoids and their control of cellular functions [5–7]. Both, AP-1 and NF-κB activities are significantly elevated in mouse skin tumors and appear to be related to the loss of the GR function [8–10].
It has been hypothesized that the transactivation properties are associated with the development of several side effects [11] like steroid diabetes, obesity, osteoporosis, skin atrophy, growth retardation, Cushing’s syndrome and many others. By contrast, the anti-inflammatory effects are due, largely, to the ability of GR to reduce the expression of pro-inflammatory genes by repressing key inflammatory transcription factors such as NF-κB. The potential separation of glucocorticoids’ side effects from their anti-inflammatory activity has stimulated considerable interest in GR ligands that can possibly dissociate the transactivation and transrepression functions [12]. It has been postulated that separation of the anti-inflammatory effects of GCs from some of the side effects could be achieved by structure modification of known glucocorticoids. Several steroidal compounds that are capable of separating transcriptional activation from repression (RU24858, RU40066, and RU24782) have been described [13]. These molecules, with in vivo anti-inflammatory properties, were able to inhibit both AP-1 and NF-κB-mediated gene induction and showed a decrease in transactivation activity of several genes in vivo. Despite their in vitro dissociated properties, in vivo they still had the same side effect profile as known steroids [14,15]. Interestingly, compounds promoting a strong GR-dependent transactivation and weak transrepression properties were unable to inhibit inflammation [15]. Since that time, several attempts to find new dissociated glucocorticoids have been undertaken but none of them have led to the replacement of the present glucocorticoids [11,16–18].
Several non-steroidal compounds interact with members of the steroid receptor subclass, including tamoxifen (estrogen receptor ligand) [19,20], casodex and flutamide (androgen receptor antagonists) [21,22], and various progesterone ligands [23,24]. Compound A, a 2-(4-acetoxyphenyl)-2-chloro-Nmethyl-ethylammonium chloride, is a stable analogue of the hydroxyl phenyl aziridine precursor found in the Namibian shrub Salsola tuberculatiformis Botschantzev [25,26]. Despite its nonsteroidal structure, CpdA (Figure 1) strongly binds both the GR and androgen receptor (AR) in competition binding assays in prostate cell lines [26–28]. CpdA is able to down regulate NF-κB-driven genes via interaction with GR but it does not induce the binding of GR to GRE-dependent genes thus reducing their expression. The anti-inflammatory mechanism involves both a reduction of the in vivo DNA-binding activity of NF-κB component, p65 protein, as well as an interference with the transactivation potential of NF-κB [26].
Figure 1.
Chemical structures of Compound A and of Fluocinolone acetonide.
In the present study, we have tested the hypothesis that only the transrepression activities of the GR are sufficient to suppress the TPA-induced skin inflammation and hyperplasia. We assessed the ability of dissociated GR agonist Compound A to suppress TPA-induced keratinocyte proliferation and inflammation in skin of SENCAR mice.
Materials and methods
Chemicals
All the chemicals used in this study, including glucocorticoid, fluocinolone acetonide (FA), tumor promoter 12-O-tetradecanoylphorbol-13-acetate (TPA) and 5-Bromo-2’-deoxyuridine (BrdU) were obtained from Sigma-Aldrich (St. Louis, MO) unless otherwise noted. Compound A was obtained from Axxora (San Diego, CA).
Animals
Female SENCAR mice, 5-week-old, were purchased from the National Cancer Institute, Frederick Cancer Research and Development Center (Frederick, MD), and placed into quarantine for one week prior to initiation of animal treatment. Mice were housed in groups of five under conditions of constant temperature and humidity and maintained on a 12-h light/dark cycle with ad libitum access to food and water. All animal procedures were performed in accordance with the National Institutes of Health Guidelines, and approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center at San Antonio. The shaved backs of mice were treated with 2 µg of TPA in 0.2 ml acetone (ACT) twice per week for 2 weeks. Compound A (0.01, 0.1 and 1.0 mg) and FA (1.5 µg) were applied 20 minutes prior to acetone control or TPA application twice per week for 2 weeks. Mice were sacrificed 24 h after the last treatment and skin samples were collected from the dosed area of each animal.
Histological evaluation
Tissues for histological evaluation were prepared by using conventional paraffin sections and hematoxylin-eosin staining. Approximately 1 cm2 of each skin was preserved in formalin for immunohistochemistry. Epithelial thickness was determined from at least 20 randomly selected sites in formalin-fixed skin samples, using Olympus BX41 clinical microscope (Olympus, PA). For proliferative index analyses, mice were given an i.p. injection of BrdU (1.5 mg per mouse) 60 min prior to sacrifice. Tissue sections were immunostained with an anti-BrdU antibody (Lab Vision Corporation, Fremont, CA). The number of BrdU-positive cells within the basal cells layer was determined from at least 20 randomly selected sites. For immunostaining of AP-1 and NF-κB complexes, we used anti-c-jun (BD Biosciences, Franklin Lakes, NJ) and anti-p50 antibodies, respectively (Santa Cruz Biotechnology Inc., Santa Cruz, CA). COX-2 immunostaining was performed using anti-COX-2 antibody (Cell Signaling Technology, Inc., Danvers, MA). Sections (4µm) of formalin-fixed, paraffin-embedded skin tissue were cut onto siliconized glass slides and deparaffinized three times with xylene for 10min each and rehydrated through a graded alcohols. The deparaffinized sections were heated and boiled twice for 6 min in 10 mM citrate buffer, pH 6.0, for antigen retrieval. To diminish nonspecific staining, each section was treated with 3% hydrogen peroxide in methanol for 15 min. For the detection, slides were incubated with 1:100 dilutions of a specific antibody overnight in Tris-buffered saline containing 0.05% Tween-20 and then developed using the HPR EnVisionTM System (Dako, Glostrup, Denmark). The peroxidase-binding sites were detected by staining with diaminobenzidine tetrahydrochloride (Dako, Glostrup, Denmark). Finally, counterstaining was performed using Mayer’s hematoxylin.
Real time PCR analysis
Total RNA was extracted using TRI Reagent (MRC, Inc. Cincinnati, OH). RNA (1 µg) was reverse transcribed with oligo(dT), using a cMaster RT kit (Eppendorf North America, Westbury, NY) according to the manufacturer's protocol. Primer sets of: 5’-TGCTCCACCGGCGG-3’, 5’-TTTGCAGACACAGCCCTGG-3’ for MT-1, 5’-ATCCTGCCAGCTCCACCG-3’, 5’-TGGTCAAATCCTGTGCTCATACAT-3’ for COX-2, 5’-CCTGTCCCCTATCGACATGG-3’, 5’-CTTTTCCGGCACTTGGAGG-3’ for c-jun and 5’-CATCCTGGCCTCGCTGTC-3’, 5’-CTCGTCGTACTCCTGCTTGGT-3’ for β-actin, were used and generated a unique product. Standard quantitative Real Time PCR (qRT-PCR) was performed in triplicate using SYBR Green RealMaster Mix (Eppendorf North America, Westbury, NY) on the Realplex MasterCycler (Eppendorf, Westbury, NY). RT-PCR cycle thresholds (Ct) of candidate genes were normalized to β-actin. The formula 2Ct(Candidate)/2Ct(Control) was used to calculate the normalized ratios.
Cytokines analysis
For analysis of levels of cytokines, skin samples were homogenized by mortar and pestle and lysed in 0.05% tween-20 in PBS containing 10 mM PMSF. Samples were centrifuged at 10,000 rpm for 15 minutes at 4°C to remove cellular debris. Supernatants were collected and protein levels were quantified by Bradford. 150 µg of protein lysate per sample was analyzed in triplicate for levels of Interleukin-2, Interleukin-4, Interleukin-6, Interleukin-10, Interleukin-17A, Interferon-γ (IFN-γ), and Tumor Necrosis Factor (TNF) using the CBA Mouse Th1/Th2/Th17 Cytokine Kit from Becton Dickinson.
Data analysis and statistics
All the results are expressed as mean ± standard deviation (SD). For comparison of the differences between the groups a two-tailed, unpaired, Student's t test was used. Differences were considered statistically significant at a P value <0.05.
Results
Effect of Compound A on TPA-induced epidermal hyperplasia
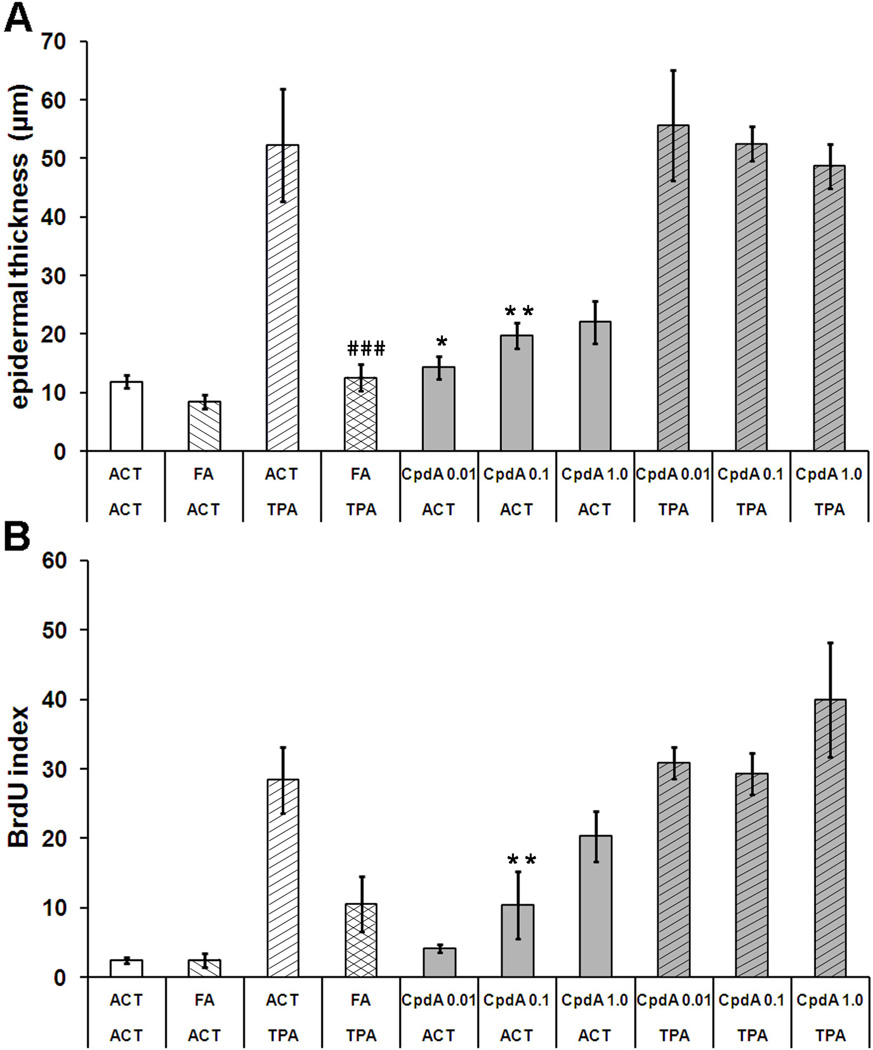
Groups of five female SENCAR mice were treated with CpdA and FA as indicated in Materials and methods. As expected, a large increase in epidermal thickness was observed after two weeks of TPA treatment (50 µm) (Figure 2A, Table 1). Average thickness of acetone or FA treated epidermis was about 10 µm. Unexpectedly, Compound A significantly increased the epidermis thickness in a dose-dependent manner, up to double the control epidermal thickness at the highest dose. However, the maximum epidermal thickness obtained with CpdA alone was still significantly less than that obtained with the TPA treatment. The full glucocorticoid FA completely reversed TPA-induced BrdU incorporation index (Figure 2B) which confirmed the results obtained from measurement of epidermis thickness. FA strongly inhibited TPA-stimulated cell proliferation. Compound A increased the proliferation rate of keratinocytes located in the basal layer of the epidermis in a dose-dependent manner. When Compound A was applied before TPA, it was unable to inhibit TPA-induced cell proliferation. Moreover, at the highest dose in combination with TPA, it appeared to enhance cell proliferation.
Figure 2. The effect of CpdA on TPA-induced epidermal hyperplasia and cell proliferation in SENCAR mice.
A - the effect of FA (1,5 µg) and CpdA (0.001, 0.1 and 1.0 mg) on the induction of epidermal hyperplasia after multiple treatments with TPA (2 µg). The results of epidermal thickness measurements are plotted as average of twenty measurements at random locations along the epidermis of the skin specimen from each treatment group. B - the effect of tested compounds on BrdU incorporation index in epidermis of SENCAR mice treated with TPA. Proliferative indexes were calculated as the mean percentage of basal layer keratinocytes having BrdU-incorporated nuclei. Approximately 20 randomly selected sites were counted for each sample. The length of skin samples evaluated for the BrdU index as well as for measurement of epidermal thickness was approximately 15 mm Presented results represent the average (±S.D.), P value <0.05(*), <0.01(**), <0.001(***) vs. acetone (ACT) group; P value <0.01 (##), P value <0.001 (###) vs. TPA group.
Table 1.
Comparison of the influence of CpdA (at 1 mg/mouse dose) and FA on the several parameters related to induced by TPA treatment skin inflammation and hyperplasia. All presented values are calculated as percent of acetone group (ACT).
| experimental group |
epidermal thickness |
BrdU index |
c-jun mRNA |
COX-2 mRNA |
p50 level |
IL-6 level |
INF-γ level |
TNF-α level |
MT-1 mRNA |
|---|---|---|---|---|---|---|---|---|---|
| ACT | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| FA | 72 | 98 | 62 | 74 | 16 | 79 | 0 | 63 | 265 |
| CpdA | 187 | 830 | 220 | 215 | 20 | 306 | 434 | 195 | 16 |
| ACT / TPA | 443 | 1000 | 320 | 558 | 252 | 604 | 88 | 290 | 195 |
| FA / TPA | 107 | 430 | 84 | 87 | 115 | 132 | 28 | 64 | 205 |
| CpdA / TPA | 412 | 1600 | 383 | 522 | 147 | 291 | 346 | 262 | 76 |
Effect of Compound A on c-jun mRNA and protein levels
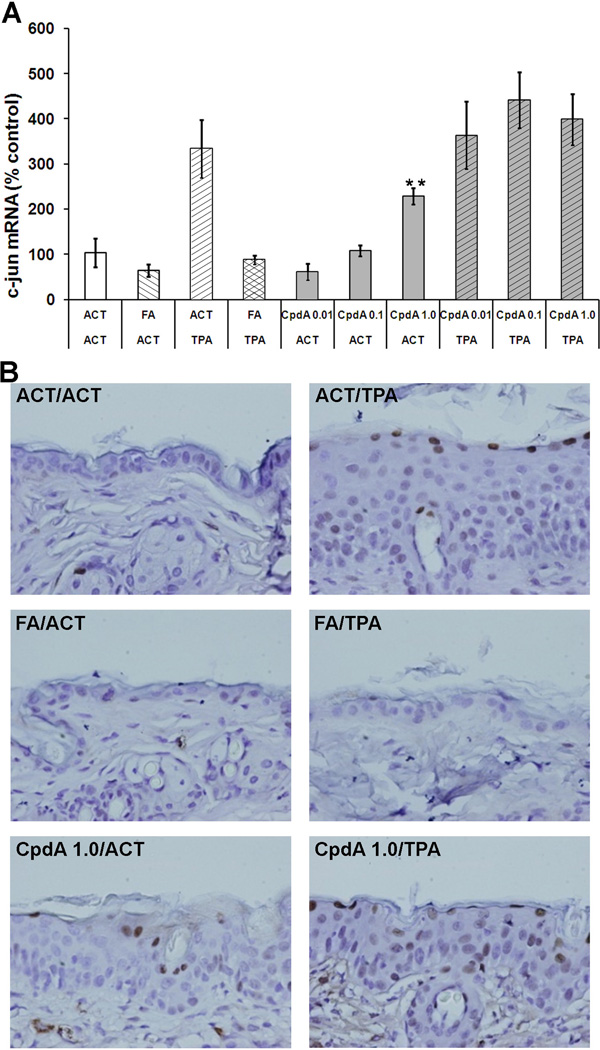
The transcriptional factor AP-1 mediates gene regulation in response to a variety of extracellular stimuli including oncogenes, growth factors, tumor promoters, carcinogens and cytokines [29]. AP-1 controls a number of cellular processes including proliferation, differentiation and apoptosis [30]. Since AP-1 has been shown to be one of the key transcription factors that is transrepressed by GR, we measured the mRNA and protein levels of AP-1 active subunit c-jun in the epidermis of mice by RT-PCR and immunohistochemistry, respectively. As expected, the TPA treatment strongly increased the c-jun expression level within the epidermal cells (Figure 3, Table 1). CpdA, when applied alone, also increased the c-jun mRNA level in a dose-dependent manner but much less than the levels obtained with TPA. When CpdA was applied before TPA, it had no effect on c-jun mRNA level. FA treatment alone resulted in a slight decrease in c-jun level. Application of FA on the skin prior to TPA, decreased TPA-induced c-jun expression to the acetone control level. Immunostaining for c-jun protein shows that an increase in mRNA level translates into c-jun protein levels as shown in representative microphotographs in Figure 3 B.
Figure 3. The effect of CpdA on c-jun mRNA and protein level.
A - the effect of CpdA and FA applied alone or together with TPA on c-jun mRNA level was measured using RT-PCR analysis. Presented results represent the average (±S.D.) from at least three independent measurements, P value <0.01(**) vs. acetone (ACT) group. B - Representative microphotographs (×400) showing c-jun protein levels by immunohistochemistry.
Effect of Compound A on COX-2 mRNA and protein levels
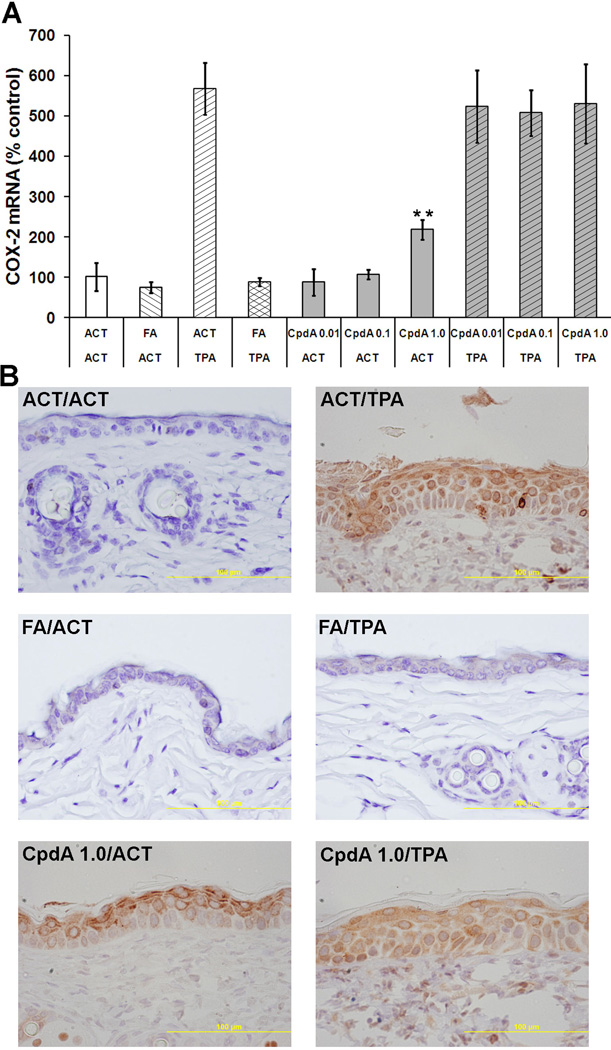
Since AP-1 plays a significant role in regulating the induction of COX-2 [31], an enzyme involved in inflammation [32] and tumorigenesis [33], the effect of FA and CpdA on COX-2 expression was also determined. As shown in Figure 4, the epidermal hyperplasia in TPA-treated skins was associated with very high levels of COX-2 mRNA and protein. Quantitative RT-PCR analysis (Figure 4A) showed that FA treatment was able to decrease the TPA elevated mRNA level of COX-2 to that of the acetone group. On the other hand, CpdA treatment increased COX-2 expression when applied alone and had no effect on the TPA-induced increase in COX-2 mRNA and protein levels in the skin (Figure 4, Table 1).
Figure 4. The effect of CpdA on COX-2 mRNA and protein level.
A - the effect of CpdA and FA applied alone or together with TPA on COX-2 mRNA level was measured using RT-PCR analysis. Presented results represent the average (±S.D.) from at least three independent measurements, P value <0.01(**) vs. aceteone (ACT) group. B - Representative microphotographs (×400) showing COX-2 protein levels by immunohistochemistry.
Effect of Compound A on NF-κB expression and intracellular localization
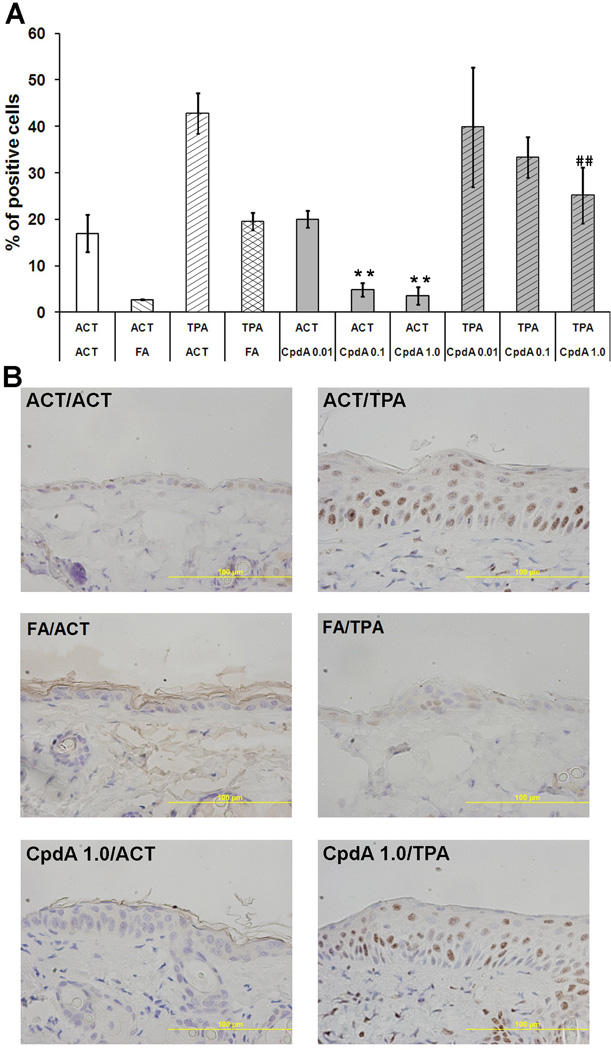
NF-κB mediated signal transduction has been implicated in the regulation of the inflammatory response, tumorigenesis and apoptosis [34]. To elucidate the effect of CpdA on the NF-κB expression and nuclear translocation in mice treated topically with TPA, we studied the expression of protein p50, a component of the NF-κB complex. The p50 protein level for each treatment group was determined by immunohistochemistry. The p50 protein nuclear positive cells were counted at 12 random locations per sample, and then expressed as a percentage of positive cells within the epidermis (Figure 5). TPA-treated epidermis yielded a very high number of p50 positive cells with strong nuclear staining. Both FA and CpdA treatments were able to significantly decrease the number of p50 positive cells (Figure 5, Table 1) compared to the group treated with TPA alone. Interestingly, the same reduction of p50 positive cells was also observed when FA and CpdA were applied alone.
Figure 5. The effect of CpdA on NF-κB expression and intracellular localization.
The p50 protein levels and localizations were assessed by immunohistochemistry. The p50 protein nuclear positive cells were counted at 12 random locations per sample, and then expressed as a percentage of positive cells within the epidermis. (**) - P value <0.01 vs. acetone (ACT) group; (##) - P value <0.01 vs. TPA group.
Cytokine analysis
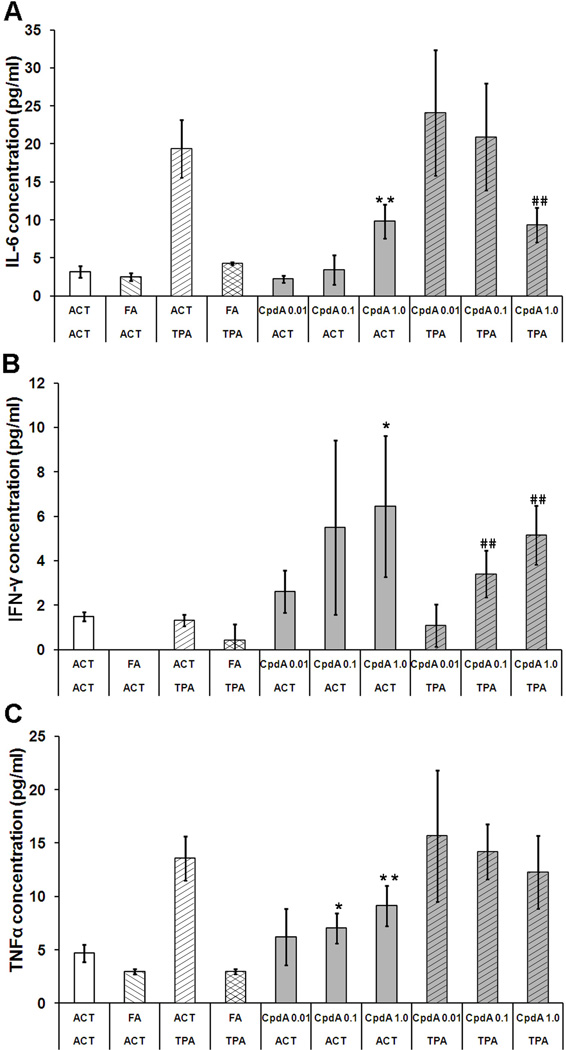
In order to determine the influence of CpdA treatment on cutaneous inflammation induced by TPA treatment, the level of several cytokines were measured in the mouse skin tissue homogenates obtained from each treatment group. Bead array technology was employed to simultaneously detect the level of multiple cytokine proteins in each sample. The CBA Mouse Th1/Th2/Th17 Cytokine Kit uses seven different beads populations to measure concentrations of interleukin-2, -4, -6, -10, -17A (IL-2, -4, -6, -10, -17A) as well as interferon gamma (IFN-γ) and tumor necrosis factor (TNF) in the same sample. All of the cytokines, except IL-4, were detectable in tissue homogenates from both control and treated groups. The TPA treatment elevated the levels of IL-6 and TNF but had no effect on IFN-γ (Figure 6). When applied alone, FA was able to decrease the basal level of IL-6, IFN-γ and TNF. In addition, treatment of mice with FA, effectively reduced the TPA-mediated increase in IL-6 and TNF protein levels in the skin. CpdA treatment alone resulted in a dose-dependent increase of IL-6, IFN-γ and TNF levels. When applied together with TPA, CpdA significantly decreased the levels of TPA-induced IL-6 levels (Figure 6A). Interestingly, CpdA showed a dose-dependent decrease in TPA-induced TNF levels; however the results were not statistically significant (Figure 6 C). The cytokines IL-2, IL-4 and IL-10 were detectable in most of the groups, but no statistically significant differences between groups were observed (data not shown).
Figure 6. The effect of CpdA and FA applied alone or together with TPA on cytokine expression.
IL-6 (A), IFNγ (B) and TNF (C) were measured in the mouse skin tissue homogenates obtained from each treatment group using CBA Mouse Th1/Th2/Th17 Cytokine Kit. (*) - P value <0.05 (**) - P value <0.01 vs. acetone (ACT) group; (##) P value <0.01 vs. TPA group.
Effect of Compound A on GR-mediated transactivation
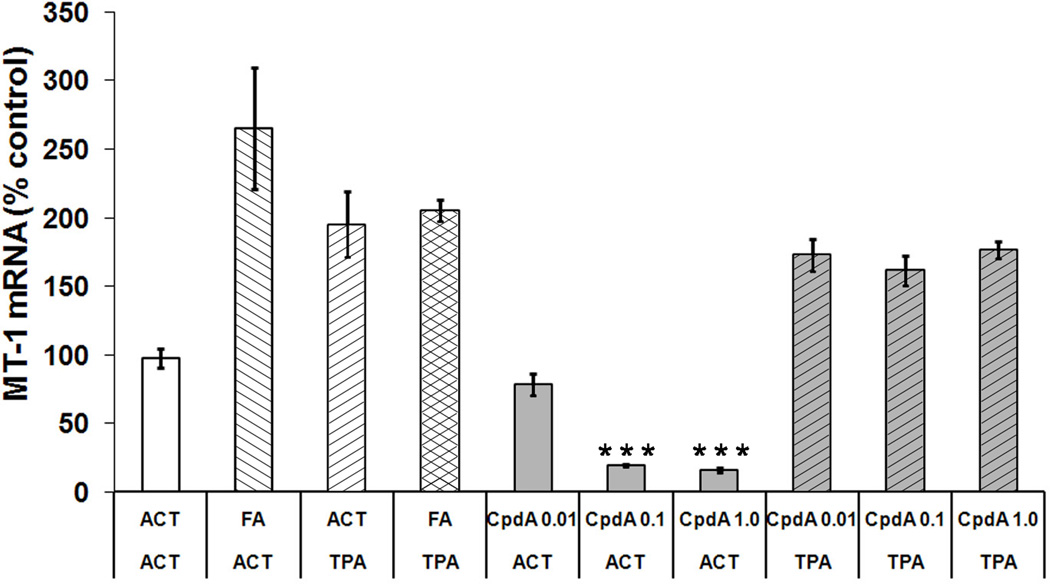
To determine whether Compound A could alter GR transcriptional activity in skin samples, the expression of an endogenous glucocorticoid-responsive gene was studied. We decided to examine metallothionein 1 (MT-1), a small metal-binding protein expressed in mouse epidermis. Expression of MT-1 gene is positively regulated by glucocorticoids in different tissues, and a sequence homologous to the GRE in the promoter region of this gene was described [35]. As expected, RT-PCR analysis of mRNA from mouse tissue showed that Compound A significantly decreased the MT-1 mRNA level in a dose-dependent manner (Figure 7 and Table 1). On the other hand, FA application caused a significant increase in MT-1 expression. Both FA and CpdA had no significant effect on MT-1 mRNA levels elevated by TPA.
Figure 7. The effect of CpdA and FA applied alone or together with TPA on MT-1 mRNA level.
MT-1 mRNA expression was measured using RT-PCR analysis. Each point with vertical bar represents the average (±S.D.) from at least three independent measurements. (***) - P value <0.001 vs. acetone (ACT) group.
DISCUSSION
The glucocorticoid hormones, such as FA, are very potent inhibitors of skin inflammation and epidermal proliferation and are effective inhibitors of mouse skin tumorigenesis induced by UV or chemical carcinogen/tumor promoter treatment. However, glucocorticoids usage is limited due to their numerous side effects [36,37]. Glucocorticoids also do not affect the growth of either established papillomas, squamous cell carcinomas (SCC), or transformed keratinocytes in vitro; skin papillomas and carcinomas become resistant to the growth inhibition by glucocorticoids and their control of cellular functions [6,38,39].
In the present study, we have employed the TPA-induced model of skin inflammation and epidermal hyperplasia to determine whether a partial activator of the glucocorticoid receptor is sufficient to reverse the effect of TPA treatment. Topical application of TPA twice per week for two weeks results in inflammation and epidermal hyperplasia associated with increased epidermal thickness and keratinocytes proliferation, all hallmarks of tumor promotion (Table 1). The TPA treatment also causes elevated levels of c-jun (AP-1 component), COX-2, p50 (NF-κB component) as well as increased levels of IL-6 and TNF in the skin. Treatment with FA, a full glucocorticoid receptor agonist, was able to completely reverse the above effects of TPA stimulation. However, the effect of CpdA treatment was less explicit. When applied alone, CpdA increased the epidermal thickness and keratinocyte proliferation in a dose-dependent manner and elevated the expression of AP-1 component c jun as well as COX-2, IL-6 and IFN-γ, but to a much lesser extent than TPA. However CpdA treatment resulted in a decrease in the number of p50 positive cells suggesting its role in inhibition of NF-κB. The same effect on p50 was observed when CpdA was applied together with TPA. CpdA also reduced IL-6 and TNF mRNA levels induced by TPA, indicating that it may have anti-inflammatory activity in the skin. The level of MT-1 mRNA was also significantly decreased in skin samples treated with CpdA, suggesting that CpdA is able to inhibit the transactivation properties and therefore the potential negative side effects of GR.
Some of our results seem to stand in contradiction to data presented earlier by Dewint et al. [40], where CpdA was able to markedly inhibit acute joint inflammation without inducing hyperinsulinemia in a murine model of collagen-induced arthritis. During systemic administration in this model, at a concentration effective in inhibiting collagen-induced arthritis in DBA/1 mice, CpdA did not down-regulate GR expression thereby retaining its anti-inflammatory effects after prolonged treatment. This finding was in sharp contrast to dexamethasone (DEX) treatment, in which prolonged DEX treatment caused a decrease in GR level, and the abolishment of inflammatory gene repression [41]. It has also been demonstrated that CpdA could effectively suppress experimental autoimmune neuritis (EAN) in rats, a helper T cell-mediated autoimmune demyelinating inflammatory disease of the peripheral nervous system, with reduced side effects [42]. CpdA treatment caused attenuation in the accumulation of macrophages and lymphocytes, demyelination, and mRNA levels of inflammatory molecules in sciatic nerves of EAN and an increase in a numbers of anti-inflammatory M2 macrophages. This treatment did not cause hyperglycemia effects in EAN rats as compared with the immunosuppressive steroid prednisolone [42]. It has been recently shown that, in several prostate cell lines, CpdA blocked AR function and strongly enhanced the anti-inflammatory functions of GR, leading to growth inhibition and apoptotic death in the AR/GR-expressing prostate cell lines. Despite the fact that CpdA was able to induce nuclear translocation of both receptors, it inhibited DNA binding and the transactivation potential of AR [28]. In addition, CpdA blocked GR-mediated transactivation but induced GR transrepression via inhibition of several transcription factors.
It has also been reported that CpdA prevented the onset of paw swelling in a zymosan-induced arthritis model [26]. Our study has shown that CpdA enhances chronic hyperplasia and keratinocytes proliferation while increasing AP-1 and COX-2 expression. On the other hand, CpdA was effective in lowering the number of p50 positive cells as well as decreasing the level of TNF and IL-6 protein induced by TPA (Figure 6). CpdA was also able to inhibit the expression of GR regulated gene (MT-1). Inhibition of NF-κB activation/translocation into keratinocytes nuclei could be caused either by direct interaction of GR activated by CpdA or by lowering the TNF level.
It has been postulated that CpdA is capable of binding to the GR and down-modulating NF-κB-regulated genes via a direct interaction with NF-κB, without modulating genes regulated by GR DNA binding, suggesting that CpdA acts as a dissociated agent [26]. In our study, CpdA was able to inhibit the activation of NF-κB without inhibiting AP-1. It even increased c-jun (AP-1 component) expression. Our results suggest that CpdA is able to inhibit transactivation of GR and selectively activate only some of the transrepressive properties of GR. The effect of CpdA may also be more complex, i.e., it might affect more than one molecular target within the cell or tissue.
In our earlier work we showed that NF-κB is constitutively activated in mouse skin tumors induced by the DMBA/TPA protocol, partially due to a strong increase in p50 (NF-κB1) expression / activation. Compounds which have only transrepression activity (responsible for the lower p50 expression) and no transactivation activity may serve as potent anti-inflammatory agents with fewer side effects. Our findings warrant future, in vivo studies to examine if CpdA treatment, and down regulation of p50 would lead to the prevention of UVB and DMBA/TPA-induced skin cancer formation, even without influencing epidermal hyperplasia. We will also examine whether this treatment can reverse the development of established skin tumors (papillomas and squamous cell carcinomas).
Acknowledgments
This study was supported by the National Cancer Institute Grant RO1 CA 0796065-09 (T.J.S.) and the Cancer Center Grant P30 CA 54174-16S1.
Abbreviations
- TPA
12-O-tetradecanoylphorbol-13-acetate
- GC
glucocorticoid
- GR
glucocorticoid receptor
- FA
fluocinolone acetonide
- RT-PCR
reverse transcriptional polymerase chain reaction
- MT-1
metallothionein-1
- ACT
acetone
- IL
interleukin
- TNF
tumor necrosis factor
- IFN-γ
interferon gamma
- AR
androgen receptor
References
- 1.Slaga TJ. Can tumour promotion be effectively inhibited? IARC Sci Publ. 1984;(56):497–506. [PubMed] [Google Scholar]
- 2.Ray A, Prefontaine KE. Physical association and functional antagonism between the p65 subunit of transcription factor NF-kappa B and the glucocorticoid receptor. Proc Natl Acad Sci U S A. 1994;91(2):752–756. doi: 10.1073/pnas.91.2.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang-Yen HF, Chambard JC, Sun YL, et al. Transcriptional interference between c-Jun and the glucocorticoid receptor: mutual inhibition of DNA binding due to direct protein-protein interaction. Cell. 1990;62(6):1205–1215. doi: 10.1016/0092-8674(90)90396-v. [DOI] [PubMed] [Google Scholar]
- 4.Tuckermann JP, Reichardt HM, Arribas R, Richter KH, Schutz G, Angel P. The DNA binding-independent function of the glucocorticoid receptor mediates repression of AP-1-dependent genes in skin. J Cell Biol. 1999;147(7):1365–1370. doi: 10.1083/jcb.147.7.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwarz JA, Viaje A, Slaga TJ. Fluocinolone acetonide: a potent inhibitor of mouse skin tumor promotion and epidermal DNA synthesis. Chem Biol Interact. 1977;17(3):331–347. doi: 10.1016/0009-2797(77)90096-5. [DOI] [PubMed] [Google Scholar]
- 6.Strawhecker JM, Pelling JC. Inhibition of mouse skin tumorigenesis by dexamethasone occurs through a Ha-ras-independent mechanism. Carcinogenesis. 1992;13(11):2075–2080. doi: 10.1093/carcin/13.11.2075. [DOI] [PubMed] [Google Scholar]
- 7.Budunova IV, Carbajal S, Kang H, Viaje A, Slaga TJ. Altered glucocorticoid receptor expression and function during mouse skin carcinogenesis. Molecular carcinogenesis. 1997;18(3):177–185. [PubMed] [Google Scholar]
- 8.Cheng Y, Li J, Martinka M, Li G. The expression of NAD(P)H:quinone oxidoreductase 1 is increased along with NF-kappaB p105/p50 in human cutaneous melanomas. Oncol Rep. 2010;23(4):973–979. doi: 10.3892/or_00000722. [DOI] [PubMed] [Google Scholar]
- 9.Cooper SJ, Bowden GT. Ultraviolet B regulation of transcription factor families: roles of nuclear factor-kappa B (NF-kappaB) and activator protein-1 (AP-1) in UVB-induced skin carcinogenesis. Curr Cancer Drug Targets. 2007;7(4):325–334. doi: 10.2174/156800907780809714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dong Z, Birrer MJ, Watts RG, Matrisian LM, Colburn NH. Blocking of tumor promoter-induced AP-1 activity inhibits induced transformation in JB6 mouse epidermal cells. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(2):609–613. doi: 10.1073/pnas.91.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schacke H, Schottelius A, Docke WD, et al. Dissociation of transactivation from transrepression by a selective glucocorticoid receptor agonist leads to separation of therapeutic effects from side effects. Proc Natl Acad Sci U S A. 2004;101(1):227–232. doi: 10.1073/pnas.0300372101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayashi R, Wada H, Ito K, Adcock IM. Effects of glucocorticoids on gene transcription. Eur J Pharmacol. 2004;500(1–3):51–62. doi: 10.1016/j.ejphar.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 13.Vayssiere BM, Dupont S, Choquart A, et al. Synthetic glucocorticoids that dissociate transactivation and AP-1 transrepression exhibit antiinflammatory activity in vivo. Mol Endocrinol. 1997;11(9):1245–1255. doi: 10.1210/mend.11.9.9979. [DOI] [PubMed] [Google Scholar]
- 14.Belvisi MG, Wicks SL, Battram CH, et al. Therapeutic benefit of a dissociated glucocorticoid and the relevance of in vitro separation of transrepression from transactivation activity. J Immunol. 2001;166(3):1975–1982. doi: 10.4049/jimmunol.166.3.1975. [DOI] [PubMed] [Google Scholar]
- 15.Belvisi MG, Brown TJ, Wicks S, Foster ML. New Glucocorticosteroids with an improved therapeutic ratio? Pulm Pharmacol Ther. 2001;14(3):221–227. doi: 10.1006/pupt.2001.0284. [DOI] [PubMed] [Google Scholar]
- 16.Rosen J, Miner JN. The search for safer glucocorticoid receptor ligands. Endocr Rev. 2005;26(3):452–464. doi: 10.1210/er.2005-0002. [DOI] [PubMed] [Google Scholar]
- 17.Schacke H, Hennekes H, Schottelius A, et al. SEGRAs: a novel class of anti-inflammatory compounds. Ernst Schering Res Found Workshop. 2002;(40):357–371. doi: 10.1007/978-3-662-04660-9_20. [DOI] [PubMed] [Google Scholar]
- 18.Miner JN, Ardecky B, Benbatoul K. Antiinflammatory glucocorticoid receptor ligand with reduced side effects exhibits an altered protein-protein interaction profile. Proc Natl Acad Sci U S A. 2007;104(49):19244–19249. doi: 10.1073/pnas.0705517104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friedman ZY. Recent advances in understanding the molecular mechanisms of tamoxifen action. Cancer Invest. 1998;16(6):391–396. doi: 10.3109/07357909809115779. [DOI] [PubMed] [Google Scholar]
- 20.Osborne CK. Tamoxifen in the treatment of breast cancer. N Engl J Med. 1998;339(22):1609–1618. doi: 10.1056/NEJM199811263392207. [DOI] [PubMed] [Google Scholar]
- 21.Schroder FH. Antiandrogens as monotherapy for prostate cancer. Eur Urol. 1998;34(Suppl 3):12–17. doi: 10.1159/000052291. [DOI] [PubMed] [Google Scholar]
- 22.Wajsman Z. Arguments for the long-term use of combined androgen blockade. Eur Urol. 1998;34(Suppl 3):25–28. doi: 10.1159/000052294. [DOI] [PubMed] [Google Scholar]
- 23.Zhi L, Tegley CM, Marschke KB, Mais DE, Jones TK. 5-Aryl-1,2,3,4-tetrahydrochromeno[3,4-f]quinolin-3-ones as a novel class of nonsteroidal progesterone receptor agonists: effect of A-ring modification. J Med Chem. 1999;42(8):1466–1472. doi: 10.1021/jm980723h. [DOI] [PubMed] [Google Scholar]
- 24.Zhi L, Tegley CM, Pio B, et al. Nonsteroidal progesterone receptor antagonists based on 6-thiophenehydroquinolines. Bioorg Med Chem Lett. 2000;10(5):415–418. doi: 10.1016/s0960-894x(00)00011-1. [DOI] [PubMed] [Google Scholar]
- 25.Louw A, Swart P, de Kock SS, van der Merwe KJ. Mechanism for the stabilization in vivo of the aziridine precursor --(4-acetoxyphenyl)-2-chloro-N-methyl-ethylammonium chloride by serum proteins. Biochem Pharmacol. 1997;53(2):189–197. doi: 10.1016/s0006-2952(96)00661-2. [DOI] [PubMed] [Google Scholar]
- 26.De Bosscher K, Vanden Berghe W, Beck IM, et al. A fully dissociated compound of plant origin for inflammatory gene repression. Proc Natl Acad Sci U S A. 2005;102(44):15827–15832. doi: 10.1073/pnas.0505554102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yemelyanov A, Czwornog J, Chebotaev D, et al. Tumor suppressor activity of glucocorticoid receptor in the prostate. Oncogene. 2007;26(13):1885–1896. doi: 10.1038/sj.onc.1209991. [DOI] [PubMed] [Google Scholar]
- 28.Yemelyanov A, Czwornog J, Gera L, Joshi S, Chatterton RT, Jr, Budunova I. Novel steroid receptor phyto-modulator compound a inhibits growth and survival of prostate cancer cells. Cancer Res. 2008;68(12):4763–4773. doi: 10.1158/0008-5472.CAN-07-6104. [DOI] [PubMed] [Google Scholar]
- 29.Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010;49(11):1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Piechaczyk M, Farras R. Regulation and function of JunB in cell proliferation. Biochem Soc Trans. 2008;36(Pt 5):864–867. doi: 10.1042/BST0360864. [DOI] [PubMed] [Google Scholar]
- 31.Park SA, Kim EH, Na HK, Surh YJ. KG-135 inhibits COX-2 expression by blocking the activation of JNK and AP-1 in phorbol ester-stimulated human breast epithelial cells. Ann N Y Acad Sci. 2007;1095:545–553. doi: 10.1196/annals.1397.059. [DOI] [PubMed] [Google Scholar]
- 32.Seibert K, Masferrer JL. Role of inducible cyclooxygenase (COX-2) in inflammation. Receptor. 1994;4(1):17–23. [PubMed] [Google Scholar]
- 33.Kim SO, Kundu JK, Shin YK, et al. [6]-Gingerol inhibits COX-2 expression by blocking the activation of p38 MAP kinase and NF-kappaB in phorbol ester-stimulated mouse skin. Oncogene. 2005;24(15):2558–2567. doi: 10.1038/sj.onc.1208446. [DOI] [PubMed] [Google Scholar]
- 34.He G, Karin M. NF-kappaB and STAT3 - key players in liver inflammation and cancer. Cell Res. 2011;21(1):159–168. doi: 10.1038/cr.2010.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kelly EJ, Sandgren EP, Brinster RL, Palmiter RD. A pair of adjacent glucocorticoid response elements regulate expression of two mouse metallothionein genes. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(19):10045–10050. doi: 10.1073/pnas.94.19.10045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hengge UR, Ruzicka T, Schwartz RA, Cork MJ. Adverse effects of topical glucocorticosteroids. J Am Acad Dermatol. 2006;54(1):1–15. doi: 10.1016/j.jaad.2005.01.010. quiz 16–18. [DOI] [PubMed] [Google Scholar]
- 37.Schoepe S, Schacke H, May E, Asadullah K. Glucocorticoid therapy-induced skin atrophy. Exp Dermatol. 2006;15(6):406–420. doi: 10.1111/j.0906-6705.2006.00435.x. [DOI] [PubMed] [Google Scholar]
- 38.Li J, Johnson TA, Hanson LA, Beer DG. Loss of glucocorticoid-dependent growth inhibition in transformed mouse lung cells. Mol Carcinog. 1996;16(4):213–220. doi: 10.1002/(SICI)1098-2744(199608)16:4<213::AID-MC5>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 39.Moalli PA, Rosen ST. Glucocorticoid receptors and resistance to glucocorticoids in hematologic malignancies. Leuk Lymphoma. 1994;15(5–6):363–374. doi: 10.3109/10428199409049738. [DOI] [PubMed] [Google Scholar]
- 40.Dewint P, Gossye V, De Bosscher K. A plant-derived ligand favoring monomeric glucocorticoid receptor conformation with impaired transactivation potential attenuates collagen-induced arthritis. J Immunol. 2008;180(4):2608–2615. doi: 10.4049/jimmunol.180.4.2608. [DOI] [PubMed] [Google Scholar]
- 41.Gossye V, Elewaut D, Van Beneden K, Dewint P, Haegeman G, De Bosscher K. A plant-derived glucocorticoid receptor modulator attenuates inflammation without provoking ligand-induced resistance. Ann Rheum Dis. 2009 doi: 10.1136/ard.2008.102871. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Z, Zhang ZY, Schluesener HJ. Compound A, a plant origin ligand of glucocorticoid receptors, increases regulatory T cells and M2 macrophages to attenuate experimental autoimmune neuritis with reduced side effects. J Immunol. 2009;183(5):3081–3091. doi: 10.4049/jimmunol.0901088. [DOI] [PubMed] [Google Scholar]