Abstract
OBJECTIVES:
The objective of this study was to analyze the safety of long-term infliximab treatment, with/without concomitant immunomodulators, across Crohn's disease (CD) and ulcerative colitis (UC) clinical trials.
METHODS:
To maximize sample size, we pooled primary safety data across 10 CD or UC trials, including five randomized, controlled trials contributing data from patients who received intravenous infliximab 5 or 10 mg/kg (n=1,713; ±azathioprine) or placebo (n=406; ±azathioprine). Pooled incidences and 95% confidence intervals (CIs) were determined for mortality, infection, and malignancy. Standardized incidence ratios and 95% CIs were also determined for malignancies using the Surveillance, Epidemiology, and End Results database.
RESULTS:
We observed no increase in infections, serious infections, or malignancy with infliximab vs. placebo in these patients with inflammatory bowel disease (IBD). In patients with UC, but not CD, immunomodulator treatment (vs. treatment without immunomodulator) yielded a higher incidence (95% CI) of infections (120.07 (110.66, 130.08)/100 patient-years (pt-yrs) vs. 92.47 (84.54, 100.94)/100 pt-yrs). Among placebo-treated patients with CD, but not UC, those with immunomodulator use demonstrated a higher incidence (95% CI) of malignancy vs. no immunomodulator treatment (1.84 (0.22, 6.66)/100 pt-yrs vs. 0.00 (0.00, 0.00)/100 pt-yrs). Mortality and infection-related mortality appeared unaffected by infliximab or immunomodulator treatment.
CONCLUSIONS:
Infliximab treatment of IBD did not appear to affect incidences of infection, mortality, or malignancy. Relative to patients with no immunomodulator use, immunomodulator-treated UC patients demonstrated a higher incidence of infection and immunomodulator-plus-placebo-treated CD patients demonstrated a higher incidence of malignancy.
INTRODUCTION
Inflammatory bowel disease (IBD) is a chronic, inflammatory disorder of the gastrointestinal tract. As such, standard therapies for IBD have focused on nonspecific inhibition of inflammation with sulfasalazine, mesalazine, steroids, the thiopurines azathioprine (AZA) and 6-mercaptopurine (6-MP), and methotrexate (MTX) (1). While these agents can be moderately effective in maintaining corticosteroid-induced remission, relapse can be common (2,3). In addition, thiopurine therapy of IBD carries an increased risk of lymphoproliferative disorders (4).
On the basis of the unmet need for patients intolerant of or unresponsive to standard therapy, several antagonists of the proinflammatory cytokine tumor necrosis factor (TNF) have been developed, including adalimumab, etanercept, infliximab, and certolizumab pegol. Although TNF antagonist therapy is generally well tolerated by patients with IBD, a unique spectrum of safety issues related to blocking TNF, including life-threatening and opportunistic infection, malignancy, and mortality, must be considered. While researchers have attempted to obtain consensus on the relationship between TNF antagonist therapy and these relatively rare events, analyses have generally been limited by comparatively small study populations and short periods of patient follow-up.
The TNF antagonist infliximab has been used to treat patients with moderately-to-severely active IBD for more than a decade. As infliximab was approved for the treatment of moderate-to-severe Crohn's disease (CD), the sponsor has conducted several large, randomized, controlled phase 3 clinical trials of infliximab maintenance therapy in IBD (hereafter referred to as “pivotal phase 3 trials”), including the ACCENT I (5,6), ACCENT II (7), and SONIC (8) trials in CD and the ACT 1 and ACT 2 trials (9) that formed the basis for approval of infliximab in ulcerative colitis (UC).
Although safety findings related to each of these studies have been reported in separate publications, a pooled analysis of key safety outcomes has been recently conducted for all sponsor-initiated infliximab trials in IBD, with emphasis on the five pivotal phase 3 trials that contribute nearly 90% of the data for patients in the pooled IBD safety analyses. Given the continued need for safety data related to TNF antagonism, findings of these analyses are detailed herein.
METHODS
The 10 (7 CD, 3 UC) clinical trials included in these pooled analyses represent the totality of the sponsor's clinical safety database for infliximab in the treatment of adult patients with IBD. The pooled studies comprise five smaller trials (4 CD, 1 UC) evaluating a total of 244 patients (10,11,12,13,14,15), 22 of whom contributed data to more than one treatment group per study, and five pivotal phase 3 trials evaluating infliximab maintenance therapy in 1,644 patients with CD (5,6,7,8) and 741 patients with UC (9), 184 of whom contributed data to more than one treatment group per study (Tables 1, 2, 3, 4, 5).
Table 1. Key features of 10 sponsor-initiated studies of infliximab in IBD.
| Study (reference) | Pt. population | Study design | Treatment regimens (no. of pts. evaluated) | AE reporting period (wks) |
|---|---|---|---|---|
| Smaller trials of infliximab in IBD | ||||
| C0168T08 (15) | Severe CD (CDAI >150) refractory to corticosteroid therapy | Phase 1, SC, OL, single dose | Grp 1: Infliximab 10 mg/kg (n=8)Grp 2: Infliximab 20 mg/kg (n=2) | 8 |
| C0168T11 (10) | Moderate-to-severea CD | Phase 2, MC, OL, single dose, sequential dose-escalating trial | Grp 1: Infliximab 1 mg/kg (n=5) | 12 |
| Grp 2: Infliximab 5 mg/kg (n=5) | ||||
| Grp 3: Infliximab 10 mg/kg (n=5) | ||||
| Grp 4: Infliximab 20 mg/kg (n=6) | ||||
| C0168T16 (10,12,14) | Moderate-to-severea CD | Phase 2/3, MC, DB, PC, with initial dose-ranging treatment phase followed by repeated-treatment phase plus OL treatment for safety assessments | Initial dose-ranging phase (single dose)Grp 1: Placebo (n=25)Grp 2: Infliximab 5 mg/kg (n=27)Grp 3: Infliximab 10 mg/kg (n=28)Grp 4: Infliximab 20 mg/kg (n=28) | 16 (n=35) and 48 (n=73) |
| Open-label phase: Infliximab 10 mg/kg (n=48) | ||||
| Repeated-treatment phase (4 DB infusions)Grp 1: Infliximab 10 mg/kg q8wks (n=37)Grp 2: Placebo q8wks (n=36) | ||||
| C0168T20 (11) | Fistulizing CD | MC, DB, PC, randomized phase 3 trial | Grp 1: Infliximab 10 mg/kg at wks 0, 2, 6 (n=32) | 52 |
| Grp 2: Infliximab 5 mg/kg at wks 0, 2, 6 (n=31) | ||||
| Grp 3: Placebo at wks 0, 2, 6 (n=31) | ||||
| C0168T12 (13) | Active UC (modified Truelove and Witts score >10) | MC, DB, PC, randomized phase 2 trial | Single dose of:Grp 1: Placebo (n=3)Grp 2: Infliximab 5 mg/kg (n=3)Grp 3: Infliximab 10 mg/kg (n=3)Grp 4: Infliximab 20 mg/kg (n=2) | 12 |
| Pivotal phase 3 trials of infliximab in IBD | ||||
| ACCENT I (5,6) |
Moderate-to-severea CD |
MC, DB, PC, phase 3 randomized trialAZA, 6-MP, MTX, corticosteroids allowed but not randomized treatments |
All pts.: Infliximab 5 mg/kg at wk 0 (n=573)Grp 1: Placebo at wks 2, 6, and q8wks through wk 46 (n=188)Grp 2: Infliximab 5 mg/kg at wks 2, 6, and q8wks through wk 46 (n=192)Grp 3: Infliximab 5 mg/kg at wks 2 and 6, then 10 mg/kg q8wks through wk 46 (n=193) |
54 |
| ACCENT II (7) | Fistulizing CD | MC, DB, PC, phase 3 randomized trial | All pts.: Infliximab 5 mg/kg at wks 0, 2, 6 (n=306) | 54 |
| AZA, 6-MP, MTX, corticosteroids allowed but not randomized treatments | Grp 1: Placebo at wk 14 and q8wks through wk 46 (crossover to 5 mg/kg possible; n=143 for placebo maintenance) | |||
| Grp 2: Infliximab 5 mg/kg at wk 14 and q8wks through wk 46 (crossover to 10 mg/kg possible; n=139 for infliximab maintenance) | ||||
| SONIC (8) | Moderate-to-severeb CD | MC, DB, ACC, phase 3 randomized trial | Grp 1: AZA 2.5 mg/kg capsules/placebo infusions (n=161) | 54c |
| Naïve to IMs and biologics; patients randomized to IM treatment | Grp 2: Placebo capsules/infliximab 5 mg/kg infusions (n=163)Grp 3: AZA 2.5 mg/kg capsules/infliximab 5 mg/kg infusions (n=179)Capsules (daily)/infusions (wks 0, 2, 6, q8wks through wk 22) | |||
| ACT 1 (9) | UC (364) in pts. with Mayo score of 6–12 pts., Mayo endoscopic subscore of ≥2, and an inadequate response to or tolerance of oral corticosteroids, 6-MP, and/or AZA | MC, DB, PC, phase 3 randomized trialAZA, 6-MP, corticosteroids allowed but not randomized treatments | Grp 1: Placebo at wks 0, 2, 6, and q8wks through wk 46 (n=121)Grp 2: Infliximab 5 mg/kg at wks 0, 2, 6, and q8wks through wk 46 (n=121)Grp 3: Infliximab 10 mg/kg at wks 0, 2, 6, and q8wks through wk 46 (n=122) | 54 |
| ACT 2 (9) | UC (364) in pts. with Mayo score of 6–12 pts., Mayo endoscopic subscore of ≥2, and an inadequate response to or tolerance of 5-ASAs, oral corticosteroids, 6-MP, and/or AZA | MC, DB, PC, phase 3 randomized trialAZA, 6-MP, corticosteroids allowed but not randomized treatments | Grp 1: Placebo at wks 0, 2, 6, and q8wks through wk 22 (n=123)Grp 2: Infliximab 5 mg/kg at wks 0, 2, 6, and q8wks through wk 22 (n=121)Grp 3: Infliximab 10 mg/kg at wks 0, 2, 6, and q8wks through wk 22 (n=120) | 54c |
ACC, active-comparator-controlled; AE, adverse event; 5-ASAs, 5-aminosalicylates; AZA, azathioprine; CD, Crohn's disease; CDAI, Crohn's disease activity index; DB, double-blind; IBD, inflammatory bowel disease; IM, immunomodulators; MC, multicenter; 6-MP, 6-mercaptopurine; MTX, methotrexate; OL, open label; PC, placebocontrolled; pts., patients; q8wks, every 8 weeks; SC, single center; UC, ulcerative colitis; wks, weeks.
Baseline CDAI score between 220 and 400, inclusive.
Baseline CDAI score between 220 and 450, inclusive.
Dosing in the SONIC and ACT 2 main studies ended with the week-22 infusion. Safety data from week 30 through week 54 were collected as part of study extensions.
Table 2. Extent of exposure to infliximab in the pivotal phase 3 IBD trials through week 46.
|
Crohn's disease |
Ulcerative colitis |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
ACCENT I |
ACCENT II |
SONIC |
ACT 1 |
ACT 2 |
||||||||||
| PBOa | INF 5 mg/kg | INF 10 mg/kg | PBOb | INF 5 mg/kg | AZA+PBO | INF 5 mg/kg+PBOc | INF 5 mg/kg+AZAc | PBO | INF 5 mg/kg | INF 10 mg/kg | PBOc | INF 5 mg/kgc | INF 10 mg/kgc | |
| Pts. treated | 188 | 192 | 193 | 143 | 139 | 161 | 163 | 179 | 121 | 121 | 122 | 123 | 121 | 120 |
| Average no. of infliximab infusions | 2.2 | 6.7 | 6.8 | 4.3 | 7.5 | 0.0 | 6.1 | 6.1 | 0.0 | 6.5 | 6.3 | 0.0 | 6.4 | 6.4 |
AZA, azathioprine; IBD, inflammatory bowel disease; INF, infliximab; pts., patients; PBO, placebo infusions, except for the INF+PBO group in SONIC, in which case: PBO, placebo capsules.
In ACCENT 1, placebo patients received 5 mg/kg infliximab at week 0, and some of them also received episodic infusions of infliximab 5 mg/kg.
In ACCENT II, placebo patients received 5 mg/kg infliximab at weeks 0, 2, and 6 before randomization at week 14, and some of them also crossed over to receive infusions of infliximab 5 mg/kg.
Including infliximab infusions received during the main study and the blinded study extension.
Table 3. Summary of infections and serious infections through week 54 of the pivotal phase 3 infliximab IBD studies by treatment and immunomodulator use.
|
Crohn's diseasea |
Ulcerative colitisa |
All inflammatory bowel diseasea |
||||
|---|---|---|---|---|---|---|
| Placebob | Infliximab | Placebob | Infliximab | Placebob | Infliximab | |
| Pts. treated | 161 | 1,228 | 245 | 485 | 406 | 1,713 |
| Total/median pt-yrs of follow-up | 108/0.7 | 1,127/1.0 | 209/0.6 | 831/1.0 | 318/0.6 | 1,958/1.0 |
| No. (%) of pts. with infection | 73 (45.3%) | 603 (49.1%) | 89 (36.3%) | 243 (50.1%) | 162 (39.9%) | 846 (49.4%) |
| P-valuec | 0.402 | <0.001 | <0.001 | |||
| Total incidence | 144 | 1,352 | 224 | 876 | 368 | 2,228 |
| Incidence per 100 pt-yrs | 132.81 | 119.98 | 106.98 | 105.41 | 115.79 | 113.8 |
| 95% CId | (112.00, 156.36) | (113.67, 126.56) | (93.43, 121.94) | (98.54, 112.63) | (104.26, 128.25) | (109.12, 118.62) |
| No. (%) of pts. with serious infection | 9 (5.6%) | 55 (4.5%) | 6 (2.4%) | 26 (5.4%) | 15 (3.7%) | 81 (4.7%) |
| P-value | 0.547 | 0.085 | 0.427 | |||
| Total incidence | 9 | 86 | 6 | 42 | 15 | 128 |
| Incidence per 100 pt-yrs | 8.3 | 7.63 | 2.87 | 5.05 | 4.72 | 6.54 |
| 95% CI | (3.80, 15.76) | (6.10, 9.43) | (1.05, 6.24) | (3.64, 6.83) | (2.64, 7.78) | (5.45, 7.77) |
| System–organ class/common preferred terms (>0.20/per 100 pt-yrs per group) | ||||||
| Resistance mechanism disorder | 5.53 | 4.44 | 1.91 | 1.8 | 3.15 | 3.32 |
| Abscess | 2.77 | 3.02 | 0.96 | 0.36 | 1.57 | 1.89 |
| Fever | 0 | 0.27 | 0 | 0.24 | 0 | 0.26 |
| Infection | 0 | 0.18 | 0.48 | 0.84 | 0.31 | 0.46 |
| Sepsis | 0.92 | 0.27 | 0 | 0.12 | 0.31 | 0.2 |
| Cellulitis | 0 | 0.27 | 0 | 0 | 0 | 0.15 |
| Herpes zoster | 0 | 0.27 | 0 | 0 | 0 | 0.15 |
| Bacterial infection | 1.84 | 0.09 | 0.48 | 0 | 0.94 | 0.05 |
| Gastrointestinal system disorder | 1.84 | 1.24 | 0 | 0.84 | 0.63 | 1.07 |
| Gastroenteritis | 1.84 | 0.18 | 0 | 0.36 | 0.63 | 0.26 |
| Abdominal pain | 0 | 0.27 | 0 | 0 | 0 | 0.15 |
| Respiratory system disorder | 0.92 | 0.44 | 0.96 | 1.68 | 0.94 | 0.97 |
| Pneumonia | 0.92 | 0.44 | 0 | 0.96 | 0.31 | 0.66 |
| Sinusitis | 0 | 0 | 0.48 | 0.12 | 0.31 | 0.05 |
| Upper respiratory infection | 0 | 0 | 0.48 | 0 | 0.31 | 0 |
| Skin and appendages disorder | 0 | 0.53 | 0 | 0 | 0 | 0.31 |
| Urinary system disorder | 0 | 0.18 | 0 | 0.24 | 0 | 0.2 |
| Body as a whole–general disorder | 0 | 0.18 | 0 | 0 | 0 | 0.1 |
| Cardiovascular disorder | 0 | 0.18 | 0 | 0 | 0 | 0.1 |
| Liver and biliary system disorder | 0 | 0.09 | 0 | 0.12 | 0 | 0.1 |
| Musculoskeletal system disorder | 0 | 0.18 | 0 | 0 | 0 | 0.1 |
| Reproductive disorder | 0 | 0.09 | 0 | 0.12 | 0 | 0.1 |
| Nervous system disorder | 0 | 0.09 | 0 | 0 | 0 | 0.05 |
| Ear and hearing disorder | 0 | 0 | 0 | 0.12 | 0 | 0.05 |
| Myo-, endo-, pericardial, coronary and valve disorder |
0 |
0 |
0 |
0.12 |
0 |
0.05 |
| |
No immunomodulatore |
Immuno modulatorf |
No immunomodulatore |
Immunomodulatorf |
No immunomodulatore |
Immunomodulatorf |
| Pts. treated | 776 | 613 | 394 | 334 | 1,170 | 947 |
| Total/median pt-yrs of follow-up | 715/1.0 | 520/1.0 | 541/0.8 | 500/1.0 | 1,256/1.0 | 1,020/1.0 |
| Number (%) of pts. with infection | 397 (51.2%) | 279 (45.5%) | 169 (42.9%) | 163 (48.8%) | 566 (48.4%) | 442 (46.7%) |
| P-value | 0.04 | 0.117 | 0.457 | |||
| Total incidence | 918 | 578 | 500 | 600 | 1,418 | 1,178 |
| Incidence per 100 pt-yrs | 128.42 | 111.07 | 92.47 | 120.07 | 112.93 | 115.48 |
| 95% CI | (120.24, 137.00) | (102.20, 120.51) | (84.54, 100.94) | (110.66, 130.08) | (107.13, 118.97) | (108.98, 122.27) |
| Number (%) of pts. with serious infections | 36 (4.6%) | 28 (4.6%) | 14 (3.6%) | 18 (5.4%) | 50 (4.3%) | 46 (4.9%) |
| P-value | 1 | 0.277 | 0.53 | |||
| Total incidence | 63 | 32 | 18 | 30 | 81 | 62 |
| Incidence per 100 pt-yrs | 8.81 | 6.15 | 3.33 | 6 | 6.45 | 6.08 |
| 95% CI | (6.77, 11.28) | (4.21, 8.68) | (1.97, 5.26) | (4.05, 8.57) | (5.12, 8.02) | (4.66, 7.79) |
AZA, azathioprine; CI, confidence interval; IBD, inflammatory bowel disease; 6-MP, 6-mercaptopurine; MTX, methotrexate; pts., patients; pt-yrs, patient-years.
Includes 3 Crohn's disease and 2 ulcerative colitis, and thus a total of 5, pivotal phase 3 IBD studies.
With or without concomitant conventional therapy.
P-values comparing treatment or immunomodulator use subgroups were calculated with the use of Fisher's exact test.
95% CIs based on an exact method.
No receipt of AZA, 6-MP, or MTX at baseline.
Receipt of AZA, 6-MP, or MTX at baseline.
Table 4. Summary of malignancies (excluding nonmelanoma skin cancers) by treatment both overall and during the main portions of all infliximab IBD studiesa and by immunomodulator use during the controlled portions of the pivotal phase 3 IBD trialsb.
|
Crohn's disease |
Ulcerative colitis |
All inflammatory bowel disease |
||||
|---|---|---|---|---|---|---|
| Placeboc | Infliximab | Placeboc | Infliximab | Placeboc | Infliximab | |
| Overall among all infliximab IBD studiesa | ||||||
| Pts. treated | 217 | 1,427 | 248 | 493 | 465 | 1,920 |
| Total/median pt-yrs of follow-up | 124/0.5 | 1,229/1.0 | 210/0.6 | 832/1.0 | 334/0.6 | 2,061/1.0 |
| All malignancies | ||||||
| No. (%) of pts. with malignancy | 2 (0.9%) | 6 (0.4%) | 0 (0.0%) | 5 (1.0%) | 2 (0.4%) | 11 (0.6%) |
| P-valued | 0.286 | 0.175 | 1 | |||
| Incidence per 100 pt-yrs | 1.61 | 0.49 | 0 | 0.6 | 0.6 | 0.53 |
| 95% CIe | (0.19, 5.82) | (0.18, 1.06) | (0.00, 1.43) | (0.20, 1.40) | (0.07, 2.16) | (0.27, 0.95) |
| Lymphoma | ||||||
| No. (%) of pts. with malignancy | 0 (0.0%) | 2 (0.1%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (0.1%) |
| P-value | 1 | 0 | 1 | |||
| Incidence per 100 pt-yrs | 0 | 0.16 | 0 | 0 | 0 | 0.1 |
| 95% CI | (0.00, 2.41) | (0.02, 0.59) | (0.00, 1.43) | (0.00, 0.36) | (0.00, 0.90) | (0.01, 0.35) |
| Non-lymphoma malignancies | ||||||
| No. (%) of pts. with malignancy | 2 (0.9%) | 4 (0.3%) | 0 (0.0%) | 5 (1.0%) | 2 (0.4%) | 9 (0.5%) |
| P-value | 0.182 | 0.175 | 1 | |||
| Incidence per 100 pt-yrs | 1.61 | 0.33 | 0 | 0.6 | 0.6 | 0.44 |
| 95% CI | (0.19, 5.82) | (0.09, 0.83) | (0.00, 1.43) | (0.20, 1.40) | (0.07, 2.16) | (0.20, 0.83) |
| Controlled portions of all infliximab IBD studiesa | ||||||
| Pts. treated | 217 | 488 | 245 | 483 | 462 | 971 |
| Total/median pt-yrs of follow-up | 121/0.5 | 298/0.6 | 137/0.6 | 333/0.6 | 258/0.6 | 631/0.6 |
| All malignancies | ||||||
| No. (%) of pts. with malignancy | 2 (0.9%) | 0 (0.0%) | 0 (0.0%) | 2 (0.4%) | 2 (0.4%) | 2 (0.2%) |
| P-value | 0.094 | 0.553 | 0.598 | |||
| Incidence per 100 pt-yrs | 1.65 | 0 | 0 | 0.6 | 0.77 | 0.32 |
| 95% CI | (0.20, 5.97) | (0.00, 1.00) | (0.00, 2.18) | (0.07, 2.17) | (0.09, 2.80) | (0.04, 1.15) |
| Lymphoma | ||||||
| No. (%) of pts. with malignancy | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Incidence per 100 pt-yrs | 0 | 0 | 0 | 0 | 0 | 0 |
| 95% CI | (0.00, 2.48) | (0.00, 1.00) | (0.00, 2.18) | (0.00, 0.90) | (0.00, 1.16) | (0.00, 0.47) |
| Non-lymphoma malignancies | ||||||
| No. (%) of pts. with malignancy | 2 (0.9%) | 0 (0.0%) | 0 (0.0%) | 2 (0.4%) | 2 (0.4%) | 2 (0.2%) |
| P-value | 0.094 | 0.553 | 0.598 | |||
| Incidence per 100 pt-yrs | 1.65 | 0 | 0 | 0.6 | 0.77 | 0.32 |
| 95% CI |
(0.20, 5.97) |
(0.00, 1.00) |
(0.00, 2.18) |
(0.07, 2.17) |
(0.09, 2.80) |
(0.04, 1.15) |
| |
No immunomodulatorf |
Immuno modulatorg |
No immunomodulatorf |
Immuno modulatorg |
No immunomodulatorf |
Immunomodulatorg |
| Controlled portions of 5 pivotal IBD studiesb | ||||||
| Pts. treated | 166 | 337 | 394 | 334 | 560 | 671 |
| All malignancies | ||||||
| Total/median pt-yrs of follow-up | 129/1.0 | 250/0.9 | 250/0.6 | 220/0.6 | 378/0.6 | 470/0.7 |
| No. (%) of pts. with malignancy | 0 (0.0%) | 2 (0.6%) | 1 (0.3%) | 1 (0.3%) | 1 (0.2%) | 3 (0.5%) |
| P-value | 1 | 1 | 0.631 | |||
| Incidence per 100 pt-yrs | 0 | 0.8 | 0.4 | 0.45 | 0.26 | 0.64 |
| 95% CI | (0.00, 2.33) | (0.10, 2.89) | (0.01, 2.23) | (0.01, 2.53) | (0.01, 1.47) | (0.13, 1.87) |
| Expected no. of pts.h | 0.43 | 0.71 | 1.22 | 0.88 | 1.65 | 1.6 |
| SIRi |
0 |
2.8 |
0.82 |
1.13 |
0.61 |
1.88 |
| SIR 95% CI | (0.00, 6.92) | (0.34, 10.11) | (0.02, 4.58) | (0.03, 6.30) | (0.02, 3.38) | (0.39, 5.48) |
| |
No immunomodulatorf |
Immuno modulatorg |
No immunomodulatorf |
Immuno modulatorg |
No immunomodulatorf |
Immunomodulatorg |
| Placebo | ||||||
| Pts. treated | 0 | 161 | 137 | 108 | 137 | 269 |
| Total/median pt-yrs of follow-up | 0/0.0 | 108/0.7 | 75/0.6 | 62/0.6 | 75/0.6 | 170/0.6 |
| No. of pts. (%) with malignancy | 0 (0.0%) | 2 (1.2%) | 0 (0.0%) | 0 (0.0%) | 0 (0. 0%) | 2 (0.7%) |
| P-value | 0 | 0 | 0.552 | |||
| Incidence per 100 pt-yrs | 0 | 1.84 | 0 | 0 | 0 | 1.17 |
| 95% CI | (0.00, 0.00) | (0.22, 6.66) | (0.00, 3.97) | (0.00, 4.85) | (0.00, 3.97) | (0.14, 4.24) |
| Expected no. of pts. | 0 | 0.35 | 0.35 | 0.23 | 0.36 | 0.59 |
| SIR | 0 | 5.7 | 0 | 0 | 0 | 3.41 |
| SIR 95% CI | (0.00, 0.00) | (0.69, 20.59) | (0.00, 8.59) | (0.00, 12.76) | (0.00, 8.24) | (0.41, 12.33) |
| Infliximab | ||||||
| Pts. treated | 166 | 176 | 257 | 226 | 423 | 402 |
| Total/median pt-yrs of follow-up | 129/1.0 | 142/1.0 | 174/0.6 | 158/0.6 | 303/0.6 | 300/0.7 |
| No. of pts. (%) with malignancy | 0 (0.0%) | 0 (0.0%) | 1 (0.4%) | 1 (0.4%) | 1 (0.2%) | 1 (0.3%) |
| P-value (infliximab vs. placebo) | 0 | 0.228 | 1 | 1 | 1 | 0.568 |
| P-value (no immunomodulator vs. immunomodulator) | 0 | 1 | 1 | |||
| Incidence per 100 pt-yrs | 0 | 0 | 0.57 | 0.63 | 0.33 | 0.33 |
| 95% CI | (0.00, 2.33) | (0.00, 2.12) | (0.01, 3.20) | (0.02, 3.52) | (0.01, 1.84) | (0.01, 1.86) |
| Expected no. of pts. | 0.43 | 0.36 | 0.82 | 0.65 | 1.29 | 1.01 |
| SIR | 0 | 0 | 1.22 | 1.54 | 0.78 | 0.99 |
| SIR 95% CI | (0.00, 6.92) | (0.00, 8.24) | (0.03, 6.82) | (0.04, 8.57) | (0.02, 4.33) | (0.02, 5.50) |
| Lymphoma | ||||||
| No. (%) of pts. with malignancy | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Incidence per 100 pt-yrs | 0 | 0 | 0 | 0 | 0 | 0 |
| 95% CI | (0.00, 2.33) | (0.00, 2.12) | (0.00, 1.72) | (0.00, 1.89) | (0.00, 0.99) | (0.00, 1.00) |
| Expected no. of pts. | 0.02 | 0.02 | 0.04 | 0.03 | 0.05 | 0.05 |
| SIR | 0 | 0 | 0 | 0 | 0 | 0 |
| SIR 95% CI | (0.00, 157.21) | (0.00,163.60) | (0.00, 83.69) | (0.00, 101.69) | (0.00, 54.61) | (0.00, 62.71) |
| Non-lymphoma malignancies | ||||||
| No. (%) of pts. with malignancy | 0 (0.0%) | 0 (0.0%) | 1 (0.4%) | 1 (0.4%) | 1 (0.2%) | 1 (0.3%) |
| P-value | 0 | 1 | 1 | |||
| Incidence per 100 pt-yrs | 0 | 0 | 0.57 | 0.63 | 0.33 | 0.33 |
| 95% CI | (0.00, 2.33) | (0.00, 2.12) | (0.01, 3.20) | (0.02, 3.52) | (0.01, 1.84) | (0.01, 1.86) |
| Expected no. of pts. | 0.41 | 0.35 | 0.82 | 0.62 | 1.23 | 0.97 |
| SIR | 0 | 0 | 1.22 | 1.61 | 0.81 | 1.03 |
| SIR 95% CI | (0.00, 7.24) | (0.00, 8.67) | (0.03, 6.82) | (0.04, 8.98) | (0.02, 4.53) | (0.03, 5.77) |
AZA, azathioprine; CI, confidence interval; IBD, inflammatory bowel disease; 6-MP, 6-mercaptopurine; MTX, methotrexate; pts., patients; pt-yrs, patient-years; SEER, Surveillance, Epidemiology, and End Results; SIR, standardized incidence ratio.
Includes 7 Crohn's disease and 3 ulcerative colitis, and thus a total of 10, IBD studies.
Includes 3 Crohn's disease and 2 ulcerative colitis, and thus a total of 5 pivotal phase 3 IBD studies.
With or without concomitant conventional therapy.
P-values comparing treatment or immunomodulator use subgroups were calculated with the use of Fisher's exact test. Note that P-values cannot be computed when a group has no patients or when neither group has such an event.
95% CIs based on an exact method.
No receipt of AZA, 6-MP, or MTX at baseline.
Receipt of AZA, 6-MP, or MTX at baseline.
Based on the SEER database (2002) adjusted for age, sex, and race.
Calculated as the quotient of the observed and expected numbers of patients with malignancy.
Table 5. Summary of mortality through week 54 by treatment (all IBD studies) and immunomodulator use status (pivotal phase 3 IBD trials).
|
Crohn's diseasea |
Ulcerative colitisa |
All inflammatory bowel diseasea |
||||
|---|---|---|---|---|---|---|
| Placebob | Infliximab | Placebob | Infliximab | Placebob | Infliximab | |
| Pts. treated | 217 | 1,427 | 248 | 493 | 465 | 1,920 |
| Total/median pt-yrs of follow-up | 124/0.5 | 1,230/1.0 | 210/0.6 | 833/1.0 | 334/0.6 | 2,063/1.0 |
| Deaths | ||||||
| No. (%) of pts. | 1 (0.5%) | 3 (0.2%) | 0 (0.0%) | 1 (0.2%) | 1 (0.2%) | 4 (0.2%) |
| P-valuec | 0.433 | 1 | 1 | |||
| Incidence/100 pt-yrs | 0.8 | 0.24 | 0 | 0.12 | 0.3 | 0.19 |
| 95% CId | (0.02, 4.48) | (0.05, 0.71) | (0.00, 1.43) | (0.00, 0.67) | (0.01, 1.67) | (0.05, 0.50) |
| Infection-related deaths | ||||||
| No. (%) of pts. | 1 (0.5%) | 2 (0.1%) | 0 (0.0%) | 1 (0.2%) | 1 (0.2%) | 3 (0.2%) |
| P-value | 0.346 | 1 | 0.58 | |||
| Incidence/100 pt-yrs | 0.8 | 0.16 | 0 | 0.12 | 0.3 | 0.15 |
| 95% CI |
(0.02, 4.48) |
(0.02, 0.59) |
(0.00, 1.43) |
(0.00, 0.67) |
(0.01, 1.67) |
(0.03, 0.43) |
| |
No immunomodulatore |
Immunomodulatorf |
No immunomodulatore |
Immunomodulatorf |
No immunomodulatore |
Immunomodulatorf |
| Pts. treated | 776 | 613 | 394 | 334 | 1,170 | 947 |
| Total/median pt-yrs of follow-up | 715/1.0 | 520/1.0 | 541/0.8 | 500/1.0 | 1,256/1.0 | 1,020/1.0 |
| Deaths | ||||||
| No. (%) of pts. | 2 (0.3%) | 2 (0.3%) | 0 (0.0%) | 1 (0.3%) | 2 (0.2%) | 3 (0.3%) |
| P-value | 1 | 0.459 | 0.662 | |||
| Incidence/100 pt-yrs | 0.28 | 0.38 | 0 | 0.2 | 0.16 | 0.29 |
| 95% CI | (0.03, 1.01) | (0.05, 1.39) | (0.00, 0.55) | (0.01, 1.11) | (0.02, 0.58) | (0.06, 0.86) |
AZA, azathioprine; CI, confidence interval; IBD, inflammatory bowel disease; 6-MP, 6-mercaptopurine; MTX, methotrexate; pts., patients; pt-yrs, patient-years.
Includes 7 Crohn's disease and 3 ulcerative colitis, or a total of 10, IBD studies when summarized by treatment and 3 Crohn's disease and 2 ulcerative colitis, or a total of 5, pivotal phase 3 IBD studies when summarized by baseline immunomodulator use.
With or without concomitant conventional therapy.
P-values comparing treatment or immunomodulator use subgroups were calculated with the use of Fisher's exact test.
95% CIs based on an exact method.
No receipt of AZA, 6-MP, or MTX at baseline.
Receipt of AZA, 6-MP, or MTX at baseline.
The five pivotal phase 3 trials, i.e., ACCENT I, ACCENT II, SONIC, ACT 1, and ACT 2, contributed approximately 89% (2,119/2,385) of all patients with data in the overall pooled analyses and were generally consistent in terms of study designs, in that they were all randomized, multicenter, double-blind trials that included a control group (Table 1). Note that for four of the five pivotal phase 3 trials (ACCENT I, ACCENT II, ACT 1, and ACT 2), treatment with the immunomodulators AZA, 6-MP, and MTX or with corticosteroids was allowed during study participation, but such use was not a randomized study treatment. Each of these study protocols stipulated that patients would continue a stable regimen of baseline immunomodulator therapy throughout study participation. Conversely, the SONIC trial enrolled exclusively immunomodulator-naïve patients, and these patients were randomized to receive AZA, 5 mg/kg of infliximab, or AZA plus 5 mg/kg of infliximab (8).
All study protocols were approved by the institutional review board at each participating site, and all patients provided written informed consent before beginning study participation. Janssen Biotech, Inc. (Horsham, PA) provided infliximab, active comparator (AZA in the SONIC trial), and placebo (as appropriate) for intravenous infusion.
To evaluate the occurrence of uncommon events, safety data from the seven studies in CD (with the majority of the data coming from the pivotal ACCENT I, ACCENT II, and SONIC studies) were pooled and are reported as ‘CD studies'. When pooled with data from the three ‘UC studies' (with the majority of the data deriving from the pivotal ACT 1 and ACT 2 studies), they are reported as ‘IBD studies'. Safety data from the pivotal phase 3 trials in IBD, i.e., ACCENT I, ACCENT II, SONIC, ACT 1, and ACT 2, were also separately pooled across the three CD, two UC, and all five pivotal phase 3 IBD studies. Summaries of key design features of these studies are provided in Table 1.
The incidences of adverse events per 100 patient-years (pt-yrs) of follow-up were calculated for infections, malignancies (including both solid tumors and hematological malignancies and excluding nonmelanoma skin cancers) and deaths by treatment group (infliximab vs. placebo) and also by immunomodulator use (treatment vs. no treatment) for infections and malignancies as the quotient of the total number of events and pt-yrs of follow-up multiplied by 100; exact 95% confidence intervals (CIs) were also calculated.
For malignancies (excluding nonmelanoma skin cancer), standardized incidence ratios (SIRs) were also calculated as the quotient of the observed and expected numbers of patients with malignancy; 95% CIs were determined using exact methodology. The expected numbers of malignancy were derived using data adjusted for age, sex, and race from the general US population in the Surveillance, Epidemiology, and End Results (SEER) database (16).
Fisher's exact test was used to compare the proportions of patients who experienced an adverse event of interest (e.g., infection, malignancy, or death) between treatment groups. Because a large number of safety parameters were evaluated, the Fisher's exact test is employed not for hypothesis testing but rather as an aid in signal detection to highlight differences requiring closer examination.
All patients in ACCENT I and ACCENT II received infliximab 5 mg/kg at week 0 and were therefore counted in the infliximab column in the calculation of pt-yrs of follow-up. Pt-yrs of follow-up for placebo were determined for the 161 placebo plus AZA-treated CD patients in the SONIC study plus additional placebo-treated patients from other CD studies (T08, T11, T16, and T20; see Table 1) as applicable to the subpopulation being assessed. Note that infliximab use, both in combination with immunomodulators and alone, and immunomodulator use, both in combination with infliximab and alone, are pooled in these analyses such that infliximab use refers to any use of infliximab and immunomodulator use refers to any use of immunomodulators. Also note that the placebo group includes only patients who never received infliximab.
As noted above, infliximab treatment was generally randomized and blinded, while immunomodulator treatment, with the exception of the SONIC trial, reflects immunomodulator use at baseline, i.e., immunomodulator use was not randomized or blinded and assumes that such use continued during the study. Protocols for these four of five pivotal phase 3 trials mandated that patients receiving a stable immunomodulator regimen at baseline would continue such use throughout study participation.
RESULTS
Analysis groups and extent of exposure
Across the 10 sponsor-initiated infliximab trials in IBD (five smaller studies and five pivotal phase 3 studies conducted following infliximab's initial approval), safety data for 2,385 patients were available for pooled safety analyses (see Tables 3, 4, 5). Note that some patients presented in Tables 1 and 2 contributed data to more than one treatment group in the analyses presented in Tables 3, 4, 5, e.g., 22 of the 244 patients in the five smaller studies and 33 of the 2,086 patients in the five pivotal trials. Data from the five pivotal trials were pooled for additional analyses, both across all five studies (n=2,119) and across the three CD studies (n=1,389) or 2 UC (n=730) studies due to similarities in study design and homogeneity of the patient populations. In the 5 pivotal IBD trials, 406 and 1,713 patients were treated with placebo and infliximab, respectively (Table 3). Among the 2,117 patients with documentation of immunomodulator use at baseline (yes/no), 947 patients did and 1,170 patients did not receive the immunomodulators AZA, 6-MP, or MTX (Tables 3 and 5).
The extent of exposure to individual study agents for each of the pivotal phase 3 trials is summarized in Table 2, which includes infliximab infusions received during the main studies, as well as the blinded study extensions of SONIC and ACT 2. Excluding patients who initially received infliximab but who were later randomized to placebo maintenance treatment, CD patients received an average of 6.1–7.5 infliximab infusions and UC patients received an average of 6.3–6.5 infliximab infusions. Note that study agent administration in the SONIC and ACT 2 main studies ended with the week-22 infusion and that safety data from week 30 through week 54 were collected as part of a blinded study extension, during which patients continued to receive blinded study agent.
Infections
The incidences of infections and serious infections were determined across the five pivotal phase 3 IBD trials. A larger proportion of infliximab- than placebo-treated UC patients (50.1% vs. 36.3% P<0.001) had at least one infection. Among CD patients, however, the proportions of patients who experienced at least one infection were similar between the infliximab- and placebo-treated patients (49.1% vs. 45.3% P=0.402). The proportions of patients who experienced at least one serious infection were also similar between placebo- and infliximab-treated patients (Table 3). When expressed on the basis of length of patient follow-up, the incidences (95% CIs) per 100 pt-yrs of infections were 132.81 (112.00, 156.36) in placebo- vs. 119.98 (113.67, 126.56) in infliximab-treated CD patients; 106.98 (93.43, 121.94) in placebo- vs. 105.41 (98.54, 112.63) in infliximab-treated UC patients; and 115.79 (104.26, 128.25) in placebo- vs. 113.80 (109.12, 118.62) in infliximab-treated IBD patients. Similar patterns of overlapping 95% CIs between the placebo and infliximab groups were also observed for serious infections (Table 3).
When assessed by the patient's baseline immunomodulator treatment (yes/no), the 95% CIs surrounding the incidences of infections and serious infections overlapped between patients treated with immunomodulators and those not treated with immunomodulators in all patient populations (CD, UC, all IBD), with one exception. In patients with UC, but not CD, immunomodulator treatment (vs. no treatment) yielded a higher incidence (95% CI) of infections (120.07 (110.66, 130.08)/100 pt-yrs vs. 92.47 (84.54, 100.94)/100 pt-yrs) (Table 3).
A summary of the incidences of serious infections (per 100 pt-yrs of follow-up) by system–organ class and preferred term is also provided in Table 3. The most common serious infections were those considered resistance mechanism disorders (3.15 and 3.32/100 pt-yrs in placebo- and infliximab-treated IBD patients, respectively), gastrointestinal disorders (0.63 and 1.07/100 pt-yrs), and respiratory system disorders (0.94 and 0.97/100 pt-yrs). Within these three system–organ classes, the most common serious infections were abscess (1.57 and 1.89/100 pt-yrs in placebo- and infliximab-treated IBD patients, respectively), gastroenteritis (0.63 and 0.26/100 pt-yrs), and pneumonia (0.31 and 0.66/100 pt-yrs) (Table 3).
Malignancy
Across the 10 IBD trials and excluding nonmelanoma skin cancers, 13 patients (two placebo-treated, 11 infliximab-treated) had a malignancy during study participation. Of the malignancies, 11 were non-lymphoma, while two patients had lymphoma. Both patients with lymphoma were infliximab-treated CD patients. One patient who received infliximab 5 mg/kg at week 0 followed by placebo maintenance and AZA had natural killer cell lymphoma diagnosed after study participation ended. A second patient with a history of AZA use received a single infusion of infliximab 10 mg/kg and was diagnosed with intravascular B-cell lymphoma 9.5 months after the single infliximab infusion. The non-lymphoma malignancies included breast (n=2), colon (n=2), prostate (n=2), bladder (n=1), lung (n=1), renal (n=1), skin (n=1), and rectal (n=1) cancers (9 infliximab-treated, 2 placebo).
When expressed on the basis of incidence (95% CI) per 100 pt-yrs of follow-up, overlapping 95% CIs indicated that the incidences of malignancies were similar in the placebo- and infliximab-treated patients with CD (1.61 (0.19, 5.82) vs. 0.49 (0.18, 1.06), respectively) and with UC (0.00 (0.00, 1.43) vs. 0.60 (0.20, 1.40), respectively). Findings observed within the lymphoma and non-lymphoma malignancy subcategories were similar (Table 4).
The incidences of malignancies during only the controlled portions of the 10 IBD trials were also determined. Four patients, two placebo-treated and two infliximab-treated, had a malignancy during the controlled study phases. No cases of lymphoma were documented during the controlled portions of the 10 IBD studies. When comparing the proportions of patients who had malignancy diagnosed during the studies (both overall and during the controlled portions), results of Fisher's exact testing indicated no significant difference between infliximab- and placebo-treated patients (Table 4).
Further, when expressed as incidence (95% CI) per 100 pt-yrs of follow-up, the incidences of malignancy appeared similar in placebo- vs. infliximab-treated patients with CD (1.65 (0.20, 5.97) vs. 0.00 (0.00, 1.00), respectively) and UC (0.00 (0.00, 2.18) vs. 0.60 (0.07, 2.17), respectively). Consistent findings were observed within the lymphoma and non-lymphoma malignancy subcategories (Table 4).
The incidence of malignancy was also assessed by immunomodulator use in the controlled portions of the five pivotal phase 3 IBD trials. When comparing the proportions of patients who had malignancy diagnosed during the studies (both overall and during the controlled portions), results of Fisher's exact testing indicated no significant difference between infliximab- and placebo-treated patients or between patients with and without immunomodulator use (Table 4).
Further, in all three of the patient populations (CD, UC, and all IBD), the 95% CIs surrounding the incidences of malignancy overlapped when compared between patients who were treated vs. those who were not treated with immunomodulators, with one exception. Among placebo-treated patients with CD, but not UC, those with immunomodulator use demonstrated a higher incidence (95% CI) of malignancy vs. no immunomodulator treatment (1.84 (0.22, 6.66)/100 pt-yrs vs. 0.00 (0.00, 0.00)/100 pt-yrs).
In a separate analysis comparing the observed incidences of malignancy with rates expected in the general US population based on the SEER database, all 95% CIs surrounding the malignancy SIRs contained 1 in all analysis groups (i.e., placebo and infliximab, with and without baseline immunomodulator use). This indicates that the incidences of malignancy observed in the controlled portions of the pivotal phase 3 trials are not significantly different from the expected rates in the general US population. Similar findings were observed within the lymphoma and non-lymphoma malignancy subcategories (Table 4).
In addition to the malignancies discussed above, among the 2,385 patients with IBD included in these analyses (465 placebo, 1,920 infliximab), five patients (3 infliximab-treated, 2 placebo-treated) had basal cell carcinoma and two patients (both infliximab-treated) had malignant skin neoplasm.
Mortality
Five patients died during the 10 IBD trials. The deaths included a patient (63-year-old female with baseline immunomodulator use) with CD in SONIC who received AZA monotherapy and died of sepsis following a colectomy; three infliximab-treated patients with CD in ACCENT I who died of septic shock (35-year-old female with no baseline immunomodulator use), sepsis (57-year-old female with no baseline immunomodulator use), and myocardial infarction (37-year-old male with baseline immunomodulator use); and one infliximab-treated patient with UC in ACT 2 (56-year-old male with baseline immunomodulator use) who died following diagnosis of pulmonary histoplasmosis during the open-label, long-term, follow-up period.
Given the longer follow-up periods for infliximab-treated patients, when summarized as incidence (95% CI) per 100 pt-yrs of follow-up, overlapping CIs indicated no increase in mortality with infliximab vs. placebo treatment among patients with CD (0.24 (0.05, 0.71) vs. 0.80 (0.02, 4.48), respectively), UC (0.12 (0.00, 0.67) vs. 0.00 (0.00, 1.43), respectively), or IBD (0.19 (0.05, 0.50) vs. 0.30 (0.01, 1.67), respectively). The same was true for infection-related deaths, which accounted for four of the five deaths. Overlapping 95% CIs were also observed in a separate analysis of mortality by immunomodulator use in the five pivotal phase 3 IBD trials (Table 5).
DISCUSSION
Since receiving marketing authorization for the treatment of CD more than a decade ago, infliximab has gained wide acceptance as a highly effective treatment option for IBD. As a result, longer-term safety data are becoming available. No difference in the incidence of neoplasia between adult patients with CD who were (n=404) and were not (n=404) treated with infliximab was reported by Biancone et al (17). An additional 4 years of follow-up of 591 of the patients in this matched-pair study yielded consistent findings, with 3.9% (12/304) of infliximab-treated patients and 4.2% (12/287) of patients not treated with infliximab diagnosed with neoplasia (P=0.95) (18). Fidder et al. (19), who retrospectively examined medical records of 734 infliximab-treated IBD patients and 666 controls, also observed no difference between the two groups in mortality, malignancy including lymphoma, or infection rate. In a retrospective safety analysis of 799 German IBD patients treated with thiopurines and/or TNF antagonists between 2002 and 2010, an elevated risk of malignancy (4.2% vs. 1.5%, P=0.024, odds ratio=2.86), but not infection (14.4% vs. 15.5%, P=0.69), was observed in patients treated with only thiopurines relative to patients treated with TNF antagonists with or without thiopurines (20). Results of several large randomized phase 3 clinical trials of infliximab maintenance therapy in IBD, including the ACCENT I (5,6), ACCENT II 7 and SONIC (8) trials in CD and the ACT 1 and ACT 2 trials (9) in UC also contribute to the growing body of infliximab safety data.
Findings presented herein from a pooled analysis of key safety outcomes, derived from 10 sponsor-conducted IBD studies with large cohorts of IBD patients treated by referral centers in daily practice, are generally consistent with conclusions drawn by Biancone et al. (17,18) and Fidder et al. (19). Specifically, results of our pooled analyses of infliximab safety data in the treatment of IBD indicate no increase in infections or serious infections with infliximab vs. placebo treatment in patients with CD or UC. Independent of infliximab use, immunomodulator treatment did not appear to increase the incidence of infections or serious infections in patients with CD but did yield a higher incidence of infection vs. no immunomodulator treatment in patients with UC. One explanation for the lack of an increase in the incidence of serious infections with either infliximab or immunomodulators in CD is that many of the complications of CD are inherently infectious in nature and may be decreased by effective CD therapy. Results derived from the TREAT Registry of CD patients, which included assessment of the role of corticosteroids in infectious complications, have shown that infliximab allows for steroid tapering and discontinuation in CD (21). Several of the protocols for the pivotal phase 3 trials included in this report mandated steroid tapering during the early phase of the trial. The lower steroid consumption in the reported CD trials may also contribute to the relatively low incidence of serious infections, regardless of immunomodulator use, as such an effect could offset any small increase in serious infections that might be associated with the immunosuppressive nature of these drugs. These findings are also consistent with additional data from the TREAT Registry that indicated that the risk for serious infection in infliximab-treated CD patients is similar to that for patients receiving conventional immunomodulators (21). Despite our observation of no increase in serious infections, all patients should be screened for pre-existing infections before the start of any immunosuppressive therapy.
Overall, 13 patients (2 placebo-treated, 11 infliximab-treated) had a malignancy (excluding nonmelanoma skin cancer) during the 10 IBD trials, equating to incidences of 0.60 and 0.53/100 pt-yrs, among placebo- and infliximab-treated IBD patients, respectively. Two of the malignancies in infliximab-treated CD patients were lymphomas; the 11 other malignancies across both cohorts were non-lymphoma.
During the controlled portions of the 10 IBD trials, four patients (2 placebo, 2 infliximab) had a malignancy, all non-lymphoma, equating to incidences of 0.77 and 0.32/100 pt-yrs, respectively, of placebo- and infliximab-treated IBD patients. The incidence of malignancy was not higher with infliximab treatment, nor was it significantly impacted by immunomodulator use. In a separate analysis comparing the observed incidences of malignancy with rates expected in the general US population based on the SEER database, the incidence of malignancy was not significantly different.
A higher incidence of malignancy was observed in placebo-treated patients receiving vs. those not receiving immunomodulators. These findings may support others suggesting that the thiopurines AZA and 6-MP are associated with a moderately increased risk of malignancy, particularly lymphoproliferative disease. Specifically, in a French cohort of nearly 20,000 patients with IBD (60% with CD, 40% with UC or unclassified IBD) followed for an average of 35 months, the risk of lymphoproliferative disorder was 5 times higher in patients exposed to thiopurines than in those never exposed to these drugs. Older age, male sex, and longer duration of IBD were also associated with increased risk of lymphoproliferative disorder in the French cohort (22). Also, as noted above, results of a retrospective safety analysis of 799 German IBD patients treated with thiopurines and/or TNF antagonists between 2002 and 2010 indicated an elevated risk of malignancy (4.2% vs. 1.5%, P=0.024, odds ratio=2.86) in patients treated with only thiopurines relative to patients treated with TNF antagonists with or without thiopurines (20).
We also determined malignancy SIRs within the groups of patients with and without baseline immunomodulator use. Results of these pooled analyses indicated no significant difference from the expected rates in the general US population. Conversely, recently reported results of a meta-analysis of 26 studies of infliximab, adalimumab, and certolizumab, including almost 9,000 CD patients, indicated that use of anti-TNF agents with immunomodulators is associated with an increased, albeit small (6.1/10,000 pt-yrs), risk of non-Hodgkin's lymphoma relative to the expected rate of non-Hodgkin's lymphoma derived from the SEER database, i.e., 1.9/10,000 pt-yrs (SIR (95% CI)=3.23 (1.5, 6.9)), and also relative to the expected rate for CD patients treated with only immunomodulators, i.e., 4.0/10,000 pt-yrs (SIR (95% CI)=1.70 (0.5, 7.1)) (23). As noted above, no cases of lymphoma were reported during the controlled portions of the trials comprising our pooled analyses. In addition to the malignancies discussed above, among the 2,385 patients with IBD included in these analyses (465 placebo, 1,920 infliximab), five patients (3 infliximab-treated, 2 placebo-treated) had basal cell carcinoma and two patients (both infliximab-treated) had nonmelanoma malignant skin neoplasm. It is still not certain whether or not infliximab use increases the risk of malignancy, but it is possible that the impact of infliximab is no worse than that of conventional immunomodulators and that, by effectively controlling inflammation, infliximab may contribute to a lower malignancy risk in IBD. The latter possibility requires further confirmation with longer-term data.
Consistent with published reports citing no evidence of increased mortality in CD patients treated with anti-TNF agents (19,20,24), we observed no difference in mortality between placebo- and infliximab-treated patients with either CD or UC. The same was true for infection-related deaths, which accounted for four of the five deaths. Immunomodulator treatment was also unassociated with increases in mortality in these IBD patients.
One point to note in the interpretation of these data is that, with the exception of the SONIC trial (8), immunomodulator treatment was not randomized, blinded, or controlled and reflects patient treatment at the time of study entry (as study protocols stipulated that any baseline immunomodulator treatment regimen would remain stable throughout study participation). It therefore remains possible that any higher event incidence in the immunomodulator-treated patients reflects their having more severe IBD rather than immunomodulator use itself. For this reason, any comparison between event rates between infliximab vs. immunomodulators must be made with caution. Still, the data of Fidder et al. (19) showed no difference in the rates of infection, malignancy including lymphoma, and mortality between IBD patients treated with infliximab and IBD patients treated with conventional therapies. It should also be noted, however, that the overall pooled results do not differ from those of the SONIC trial, in which both treatment with infliximab and treatment with immunomodulators were randomized in a controlled trial. It is also important to note that the relatively short period of follow-up, along with the relative lack of power inherent in these clinical trial data for determining treatment group differences in rare safety events, limit our ability to draw definitive conclusions from these analyses. The powering, however, is fairly good for detecting a doubling or tripling of the malignancy rate, both of which are clinically important to exclude. The 2,061 pt-yrs of follow-up from the infliximab-treated IBD cohort in this paper would yield 71% or 99% power to detect a doubling or tripling in malignancy incidence, respectively. Representing data derived from rigorous clinical trials, therefore, the current data are somewhat reassuring and have merit when assessing the overall safety of anti-TNF agents.
When taken together, results of these pooled analyses indicate no increase in serious infection, mortality, or malignancy, including lymphoma in association with infliximab treatment of IBD. In addition, the safety of infliximab in these analyses appears comparable to that of conventional immunomodulators.
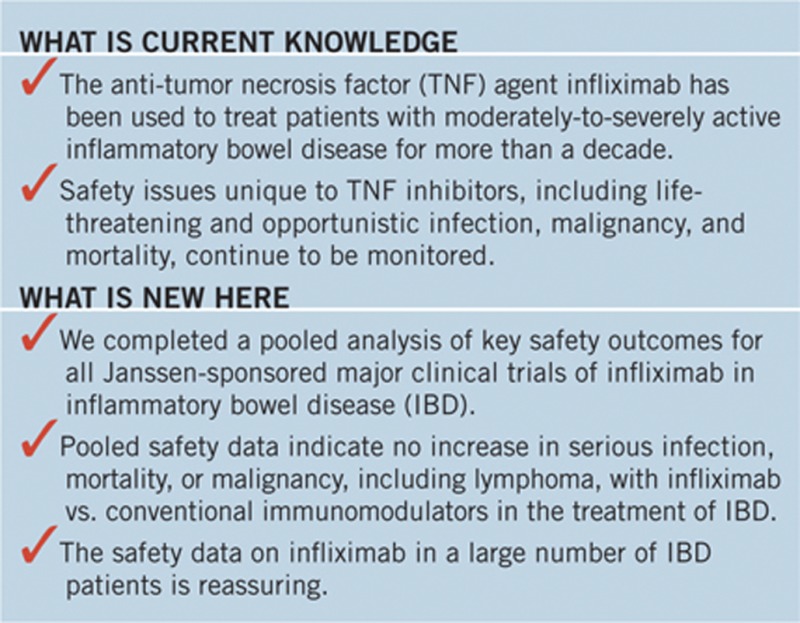
STUDY HIGHLIGHTS
Acknowledgments
We thank James P. Barrett and Mary Ann Thomas of Janssen Biotech, Inc., for their work on the study protocols, Kathryn Mingione of Janssen for her work pertaining to data interpretation, and Michelle Perate and Mary H. Whitman of Janssen Biotech, Inc., for their writing support.
Guarantor of the article: Gary R. Lichtenstein, MD.
Specific author contributions: Design and conduct of clinical trials contributing data to these pooled analyses: Gary R. Lichtenstein, Paul Rutgeerts, William J. Sandborn, Bruce E. Sands, and Robert H. Diamond; data collection and analysis: Robert H. Diamond, Linda Tang, Jennifer Montello, and Freddy Cornillie; and all authors provided critical content review and final approval of this manuscript.
Financial support: Funding for the clinical trials contributing data to these pooled analyses was provided by Janssen Research & Development, a Johnson & Johnson (J&J) pharmaceutical company.
Potential competing interests: Dr Lichtenstein has received research grants and/or has served as a consultant for Abbott Corporation, Alaven, Bristol-Myers Squibb, Janssen, Elan, Ferring, Meda Pharmaceuticals, Merck/Schering-Plough, Millennium Pharmaceuticals, Pfizer Pharmaceuticals, Proctor and Gamble, Prometheus Laboratories, Salix Pharmaceuticals, Santarus, Shire Pharmaceuticals, UCB, Warner Chilcotte, and Wyeth. Professor Rutgeerts has received research support from Janssen, Merck/Schering-Plough, UCB, and Abbott Laboratories, as well as consulting and/or speaking honoraria from Janssen, Merck/Schering-Plough, UCB, Abbott Laboratories, Elan-Biogen, NovImmune, Italfarmako, Bristol-Myers Squibb, Millennium Pharmaceuticals, Tillots, Glaxo SmithKline, and ChemoCentryx. Dr Sandborn has received has received consulting fees and research support from Abbott Laboratories, Janssen, and UCB Pharma, as well as consulting fees from Merck/Schering-Plough. Dr Sands has received consulting fees from Abbott Immunology, Axcan Pharma, Avaxia Biologics, Bristol-Myers Squibb, Elan Pharmaceuticals, Emmi Solutions, Janssen, Glaxo Wellcome SmithKline, Millennium Pharmaceuticals/Takeda, Novartis Pharmaceuticals, Pfizer, Prometheus Laboratories, and owns common stock in Avaxia Biologics, a company that is not publicly traded. Professor Colombel has received consulting fees and has participated in continuing medical education events supported by unrestricted educational grants from Janssen and Merck/Schering-Plough. Dr Diamond and Linda Tang are employed by Janssen Research & Development, a J&J pharmaceutical company. Jennifer Montello is an employee of J&J. Freddy Cornillie is an employee of Janssen Biologics BV, a J&J pharmaceutical company.
References
- Ferkolj I. How to improve the safety of biologic therapy in Crohn's disease. J Physiol Pharmacol. 2009;60 (Suppl 7:67–70. [PubMed] [Google Scholar]
- Biancone L, Tosti C, Fina D, et al. Review article: maintenance treatment of Crohn's disease. Aliment Pharmacol Ther. 2003;17 (Suppl 2:31–37. doi: 10.1046/j.1365-2036.17.s2.20.x. [DOI] [PubMed] [Google Scholar]
- Patel V, MacDonald JK, MacDonald JW, et al. Methotrexate for maintenance of remission in Crohn's disease. Cochrane Database Sys Rev. 2009;4:CD006884. doi: 10.1002/14651858.CD006884.pub2. [DOI] [PubMed] [Google Scholar]
- Ha C, Dassopoulos T. Thiopurine therapy in inflammatory bowel disease. Expert Rev Gastroenterol Hepatol. 2010;4:575–588. doi: 10.1586/egh.10.59. [DOI] [PubMed] [Google Scholar]
- Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet. 2002;359:1541–1549. doi: 10.1016/S0140-6736(02)08512-4. [DOI] [PubMed] [Google Scholar]
- Rutgeerts P, Feagan BG, Lichtenstein GR, et al. Comparison of scheduled and episodic treatment strategies of infliximab in Crohn's disease. Gastroenterology. 2004;126:402–413. doi: 10.1053/j.gastro.2003.11.014. [DOI] [PubMed] [Google Scholar]
- Sands BE, Anderson FH, Bernstein CN, et al. Infliximab maintenance therapy for fistulizing Crohn's disease. N Engl J Med. 2004;350:876–885. doi: 10.1056/NEJMoa030815. [DOI] [PubMed] [Google Scholar]
- Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med. 2010;362:1383–1395. doi: 10.1056/NEJMoa0904492. [DOI] [PubMed] [Google Scholar]
- Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis N Engl J Med 20053532462–2476.Erratum in: N Engl J Med 2006;354:2200. Comment/author reply in: N Engl J Med 2006;354:1424–6; J Pediatr Gastroenterol Nutr 2006;42:589–90. [DOI] [PubMed] [Google Scholar]
- Cornillie F, Shealy D, D'Haens G, et al. Infliximab induces potent anti-inflammatory and local immunomodulatory activity but no systemic immune suppression in patients with Crohn's disease. Aliment Pharmacol Ther. 2001;15:463–473. doi: 10.1046/j.1365-2036.2001.00956.x. [DOI] [PubMed] [Google Scholar]
- Present DH, Rutgeerts P, Targan S, et al. Infliximab for the treatment of fistulas in patients with Crohn's disease. N Engl J Med. 1999;340:1398–1405. doi: 10.1056/NEJM199905063401804. [DOI] [PubMed] [Google Scholar]
- Rutgeerts P, D'Haens G, Targan S, et al. Efficacy and safety of retreatment with anti-tumor necrosis factor antibody (infliximab) to maintain remission in Crohn's disease. Gastroenterology. 1999;117:761–769. doi: 10.1016/s0016-5085(99)70332-x. [DOI] [PubMed] [Google Scholar]
- Sands BE, Tremaine WJ, Sandborn WJ, et al. Infliximab in the treatment of severe, steroid-refractory ulcerative colitis: a pilot study. Inflamm Bowel Dis. 2001;7:83–88. doi: 10.1097/00054725-200105000-00001. [DOI] [PubMed] [Google Scholar]
- Targan SR, Hanauer SB, van Deventer SJ, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn's disease. Crohn's Disease cA2 Study Group. N Engl J Med. 1997;337:1029–1035. doi: 10.1056/NEJM199710093371502. [DOI] [PubMed] [Google Scholar]
- van Dullemen HM, van Deventer SJ, Hommes DW, et al. Treatment of Crohn's disease with anti-tumor necrosis factor chimeric monoclonal antibody (cA2) Gastroenterology. 1995;109:129–135. doi: 10.1016/0016-5085(95)90277-5. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute Surveillance, Epidemiology and End Results 2002 . http://seer.cancer.gov .
- Biancone L, Orlando A, Kohn A, et al. Infliximab and newly diagnosed neoplasia in Crohn's disease: a multicentre matched pair study. Gut. 2006;55:228–233. doi: 10.1136/gut.2005.075937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biancone L, Petruzziello C, Orlando A, et al. Cancer in Crohn's disease patients treated with infliximab: a long-term multicenter matched pair study. Inflamm Bowel Dis. 2011;17:758–766. doi: 10.1002/ibd.21416. [DOI] [PubMed] [Google Scholar]
- Fidder H, Schnitzler F, Ferrante M, et al. Long-term safety of infliximab for the treatment of inflammatory bowel disease: a single center cohort study. Gut. 2009;58:501–508. doi: 10.1136/gut.2008.163642. [DOI] [PubMed] [Google Scholar]
- Ochsenkühn T, Steinborn A, Beigel F, et al. Rate of malignancies and infections in a large single center cohort of IBD patients treated with thiopurines and anti-TNF-antibodies Dig Dis Week 2011. May 7–10:Abstract Tu1230.
- Lichtenstein GR, Feagan BG, Cohen RD, et al. Serious infections and mortality in association with therapies for Crohn's disease: TREAT Registry Clin Gastroenterol Hepatol 20064621–630.Erratum in Clin Gastroenterol Hepatol 2006;4:931. Comment in: Inflamm Bowel Dis 2007;13:933–4. [DOI] [PubMed] [Google Scholar]
- Beaugerie L, Brousse N, Bouvier AM, for the CESAME Study Group et al. Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Lancet. 2009;374:1617–1625. doi: 10.1016/S0140-6736(09)61302-7. [DOI] [PubMed] [Google Scholar]
- Siegel CA, Marden SM, Persing SM, et al. Risk of lymphoma associated with combination anti-tumor necrosis factor and immunomodulator therapy for the treatment of Crohn's disease: a meta-analysis. Clin Gastroenterol Hepatol. 2009;7:874–881. doi: 10.1016/j.cgh.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombel JF, Sandborn WJ, Panaccione R, et al. Adalimumab safety in global clinical trials of patients with Crohn's disease. Inflamm Bowel Dis. 2009;15:1308–1319. doi: 10.1002/ibd.20956. [DOI] [PubMed] [Google Scholar]


