Abstract
Co-stimulatory molecules are important for regulating T cell activation and immune response. CD274 [programmed death ligand 1 (PD-L1), B7-H1] has emerged as an important immune modulator that can block T cell receptor signalling. We have investigated whether PD-L1 and other co-stimulatory ligands could be expressed in human B cells stimulated by cytosine–phosphate–guanosine (CpG)-DNA. CpG-DNA strongly induced the co-inhibitory molecule ligand, PD-L1, of human B cells. Results show that nuclear factor-kappa B (NF-κB) signalling is involved directly in CpG-DNA-induced PD-L1 expression in human B cells. We sought to determine the effect of CpG-DNA-treated B cells on T helper type 2 (Th2) cytokine production in Cry j 1 (Japanese pollen antigen)-stimulated human CD4-positive cells from patients with seasonal allergic rhinitis caused by Japanese cedar pollen. CpG-DNA-treated B cells reduced Cry j 1-induced interleukin (IL)-5 and IL-13 production in CD4-positive cells. When the binding of PD-1 to PD-L1 was inhibited by PD-1-immunoglobulin (Ig), this chimera molecule reversed the previously described reductions in IL-5 and IL-13 production. In contrast, the CpG B-treated B cells increased both interferon (IFN)-γ and IL-12 production in the presence of Cry j 1-stimulated CD4-positive cells. CpG-DNA simultaneously reduced the expression of B7RP-1 [also known as inducible co-stimulator ligand (ICOSL), B7-H2] and the ligand of CD30 (CD30L). These results indicate that CpG-DNA induces co-inhibitory molecule ligand PD-L1 expression in human B cells and PD-L1 can suppress Th2 cytokine production in Cry j 1-stimulated CD4-positive cells, while CpG-DNA increased Th1 cytokine production and reduced the expression of co-stimulatory molecule ligands that can promote Th2 inflammatory responses.
Keywords: CD274, CpG, Cry j 1, human B cells, Th2 cytokine
Introduction
Although the immunoglobulin (Ig) superfamily of co-stimulatory and co-inhibitory ligands could play critical roles in modulating the immune responses of T helper type 2 (Th2) cells [1], it is unknown how these molecules are expressed in human B cells and whether they affect the function of Th2 cells during allergic reactions. Programmed death 1 (PD-1) belongs to the B7 family of co-inhibitory proteins. Its ligand CD274 (also known as PD-L1 and B7-H1) is expressed on immune cells and non-haematopoietic cells. PD-L1, expressed on lymphocytes, inhibits their cytolytic function by phosphorylating immunoreceptor tyrosine-based switch motifs and blockading T cell receptor signalling. PD-1 and PD-L1 interactions are necessary for maintaining peripheral immune tolerance and for modulating T cell activation [2]. The expression levels of PD-L1 in human primary bronchial epithelial cells are increased by stimulation of the synthetic dsRNA analogue containing Toll-like receptor 3 (TLR-3) [3]. Monosphoryl lipid A, which ligates TLR-4, up-regulates PD-L1 expression in human Langerhans cells [4].
TLRs, which recognize pathogen-associated molecular patterns, play a key role in innate immunity. It remains unclear which TLR ligands induce or inhibit the ligands of co-stimulatory or co-inhibitory molecule expression in human B cells. DNA containing cytosine–phosphate–guanosine (CpG) motifs (CpG-DNA) involving TLR-9 has immunomodulatory effects, including the suppression of allergic responses mediated by Th2 cells. They directly induce the expression of T-bet mRNA and inhibit IgG1 and IgE switching in purified murine B cells [5]. In humans, inhibition of IgE production is mediated by both interferon (IFN)-γ and interleukin (IL)-12 [6]. CpG-DNA increases synergistically the activation of human B cells induced by Epstein–Barr virus (EBV) [7] and an agonistic CD40 antibody [8], whereas CpG-DNA directly induces expression of the beta-defensin-2 gene in human B cells [9]. Although the ligands of co-stimulatory or co-inhibitory molecule expression in human B cells are important, because they are involved in adaptive immune responses, the effects of CpG-DNA stimulation on co-stimulatory or co-inhibitory ligands in human B cells remain unclear.
In this study, we investigated whether CpG-DNA affects the expression of co-stimulatory or co-inhibitory ligands in human B cells. We sought to determine the effects of CpG-DNA-treated B cells on IL-5, IL-13, IFN-γ and IL-12 production in Cry j 1-stimulated human CD4-positive cells from patients with seasonal allergic rhinitis (SAR) caused by Japanese cedar (JC). We also examined whether PD-L1 is involved in IL-5, IL-13, IFN-γ and IL-12 production from CD4-positive cells with CpG-B-treated B cells using PD-1-Ig, a chimera molecule that inhibits the binding of PD-1 to PD-L1. Furthermore, to demonstrate the intracellular pathways involved in PD-L1 expression, we used specific inhibitors of nuclear factor (NF)-κB, p38 mitogen-activated protein kinase (p38 MAPK), c-Jun N-terminal kinase (JNK) and extracellular signal-related kinase (ERK) signalling in these events.
Materials and methods
Reagents
The following reagents were used: SP600125 (Biomol Research Laboratories Inc., Plymouth Meeting, PA, USA) as a specific inhibitor of JNK, SB203580 (Promega Corp., Madison, WI, USA) as a specific inhibitor of p38 MAPK, U0126 (Promega) as a specific inhibitor of MEK-1, Bay 117082 (Calbiochem, Darmstadt, Germany) and NEMO-binding domain (NBD) peptide (Enzo Life Sciences, Farmingdale, NY, USA) as specific inhibitors of NF-κB signalling, the Japanese cedar pollen allergen Cry j 1 (Hayashibara Biochemical Laboratories, Okayama, Japan) or recombinant human PD-1/Fc chimera (R&D Systems, Inc., Minneapolis, MN, USA).
Oligodeoxynucleotides (ODN)
The following ODN were synthesized at Hokkaido System Science Co., Ltd (Sapporo, Japan) (small letters: phosphorothioate linkage; capital letters: phosphodiester linkage): ODN 2216 (A type CpG-DNA) 5′-ggGGGACGATCGTCgggggG-3′, ODN 2243 (GpC control to CpG 2216) 5′-ggGGGAGCATGCTCgggggG-3′, ODN 2006 (B type CpG-DNA) 5′-tcgtcgttttgtcgttttgtcgtt-3′ and ODN 2137 (GpC control for ODN 2006) 5′-tgctgcttttgtgcttttgtgctt-3 [10].
Cells, cell lines and cell culture
Peripheral blood mononuclear cells (PBMC) were isolated from the peripheral blood of healthy volunteers or patients with SAR caused by JC, who were diagnosed on the basis of an elevated score on the capsulated hydrophobic carrier polymer-radioallergosorbent test against major JC allergen Cry j 1 [11]. All patients had a JC-specific CAP-RAST score of 2 or more. A low-density fraction was separated from the PBMC using Lymphoprep™ (Axis-Shield, Oslo, Norway). The cell suspension was centrifuged and PBMC were obtained. T cells and B cells were isolated from PBMC by magnetic affinity cell sorting (MACS) separation (Miltenyi Biotec, Gladbach, Germany). CD19 MicroBeads were used for B cell separation and CD4 MicroBeads were used for T cell separation. The PBMC were then resuspended in buffer, and CD19 or CD4 MicroBeads were added and incubated for 15 min at 6–12°C. The cells were then washed and centrifuged; the resultant cell pellet was resuspended in buffer and the cells were separated magnetically using autoMACS. Magnetically labelled cells were collected and centrifuged. The cells were then resuspended and cultured in RPMI-1640 medium (Nissui Pharmaceutical, Tokyo, Japan), supplemented with 10% heat-inactivated fetal calf serum (FCS) (Gibco, Grand Island, NY, USA), 0·29 mg/ml glutamine, 100 U/ml penicillin and 100 µg/ml streptomycin at 37°C in 5% CO2 and humidified air. The human B lymphoma cell line Ramos 2G6 (American Type Culture Collection, Rockville, MD, USA) was maintained and cultured in complete RPMI-1640.
Real-time reverse transcription–polymerase chain reaction (RT–PCR)
Total RNA was extracted using a total RNA isolation kit (NucleoSpin™ RNA II; Machery-Nagel, Düren, Germany). Reverse transcription reaction was performed with TaqMan® RT Reagents (Applied Biosystems Japan, Tokyo, Japan) using random hexamer primers. Amplification of TLR and β2 microglobulin cDNA was performed in a MicroAmp optical 96-well reaction plate (Applied Biosystems). All TaqMan® probe/primer combinations used for this study were TaqMan® Gene Expression Assay products purchased from Applied Biosystems. β2-microglobulin was chosen as the reference housekeeping gene because it is convenient to assay and is highly expressed. Furthermore, to select the housekeeping gene we evaluated it using a TaqMan® human endogenous control plate, which was the most suitable. TaqMan® PCR was performed in a 20-µl volume using TaqMan® Universal PCR master mix (Applied Biosystems). The reaction was performed (ABI PRISM 7000 Sequence Detection System; Applied Biosystems), and the reaction mixtures were pre-incubated for 2 min at 50°C. The PCR programme involved 10 min of Taq Gold activation at 95°C with 40 subsequent cycles of 15 s at 95°C and 1 min at 60°C (maximum ramping speed between temperatures). Human cDNA equivalent to 50 ng of total RNA from each sample was assayed in each tube. The threshold cycle number (Ct) was determined with Sequence Detector Software (version 1·1; Applied Biosystems) and transformed using comparative Ct methods as described by the manufacturer, with β2-microglobulin used as the calibrator gene.
Flow cytometry
Human B cells were Fc-blocked with human IgG (R&D Systems, Inc., Minneapolis, MN, USA) for 15 min at room temperature. After blocking, B cells were stained for 30 min at room temperature in staining buffer (0·1% FCS and 0·01% sodium azide) and unbound antibody was removed by washing the cells in staining buffer. PD-L1 expression was analysed on a FACSCaliber (FACSCanto2; Becton Dickinson, Franklin Lakes, NJ, USA). Allophycocyanin (APC) anti-human CD274 (PD-L1) antibody and APC mouse IgG2b (κ isotype control) (Biolegend, San Diego, CA, USA) were used for staining.
Enzyme-linked immunosorbent assay (ELISA)
The cytokine concentrations of cell-free culture supernatants were measured using ELISA kits (Biosource International, Inc., Camarillo, CA, USA) according to the manufacturer's instructions.
Western blot
Whole cell lysates were electrophoresed on 10% sodium dodecyl sulphate (SDS)-polyacrylamide gels and transferred to polyvinylidene fluoride membranes (Millipore Corp., Bedford, MA, USA). The transblotted membranes were blocked with skimmed milk and stained with goat polyclonal antibody against PD-L1 (R&D Systems, Inc.) or anti-phosphorylated-inhibitor-kappa Bα (phospho-IκBα) monoclonal antibody (Cell Signaling, Beverly, MA, USA), before being stained with anti-goat or anti-mouse IgG-horseradish peroxidase (HRP) antibody (R&D Systems, Inc.). Signals were detected using the enhanced chemiluminescence (ECL) Plus Western Blotting Detection System (Amersham, Buckinghamshire, UK) and scanned using a FluoroChem Digital Imaging System (Alpha Innotech Corp., San Leonardo, CA, USA).
Data and statistical analysis
Statistical analysis was performed using the Wilcoxon signed-rank test to assess the significance of results.
Results
CpG-DNA induces PD-L1 expression in human B cells
To determine whether co-inhibitory molecules are expressed in human B cells, we screened the stimulated human B cells from healthy volunteers and human B cell line Ramos 2G6 cells for their expression using real-time RT–PCR. Figure 1a shows that mRNA expression of PD-L1 in human B cells was induced markedly in the presence of B type CpG-DNA (CpG-B) to a level 10 times higher than that observed in the absence of CpG-DNA (P < 0·05). CpG-B also induced PD-L1 expression in Ramos 2G6 cells (Fig. 1a). The exposure of B cells to CpG-B triggered rapid expression of PD-L1-mRNA at 6 h, which decreased thereafter. PD-L1 expression was increased eightfold at 24 h and by twofold (P < 0·05) at 48 h (data not shown). In contrast, although 10-fold induction of PD-L1 expression was detected (P < 0·05) in human B cells after treatment with 1 µM of CpG-B, fivefold induction of PD-L1 expression was detected after treatment with 1 µM of A type CpG-DNA (CpG-A). No induction was detected in Ramos 2G6 cells, even after treatment with 1 µM of CpG-A.
Fig. 1.
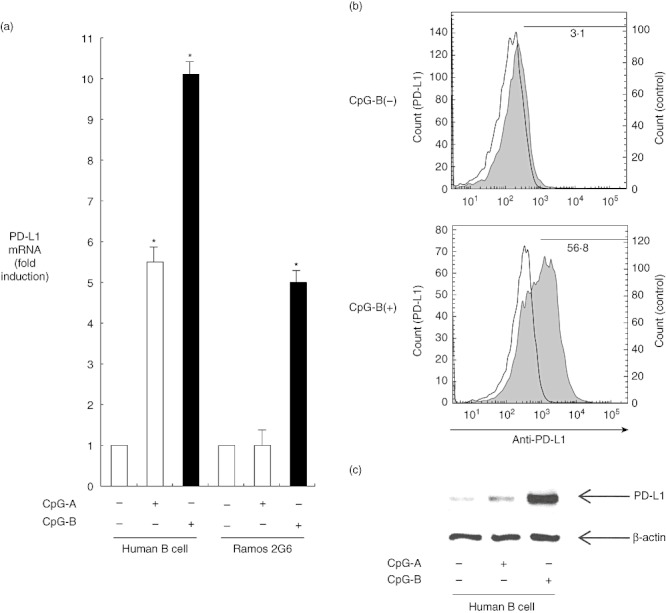
(a) Programmed death ligand 1 (PD-L1) expression induced by cytosine–phosphate–guanosine (CpG)-DNA in human B cells. Human B cells from peripheral blood mononuclear cells (PBMC) and Ramos 2G6 cells were incubated in the presence or absence of 1 µM oligodeoxynucleotide (ODN) 2216 (CpG-A) or ODN 2006 (CpG-B) for 6 h. The mRNA was reverse-transcribed to cDNA, which was then used for real-time polymerase chain reaction (PCR). All reactions were performed in triplicate. The results were normalized to the levels of β2 microglobulin mRNA. The data are presented as the mean ± standard error of the mean fold increase relative to the control (n = 6); *P < 0·05. (b) CpG-DNA induces PD-L1 surface expression on human B cells. Human B cells from PBMC were incubated in the presence or absence of 1 µM ODN 2006 (CpG-B) for 48 h. Cells were harvested and the expression of PD-L1 was analysed using a fluorescence activated cell sorter (FACS)Caliber. The open histogram indicates staining with isotype control, and the closed histogram indicates staining with allophycocyanin (APC)-anti-PD-L1 antibody. (c) Human B cells from PBMC were cultured with medium in the presence or absence of 1 µM ODN 2216 (CpG-A) or ODN 2006 (CpG-B) for 24 h. After the cells had been harvested, the samples were separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and electroblotted. The presence of PD-L1 and β-actin was monitored by Western blot/enhanced chemiluminescence (ECL) reaction using anti-PD-L1 antibody and anti-β-actin antibody. The positions of PD-L1 and β-actin are indicated by arrows.
Similar to other co-stimulatory members, PD-L1 is generally expressed as a transmembrane protein. In order to detect the surface levels of PD-L1 on human B cells, we assessed the effect of CpG-B on PD-L1 surface expression of human B cells by flow cytometry using APC anti-PD-L1-specific antibody. Figure 1b shows that the surface expression of PD-L1 in human B cells was induced markedly in the presence of CpG-B to 18 times higher than that observed in the absence of CpG-DNA. The protein expression of PD-L1 was also examined using Western blotting. Figure 1c shows that the CpG-B-induced form of PD-L1-protein in human B cells weighs 39 kDa. Furthermore, we have also confirmed that CpG-B induces PD-L1 expression on human B cells using confocal laser scanning microscopy (data not shown).
CpG-DNA-treated human B cells suppress IL-5 production in Cry j 1-stimulated CD4-positive cells
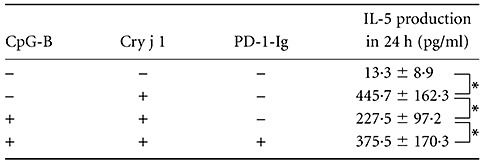
CD4-positive cells acquire ‘Th2 cell identity’, the capability to produce a large amount of Th2 cytokines selectively, and play an important role in humoral immunity and allergic reactions. Having shown that CpG-B induces PD-L1 expression strongly in human B cells, we investigated its effect on IL-5 production in Cry j 1-stimulated human CD4-positive cells using CpG-B-treated B cells from patients with seasonal allergic rhinitis. After the CD4-positive cells had been treated with Cry j 1 for 24 h, IL-5 protein production was detected using ELISA. IL-5 production was induced by Cry j 1 in the presence of non-treated B cells (P < 0·05), and CpG-B-treated B cells reduced Cry j 1-induced IL-5 production by 50% (P < 0·05) (Table 1). PD-1 is a cell surface molecule that regulates the adaptive immune response. The engagement of PD-1 by its ligand PD-L1 transduces a signal that inhibits cytokine production by T cells. We sought to determine whether PD-1 is involved directly in IL-5 production by CD4-positive cells using CpG-B-treated B cells; we then tested the ability of PD-1-Ig, a chimera molecule that inhibits the binding of PD-1 to PD-L1. Table 1 shows that PD-1-Ig reversed its reduction of IL-5 production by 68% (148·0 pg/ml) compared with reduction (218·2 pg/ml) by the CpG-B-treated B cells without PD-1-Ig (P < 0·05).
Table 1.
Cytosine–phosphate–guanosine (CpG)-DNA-treated human B cells suppressed interleukin (IL)-5 production in Cry j 1-stimulated CD4-positive cells. B cells and CD4-positive cells were separated from patients with seasonal allergic rhinitis. The B cells were pretreated with or without 1 µM oligodeoxynucleotide (ODN) 2006 (CpG-B) for 12 h, washed and co-incubated with CD4-positive cells. The resultant supernatants were harvested 24 h after stimulation with Cry j 1 (10 µg/ml) in the presence or absence of soluble programmed death ligand 1-immunoglobulin (PD-1-Ig) (1 µg/ml); the levels of IL-5 were then determined using enzyme-linked immunosorbent assay. The results are shown as the mean ± standard error of the mean (n = 6); *P < 0·05.
CpG-DNA-treated human B cells suppress IL-13 production in Cry j 1-stimulated CD4-positive cells
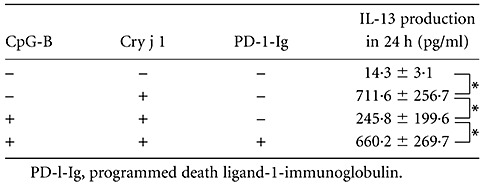
We next investigated the effect of CpG-B on IL-13 production in Cry j 1-stimulated human CD4-positive cells using CpG-B-treated B cells from patients with seasonal allergic rhinitis. IL-13 production was induced by Cry j 1 in the presence of non-CpG-B-treated B cells (P < 0·05), and CpG-B-treated B cells reduced Cry j 1-induced IL-13 production by 66% (P < 0·05).
We also examined whether PD-1 is involved in IL-13 production by CD4-positive cells using CpG-B-treated B cells and PD-1-Ig, which inhibits PD-1-induced signalling. Table 2 shows that PD-1-Ig reversed its reduction of IL-13 production by 89% (414·4 pg/ml), compared with reduction (465·8 pg/ml) by the CpG-B-treated B cells without PD-1-Ig (P < 0·05).
Table 2.
Cytosine–phosphate–guanosine (CpG)-DNA-treated human B cells suppress interleukin (IL)-13 production in Cry j 1-stimulated CD4-positive cells. Samples were prepared as shown in Table 1. The IL-13 levels were determined using enzyme-linked immunosorbent assay. Results are shown as the mean ± standard error of the mean (n = 6); *P < 0·05.
CpG-B-treated B cells could not reduce Cry j 1-induced IL-4 production significantly, although IL-4 production was induced by Cry j 1 in the presence of non-CpG-B-treated B cells (P < 0·05) (data not shown).
IFN-γ and IL-12 production from Cry j 1-stimulated CD4-positive cells with CpG-DNA-treated human B cells
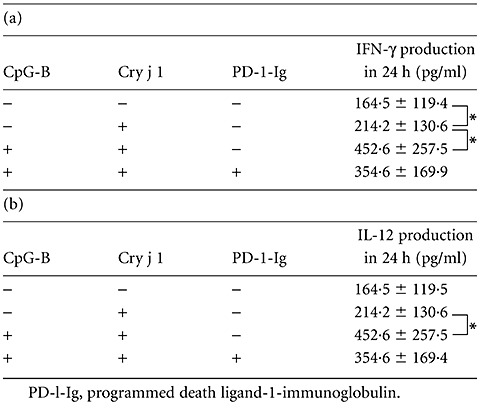
CpG-DNA is a potent inducer of Th1 cytokines that also play important roles in allergic reactions, and we investigated IFN-γ and IL-12 production from Cry j 1-stimulated CD4-positive cells and CpG-DNA-treated human B cells from patients with seasonal allergic rhinitis (Table 3). While IFN-γ production was induced by Cry j 1 (P < 0·05), we could not detect a significant increase of IL-12 production from Cry j 1-stimulated CD4-positive cells. Conversely, the CpG-B-treated B cells increased both IFN-γ (P < 0·05) (Table 3a) and IL-12 production (P < 0·05) (Table 3b) in the presence of Cry j 1-stimulated CD4-positive cells. At the same time, we examined whether PD-1 is involved in the production of these cytokines using the chimera molecule, PD-1-Ig. Table 3 shows that PD-1-Ig could not reverse the increase of IFN-γ and IL-12 production compared with the situation without PD-1-Ig.
Table 3.
Interferon (IFN)-γ and interleukin (IL)-12 production from Cry j 1-stimulated CD4-positive cells with cytosine–phosphate–guanosine (CpG)-DNA-treated human B cells. Samples were prepared as shown in Table 1. The levels of IFN-γ (a) and IL-12 (b) were determined using enzyme-linked immunosorbent assay. Results are shown as the mean ± standard error of the mean (n = 6); *P < 0·05.
Effect of CpG-DNA on co-stimulatory molecule ligands in human B cells
While CpG-DNA strongly induced the co-inhibitory molecule ligand PD-L1, we also screened the effect of CpG-DNA on co-stimulatory molecule ligands in human B cells by real-time RT–PCR. As shown in Fig. 2a, the expression of B7-related protein-1 (B7RP-1) in human B cells was suppressed in the presence of CpG-B by 67%, and CpG-A suppressed its expression by 49%. This ligand co-stimulatory molecule, via the B7RP-1 (ICOSL)-ICOS pathway, is involved in the development of airway allergic inflammation [12]. At the same time, CpG-B suppressed the expression of CD30L in human B cells by 70% (Fig. 2b). CD30L plays an important role in allergic rhinitis. Symptoms of allergic rhinitis, levels of antigen-specific IgE in the sera and the Th2 response in lymphoid tissues were diminished drastically in antigen-sensitized CD30L knock-out mice following intranasal challenge with antigen [13]. CpG-B not only induced the expression of PD-L1 that inhibited Th2 cytokines, but also reduced the expression of B7RP-1 and CD30L, which could promote Th2 inflammatory responses.
Fig. 2.
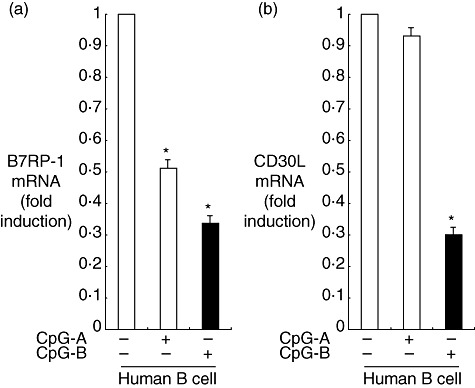
Effect of cytosine–phosphate–guanosine (CpG)-DNA on expression levels of the ligand of co-stimulatory molecules; (a) B7-related protein-1 (B7RP-1), (b) CD30L. Human B cells from peripheral blood mononuclear cells (PBMC) were incubated in the presence or absence of 1 µM oligodeoxynucleotide (ODN) 2216 (CpG-A) or ODN 2006 (CpG-B) for 6 h. The mRNA was reverse-transcribed to cDNA, which was then used for real-time polymerase chain reaction (PCR). All reactions were performed in triplicate. The results were normalized to the levels of β2 microglobulin mRNA. Data are presented as the mean ± standard error of the mean fold increase relative to the control (n = 6); *P < 0·05.
The effect of CpG-DNA on NF-κB signalling related to PD-L1 expression in human B cells
CpG-B induces PD-L1 expression, mainly through TLR-9 signalling. NF-κB and MAPKs participate in CpG-DNA-related or TLR-9-related intracellular signalling [10]. To determine whether NF-κB signalling or MAPK signalling is involved directly in CpG-B-induced PD-L1 expression, we tested the abilities of NBD peptide and Bay 11-7082 (NF-κB signalling inhibitors), SB203580 (a specific inhibitor of p38 MAPK signalling), SP600125 (JNK inhibitor) or U0126 (a specific inhibitor of ERK signalling) to affect the PD-L1 expression of human B cells stimulated with CpG-B (Fig. 3a).
Fig. 3.
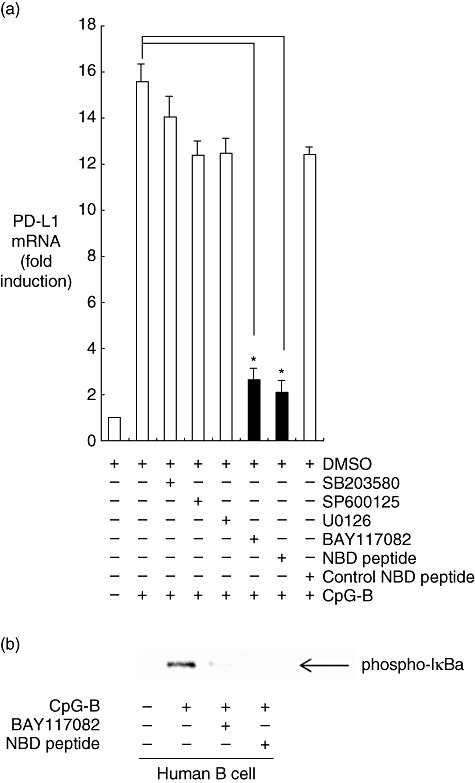
The effect of cytosine–phosphate–guanosine (CpG)-DNA on nuclear factor (NF)-κB signalling related to programmed death ligand 1 (PD-L1) expression in human B cells. (a) Effects of signal transduction inhibitors on CpG-DNA-induced PD-L1 expression. Human B cells from peripheral blood mononuclear cells (PBMC) were pre-incubated with 10% dimethylsulphoxide (DMSO) (a vehicle control), 10 µM SP600125 [a c-Jun N-terminal kinase (JNK) inhibitor], 10 µM SB203580 [a p38 mitogen-activated protein kinase (MAPK) inhibitor], 10 µM U0126 [an extracellular signal-related kinase (ERK) inhibitor], 10 µM Bay 117082 (a NF-κB signalling inhibitor), 100 µM NEMO-binding domain (NBD) peptide (an NF-κB signalling inhibitor) or control NBD peptide for 30 min. The cells were then stimulated with 1 µM oligodeoxynucleotide (ODN) 2006 (CpG-B) for 6 h. The mRNA was reverse-transcribed to cDNA, which was then used for real-time polymerase chain reaction (PCR). All reactions were performed in triplicate. The results were normalized to the expression of β2 microglobulin mRNA. Data are expressed as the mean ± standard error of the mean fold increase relative to the control (n = 6); *P < 0·05. (b) The phosphorylation of inhibitor kappa B α(IκBα) induced by CpG-DNA. After pre-incubation with Bay 11-7082 (10 µM) or NBD peptide (100 µM) NF-κB signalling inhibitors for 30 min, the human B cells were stimulated with or without CpG-B (1 µM) for 30 min. Then the cells were harvested, lysed, applied to each lane and blotted with anti-phosphorylated IκBα antibody. The position of phosphorylated IκBα is indicated on the right by the arrow.
Bay 11-7082 and NBD peptide decreased the expression of PD-L1 markedly in CpG-B-stimulated cells, from 15·6-fold to 2·6-fold (P < 0·05) and to 2·1-fold (P < 0·05), respectively. The expression of PD-L1 was not affected significantly by the JNK inhibitor SP600125, the specific inhibitor of ERK signalling U0126, the p38 MAPK inhibitor SB203580 and control NBD peptide. No differences were found in cell shape or viability among the cells treated with the five inhibitors (data not shown).
In order to confirm the activation of NF-κB signalling cascades in the CpG-DNA-treated human B cells from patients with seasonal allergic rhinitis, the cell lysates were then analysed using Western blotting for phosphorylation of inhibitor kappa Bα (IkBα), which is required critically for NF-κB activation. As shown in Fig. 3b, CpG-B increased phosphorylation of IkBα markedly. In contrast, Bay 11-7082 (selective inhibitor of IkBα phosphorylation) and NBD peptide (IκB kinase inhibitor) decreased CpG-B-induced phosphorylation of IkBα.
Discussion
This study demonstrated that CpG-DNA strongly induced co-inhibitory molecule ligand PD-L1 expression in human B cells. CpG-B-treated B cells reduced Cry j 1-induced IL-5 and IL-13 production in CD4-positive cells. When the binding of PD-1 to PD-L1 was inhibited using PD-1-Ig, this chimera molecule reversed the reductions in IL-5 and IL-13 production induced by CpG-B-treated B cells. In contrast, the CpG-B-treated B cells increased both IFN-γ and IL-12 production in the presence of Cry j 1-stimulated CD4-positive cells. At the same time, CpG-DNA reduced the expression of co-stimulatory molecule ligands B7RP-1 and CD30L on human B cells. CpG-DNA-induced PD-L1 expression of human B cells has not been reported previously. Its intracellular signalling mechanism also remains unclear. Because CpG-B activated NF-κB signalling in human B cells, and pre-incubation with NF-κB signalling inhibitor reversed the CpG-DNA-induced expression of PD-L1, NF-κB signalling is involved directly in CpG-induced PD-L1 expression.
The initial gene up-regulation corresponded to a period when TLR-9 ligation stimulated genes that were functionally associated with the generation of innate and adaptive immune responses [14]. The ligation of TLR-9 to CpG-DNA activates memory B cells, enhances EBV-mediated transformation of naive B cells [7], augments CD40 cross-linking-induced activation of B cells [8] and enhances antigen-specific B cell proliferation and differentiation, leading to the formation of extrafollicular plasma cells. CpG-DNA enhances the kinetics, magnitude and longevity of the vaccine-specific memory B cell pool [15]. Direct conjugation of antigen and CpG-DNA reveals a mechanism that operates during the initiation of primary immune responses, which is useful as a strategy for the design of adjuvants suitable for vaccinations [16].
However, CpG-DNA has immunomodulatory effects that trigger anti-allergic immune responses by directly regulating T-bet expression in mouse B cells via a signalling pathway that is dependent upon TLR-9 and inhibiting the IgG1 and IgE switching induced by IL-4 and CD40 signalling [5]. The ligation of CpG-DNA to an antigen elicits a lower IgE/IgG2a ratio than that induced by the allergen alone [17]. In an in-vitro explant model composed of sinonasal tissue, CpG-DNA reduced the expression of the proinflammatory cytokine IL-5, although no significant difference was found in the expression levels of IL-12, IFN-γ or TLR-9 [18]. We have shown that CpG-DNA-treated B cells reduced Th2 cytokine production in Cry j 1-stimulated CD4-positive cells. PD-L1 expressed on human ocular cells reportedly plays a role in controlling ocular inflammation by inhibiting the production of proinflammatory and Th2 cytokines by activated T cells [19]. PD-1, an immunoreceptor belonging to the CD28/cytotoxic T lymphocyte antigen (CTLA)-4 family, regulates antigen receptor signalling negatively by recruiting a protein tyrosine phosphatase, src homology 2 domain-containing protein tyrosine phosphatase 2 (SHP-2), upon interacting with its ligand PD-L1 [20]. We have shown that CpG-B induces PD-L1 expression in human B cells by suppressing Th2 cytokine production in Cry j 1-stimulated CD4-positive cells, mainly through this negative signalling regulation. Conversely, CpG-DNA provokes an abundance of Th1-skewing cytokines, including IFN-γ and IL-12 [21], and in our system CpG-B also increased IFN-γ and IL-12 production. This increase was not reversed by PD-1-Ig.
TLRs play key roles in innate immunity by recognizing pathogen-associated molecular patterns. The exposure of TLR-3 ligand dsRNA to BEAS2B and human primary bronchial epithelial cells caused increased levels of cell-surface and mRNA expression of PD-L1 (B7-H1), but not of B7-H2 or B7-H3 [3]. Human oral Langerhans cells express TLR-4, and its ligation to monosphoryl lipid A up-regulated the expression of PD-L1 [4]. Here, we show that TLR-9 ligand CpG-DNA induces PD-L1 expression in human B cells.
CpG-DNA triggers anti-allergic immune responses in B cells that are dependent upon TLR-9 [5]. In a previous study, gene expression analysis showed an association between CpG-DNA-stimulated genes and immune responses including the NF-κB and B cell-receptor pathways [14]. In another study, the suppression of myeloid differentiation primary response gene 88 (MyD88) function and the inhibition of NF-κB nuclear localization, or treatment with NF-κB pathway inhibitors, blocked the cellular function of CpG-DNA in a human B cell line [9]. Regulatory molecules of the B7-H-family are important for immune homeostasis, but their mechanism of regulation remains largely unknown. In this study, we have shown that CpG-B markedly increased phosphorylation of IkBα in human B cells from patients with seasonal allergic rhinitis. Bay 11-7082 and NBD peptide decreased CpG-B-induced phosphorylation of IkBα, and pre-incubation with these NF-κB signalling inhibitors strongly suppressed CpG-DNA-induced PD-L1 expression. The phosphorylation on IκBα leads inhibitor molecules to be degraded by the proteasome. With the degradation of IκBα, the NF-κB complex is then freed to enter the nucleus and can turn on the expression of specific genes. PD-L1 expression is controlled by signal transducer and activator of transcription-3 (STAT-3) on APCs or lymphoma cells [22],[23], as STAT-3 binds to the CD274 gene promoter. In human B cells, CpG-B affected the phosphorylation of STAT-3 and its effect was reversed by these NF-κB signalling inhibitors (data not shown). STAT-3 might also participate in CpG-B-induced PD-L1 expression on human B cells, although further studies need to be performed.
CpG-DNA is currently used in clinical trials. After treating allergic patients with CpG-DNA as an adjuvant for subcutaneous immunotherapy, together with a house dust mite allergen extract, the symptoms of rhinitis and allergic asthma decreased significantly [24],[25]. In addition, when CpG-DNA was used as a pro-Th1 adjuvant, it decreased the risk of allergen sensitization and IgE induction and prevented anaphylactic shock after allergen provocation in a mouse model [17]. Although CpG-DNA provokes the expression of an abundance of Th1-skewing cytokines, including IL-12, IFN-α and IFN-γ, the administration of CpG-DNA to IFN-γ-deficient mice inhibited IgE production and prevented antigen-induced anaphylaxis. CpG-DNA acts directly on B cells via a T cell-independent mechanism [21]. PD-L1 participates in various disorders; its pathway is a candidate for novel therapeutic approaches to certain diseases. Because PD-L1 suppresses host immunity in T cell lymphoproliferative disorders [20], interaction between PD-1 and PD-L1 regulates antigen receptor signalling negatively and is involved in almost every aspect of immune responses, including allergy-related and infectious immunity, suggesting a promising future for the clinical application of PD-1 agonists and antagonists [26]. The superantigen Staphylococcus aureus enterotoxin B causes an increase in IL-5 levels that is dampened when CpG-ODN is added to an in-vitro explant model composed of sinonasal tissue [18].
The typical Th2 cytokines are synthesized mainly by Th2 effector T cells and are essential in driving the allergic inflammatory process. The various steps in which naive CD4-positive T cells differentiate to allergen-specific, activated T cells of the Th2 type play a pivotal role in the pathogenesis of chronic allergic airway disease. Aiming at a concept for highly specific therapy of this disease, various T cell co-stimulatory molecules and ligands expressed on activated Th2 effector T cells have been discussed as potential targets for an antibody-based therapy [27]. The Th2-cell co-stimulators and ligands that are expressed de novo are of greater interest: the positive co-stimulators/ligands, ICOS/B7RP-1 and CD30/CD30L, and the co-stimulator/ligand, PD-1/PDl-1. Although the negative co-stimulator is unsuitable for blockade, CpG-B not only reduced the expression of B7RP-1 and CD30L but also induced the expression of PD-L1. It also inhibited IL-5 and IL-13 production and increased IFN-γ and IL-12 production. These results might contribute to a better understanding of the therapeutic effects of CpG-DNA in various human immune inflammatory or allergic diseases.
Acknowledgments
We thank M. Masuyama, M. Imamura, Y. Ishikawa and K. Uno for their excellent technical assistance. This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Health and Welfare, Japan (H20-Immunology-001), and from the Ministry of Education, Culture, Sports, Science and Technology of Japan (22791581, 22591896, 20390441).
Disclosure
None of the authors has conflicts of interest to declare, or any relevant financial interest, in any company or institution that might benefit from this publication.
References
- 1.Lombardi V, Singh AK, Akbari O. The role of costimulatory molecules in allergic disease and asthma. Int Arch Allergy Immunol. 2010;151:179–89. doi: 10.1159/000242355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Folkl A, Bienzle D. Structure and function of programmed death (PD) molecules. Vet Immunol Immunopathol. 2010;134:33–8. doi: 10.1016/j.vetimm.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Heinecke L, Proud D, Sanders S, Schleimer R, Kim J. Induction of B7-H1 and B7-DC expression on airway epithelial cells by the Toll-like receptor 3 agonist double-stranded RNA and human rhinovirus infection: in vivo and in vitro studies. J Allergy Clin Immunol. 2008;121:1155–60. doi: 10.1016/j.jaci.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allam J, Peng W, Appel T, et al. Toll-like receptor 4 ligation enforces tolerogenic properties of oral mucosal Langerhans cells. J Allergy Clin Immunol. 2008;121:368–74. doi: 10.1016/j.jaci.2007.09.045. [DOI] [PubMed] [Google Scholar]
- 5.Liu N, Ohnishi N, Ni L, Akira S, Bacon K. CpG directly induces T-bet expression and inhibits IgG1 and IgE switching in B cells. Nat Immunol. 2003;4:687–93. doi: 10.1038/ni941. [DOI] [PubMed] [Google Scholar]
- 6.Fujieda S, Iho S, Kimura Y, Yamamoto H, Igawa H, Saito H. Synthetic oligodeoxynucleotides inhibit IgE induction in human lymphocytes. Am J Respir Crit Care Med. 2000;162:232–9. doi: 10.1164/ajrccm.162.1.9906136. [DOI] [PubMed] [Google Scholar]
- 7.Iskra S, Kalla M, Delecluse H, Hammerschmidt W, Moosmann A. Toll-like receptor agonists synergistically increase proliferation and activation of B cells by Epstein–Barr virus. J Virol. 2010;84:3612–23. doi: 10.1128/JVI.01400-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carpenter EL, Mick R, Rüter J, Vonderheide RH. Activation of human B cells by the agonist CD40 antibody CP-870,893 and augmentation with simultaneous Toll-like receptor 9 stimulation. J Transl Med. 2009;7:93. doi: 10.1186/1479-5876-7-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han SH, Kim YE, Park JA, et al. Expression of human beta-defensin-2 gene induced by CpG-DNA in human B cells. Biochem Biophys Res Commun. 2009;389:443–8. doi: 10.1016/j.bbrc.2009.08.162. [DOI] [PubMed] [Google Scholar]
- 10.Osawa Y, Iho S, Takauji R, et al. Collaborative action of NF-kappaB and p38 MAPK is involved in CpG DNA-induced IFN-alpha and chemokine production in human plasmacytoid dendritic cells. J Immunol. 2006;177:4841–52. doi: 10.4049/jimmunol.177.7.4841. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto H, Yamada T, Takabayashi T, et al. Platelet derived endothelial cell growth factor/thymidine phosphorylase enhanced human IgE production. Allergol Int. 2011;60:79–85. doi: 10.2332/allergolint.10-OA-0220. [DOI] [PubMed] [Google Scholar]
- 12.Chen YQ, Shi HZ. CD28/CTLA-4–CD80/CD86 and ICOS–B7RP-1 costimulatory pathway in bronchial asthma. Allergy. 2006;61:15–26. doi: 10.1111/j.1398-9995.2006.01008.x. [DOI] [PubMed] [Google Scholar]
- 13.Fuchiwaki T, Sun X, Fujimura K, et al. The central role of CD30L/CD30 interactions in allergic rhinitis pathogenesis in mice. Eur J Immunol. 2011;41:2947–54. doi: 10.1002/eji.201141423. [DOI] [PubMed] [Google Scholar]
- 14.Klaschik S, Tross D, Shirota H, Klinman DM. Short- and long-term changes in gene expression mediated by the activation of TLR9. Mol Immunol. 2010;47:1317–24. doi: 10.1016/j.molimm.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crompton PD, Mircetic M, Weiss G, et al. The TLR9 ligand CpG promotes the acquisition of Plasmodium falciparum-specific memory B cells in malaria-naive individuals. J Immunol. 2009;182:3318–26. doi: 10.4049/jimmunol.0803596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eckl-Dorna J, Batista FD. BCR-mediated uptake of antigen linked to TLR9 ligand stimulates B-cell proliferation and antigen-specific plasma cell formation. Blood. 2009;113:3969–77. doi: 10.1182/blood-2008-10-185421. [DOI] [PubMed] [Google Scholar]
- 17.San Román B, Irache JM, Gómez S, Gamazo C, Espuelas S. Co-delivery of ovalbumin and CpG motifs into microparticles protected sensitized mice from anaphylaxis. Int Arch Allergy Immunol. 2009;149:111–8. doi: 10.1159/000189193. [DOI] [PubMed] [Google Scholar]
- 18.Tan L, Rogers TJ, Hatzirodos N, Baker LM, Ooi E, Wormald PJ. Immunomodulatory effect of cytosine–phosphate–guanosine (CpG)-oligonucleotides in nonasthmatic chronic rhinosinusitis: an explant model. Am J Rhinol Allergy. 2009;23:123–9. doi: 10.2500/ajra.2009.23.3279. [DOI] [PubMed] [Google Scholar]
- 19.Yang W, Li H, Chen PW, et al. PD-L1 expression on human ocular cells and its possible role in regulating immune-mediated ocular inflammation. Invest Ophthalmol Vis Sci. 2009;50:273–80. doi: 10.1167/iovs.08-2397. [DOI] [PubMed] [Google Scholar]
- 20.Wilcox RA, Feldman AL, Wada DA, et al. B7-H1 (PD-L1, CD274) suppresses host immunity in T-cell lymphoproliferative disorders. Blood. 2009;114:2149–58. doi: 10.1182/blood-2009-04-216671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu W, Tamura T, Takatsu K. CpG ODN mediated prevention from ovalbumin-induced anaphylaxis in mouse through B cell pathway. Int Immunopharmacol. 2008;8:351–61. doi: 10.1016/j.intimp.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 22.Marzec M, Zhang Q, Goradia A, et al. Oncogenic kinase NPM/ALK induces through STAT3 expression of immunosuppressive protein CD274 (PD-L1, B7-H1) Proc Natl Acad Sci USA. 2008;105:20852–7. doi: 10.1073/pnas.0810958105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wölfle SJ, Strebovsky J, Bartz H, et al. PD-L1 expression on tolerogenic APCs is controlled by STAT-3. Eur J Immunol. 2011;41:413–24. doi: 10.1002/eji.201040979. [DOI] [PubMed] [Google Scholar]
- 24.Senti G, Johansen P, Haug S, et al. Use of A-type CpG oligodeoxynucleotides as an adjuvant in allergen-specific immunotherapy in humans: a phase I/IIa clinical trial. Clin Exp Allergy. 2009;39:562–70. doi: 10.1111/j.1365-2222.2008.03191.x. [DOI] [PubMed] [Google Scholar]
- 25.Carnés J, Robinson DS. New strategies for allergen immunotherapy. Recent Pat Inflamm Allergy Drug Discov. 2008;2:92–101. doi: 10.2174/187221308784543610. [DOI] [PubMed] [Google Scholar]
- 26.Okazaki T, Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol. 2007;19:813–24. doi: 10.1093/intimm/dxm057. [DOI] [PubMed] [Google Scholar]
- 27.Kroczek R, Hamelmann E. T-cell costimulatory molecules: optimal targets for the treatment of allergic airway disease with monoclonal antibodies. J Allergy Clin Immunol. 2005;116:906–9. doi: 10.1016/j.jaci.2005.07.005. [DOI] [PubMed] [Google Scholar]


