Abstract
Studies of multiple sclerosis (MS) have concentrated mainly on antigen presentation of peptides derived from the myelin sheath, while the implication of lipid antigen has been less explored in this pathology. As the extracellular environment regulates expression of the lipid antigen-presenting molecule CD1, we have examined whether sera from patients alters CD1 surface expression in monocyte-derived dendritic cells. We have shown that: (i) CD1 group 1 proteins were highly expressed in the presence of MS sera; (ii) sera from MS patients differentially regulated CD1 group 1 versus CD1 group 2 molecular expression; and (iii) CD1 was expressed strongly in monocytes from MS patients under immunosuppressive treatment. Overall, these results reveal that CD1 expression is modified in MS and provide novel information on the regulation of lipid antigen presentation in myeloid cells.
Keywords: autoimmunity, dendritic cells, monocytes, multiple sclerosis, serum
Introduction
Multiple sclerosis (MS) is an autoimmune chronic pathology involving antigen-presenting cells (APCs) including dendritic cells (DC), as has been established by studies of animal models of MS such as experimental autoimmune encephalomyelitis (EAE), and by the detection of myelin-reactive cells in MS [1]–[3]. A large majority of patients (70%) develop acute exacerbation of the disease, with intervals of remission defining the relapsing–remitting (RR) form of disease, while other patients have primary–progressive (PP) or secondary–progressive (SP) forms. DC are essential for antigen presentation leading to CD4+ T lymphocyte activation mandatory for disease development, and both T helper type 1 (Th1) and Th17 inflammatory CD4+ T lymphocyte subpopulations have been implicated [4],[5]. The autoimmune response can be limited by the action of regulatory T lymphocyte subsets induced either by ‘regulatory’ DC or particular cytokine combinations. DC thus control the equilibrium between inflammatory and regulatory CD4+ T lymphocyte populations and can present a large variety of antigens, including lipid antigens. Large-scale lipid microarray analysis of sera and cerebrospinal fluid has identified the glycolipid sulphatide, a major constituent of the myelin sheath, as a principal target of the humoral response in patients with MS [6]. This suggests strongly that lipid antigen recognition activates immune responses involved in the physiopathology of MS.
Lipid antigen presentation to specific T cells is carried out by members of the CD1 protein family, which are expressed on APCs, including DC [7],[8]. CD1 glycoproteins include group I molecules (CD1a, CD1b and CD1c) and the group II molecule CD1d. Two major classes of lipid antigen can be presented by CD1 molecules: exogenous lipids derived from the wall of Mycobacterium sp. and endogenous lipids such as gangliosides and sulphatide found abundantly in the central nervous system (CNS). Moreover, the recognition of glycolipids by autoreactive T lymphocytes has been revealed in patients with MS [9], and antibodies directed against the glycolipid component of myelin have been described [10].
The regulation of CD1 expression and its role at the antigen-presenting cell surface in MS have received limited attention. Previous studies have shown that the spectrum of serum lipids can modify CD1 expression and its antigen-presentation function, indicating that the lipid microenvironment can modulate DC function via CD1 [11]. Alterations in the lipid composition of sera from MS patients have been described [12], but the effects of these changes on DC functions have not been investigated.
To understand more clearly the role of CD1 molecules in the presentation of CNS-derived lipids in MS, the present study was carried out to determine: (1) if constituents of serum from patients with MS influence and modify CD1 expression in monocyte-derived DC; and (2) whether the expression of CD1 molecules is regulated differentially in monocytes from patients with MS in comparison with cells from healthy donors.
Materials and methods
Patients and controls
Neurological patients suffering from RR, PP and SP were recruited at the Department of Neurology in the Timone Hospital (Marseille, France). Protocols were validated by the ethics committee of the Timone University Hospital (Marseille, France). Two dry tubes and two sodium citrate tubes of blood were collected. Two subtypes of patients were studied: (i) patients with active RR MS: at least one relapse during the past year and two within the past 3 years (n = 6); and (ii) PP MS in progression of at least two expanded disability status scale (EDSS) points within the last 2 years (n = 8). Patients with a residual EDSS superior or equal to 5 within less than 5 years at inclusion time were included preferentially. Patients suffering from RR or PP forms of MS were assessed twice [13]–[15] (Table 1). Monocytes from peripheral blood of healthy donors were analysed as controls.
Table 1.
Patients characteristics.
| Patient | Type | Age (years) | Sex | EDSS | Status | Treatment | % CD1a+ (Fig. 3) |
|---|---|---|---|---|---|---|---|
| 1 | SP | 40 | F | 6 | Slow progression | Solumedrol | 8·9 |
| 2 | SP | 35 | F | 6 | Stable | Solumedrol/corticoids | 2·5 |
| 3 | SP | 72 | M | 6·5 | Stable | Immunosuppressor | 4 |
| 4 | SP | 34 | F | 8 | Slow progression | Solumedrol | 7·5 |
| 5 | SP | 58 | F | 8 | Slow progression | None | 0·1 |
| 6 | SP | 66 | F | 6·5 | Stable | Immunosuppressor | n.d. |
| 7 | SP | 46 | F | 4·5 | Stable | Solumedrol | n.d. |
| 8 | RR | 29 | M | 3 | Stable | None | 1·6 |
| 9 | RR | 38 | M | 2 | Relapsing | None | 0·1 |
| 10 | RR | 42 | F | 1 | Relapsing | None | n.d. |
| 11 | RR | 43 | F | 3·5 | Stable | None | n.d. |
| 12 | RR | 33 | F | 2 | No relapsing | None | n.d. |
| 13 | CIS | 28 | F | 0 | No relapsing | None | 2·4 |
CIS: clinically isolated syndrome; EDSS: expanded disability status scale; F: female; M: male; n.d.: not done; RR: relapsing–remitting SP: secondary-progressive.
Immunofluorescence staining and analysis
Phenotypical analysis was carried out by multi-colour flow cytometry using a fluorescence activated cell sorter (FACS) Canto II (BD Biosciences, San Jose, CA, USA) using FlowJo software. Cells were stained for 30 min at 4°C with the following fluorochrome-conjugated mouse monoclonal antibodies (mAbs): CD1a (clone HI149), CD1b (clone M-T101), CD1d (clone CD1d42), human leucocyte antigen D-related (HLA-DR) (clone L243), CD14 (clone MjP9) and CD209 (clone DCN46) obtained from BD-Pharmingen (San Diego, CA, USA) and CD1c (BDCA-1) from Miltenyi Biotec (Bergisch Gladbach, Germany). Background fluorescence was evaluated using the appropriate isotype-matched irrelevant mAbs.
Purification, culture and DC differentiation of monocytes
Monocytes were purified from peripheral blood mononuclear cells (PBMCs) isolated by Ficoll-Paque (GE Healthcare, Little Chalfont, UK) density gradient centrifugation of platelet-depleted buffy coats obtained from the local blood bank, followed by positive selection using CD14 conjugated microbeads (Miltenyi Biotec). Monocytes were cultured for 5 days at 37°C 5% CO2 in RPMI-1640 supplemented with 5% fetal calf serum (FCS), 5% AB human serum (healthy serum: HS) or 5% MS patient serum/plasma, and 1000 UI/ml human recombinant interleukin (rIL)-4 and 800 UI/ml human recombinant granulocyte–macrophage colony-stimulating factor (GM-CSF) (R&D Systems, Minneapolis, MN, USA) to induce DC differentiation.
Cell preparation for confocal microscopy, image acquisition and data processing
DC were adhered to slides precoated with poly-l-lysine (Sigma-Aldrich, St Louis, MO, USA), then washed and fixed in 2% paraformaldehyde (PFA). Fixed cells were permeabilized with saponin (Sigma-Aldrich) in phosphate-buffered saline (PBS) with 0·5% bovine serum albumin (BSA). After washing, cells were stained with either directly labelled anti-CD1a fluorescein isothiocyanate (FITC) antibody (BD Pharmingen) or anti-HLA-DR (L243) mAb followed by a secondary goat anti-mouse antibody coupled with AlexaFluor 568 (Molecular Probes, Invitrogen, Carlsbad, CA, USA). Slides were mounted in Vectashield with 4',6-diamidino-2-phenylindole (DAPI) (Vector Laboratories, Burlingame, CA, USA). After 48 h cells were analysed using an MRC 1024 confocal microscope (Bio-Rad, Hercules, CA, USA) with a ×63 lens and Zeiss LSM510 version 4·0 confocal scan. Images were obtained after sequential excitation at 488 nm, 760 nm and 543 nm. Quantification was performed by determination of the intensity of fluorescence of all cells in two large fields. Data were acquired from two images of control monocytes incubated with two different MS sera. Using ZEN 2010 software, a discoid field of 20 000 pixels was chosen to analyse each cell. The fluorescence intensities obtained from all the differentiated cells in the field were pooled in order to obtain a representative value for the entire population, and the average and standard deviation of fluorescence of each antibody was compared for different sera. The ratio between green and red fluorescence was compared in order to determine the relative expression of HLA-DR versus CD1a.
Results
Expression of CD1 molecules in DC cultured with different sera
Our objective was to determine whether serum or plasma from patients with MS altered CD1 molecule expression in professional APC, such as monocyte-derived DC. To this end we analysed the expression of CD1a, CD1b, CD1c and CD1d together with HLA-DR and CD209 [dendritic cell-specific intercellular adhesion molecule 3 (ICAM-3) grabbing non-integrin (DC-SIGN)] molecules in differentiated monocytes in the presence of FCS, HS or serum from patients with MS. Purified monocytes from healthy donors were incubated in RPMI-1640 supplemented with 5% FCS, MS sera or MS plasma during differentiation to DC.
As shown in Fig. 1a, using serum and plasma from a representative patient, differentiation in the presence of MS serum led to dramatic differences in comparison with HS, shown by very high fluorescence intensity of CD1a and large increases in the expression of CD1b and CD1c. CD1d was increased only slightly. In contrast to group 1 CD1, HLA-DR and CD209 levels of expression were not altered while CD14 expression (not shown) was lost. Group 1 CD1 expression differed in FCS and HS conditions with reduced expression of CD1a, CD1b and CD1c. Published data have suggested that peroxisome proliferator-activated receptor (PPAR)-γ, immunoglobulins and lipid from serum regulate CD1 expression and DC function [16]–[19].
Fig. 1.
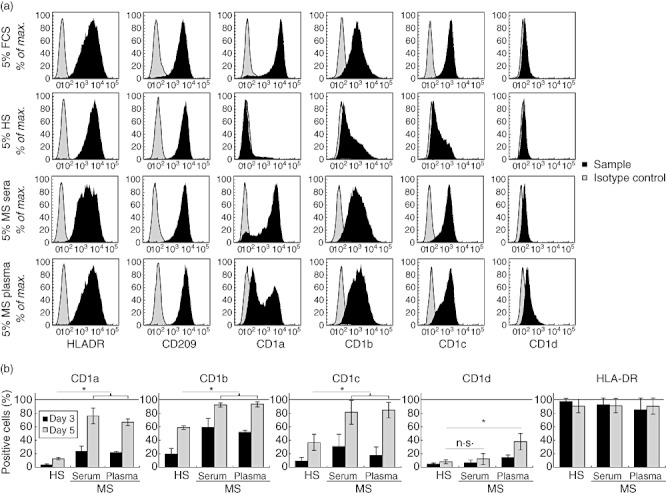
Expression of human leucocyte antigen (HLA) class II and CD1 molecules in dendritic cells (DC) differentiated in the presence of sera or plasma from multiple sclerosis (MS) patients. Purified monocytes were cultured in the presence of interleukin (IL)-4 and granulocyte–macrophage colony-stimulating factor (GM-CSF) in medium supplemented with various serum or plasma and were surface-labelled with anti-HLA-DR, -CD209, -CD1a, -CD1b, -CD1c, -CD1d or isotype control fluorochrome-conjugated monoclonal antibodies (mAbs) and analysed by flow cytometry. (a) Profiles of expression in monocytes differentiated in 5% fetal calf serum (FCS), 5% AB human serum [healthy serum (HS)] or 5% MS serum or plasma from one patient (patient 4). Results shown are representative of five independent analyses. (b) Percentage of monocytes cultured in the presence of 5% AB serum (HS) (n = 11), MS serum (n = 11) and MS plasma (n = 9) during 5 days. At days 3 and 5 of differentiation, cells were labelled with anti-CD1a, -CD1b, -CD1c, -CD1d, -HLA-DR or isotype control fluorochrome-conjugated mAbs and analysed by flow cytometry (mean ± standard deviation, Mann–Whitney U-test; n.s.: non-significant; *P-value < 0·05%).
The effects of sera and plasma from 13 MS patients were analysed similarly, and the rates of cell expression are represented in Fig. 1b. At day 3 of differentiation, four times more DC expressed CD1a in MS serum cultures, and at day 5 seven times more DC were CD1a-positive, suggesting an accelerated differentiation of monocytes to DC. The numbers of CD1b-positive DC also increased in cultures with MS serum (threefold at day 3, 1·5-fold at day 5), although less than for CD1a. In contrast, CD1c expression was highly increased in MS serum (sixfold more DC at day 3, twofold more at day 5). The numbers of DC expressing CD1d were not altered by MS serum. Similarly, HLA-DR expression did not vary in MS sera cultures. Overall, cells cultured in MS plasma had the same pattern of expression as those in MS sera.
Confocal analysis of CD1a and HLA-DR expression in monocyte-derived DC
CD1 and major histocompatibility complex (MHC)-II molecules are antigen-presenting molecules that cycle between cell surface and intracellular compartments to achieve their functions. To determine if modifications in the expression of CD1a induced by MS sera were related to differences of expression in intracellular compartments, we next analysed the localization of CD1a and HLA-DR expressed in DC after differentiation of monocytes in the presence of sera from either healthy donors or MS patients. After staining, HLA-DR (red) and CD1a (green) images were acquired by confocal microscopy. In Fig. 2a, differentiated DC with MS serum (left) or healthy serum (right) are shown. No difference in the localization of either molecule was observed initially. However, comparison of the fluorescence intensity of HLA-DR and CD1a expression revealed some modified CD1a expression. Figure 2b shows that HLA-DR staining is similar under both conditions. However, CD1a staining was significantly higher in cells which had been differentiated in the presence of MS serum. When the ratio of CD1a to HLA-DR intensities were compared, we found that CD1a expression was increased selectively in DC cultured in the presence of MS sera. Therefore, both flow cytometry and confocal analysis demonstrate a significant increase of CD1a in DC differentiated in MS serum compared to healthy donors.
Fig. 2.
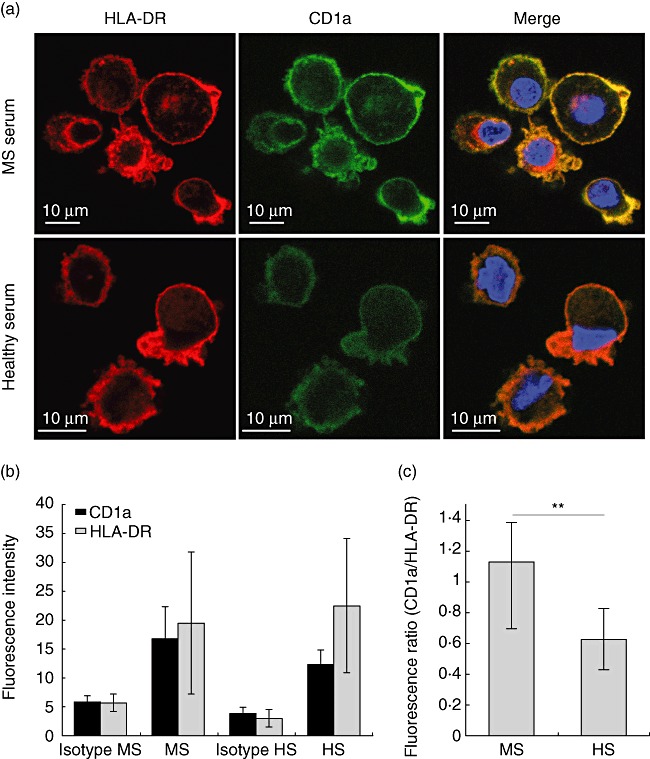
Confocal analysis of CD1a and human leucocyte antigen D-related (HLA-DR) expression in monocyte-derived immature dendritic cells (iDC). (a) Images of monocyte-derived DC obtained by confocal microscopy with CD1a in green, HLA-DR in red and nucleus in blue [4',6-diamidino-2-phenylindole (DAPI)], for iDC cultured in patient serum (left) or healthy donor serum (right); (b) fluorescence intensity was quantified in cell images captured by confocal microscopy and averaged as described in Methods. Cells cultured in multiple sclerosis (MS) or in healthy donor (HS) sera were analysed for their expression of CD1a (green channel) and HLA-DR (red channel); (c) normalized expression of CD1a with respect to HLA-DR within each DC analysed in (b) (standard deviation, Student's t-test: n.s.: non-significant, **P-value < 0·01%).
Expression of CD1 in monocytes from patients
In MS patients, the regulation of glycolipid presentation by CD1 molecules may be modified by the release of CNS-derived lipids such as sulphatide. To address this, we determined the profile of expression of antigen presentation-related molecules in monocytes from patients with MS (Fig. 3) in comparison to healthy donors. MS monocytes expressed HLA-DR, CD14 and CD86 similarly to control cells, while CD80 and CD83 were not expressed at the cell surface in either patients or controls (not shown). However, we observed that CD1 was expressed in significantly increased numbers of monocytes from patients undergoing immunosuppressive treatment (n = 4), while monocytes from untreated MS patients (n = 4) or healthy controls (n = 6) did not express any group 1 CD1 molecules (Fig. 3). In contrast, CD1d was expressed in a small percentage of monocytes from untreated patients, and immunosuppressive treatment increased significantly the numbers of monocytes expressing CD1d. Therefore, expression of group 1 CD1 molecules in monocytes is modified in treated, but not in untreated patients.
Fig. 3.
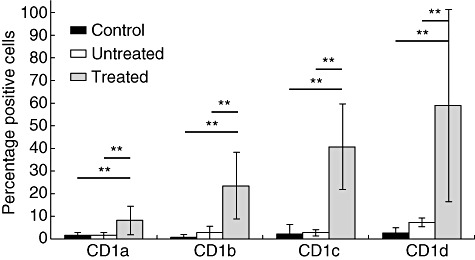
CD1 expression in monocytes from multiple sclerosis (MS) patients. CD1 molecule (CD1a, CD1b, CD1c and CD1d) phenotypes in monocytes from MS patients treated (n = 4) or untreated (n = 4) and control individuals (n = 6) were analysed by flow cytometry (standard deviation, Mann–Whitney U-test standard deviation, Mann–Whitney U-test: **P-value < 0·01%).
Discussion
Our study demonstrates that the presence of serum or plasma from patients with MS induced much higher DC expression of CD1 with a higher number of positive cells and a higher expression per cell, compared to the low level of CD1 expression in the presence of sera from healthy donors. In addition, CD1a, which is absent from the cell surface of MS monocytes ex vivo, is expressed highly in monocytes from treated patients.
Recent studies have shown that the ability of normal DC to present lipid antigen by CD1 molecules is regulated by serum components and is controlled at the transcriptional level by PPAR-γ activation [12],[20]. Lipids present in normal serum, such as lysophosphatidic acid and cardiolipin, can specifically inhibit the expression of type I CD1 isoforms [11]. The expression of CD1 induced by MS sera could result from interference with the inhibitory effects of these serum constituents. However, modifications in serum lysophosphatidic acid and cardiolipin in MS have not been reported. If these lipids were unchanged in MS, other serum components present during MS (i.e. other lipids) may blunt the inhibitory effect by competing with lysophosphatidic acid and cardiolipin for the PPAR-γ pathway. Alternatively, MS serum components may induce effects on a distinct pathway that overrides inhibition by lysophosphatidic acid and cardiolipin.
The expression of CD1a in monocyte-derived DC in MS has been reported previously [21]. Patient monocytes differentiated in standard culture conditions (with FCS as source of serum) showed increased CD1a expression, indicating a cell-intrinsic potential for increased expression of one lipid presentation molecule. Our results showed that control monocyte-derived DC differentiated in MS sera expressed higher levels of all isoforms of type I CD1 molecules. Together, these data show that both cellular and serum factors in MS promote higher expression of CD1 after differentiation of monocytes into DC. Whether or not higher CD1 expression was already present in undifferentiated monocytes was addressed in this study, showing that the expression of all CD1 isoforms was increased in treated patients. Huang et al. examined CD1a expression in MS using monocytes cultured for 2 days [22], and found higher CD1a expression in untreated patients and mixed variations in treated patients [interferon (IFN)-β and glatiramer] depending upon the cohort examined. Because cultured monocytes probably underwent some kind of unspecified differentiation, it is possible that contrasting results in the two studies depend upon differences in protocols and/or treatment.
Numerous studies in various experimental models concurred with the autoimmune character of MS with involvement of myelin-reactive T cells. Strikingly, T cells reactive to myelin components induce EAE when transferred into a naive animal [23]. Mice transgenic for myelin basic protein-specific T cell receptors (TCR) were more likely to develop EAE with characteristic lesions of the myelin sheath [24]. Activation of the T cell compartment requires the presentation of molecules present in myelin by APC. Our results indicate that components present in the sera of MS patients promote the expression of cell surface molecules that can present myelin lipids to T cells. CD1-dependent T cell activation may take place in the CNS or at the periphery, and may participate in the early stages of MS pathogenesis or in the development of effectors of myelin sheath damage.
Higher expression of CD1 may affect lipid antigen presentation in several ways. Bacterial infections can induce endogenous lipid synthesis and infected cells can acquire the ability to stimulate specific CD1-restricted T lymphocytes, a mechanism believed to be involved in the activation of autoreactive T cells and the development of autoimmune disorders such as MS [19]. Bacterial products can thus induce sulphatide synthesis rapidly by DC which can, in turn, induce IFN-γ production by specific T cell clones. Such activation is dependent upon CD1a, b and c molecules expressed at the cell surface of DC, and could lead to the emergence of autoimmune pathologies such as MS. The mechanisms, which in the course of an efficient T cell response against microbial antigens lead to induction of autoimmune responses in susceptible individuals, remain to be characterized. A second mechanism implicating increased CD1 expression would involve enhanced presentation of CNS lipids, such as sulphatide, released from lesions of the myelin sheath early in the course of MS. Enhanced presentation by CD1 may then exacerbate the disease and lead to progression.
Modification in the profile of expression of CD1 molecules have been shown to determine the capacity of APC to stimulate T cells and invariant natural killer (iNK) T cells, and thus to trigger an immune response [11]. Negative regulation of CD1 molecules in DC from healthy subjects suggests that, in steady-state conditions, the aptitude of these cells to activate lymphocytes by lipid antigen is lower than that of DC differentiated in pathological situations. Regulation of the expression and function of CD1 molecules may then have implications in inflammatory illnesses ascribing a pathogenic role to CD1. Moreover, the role of chronic inflammation may be crucial to the pathology of these disorders. Recently, Moody et al. have designed a system in which cells provide a diverse pool of lipid antigens for CD1a loading; they identified CD1a-autoreactive cells as a subset of the T cell repertoire by defining CD1a as a specific target of the Th22 helper T cell subset [25].
In this study we have documented further the alteration of CD1 expression in MS, and identify a role for serum components in variations of this expression. Future studies will determine the impact of CD1 expression on lipid-specific T cell responses such as specific T cell frequency and polarization in MS patients.
Acknowledgments
This work was supported by grants from Fondation pour la Recherche sur le Cerveau (FRC) and Institut National de la Santé et de La Recherche Médicale (INSERM). We are grateful to E. Philadelphe for technical assistance. We thank Professors Cherif and Pelletier (Hôpital de la Timone, Marseille, France) for patient recruitment.
Disclosure
None of the authors has any conflicts of interest to report.
References
- 1.Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Engl J Med. 2000;343:938–52. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- 2.Kanter JL, Narayana S, Ho PP, et al. Lipid microarrays identify key mediators of autoimmune brain inflammation. Nat Med. 2006;12:138–43. doi: 10.1038/nm1344. [DOI] [PubMed] [Google Scholar]
- 3.O'Connor AB, Schwid SR, Herrmann DN, Markman JD, Dworkin RH. Pain associated with multiple sclerosis: systematic review and proposed classification. Pain. 2008;137:96–111. doi: 10.1016/j.pain.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 4.Behar SM, Porcelli SA. CD1-restricted T cells in host defense to infectious diseases. Curr Top Microbiol Immunol. 2007;314:215–50. doi: 10.1007/978-3-540-69511-0_9. [DOI] [PubMed] [Google Scholar]
- 5.Miyake S, Yamamura T. NKT cells and autoimmune diseases: unraveling the complexity. Curr Top Microbiol Immunol. 2007;314:251–67. doi: 10.1007/978-3-540-69511-0_10. [DOI] [PubMed] [Google Scholar]
- 6.Swann JB, Coquet JMC, Smyth MJ, Godfrey DI. CD1-restricted T cells and tumor immunity. Curr Top Microbiol Immunol. 2007;314:293–323. doi: 10.1007/978-3-540-69511-0_12. [DOI] [PubMed] [Google Scholar]
- 7.De Libero G, Mori L. Recognition of lipid antigens by T cells. Nat Rev Immunol. 2005;5:485–96. doi: 10.1038/nri1631. [DOI] [PubMed] [Google Scholar]
- 8.Sloma I, Zilber M-T, Charron D, Girot R, Tamouza R, Gelin C. Upregulation and atypical expression of the CD1 molecules on monocytes in sickle cell disease. Hum Immunol. 2004;65:1370–6. doi: 10.1016/j.humimm.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 9.Shamshiev A, Donda A, Carena I, Mori L, Kappos L, De Libero G. Self glycolipids as T-cell autoantigens. Eur J Immunol. 1999;29:1667–75. doi: 10.1002/(SICI)1521-4141(199905)29:05<1667::AID-IMMU1667>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 10.Bansal AS, Abdul-Karim B, Malik RA, et al. IgM ganglioside GM1 antibodies in patients with autoimmune disease or neuropathy, and controls. J Clin Pathol. 1994;47:300–2. doi: 10.1136/jcp.47.4.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leslie DS, Dascher CC, Cembrola K, et al. Serum lipids regulate dendritic cell CD1 expression and function. Immunology. 2008;125:289–301. doi: 10.1111/j.1365-2567.2008.02842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cherayil GD. Sialic acid and fatty acid concentrations in lymphocytes, red blood cells and plasma from patients with multiple sclerosis. J Neurol Sci. 1984;63:1–10. doi: 10.1016/0022-510x(84)90104-7. [DOI] [PubMed] [Google Scholar]
- 13.Audoin B, Ibarrola D, Malikova I, et al. Onset and underpinnings of white matter atrophy at the very early stage of multiple sclerosis – a two-year longitudinal MRI/MRSI study of corpus callosum. Mult Scler. 2007;13:41–51. doi: 10.1177/1352458506071215. [DOI] [PubMed] [Google Scholar]
- 14.Desplat-Jégo S, Feuillet L, Pelletier J, Bernard D, Chérif AA, Boucraut J. Quantification of immunoglobulin free light chains in cerebrospinal fluid by nephelometry. J Clin Immunol. 2005;25:338–45. doi: 10.1007/s10875-005-5371-9. [DOI] [PubMed] [Google Scholar]
- 15.Feuillet L, Reuter F, Audoin B, et al. Early cognitive impairment in patients with clinically isolated syndrome suggestive of multiple sclerosis. Mult Scler. 2007;13:124–7. doi: 10.1177/1352458506071196. [DOI] [PubMed] [Google Scholar]
- 16.De Libero G, Moran AP, Gober H-J, et al. Bacterial infections promote T cell recognition of self-glycolipids. Immunity. 2005;22:763–72. doi: 10.1016/j.immuni.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 17.Gogolak P, Rethi B, Szatmari I, et al. Differentiation of CD1a– and CD1a+ monocyte-derived dendritic cells is biased by lipid environment and PPARgamma. Blood. 2007;109:643–52. doi: 10.1182/blood-2006-04-016840. [DOI] [PubMed] [Google Scholar]
- 18.De Libero G, Mori L. How T cells get grip on lipid antigens. Curr Opin Immunol. 2008;20:96–104. doi: 10.1016/j.coi.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 19.Smed-Sörensen A, Moll M, Cheng T-Y, et al. IgG regulates the CD1 expression profile and lipid antigen-presenting function in human dendritic cells via FcgammaRIIa. Blood. 2008;111:5037–46. doi: 10.1182/blood-2007-07-099549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manolova V, Kistowska M, Paoletti S, et al. Functional CD1a is stabilized by exogenous lipids. Eur J Immunol. 2006;36:1083–92. doi: 10.1002/eji.200535544. [DOI] [PubMed] [Google Scholar]
- 21.Huang Y-M, Kouwenhoven M, Jin YP, Press R, Huang WX, Link H. Dendritic cells derived from patients with multiple sclerosis show high CD1a and low CD86 expression. Mult Scler. 2001;7:95–9. doi: 10.1177/135245850100700204. [DOI] [PubMed] [Google Scholar]
- 22.Hussien Y, Sanna A, Söderström M, Link H, Huang Y-M. Multiple sclerosis: expression of CD1a and production of IL-12p70 and IFN-gamma by blood mononuclear cells in patients on combination therapy with IFN-beta and glatiramer acetate compared to monotherapy with IFN-beta. Mult Scler. 2004;10:16–25. doi: 10.1191/1352458504ms979oa. [DOI] [PubMed] [Google Scholar]
- 23.Genain CP, Lee-Parritz D, Nguyen MH, et al. In healthy primates, circulating autoreactive T cells mediate autoimmune disease. J Clin Invest. 1994;94:1339–45. doi: 10.1172/JCI117454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Madsen LS, Andersson EC, Jansson L, et al. A humanized model for multiple sclerosis using HLA-DR2 and a human T-cell receptor. Nat Genet. 1999;23:343–7. doi: 10.1038/15525. [DOI] [PubMed] [Google Scholar]
- 25.De Jong A, Peña-Cruz V, Cheng T-Y, Clark RA, Van Rhijn I, Moody DB. CD1a-autoreactive T cells are a normal component of the human αβ T cell repertoire. Nat Immunol. 2010;11:1102–9. doi: 10.1038/ni.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]


