Abstract
To investigate the pathogenesis of localized autoimmune damage in Sjögren's syndrome (SS) by examining the expression patterns of cytokines, chemokines and chemokine receptors at sites of autoimmune damage. mRNA expression of these molecules in the labial salivary glands (LSGs) and peripheral blood mononuclear cells (PBMCs) from 36 SS patients was examined using a real-time polymerase chain reaction-based method. Subsets of the infiltrating lymphocytes and chemokines/chemokine receptors expression in the LSG specimens were examined by immunohistochemistry. Cytokines/chemokine concentrations in the saliva were analysed using flow cytometry or enzyme-linked immunosorbent assay. mRNA expression of T helper type 1 (Th1) cytokines, chemokines and chemokine receptors was higher in LSGs than in PBMCs. In contrast, mRNA expression of Th2 cytokines, chemokines [thymus and activation-regulated chemokine (TARC/CCL17), macrophage-derived chemokine (MDC/CCL22)] and chemokine receptor (CCR4) was associated closely with strong lymphocytic accumulation in LSGs. Furthermore, TARC and MDC were detected immunohistochemically in/around the ductal epithelial cells in LSGs, whereas CCR4 was detected on infiltrating lymphocytes. The concentrations of these cytokines/chemokines were significantly higher in the saliva from SS patients than those from controls, and the concentrations of Th2 cytokines/chemokines were associated closely with strong lymphocytic accumulation in LSGs. These results suggest that SS might be initiated and/or maintained by Th1 and Th17 cells and progress in association with Th2 cells via the interaction between particular chemokines/chemokine receptors. Furthermore, the measurement of cytokines/chemokines in saliva is suggested to be useful for diagnosis and also to reveal disease status.
Keywords: chemokines, cytokines, Sjögren's syndrome, T cells
Introduction
Sjögren's syndrome (SS) is an autoimmune disease characterized by lymphocytic infiltration of the salivary and lacrimal glands with the concomitant destruction of glandular tissue. Patients typically experience the symptoms of a dry mouth (xerostomia) and dry eyes (keratoconjunctivitis sicca) [1]. Immunohistochemical studies have shown that CD4+ T helper (Th) cells predominantly infiltrate salivary glands at an early stage of SS, and may be critical in the induction and/or maintenance of the disease [2]. SS is proposed to progress through the following three stages: (i) initial infiltration of CD4+ Th cells and then B cells into the glandular tissue with the presence of serum autoantibodies; (ii) infiltration of lymphocytes into the extraglandular tissue and the promotion of pseudolymphoma and hypergammaglobulinaemia; and (iii) progression to B cell lymphoma, possibly accompanied by hypogammaglobulinaemia and immunodeficiency [1],[3]–[5]. Therefore, it has been proposed that SS is a lymphoproliferative disorder, thus it is of interest to understand the involvement of CD4+ Th cells in both the initiation and progression of the disease.
Th cell populations are comprised of functionally distinct subsets characterized by specific patterns of cytokine production. At least five subsets have been described: Th0, Th1, Th2, Th17 and regulatory T cells (Tregs). Th1 cells support cell-mediated immunity and produce interleukin (IL)-2, interferon (IFN)-γ and tumour necrosis factor (TNF)-α that induce inflammatory responses responsible for killing intracellular parasites and perpetuating autoimmune responses. However, excessive inflammatory responses can lead to uncontrolled tissue damage. Th2 cells produce IL-4, IL-5, IL-6 and IL-10, which provide help for humoral immunity and are associated with the promotion of immunoglobulin (Ig)E and eosinophilic responses. Th2 responses can counteract Th1-mediated microbicidal action. Thus, the Th1/Th2 balance plays an important role in immunoregulation. In contrast, Th0 cells are characterized by the production of both Th1 and Th2 cytokines and are considered to be precursors of Th1 and Th2 cells. Tregs control these effector T cell functions to maintain immune responses and prevent autoimmunity and inappropriate tissue inflammation. Several studies have revealed that autoimmune diseases are caused by the collapse of the Th1/Th2 balance [6]–[8]. Fox et al. [9] reported that CD4+ T cells in the salivary glands of SS patients produce IL-2, IFN-γ and IL-10, but not IL-4 or IL-5. Furthermore, we reported that IL-2, IFN-γ, IL-10, IL-6 and transforming growth factor (TGF)-β are detected consistently in all SS patients, while IL-4 and IL-5 are detected only in patients who have high levels of B cell accumulation in the salivary glands. These reports therefore suggest that Th1 cytokines, as well as IL-10, IL-6 and TGF-β, are essential for the induction and/or maintenance of SS, whereas Th2 cytokines may be involved in disease progression, especially local B cell activation [5]. Recently, the Th1/Th2 paradigm was expanded by identification of Th17 cells, a subset of CD4+ Th cells characterized by their ability to produce IL-17. These new Th subsets, Th17 cells and Tregs, are distinct from Th1 and Th2 cells and are important in the induction of autoimmunity [10].
Chemokines are important in leucocyte activation and chemotaxis [11]. Interactions between chemokines and chemokine receptors promote selective local infiltration of specific cells into inflamed areas [12]. Furthermore, chemokines are involved intimately in the maintenance of the Th1/Th2 balance and immune responses, in cardiac allograft rejection [13], autoimmune diabetes [14], atopic keratoconjunctivitis [15], cutaneous lupus erythematosus [16] and experimental autoimmune encephalomyelitis [17]. Chemokines also play a key role in the recruitment of inflammatory cells and lymphoid neogenesis in the target organs [18],[19]. In particular, Ogawa et al. [20] reported that Th1 chemokines, such as IFN-γ-inducible 10-kd protein (IP-10) and monokine induced by IFN-γ (MIG), were involved in the accumulation of T cell infiltrates in the salivary glands of SS patients.
In order to determine the involvement of cytokines, chemokines and chemokine receptors in the initiation and progression of SS, we examined the salivary glands and saliva from SS patients to identify the expression patterns of these molecules and their association with the degree of the lymphocytic infiltration and the lymphocyte subsets.
Patients and methods
Patients
Thirty-six patients with primary SS (35 female and one male) referred to the Department of Oral and Maxillofacial Surgery, Kyushu University Hospital between 2000 and 2007 were included into the study. The patients ranged in age from 21 to 84 years [mean ± standard deviation (s.d.) age: 57·6 ± 13·6 years] and in disease duration from 3 months to 15 years (mean ± s.d. age: 7·3 ± 4·7 years). The disease duration was defined as the period from the initial observation of dry mouth to the first visit and was obtained for 28 patients. All patients met the diagnostic criteria proposed by the Research Committee on Sjögren's Syndrome of the Ministry of Health and Welfare of the Japanese Government [21] and the criteria proposed by the American–European Consensus Group criteria for SS [22]. Each patient exhibited objective evidence of salivary gland involvement based on the presence of subjective xerostomia and a decreased salivary flow rate, abnormal findings on parotid sialography and focal lymphocytic infiltration of the labial salivary glands (LSGs). None of the patients were treated with steroids or any other immunodepressants. The prevalence of anti-SS-A/Ro, anti-SS-B/La and anti-nuclear antibodies were 72·2%, 33·3% and 80·6%, respectively. The LSG biopsies were performed as described by Greenspan et al. [23]. Heparinized blood samples and saliva were also obtained from the patients at the time of biopsy. As controls, LSG biopsy specimens, blood samples and saliva were also obtained from 15 patients with mucoceles who had no clinical or laboratory evidence of systemic autoimmune disease. All control LSGs were histologically normal. This study design was approved by the Ethics Committee of Kyushu University, Japan, and informed consent was obtained from all the patients and healthy controls.
Histological study of LSGs
Four-µm frozen sections of LSG specimens were prepared and stained with haematoxylin and eosin (H&E) for a conventional histological examination. The degree of lymphocytic infiltration in the specimens was judged by focus scoring [23],[24]. One standardized score is the number of focal inflammatory cell aggregates containing 50 or more mononuclear cells in each 4-mm2 area of salivary gland tissue [25].
Immunohistochemical study of LSGs
For the immunohistochemical analysis of lymphocyte subsets and the expression of the chemokines and chemokine receptors, 4-µm frozen sections were prepared and stained using a conventional avidin–biotin complex (ABC) technique, as reported previously [26]. The mouse monoclonal antibodies used to analyse lymphocyte subsets were anti-CD3, anti-CD19, anti-CD4 and anti-CD8 (Leu4, Leu12, Leu3a + 3b and Leu2a, respectively; BD Bioscience, San Jose, CA, USA). Mouse monoclonal antibodies used to analyse the chemokines and chemokine receptors were anti-thymus and activation-regulated chemokine (TARC) (R&D Systems, Minneapolis, MN, USA), anti-macrophage-derived chemokine (MDC) (R&D Systems) and anti-CC chemokine receptor 4 (CCR4) (R&D Systems). Sections were incubated sequentially with monoclonal antibodies, biotinylated anti-mouse IgG antibodies, avidin–biotin horseradish peroxidase complex and 3,3′-diaminobenzidine (all from Vector Laboratories, Burlingame, CA, USA). Mayer's haematoxylin was used for counterstaining. Photomicrographs were obtained using a light microscope equipped with a digital camera (CoolSNAP; Photometrics, Tucson, AZ, USA). Stained lymphocytes were imaged and counted in 1-mm3 sections from three different areas using image analyser software (NIH image 1·62; National Institutes of Health, Bethesda, MD, USA) [27] and the ratios of CD3+ to CD19+ cells and CD4+ to CD8+ cells were calculated.
RNA extraction and complementary DNA synthesis
Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Hypaque density gradient centrifugation. The total RNA was prepared from the LSG specimens and the PBMCs by the acidified guanidinium–phenol–chloroform method, as described previously [28],[29]. Three micrograms of the total RNA preparation were then used for the synthesis of cDNA. Briefly, RNA was incubated for 1 h at 42°C with 20 U of RNasin® ribonuclease inhibitor (Promega, Madison, WI, USA), 0·5 µg of oligo-(dT)1218 (Pharmacia, Uppsala, Sweden), 0·5 mM of each deoxyribonucleotide triphosphate (dNTP) (Pharmacia), 10 mM of dithiothreitol (DTT) and 100 U of RNase H reverse transcriptase (Life Technologies, Gaithersburg, MD, USA).
Quantitative estimation of mRNA using real-time polymerase chain reaction (PCR)
Quantitative cDNA amplification was performed according to the manufacturer's instructions and previous reports [28],[29]. The cDNA of the cytokines, chemokines and chemokine receptors was analysed by real-time PCR using LightCycler Fast Start DNA Master SYBR Green 1 (Roche Diagnostics, Mannheim, Germany) on a LightCycler real-time PCR instrument (version 3·5; Roche Diagnostics). The cytokines examined were IL-2, IFN-γ, IL-4, IL-5, IL-10, IL-12, IL-17 and TGF-β. The chemokines examined were IP-10, macrophage inflammatory protein-1α (MIP-1α), regulated upon activation normal T expressed and secreted (RANTES), TARC and MDC. The chemokine receptors examined were CXCR3, CCR4 and CCR5. The transcription factors examined were forkhead box protein 3 (FoxP3). In order to provide a meaningful comparison between different individuals or samples, we calculated the relative amounts of cytokines, chemokines and chemokine receptor PCR products relative to the amount of β-actin PCR products (for the standardization of total cellular mRNA) in each sample.
Concentrations of cytokines and chemokines in saliva assessed by enzyme-linked immunosorbent assay (ELISA)
Study participants were asked to refrain from smoking, eating and drinking for at least 2 h prior to collection of samples. Stimulated whole saliva was collected by Salivette tubes (Sarstedt, Nümbrecht, Germany) using polyester swabs from the subjects' mouths following 5 min of chewing. Swabs with absorbed saliva were returned to Salivette tubes and centrifuged for 2 min at 1000 g, yielding a clear saliva sample. Soluble molecules including Th1 and Th2 cytokines (IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12, TNF-α and IFN-γ) and chemokines (RANTES, IL-8, MIG and IP-10) were measured by flow cytometry assay using a cytometric bead array system (BD Biosciences, San Diego, CA, USA), according to the manufacturer's recommendations. The concentrations of chemokines (TARC, MDC, MIP-1α) were measured by immunoreactivity in double sandwich ELISA format using a Quatikine kit (R&D Systems), as reagents to detect these molecules by cytometric bead array are not available.
Statistical analysis
The statistical significance of the differences between the groups was determined by the Mann–Whitney U-test, Student's t-test and Spearman's rank correlation. Probabilities of P < 0·05 were considered statistically significant.
Results
Histological findings and lymphocyte subsets in the LSG specimens
All the LSGs from 18 SS patients, from whom enough LSGs were available, contained periductal lymphocytic infiltration and atrophy or destruction of the acini. The degree of lymphocytic infiltration judged by focus scoring was evaluated and its association with disease duration and lymphocyte subsets was assessed (Fig. 1). Representative histology results are shown in Fig. 2. Disease duration showed a positive correlation with the focus score (Fig. 1a). Infiltrating lymphocyte subsets in the LSGs were similar to those reported previously [26],[30]. Although a general predominance of CD3+ T cells over CD19+ B cells was observed, LSGs with strong lymphocytic infiltration showed reduced CD3+ T cell predominance over CD19+ B cells in comparison to LSGs with weak lymphocytic infiltration. In two cases with a focus score 12, significantly higher numbers of CD19+ B cells (P < 0·01) were observed compared with CD3+ T cells (Fig. 1b). A slight predominance of CD8+ T cells over CD4+ T cells was observed in LSGs with weak lymphocytic infiltration, whereas a predominance of CD4+ cells over CD8+ cells was seen in those with strong lymphocytic infiltration (Fig. 1c).
Fig. 1.
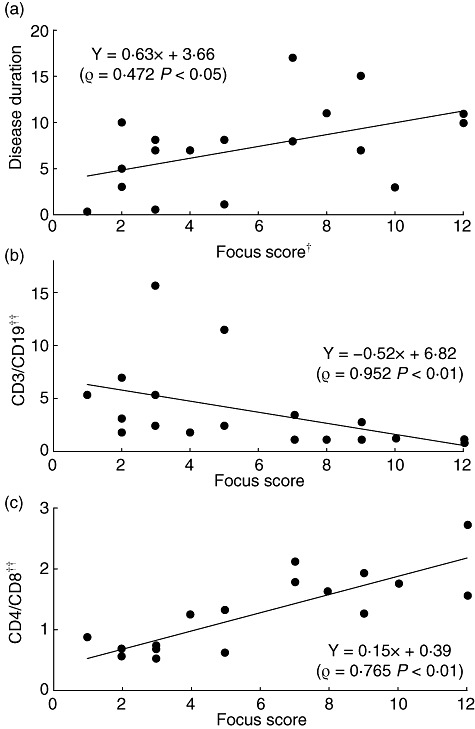
Correlation between focus score and lymphocyte subsets in the labial salivary glands (LSGs) from patients with Sjögren's syndrome (SS). †The degree of lymphocytic infiltration in the LSGs was graded from 1 to 12 by focus scoring. ††The ratio of stained lymphocytes was calculated as described in the Patients and methods section. Correlations between focus score and disease duration (a), the CD3/CD19 ratio (b) or the CD4/CD8 ratio (c) in the LSGs from SS patients were observed. Statistical significance of the differences between groups was determined by Spearman's rank correlation.
Fig. 2.
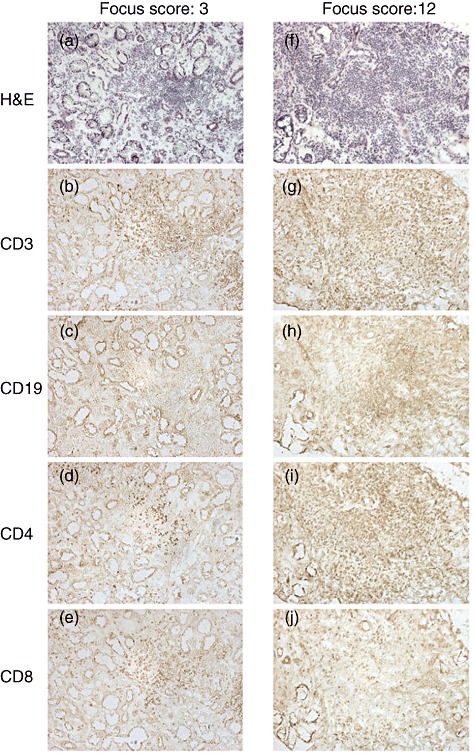
Periductal infiltration of lymphocytes in the labial salivary glands (LSGs) from two representative Sjögren's syndrome (SS) patients. Each set of serial sections was stained with haematoxylin and eosin (H&E) (a,f), anti-CD3 (b,g), anti-CD19 (c,h), anti-CD4 (d,i) and anti-CD8 (e,j) monoclonal antibodies, as described in the Patients and methods section. The degrees of lymphocytic infiltration in the LSGs from the two patients had focus scores 3 and 12. There was a predominance of CD3+ and CD8+ T cells compared with CD19+ B cells and CD4+ T cells, respectively, with focus score 3 LSGs (a–e). However, there was a predominance of CD19+ B cells and CD4+ T cells compared with CD3+ T cells and CD8+ T cells, respectively, with focus score 12 LSGs (f–j) (original magnification ×100).
Cytokine, chemokine and chemokine receptor mRNA expression in LSGs
Relative levels of mRNA expression of IL-2, IFN-γ, IP-10, CXCR3, MIP-1α, RANTES, IL-4, IL-10, TARC, MDC, CCR4 and IL-17 in the LSGs from SS patients were higher than those in the control LSGs (Fig. 3a). In PBMCs from SS patients, only IP-10 and CXCR3 were expressed at higher levels than those in the control PBMCs (Fig. 3b). In addition to being significantly higher than LSG controls, the mRNA expression of IL-2, IFN-γ, TARC and MDC in the LSGs from SS patients was also significantly higher than that in PBMCs from the SS patients.
Fig. 3.
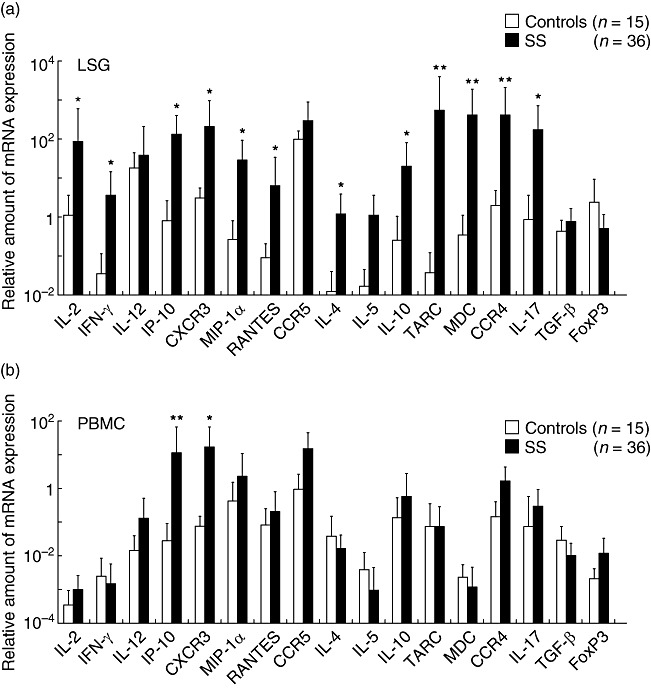
The mRNA expression of cytokines, chemokines and chemokine receptors in the labial salivary glands (LSGs) and peripheral blood mononuclear cells (PBMCs) from Sjögren's syndrome (SS) patients. Real-time polymerase chain reaction (PCR) products of interferon (IFN)-γ, interleukin (IL)-2, IL-4, IL-5, IL-10, IL-12, inducible protein (IP)-10, macrophage inflammatory protein (MIP-1α), regulated upon activation normal T cell expressed and secreted (RANTES), thymus and activation-regulated chemokine (TARC), macrophage-derived chemokine (MDC), CXCR3, CCR4 and CCR5 were estimated quantitatively, as described in the Patients and methods section. The mRNA expression in the LSGs from SS patients was compared with that from control LSGs (a) and the mRNA expression in the PBMCs from SS patients was compared with that from control PBMCs (b). The bar shows the mean value ± standard deviation. Statistical significance of the differences between groups was determined by the Mann–Whitney U-test (*P < 0·05; **P < 0·01).
Association of cytokine, chemokine and chemokine receptor mRNA expression with the degree of lymphocytic infiltration in LSGs
As shown in Fig. 4, mRNA expression of Th1 cytokines such as IL-2, IFN-γ and IL-12, chemokines such as IP-10, RANTES and MIP-1α and chemokine receptors such as CXCR3 and CCR5 was detected consistently in the LSGs from all SS patients. The SS patients were divided into two groups: (i) those with weak lymphocytic infiltration of LSGs (focus scores ranging from 1 to 6, mean ± s.d. score: 2·7 ± 1·5) and (ii) those with strong lymphocytic infiltration (focus scores ranging from 7 to 12, mean ± s.d. score: 8·8 ± 1·7). The mRNA expression of Th1, Th17 and Treg molecules showed no relationship to the degree of lymphocytic infiltration in the LSGs from SS patients. In contrast, the mRNA expression of Th2 cytokines such as IL-4 and IL-5, chemokines such as TARC and MDC and a chemokine receptor, CCR4, was significantly higher in LSGs with strong lymphocytic infiltration in comparison to those with weak lymphocytic infiltration (Fig. 4a). However, PBMC mRNA expression of all these molecules showed no significant relationship to the degree of lymphocytic infiltration in the LSGs from SS patients (Fig 4b). Interestingly, disease duration was significantly longer in patients with strong lymphocytic infiltration of the LSGs (10·3 ± 4·4 years) in comparison to those with weak lymphocytic infiltration (5·0 ± 3·6 years).
Fig. 4.
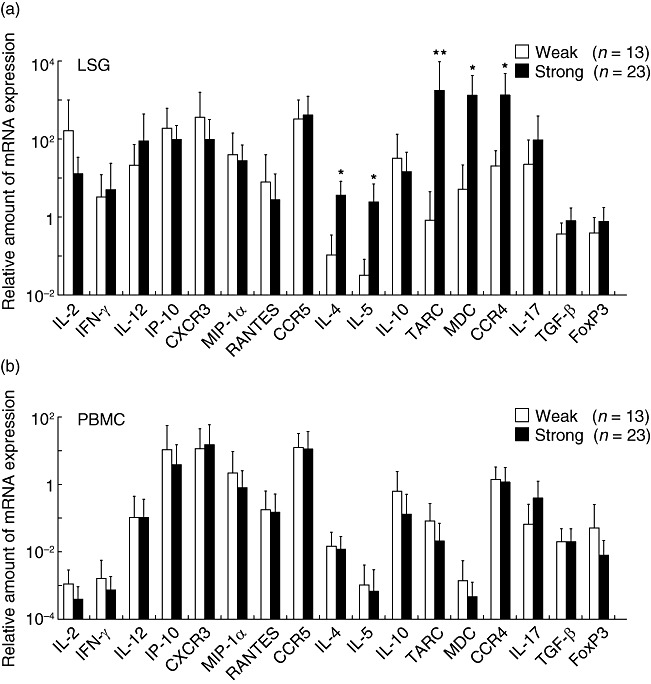
Association between cytokine, chemokine and chemokine receptor mRNA expression and the degree of lymphocytic infiltration in the labial salivary glands (LSGs) from Sjögren's syndrome (SS) patients. Real-time polymerase chain reaction (PCR) products for interferon (IFN)-γ, IL-2, interleukin (IL)-4, IL-5, IL-10, IL-12, inducible protein (IP)-10, macrophage inflammatory protein (MIP)-1α, regulated upon activation normal T cell expressed and secreted (RANTES), thymus and activation-regulated chemokine (TARC), macrophage-derived chemokine (MDC), CXCR3, CCR4 and CCR5 were estimated quantitatively, as described in the Patients and methods section. The degree of lymphocytic infiltration in the LSGs was graded from 1 to 12 by focus scoring, and was then divided into two groups: (i) those with focus scores ranging from 1 to 6 were categorized as having weak infiltration, and (ii) those with scores from 7 to 12 were categorized as having strong infiltration. The bar shows the mean value ± standard deviation. Statistical significance of the differences between groups was determined by the Mann–Whitney U-test (*P < 0·05; **P < 0·01).
Distribution of TARC, MDC and CCR4 in the LSGs
As the expression of TARC, MDC and CCR4 was associated closely with the degree of lymphocytic infiltration in the LSGs from SS patients, we examined the distribution of these proteins in the LSGs from seven patients (four patients with weak lymphocytic infiltration with focus scores of 1 or 2, and three patients with strong lymphocytic infiltration with focus scores of 10 or 12). As shown in Fig. 5, TARC and MDC were detected more strongly in/around the ductal epithelial cells in the LSGs with strong lymphocytic infiltration. CCR4 was detected in higher numbers of infiltrating lymphocytes in the LSGs with strong lymphocytic infiltration in comparison to those with weak lymphocytic infiltration.
Fig. 5.
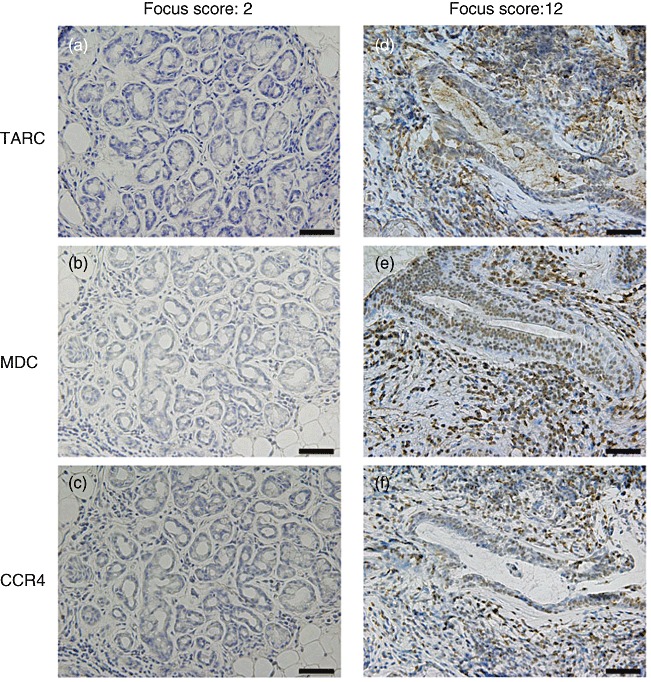
The distribution of thymus and activation-regulated chemokine (TARC), macrophage-derived chemokine (MDC) and CCR4 in the labial salivary glands (LSGs) from two representative Sjögren's syndrome (SS) patients. Each set of serial LSG sections was stained with anti-TARC (a,d), anti-MDC (b,e) and anti-CCR4 (c,f) monoclonal antibodies (brown stain), as described in the Patients and methods section. Counterstaining with Mayer's haematoxylin and eosin was performed subsequently (blue stain). TARC and MDC were detected in or around ductal epithelial cells in the LSGs with a focus score of 12, but not in those with a focus score of 2. CCR4 was detected frequently on the infiltrating lymphocytes in the LSGs with a focus score of 12, but not in those with a focus score of 2 (scale bar = 50 µm; original magnification ×200).
Cytokine and chemokine concentrations in saliva
As cytokines and chemokines are soluble factors secreted in the salivary glands, we examined their concentration in saliva using flow cytometry and ELISA. The concentrations of IL-1β, IL-8, IL-4, IL-6, IL-10 and MDC in saliva from SS patients were significantly higher than in the controls (Fig. 6a).
Fig. 6.
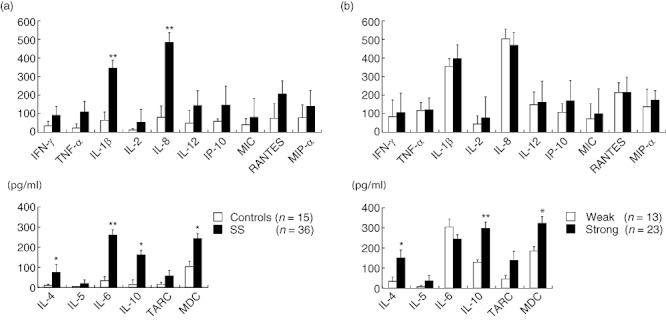
The concentration and the secretion volume per unit time of cytokines and chemokines in the saliva from Sjögren's syndrome (SS) patients. The concentrations of interferon (IFN)-γ, tumour necrosis factor (TNF)-α, interleukin (IL)-1β, IL-2, IL-4, IL-5, IL-6, ILl-10, IL-12, inducible protein (IP)-10, macrophage inflammatory protein (MIP)-1α, regulated upon activation normal T cell expressed and secreted (RANTES), thymus and activation-regulated chemokine (TARC) and macrophage-derived chemokine (MDC) were estimated quantitatively, as described in the Patients and methods section. Concentrations in the saliva from SS patients were compared with those in the controls (a). Furthermore, the concentrations in the saliva from SS patients with strong lymphocytic infiltration were compared with those with weak lymphocytic infiltration (b). The bar shows the mean value ± standard deviation. Statistical significance of the differences between groups was determined by Student's t-test (*P < 0·05; **P < 0·01).
Association of cytokine and chemokine concentrations in saliva with the degree of lymphocytic infiltration in the LSGs
As shown in Fig. 6b, the concentrations of Th1 type molecules showed no relationship to the degree of lymphocytic infiltration in the LSGs from SS patients. In contrast, the concentrations of Th2 type molecules such as IL-4, IL-10 and MDC was significantly higher in the LSGs with strong lymphocytic infiltration compared to those with weak lymphocytic infiltration.
Discussion
It is generally accepted that CD4+ Th cells play a crucial role in the pathogenesis of SS. Several studies on autoimmune diseases in mice and humans have indicated a pathogenic role for Th1 cells and a possible protective role for Th2 cells [7],[8]. However, relatively little is known about the functional activity of CD4+ Th cells at the site of autoimmune damage in human autoimmune diseases because of the difficulty in locating these sites during early disease [9],[31],[32]. Our previous studies suggested a pathogenic model of SS [5],[30],[33]. The mutual stimulation of Th1 cells and the target organ via the production of various cytokines (IL-1, IL-2, IL-6, IFN-γ and TNF), co-stimulatory molecules and Fas/Fas-ligand signalling pathways play a key role in the induction and/or maintenance of the disease and results in the eventual destruction of the target organ. The lymphoaggressiveness of SS is affected by the stimulation of B cells by Th2 cells via differentiation, proliferation and production of immunoglobulins. Furthermore, the salivary gland environment in association with tissue tropic viruses such as the Epstein–Barr virus might increase the risk of pseudolymphoma and hypergammaglobulinaemia, and might consequently hasten the progression to B cell lymphoma. Therefore, as Th2 cells may have a role in the induction of B cell abnormalities, they might have a harmful, rather than a protective, effect in SS patients. We have reported previously that the mRNA levels of Th1 and Th2 cytokines/chemokines in the LSGs from SS patients with strong lymphocytic infiltration were significantly higher than in controls [34]. Furthermore, Theander et al. [35] proposed that detection of germinal centre-like structures (B cell accumulation) in LSG biopsy specimens from primary SS patients might be a highly predictive and easy-to-obtain marker for non-Hodgkin's lymphoma development. Recently, CD4+ Th17 cells have been shown to be tissue-seeking and intimately involved in the initiation of SS [36]. The results of the present study concerning lymphocyte subsets and cytokine production in the LSGs from SS patients are consistent with this model.
The interactions between chemokines and chemokine receptors play an important role in the induction of selective local infiltration of specific cells in various diseases [12]–[17],[37]. The mRNA expression of chemokines such as IP-10, RANTES and MIP-1α, and chemokine receptors such as CXCR3 and CCR5, which are involved in Th1 cell migration [38], was consistent in the LSGs examined and was not associated with the degree of lymphocytic infiltration or the type of lymphocyte subsets present. IP-10 binds to CXCR3 and induces a chemotactic gradient for monocytes and Th1 cells. IP-10 is produced by fibroblasts and vascular endothelial cells [38],[39], while CXCR3 and CCR5 are specific chemokine receptors expressed by Th1 cells [40]. The interaction between CXCR3 and IP-10 plays a critical role in the induction of SS [20],[41]. Our results obtained in the present study are consistent with these reports. IP-10 production is induced strongly by IFN-γ[42]. As we observed increased IFN-γ expression in LSGs from SS patients, it may be involved in the local production of IP-10.
The mRNA expression of chemokines such as TARC and MDC, and chemokine receptors such as CCR4, which are all involved in Th2 cell migration [38], was also detected in LSGs and associated closely with strong lymphocytic infiltration, CD4+ T cell and B cell accumulation and Th2 cytokine production.
Immunohistochemical staining indicated that MDC and TARC were detectable in/around the ductal epithelial cells, while CCR4 was expressed on the infiltrating lymphocytes in the LSGs with strong lymphocytic infiltration. The interaction of CCR4 with MDC and TARC may play a critical role in the accumulation of Th2 cells and consequently the progression of SS. TARC and MDC are natural ligands for CCR4 on Th2 cells [43],[44]. TARC is produced by vascular endothelial cells and dendritic cells, and MDC is produced by antigen-presenting cells [45]. TARC and MDC are detectable in/around the ductal epithelial cells, which may act as antigen-presenting cells in SS [33]. Further studies are required to determine whether salivary gland epithelial cells produce TARC and MDC and, if so, what stimulates the production of these molecules. As SS preferentially affects females, recent reports have suggested that oestrogen may regulate expression of the CCR gene [46] and that of Th2 chemokines such as TARC and MDC [47]. Furthermore, we also focused on infiltrating lymphocytes, especially Th subsets, around the ductal epithelial cells and ectopic GCs in LSGs. Using a laser capture microdissection technique to obtain tissue samples exclusively from specific regions of interest, we observed that Th2 cytokine levels were higher in infiltrating lymphocytes from LSGs with ectopic GC than those around the ductal epithelial cells (manuscript in preparation). These results suggested that Th1 and Th17 cytokines/chemokines are essential in the induction and/or maintenance of SS, while Th2 cytokines/chemokines are involved in the progression of disease process, especially local B cell activation.
The findings in this study provided additional information for the development of a model of SS pathogenesis. However, it is still necessary to elucidate the mechanisms underlying Th1/Th2 cell induction, which might eventually lead to novel therapeutic strategies to inhibit the progression of SS. A more thorough understanding of the complex mechanisms of SS might also lead to pharmacological strategies to inhibit or block the interactions between chemokines and chemokine receptors or to disrupt the cytokine network as a means to inhibit the initiation and/or progression of the disease.
Saliva constituents have been used to monitor systemic conditions. Denny et al. [48] reported that a catalogue containing the salivary proteome of healthy individuals allows the analyses of salivary samples from individuals with oral and systemic diseases such as neurodegenerative conditions (Alzheimer's, Huntington's and Parkinson's diseases) and type I/II diabetes, with the goal of identifying biomarkers with diagnostic potential. IFN-regulated proteomic and genomic biomarkers in whole saliva are highly discriminatory of SS patients [49]. In this study, we found that soluble molecules such as cytokines and chemokines are detectable in the saliva of SS patients and thus have a close association with those in the salivary glands, as they probably originated there.
It is a matter of great regret that this study performed neither a prospective cohort study nor multiple biopsies over time, and could not evaluate the initiation and progression of SS properly. From a practical viewpoint, we are collecting saliva samples over the long term and performing continuous follow-up of SS patients with the aim of evaluating the initiation and progression of SS. As repetitive saliva samples are easily obtained in a non-invasive manner, the measurements of saliva cytokines and chemokines could be a useful test for the diagnosis of SS. In addition, this method would allow the monitoring of disease status, including B cell lymphoma development, by focusing on the Th1/Th2 balance, particularly the involvement of Th2 cytokines/chemokines.
Acknowledgments
This work was supported in part by grants from the Ministry of Education, Culture, Sports, Science, and Technology of Japan and the Research Program of Intractable Disease provided by the Ministry of Health, Labor and Welfare of Japan.
Disclosure
The authors have declared no conflicts of interest concerning this paper.
References
- 1.Tatal N, Moutsopoulos HM, Kassan SS. Sjögren's syndrome – clinical and immunological aspects. Berlin: Springer-Verlag; 1987. [Google Scholar]
- 2.Adamson TC, Fox RI, Frisman DM, Howell FV. Immunohistologic analysis of lymphoid infiltrates in primary Sjögren's syndrome using monoclonal antibodies. J Immunol. 1983;130:203–8. [PubMed] [Google Scholar]
- 3.Sugai S, Shimizu S, Konda S. Lymphoproliferative disorders in Japanese patients with Sjögren's syndrome. Scand J Rheumatol Suppl. 1986;61:118–22. [PubMed] [Google Scholar]
- 4.Prochorec-Sobieszek M, Wagner T. Lymphoproliferative disorders in Sjögren's syndrome. Otolaryngol Pol. 2005;59:559–64. [PubMed] [Google Scholar]
- 5.Ohyama Y, Nakamura S, Matsuzaki G, et al. Cytokine messenger RNA expression in the labial salivary glands of patients with Sjögren's syndrome. Arthritis Rheum. 1996;39:1376–84. doi: 10.1002/art.1780390816. [DOI] [PubMed] [Google Scholar]
- 6.Merrill J, Kono D, Clayton J, Ando D, Hinton D, Hofman F. Inflammatory leukocytes and cytokines in the peptide-induced disease of experimental allergic encephalomyelitis in SJL and B10.PL mice. Proc Natl Acad Sci USA. 1992;89:574–8. doi: 10.1073/pnas.89.2.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kennedy M, Torrance D, Picha K, Mohler K. Analysis of cytokine mRNA expression in the central nervous system of mice with experimental autoimmune encephalomyelitis reveals that IL-10 mRNA expression correlates with recovery. J Immunol. 1992;149:2496–505. [PubMed] [Google Scholar]
- 8.Rapoport MJ, Jaramillo A, Zipris D, et al. Interleukin 4 reverses T cell proliferative unresponsiveness and prevents the onset of diabetes in nonobese diabetic mice. J Exp Med. 1993;178:87–99. doi: 10.1084/jem.178.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fox RI, Kang H, Ando D, Abrams J, Pisa E. Cytokine mRNA expression in salivary gland biopsies of Sjögren's syndrome. J Immunol. 1994;152:5532–9. [PubMed] [Google Scholar]
- 10.Infante-Duarte C, Horton HF, Byrne MC, Kamradt T. Microbial lipopeptides induce the production of IL-17 in Th cells. J Immunol. 2000;165:6107–15. doi: 10.4049/jimmunol.165.11.6107. [DOI] [PubMed] [Google Scholar]
- 11.Kelner GS, Kennedy J, Bacon KB, et al. Lymphotactin: a cytokine that represents a new class of chemokine. Science. 1994;266:1395–9. doi: 10.1126/science.7973732. [DOI] [PubMed] [Google Scholar]
- 12.Murdoch C, Finn A. Chemokine receptors and their role in inflammation and infectious diseases. Blood. 2000;95:3032–41. [PubMed] [Google Scholar]
- 13.Christopher AH, Wayne WH, David WG, et al. Targeted deletion of CX3CR1 reveals a role for fractalkine in cardiac allograft rejection. J Clin Invest. 2001;108:679–88. doi: 10.1172/JCI12976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soon HK, Mary MC, Howard SF, David C, Nora S. CCR4-bearing T cells participate in autoimmune diabetes. J Clin Invest. 2002;110:1675–86. doi: 10.1172/JCI15547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Satoru Y, Nobuyuki E, Seiichi Y, Shiro A. Chemokine receptor gene expression in giant papillae of atopic keratoconjunctivitis. Mol Vis. 2005;11:192–200. [PubMed] [Google Scholar]
- 16.Joerg W, Stephanie H, Eva W, et al. Role of the chemokine receptor CCR4 and its ligand thymus- and activation-regulated chemokine/CCL17 for lymphocyte recruitment in cutaneous lupus erythematosus. J Invest Dermatol. 2005;124:1241–8. doi: 10.1111/j.0022-202X.2005.23755.x. [DOI] [PubMed] [Google Scholar]
- 17.Sorensen TL, Ransohoff RM, Strieter RM, Sellbjerg F. Chemokine CCL2 and chemokine receptor CCR2 in early active multiple sclerosis. Eur J Neurol. 2004;11:445–9. doi: 10.1111/j.1468-1331.2004.00796.x. [DOI] [PubMed] [Google Scholar]
- 18.Amft N, Curnow SJ, Scheel-Toellner D, et al. Ectopic expression of the B cell-attracting chemokine BCA-1 (CXCL13) on endothelial cells and within lymphoid follicles contributes to the establishment of germinal center-like structures in Sjögren's syndrome. Arthritis Rheum. 2001;44:2633–41. doi: 10.1002/1529-0131(200111)44:11<2633::aid-art443>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 19.Szodoray P, Alex P, Brunz JG, Centolay M, Jonsson R. Circulating cytokines in primary Sjögren's syndrome determined by a multiplex cytokine array system. Scand J Immunol. 2004;59:592–9. doi: 10.1111/j.0300-9475.2004.01432.x. [DOI] [PubMed] [Google Scholar]
- 20.Ogawa N, Ping L, Zhenjun L, Takada Y, Sugai S. Involvement of the interferon-γ-induced T cell-attracting chemokines, interferon-γ-inducible 10-kd protein (CXCL10) and monokine induced by interferon-γ (CXCL9), in the salivary gland lesions of patients with Sjögren's syndrome. Arthritis Rheum. 2002;46:2730–41. doi: 10.1002/art.10577. [DOI] [PubMed] [Google Scholar]
- 21.Fujibayashi T, Sugai S, Miyasaka N, Hayashi Y, Tsubota K. Revised Japanese criteria for Sjögren's syndrome (1999): availability and validity. Mod Rheumatol. 2004;14:425–34. doi: 10.3109/s10165-004-0338-x. [DOI] [PubMed] [Google Scholar]
- 22.Vitali C, Bombardieri S, Jonsson R, et al. Classification criteria for Sjögren's syndrome: a revised version of the European criteria proposed by the American–European Consensus Group. Ann Rheum Dis. 2002;61:554–8. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greenspan JS, Daniels TE, Talal N, Sylvester RA. The histopathology of Sjögren's syndrome in labial salivary gland biopsies. Oral Surg. 1974;37:217–29. doi: 10.1016/0030-4220(74)90417-4. [DOI] [PubMed] [Google Scholar]
- 24.Daniels TE, Whitcher JP. Association of patterns of labial salivary gland inflammation with keratoconjunctivitis sicca. Analysis of 618 patients with suspected Sjögren's syndrome. Arthritis Rheum. 1994;37:869–77. doi: 10.1002/art.1780370615. [DOI] [PubMed] [Google Scholar]
- 25.Szodoray P, Alex P, Jonssonc MV, et al. Distinct profiles of Sjögren's syndrome patients with ectopic salivary gland germinal centers revealed by serum cytokines and BAFF. Clin Immunol. 2005;117:168–76. doi: 10.1016/j.clim.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 26.Hiroki A, Nakamura S, Shinohara M, et al. A comparison of glandular involvement between chronic graft-versus-host disease and Sjögren's syndrome. Int J Oral Maxillofac Surg. 1996;25:298–307. doi: 10.1016/s0901-5027(06)80062-7. [DOI] [PubMed] [Google Scholar]
- 27.Hino K, Nishikawa M, Sato E, Inoue M. L-Carnitine inhibits hypoglycemia-induced brain damage in the rat. Brain Res. 2005;1053:77–87. doi: 10.1016/j.brainres.2005.06.062. [DOI] [PubMed] [Google Scholar]
- 28.Ohyama Y, Nakamura S, Matsuzaki G, et al. T-cell receptor Vα and Vβ gene use by infiltrating T cells in labial glands of patients with Sjögren's syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;79:730–7. doi: 10.1016/s1079-2104(05)80308-7. [DOI] [PubMed] [Google Scholar]
- 29.Sasaki M, Nakamura S, Ohyama Y, et al. Accumulation of common T cell clonotypes in the salivary glands of patients with human T lymphotropic virus type I-associated and idiopathic Sjögren's syndrome. J Immunol. 2000;164:2823–31. doi: 10.4049/jimmunol.164.5.2823. [DOI] [PubMed] [Google Scholar]
- 30.Nakamura S, Ikebe-Hiroki A, Shinohara M, et al. An association between salivary gland disease and serological abnormalities in Sjögren's syndrome. J Oral Pathol Med. 1997;26:426–30. doi: 10.1111/j.1600-0714.1997.tb00243.x. [DOI] [PubMed] [Google Scholar]
- 31.Firestein GS, Alvaro-Garcia JM, Maki R. Quantitative analysis of cytokine gene expression in rheumatoid arthritis. J Immunol. 1990;144:3347–53. [PubMed] [Google Scholar]
- 32.Wucherpfennig KW, Newcombe J, Li H, Keddy C, Cuzner ML, Hafler DA. T cell receptor Vα-Vβ repertoire and cytokine gene expression in active multiple sclerosis leisions. J Exp Med. 1992;175:993–1002. doi: 10.1084/jem.175.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsunawaki S, Nakamura S, Ohyama Y, et al. Possible function of salivary gland epithelial cells as nonprofessional antigen-presenting cells in the development of Sjögren's syndrome. J Rheumatol. 2002;29:1884–96. [PubMed] [Google Scholar]
- 34.Tanaka A, Moriyama M, Nakashima H, et al. Th2 and regulatory immune reactions contributes to IgG4 production and the initiation of Mikulicz's disease. Arthritis Rheum. 2012;64:254–63. doi: 10.1002/art.33320. [DOI] [PubMed] [Google Scholar]
- 35.Theander E, Vasaitis L, Baecklund E, et al. Lymphoid organisation in labial salivary gland biopsies is a possible predictor for the development of malignant lymphoma in primary Sjögren's syndrome. Ann Rheum Dis. 2011;70:1363–8. doi: 10.1136/ard.2010.144782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nguyen CQ, Hu MH, Li Y, Stewart C, Peck AB. Salivary gland tissue expression of interleukin-23 and interleukin-17 in Sjögren's syndrome. Arthritis Rheum. 2008;58:734–43. doi: 10.1002/art.23214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akpek EK, Jabs DA, Gerard HC, et al. Chemokines in autoimmune lacrimal gland disease in MRL/MpJ mice. Invest Ophthalmol Vis Sci. 2004;45:185–90. doi: 10.1167/iovs.03-0812. [DOI] [PubMed] [Google Scholar]
- 38.Wysocki C, Panoskaltsis-Mortari A, Blazar B, Serody J. Leukocyte migration and graft-versus-host disease. Blood. 2005;105:4191–9. doi: 10.1182/blood-2004-12-4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qin S, Rottman JB, Myers P, et al. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Invest. 1998;101:746–54. doi: 10.1172/JCI1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Welniak LA, Wang Z, Sun K, et al. An absence of CCR5 on donor cells results in acceleration of acute graft-vs-host disease. Exp Hematol. 2004;32:318–24. doi: 10.1016/j.exphem.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 41.Lee YJ, Scofield RH, Hyon JY, et al. Salivary chemokine levels in patients with primary Sjogren's syndrome. Rheumatology. 2010;49:1747–52. doi: 10.1093/rheumatology/keq121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ichiba T, Teshima T, Kuick R, et al. Early changes in gene expression profiles of hepatic GVHD uncovered by oligonucleotide microarrays. Blood. 2003;102:763–71. doi: 10.1182/blood-2002-09-2748. [DOI] [PubMed] [Google Scholar]
- 43.Imai T, Nagira M, Takagi S, et al. Selective recruitment of CCR4-bearing Th2 cells toward antigen-presenting cells by the CC chemokines thymus and activation-regulated chemokine and macrophage-derived chemokine. Int Immunol. 1999;11:81–8. doi: 10.1093/intimm/11.1.81. [DOI] [PubMed] [Google Scholar]
- 44.Imai T, Baba M, Nishimura M, Kakizawa M, Takagi S, Yoshie O. The T cell-directed CC chemokine TARC is a highly specific biological ligand for CC chemokine receptor 4. J Biol Chem. 1997;272:15036–42. doi: 10.1074/jbc.272.23.15036. [DOI] [PubMed] [Google Scholar]
- 45.Bonecchi R, Bianchi G, Bordignon PP, et al. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J Exp Med. 1998;187:129–34. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mo R, Chen J, Grolleau-Julius A, Murphy HS, Richardson BC, Yung RL. Estrogen regulates CCR gene expression and function in lymphocytes. J Immunol. 2005;174:6023–9. doi: 10.4049/jimmunol.174.10.6023. [DOI] [PubMed] [Google Scholar]
- 47.Bengtsson AK, Ryan EJ, Giordano D, Magaletti DM, Clark EA. 17β-Estradiol (E2) modulates cytokine and chemokine expression in human monocyte-derived dendritic cells. Blood. 2004;104:1404–10. doi: 10.1182/blood-2003-10-3380. [DOI] [PubMed] [Google Scholar]
- 48.Denny P, Hagen FK, Hardt M, et al. The proteomes of human parotid and submandibular/sublingual gland salivas collected as the ductal secretions. J Proteome Res. 2008;7:1994–2006. doi: 10.1021/pr700764j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hu S, Wang J, Meijer J, et al. Salivary proteomic and genomic biomarkers for primary Sjögren's syndrome. Arthritis Rheum. 2007;56:3588–600. doi: 10.1002/art.22954. [DOI] [PMC free article] [PubMed] [Google Scholar]


