Abstract
The pattern-recognition molecules mannan-binding lectin (MBL) and the three ficolins circulate in blood in complexes with MBL-associated serine proteases (MASPs). When MBL or ficolin recognizes a microorganism, activation of the MASPs occurs leading to activation of the complement system, an important component of the innate immune system. Three proteins are produced from the MASP1 gene: MASP-1 and MASP-3 and MAp44. We present an assay specific for MASP-1, which is based on inhibition of the binding of anti-MASP-1-specific antibody to MASP-1 domains coated onto microtitre wells. MASP-1 was found in serum in large complexes eluting in a position corresponding to ∼600 kDa after gel permeation chromatography in calcium-containing buffer and as monomers of ∼75 kDa in dissociating buffer. The concentration of MASP-1 in donor sera (n = 105) was distributed log-normally with a median value of 11 µg/ml (range 4–30 µg/ml). Serum and citrate plasma levels were similar, while the values in ethylenediamine tetraacetic acid plasma were slightly lower and in heparin plasma were 1·5 times higher than in serum. MASP-1 was present at adult level at 1 year of age, while it was 60% at birth. In normal healthy individuals the level of MASP-1 was stable throughout a 2-month period. After induction of an acute-phase reaction by operation we found an initial short decrease, concomitant with an increase in C-reactive protein levels, followed by an increase, doubling the MASP-1 concentration after 2 days. The present data prepare the ground for studies on the associations of MASP-1 levels with disease.
Keywords: complement, inflammation, innate immunity, lectin pathway
Introduction
The innate immune system comprises a number of recognition and effector mechanisms. The complement system is an important component of these systems. It consists of more than 30 proteins, some of which are able to recognize foreign or altered structures, while others are pro-enzymes poised to be activated by the recognition molecules [1],[2]. When complement is activated it mediates inflammatory reactions leading to the elimination of infectious microorganisms, but the destruction of self-tissues can be a side effect of complement-initiated inflammation [3].
The lectin pathway of the complement system is initiated when mannan-binding lectin (MBL) or one of the three ficolins (H-ficolin, L-ficolin or M-ficolin), in complex with the three MBL-associated serine proteases (MASPs: MASP-1, MASP-2 and MASP-3) and MBL-associated proteins (MAp19 and MAp44), binds to appropriate targets [4],[5]. Suitable targets for MBL display patterns of adequately spaced terminal carbohydrates with horizontal 3- and 4-OH groups, whereas targets for the ficolins may be carbohydrates with N-acetyl groups or, indeed, other compounds with a suitable pattern of acetyl groups [4],[6]. The exact composition of the MBL/MASP- or ficolin/MASP-complexes remains unsolved, but it is generally agreed that MASP-2 plays a most significant role in the generation of the C3 convertase, C4bC2a [7],[8]. While the role of MASP-2 appears reasonably well established, the roles of MASP-1, MASP-3, MAp19 and MAp44 are still debated [4],[9]–[11]. However, results have indicated that MASP-1 will accelerate while MASP-3 and MAp44 will inhibit the generation of the C3 convertase [9]–[11]. Recent studies have demonstrated that, while MASP-2 is indeed capable of autoactivating and initiating complement on its own, MASP-1 may play an important role both in activating MASP-2 and cleaving C2 [8],[9],[12],[13]. Although MASP-3 has been reported to have an enzymatic activity towards insulin-like growth factor-binding protein-5, the functional activity of MASP-3 and MAp44 has so far been ascribed primarily to an inhibitory activity on the activation of the lectin pathway [10], although very recently an activity of MASP-3 in accelerating cleavage of factor B and factor D has been presented [14]. Conversely, MASP-1 is clearly an active enzyme which may initiate cleavage of several substrates, some being members of the complement system but others belonging more traditionally to other physiological systems, i.e. a thrombin-like activity in cleaving fibrinogen and factor XIII and the protease activated receptor 4 (PAR4) [13],[15],[16]. Also of note, the MASP1 gene has been implicated in the aetiology of the 3MC syndrome, although the mechanism remains unknown [17],[18]. An assay for MASP-1 will thus be of importance in a number of scientific fields.
The role of MBL was discovered through the study of patients with unexplained susceptibility to infections and opsonin deficiency, as such patients were found to be MBL-deficient [19]. Previously we have described a patient lacking MASP-2, and thus a functional lectin pathway [20]. It seems plausible that elucidating the role(s) of the MASPs as well as those of the MBL-associated small, non-enzymatic splice products, MAp44 and MAp19 [11],[21] may well benefit from epidemiological investigations on selected patient populations. We thus decided to construct assays for these components. We have presented assays previously for MASP-2, MASP-3, MAp44 and MAp19 [11],[21],[22]. Similarly, we have generated assays for the recognition molecules associating with the MASPs/MBL-associated proteins (Maps), i.e. MBL, H-, L- [23] and M-ficolin [24].
The development of the assay for MASP-1 presented here was hampered by the difficulty in raising selective monoclonal antibodies (mAb) due to the extensive sharing of domains between the proteins of the MASP1 gene, which encodes three alternative splice products giving rise to the three proteins MASP-1, MASP-3 and MAp44 [25]. MASP-1 and MASP-3 share five domains (constituting the so-called A-chain), whereas they have unique protease domains (SP domains or B-chains), and the protein MAp44 shares its first four domains with MASP-1 and MASP-3 but has an additional 17 unique amino acid residues C-terminally.
We have now developed specific anti-MASP-1 antibodies and present here a microtitre well-based inhibition assay which is used for the estimation of some basic parameters as a foundation for future clinical investigations. This, in turn, allows us to explore the relative abundances of the MASPs/MAps and the soluble pattern recognition molecules (PRMs) and hence the physiological equilibrium between these.
Methods
Biological reagents
Serum and plasma were obtained from Danish blood donors. The study was approved by local Ethics Committees and informed consent was obtained from the donors. In the following sections references are given to papers which have used the same samples for other purposes. A recombinant fragment of MASP-1 comprising the CCP1-CCP2-SP domains (rCCP1-CCP2-SP) was produced in Escherichia coli, and refolded and purified as described previously [13].
Anti-MASP-1 antibody
A synthetic peptide representing the 15 C-terminal amino acid residues of human MASP-1 (CHHNKDWIQRVTGVR) was coupled to keyhole limpet haemocyanin. Three Wistar rats were immunized four times subcutaneously with 10 µg of this conjugate, emulsified first in complete Freund's adjuvant and then in incomplete Freund's adjuvant for boosts. Sera from the animals were tested for reactivity towards rCCP1-CCP2-SP coated onto microtitre wells. All rats responded and the rat with the highest titre was selected. IgG was purified from the anti-serum by affinity chromatography on Protein G-coupled beads. The serum was diluted 1/1 in phosphate-buffered saline (PBS; 137 mM NaCl, 2·7 mM KCl, 1·5 mM KH2PO4, 8·1 mM Na2HPO4, pH 7·4) containing 10 mM EDTA (PBS/EDTA), and passed through the beads. After washing, the bound IgG was eluted with 0·1 M glycine, pH 2·4. The immunoglobulin concentration was determined by spectroscopy at 280 nm. The purified IgG was biotinylated by standard procedure [26] using 167 µg biotin-N-hydroxysuccinimide ester (Sigma-Aldrich, St Louis, MO, USA) per mg antibody.
Reactivity of antibody
The anti-MASP-1 anti-serum was tested by Western blotting. MBL/MASP complexes were purified from serum by affinity chromatography on mannan coupled to Sepharose beads, as described previously [8]. The complexes, as well as a preparation of rCCP1-CCP2-SP, were separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) in non-reducing conditions followed by blotting onto a membrane. For the preparation of Western blotting strips, MBL/MASP complexes corresponding to 30 µg MBL were loaded onto a single-well XT-Criterion pre-cast 4–12% gradient Bis-Tris polyacrylamide gel (Bio-Rad, Copenhagen, Denmark) and cut into 2·5-mm-wide strips after blotting, resulting in approximately 1 µg MBL (+ associated proteins) per strip. The proteins were blotted onto a nitrocellulose membrane (Hybond-ECN; GE Healthcare, Hilleroed, Denmark) in transfer buffer (25 mM Tris, 0·192 M glycine, 20% v/v ethanol, 0·1% w/v SDS, pH 8·3) for 500 volt-hours. The membrane was blocked in 0·1% Tween 20 in Tris-buffered saline (TBS) (10 mM Tris–HCl, 140 mM NaCl, 1·5 mM NaN3, pH 7·4) before being cut into strips. The strips were incubated with primary antibodies (normal rat IgG or rat anti-MASP-1 antibody) diluted in primary buffer (TBS with 0·05% Tween 20 (TBS/Tw), 1 mM EDTA, with 1 mg human serum albumin (HAS) and 100 µg normal human IgG (hIgG) added per ml) in eight-well trays (Octaline, Pateof, Denmark) for 2·5 h on a rocking table. The strips were washed, incubated with secondary antibody, horseradish peroxidase-labelled goat anti-rat Ig (P0450; Dako, Glostrup, Denmark) in secondary buffer (TBS/Tw, no azide, 1 mM EDTA, 100 µg hIgG/ml) for 1·5 h and washed before being developed with SuperSignal West Dura Extended Duration Substrate (Pierce, Rockford, IL, USA). Images were taken using a CCD camera (LAS-3000; Fuji, Dusseldorf, Germany) and analysed with the software supplied with the camera.
The antibody specificity was explored further in the assay described below, where the addition of possible competing molecules was tested and the molecular size of the antigen was determined (see below).
MASP-1 assay
The human MASP-1 assay was based on competition from MASP-1 in serum with the interaction between anti-MASP-1 antibody and a fragment of MASP-1 (rCCP1-CCP2-SP) coated onto microtitre wells. The procedure described below leads to the measurement of europium as the label on the detecting reagents, and the procedure as such is termed a time-resolved immunofluorimetric assay (TRIFMA). Microtitre wells were coated with 100 ng rCCP1-CCP2-SP in 100 µl coating buffer (15 mM Na2CO3, 35 mM NaHCO3, 1·5 mM NaN3, pH 9·6) overnight at 4°C. Residual binding sites were blocked by incubation with HSA at 1 mg/ml TBS and washed with TBS/Tw. To test for MASP-1 the wells next received 100 µl of samples (e.g. normal human plasma or serum), which had been diluted in assay buffer (1 M NaCl, 10 mM Tris-HCl, 5 mM CaCl2, 15 mM NaN3, pH 7·4, 0·05% (v/v) Tween 20), mixed with rat anti-MASP-1 anti-serum and incubated for 15 min to ensure binding of anti-MASP-1 antibody to MASP-1 in the sample, before transfer to the microtitre wells. Routinely, serum or plasma was tested at a final concentration of 1·6% (60-fold dilution) and the anti-MASP-1 anti-serum was diluted 5000-fold. Following incubation overnight at 4°C, the wells were washed with TBS/Tw/Ca and incubated with 1 µg/ml biotinylated anti-rat-Ig in 100 µl of TBS/Tw/Ca for 2 h at room temperature. The wells were washed and subsequently incubated with europium-labelled streptavidin (Perkin Elmer, Skovlunde, Denmark) diluted to 0·25 µg/ml in TBS/Tw containing 25 µM EDTA. After incubating for 1 h the wells were washed, and bound europium in the wells was measured by time-resolved fluorimetry (Victor3; Perkin Elmer) after the addition of enhancement solution (Perkin Elmer). The readings are given as photon counts per second.
For each plate, a standard curve was made from a pool of plasma from healthy adult blood donors. The plasma was diluted 1/10 followed by twofold dilutions (seven times). In addition, for quality control each microtitre plate included three different citrate plasma samples diluted 60-fold.
The standard plasma pool was found to have a concentration of 5·7 µg MASP-1 per ml by comparison with dilutions of rCCP1-CCP2-SP. This reagent weighs 45·073 g/mol compared to 79·247 g/mol for MASP-1 (both based on amino acid sequence of the polypeptide chains and not taking into account post-translational modifications). The inhibition obtained by the number of molecules in 1 µg rCCP1-CCP2-SP per ml was thus said to be equivalent to the number of molecules in 1·76 (79 247/45 073) µg MASP-1 per ml. We added the rCCP1-CCP2-SP to 10% fetal calf serum before performing the dilutions in order to obtain a similar matrix and to obtain comparable slopes of the dilution curve of the standard plasma and the recombinant material (the antibodies employed do not cross-react with bovine MASP-1).
To test for the specificity of the assay, purified rMAp44 or rMASP-3 (produced and purified as described in Degn et al. [21]) was added to the MASP-1 assay at a concentration of 10 µg/ml for rMAp44 and 2·5 µg/ml for rMASP-3 at the highest concentration and dilutions thereof. The addition of rMAp44 or rMASP-3 did not influence the signal.
To characterize the assay further and to study the association of MASP-1 with other serum components, serum was subjected to gel permeation chromatography (GPC) on a 1 × 30 cm Superose 6 HR column (GE Healthcare). The running buffer was TBS, 0·01% (v/v) Tween 20 containing either 5 mM Ca2+ or 10 mM EDTA + 860 mM NaCl (to reach a total of 1 M NaCl). This buffer dissociates MBL/MASP complexes [27]. The column was loaded with 50 µl normal human serum diluted with one volume of column buffer. Fractions of 0·25 ml were collected in polystyrene microtitre plates (Nunc, Roskilde, Denmark) pre-blocked by short incubation for 10 min with TBS/Tw followed by washing with water and drying the wells. The fractions were tested in the MASP-1 assay described above after 2·5-fold dilution in the assay buffer. The EDTA-containing samples were diluted in assay buffer with extra CaCl2 (20 mM) added to overcome the chelating effect of the EDTA. MBL, M- and H-ficolin were quantified in the fractions by TRIFMAs, as described previously [23],[24]. In order to establish relevant molecular size markers, fractions were also analysed for IgM, IgG and HSA.
Variation of MASP-1 levels over time
Serum samples from four healthy individuals were collected over a 50-day period. For the first week, the samples were collected each day, followed by weekly collections. The samples were kept at −80°C and MASP-1 was measured as described above.
Ontogeny
MASP-1 levels in infants were determined in samples obtained from the umbilical cords at term, and sequentially at 6, 9 and/or 12 months after birth. The samples have been described previously in detail [28]. Samples were kept at −80 C and freeze–thaw cycles kept to a minimum.
Acute-phase reaction
To estimate the MASP-1 levels after the induction of an acute-phase response we tested samples from patients undergoing surgery. The samples were obtained from colorectal cancer patients prior to surgery and sequentially at 12 h, 24 h, 2, 3, 4 and 5 days post-surgery, and at additional time-points up to 35 days after surgery. The samples have been described previously [29].
Statistics
The MASP-1 concentrations are presented by the median, quartiles and range. The associations between the variables MASP-1, MASP-3 and MAp44 were estimated by Spearman's rank correlation. All P-values <5% were considered significant.
Results
Anti-MASP-1 antibody
Wistar rats were immunized with a complex consisting of the 15 C-terminal amino acid residues of MASP-1 (i.e. a sequence not shared by MASP-3 and MAp44) coupled to keyhole limpet haemocyanin (KLH). The sera were tested for reactivity to rCCP1-CCP2-SP coated in microtitre wells. The specificity of the preferred serum was examined further by application to blots of MBL/MASP complexes purified from human serum. A Western blot is shown in Fig. 1a, lane 1, where reaction with protein can be seen at a position corresponding to the previously observed mobility of pro-enzyme MASP-1 (Mr ∼75 kDa). We saw no reactivity with material at the positions of MASP-3 (Mr ∼105 kDa), MASP-2 (Mr ∼70 kDa), MAp44 (Mr ∼44 kDa) or MAp19 (Mr ∼19 kDa), which were revealed by incubating parallel strips with the relevant antibodies (not shown), as described previously in detail [10],[21]. No reactivity of a normal rat serum is seen (Fig. 1a, lane 2). The anti-MASP-1 serum was tested further on Western blots of rCCP1-CCP2-SP. A reactivity corresponding to ∼45 kDa was seen, which is the expected size of the construct (calculated at 45 073 g/mol) (Fig. 1a, lane 4). No reactivity was seen by a normal rat serum (Fig. 2a, lane 3). The reaction of the rat anti-MASP-1 anti-serum was also evaluated by TRIFMA, as described below.
Fig. 1.
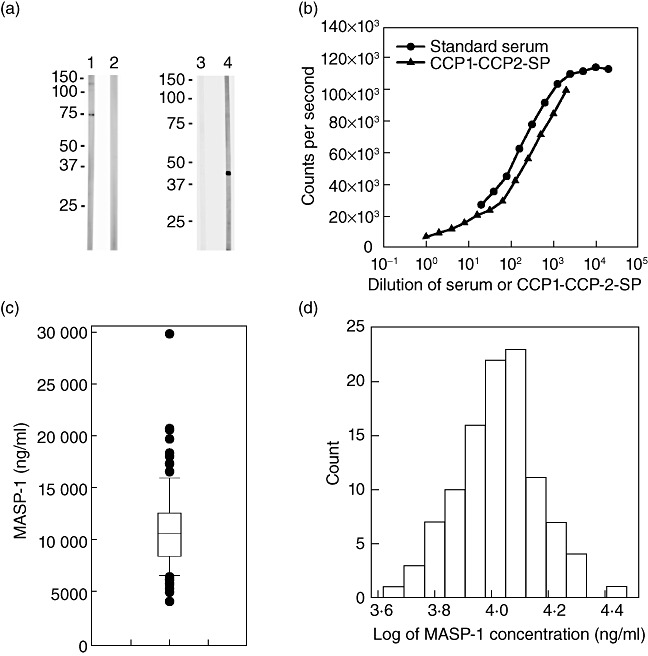
Anti-mannan-binding lectin (MBL)-associated serine protease-1 (MASP-1) antibodies and MASP-1 assay. (a) Analysis of reactivity of polyclonal rat anti-MASP-1 antibody by Western blotting. Lane 1 shows the reaction of anti-MASP-1 antibody on a strip representing non-reduced MBL/MASP complex, whereas lane 2 shows the result when using normal rat immunoglobulin (Ig)G. Lane 3 shows development with normal rat IgG on strips of non-reduced rCCP1-CCP2-SP and lane 4 shows development with anti-MASP-1 antibody. The positions of molecular size markers are indicated. (b) The responses obtained in the MASP-1 assay when applying a dilution series of the standard plasma pool (●) as well as the responses when applying dilutions of purified rCCP1-CCP2-SP (▴). (c) A plot of MASP-1 concentrations in 105 healthy blood donors on a linear scale. The statistical distribution with median (10·7 µg/ml, line in the box), box indicating the 25–75 percentiles (8·5–12·6 µg/ml), whiskers the 5–95 percentile and arithmetic mean (11·1 ng/ml, cross inside the box). (d) The distribution of log-transformed MASP-1 levels in column format on a log scale.
Fig. 2.
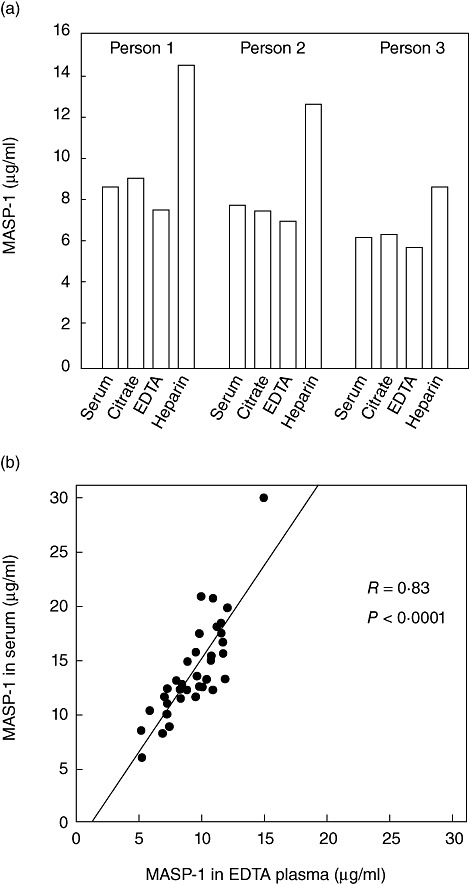
Mannan-binding lectin (MBL)-associated serine protease-1 (MASP-1) levels in serum and plasma. (a) Serum and citrate, ethylenediamine tetraacetic acid (EDTA) and heparin plasma were obtained from three individuals. The estimated MASP-1 levels are plotted. (b) The results obtained when comparing corresponding serum (y-axis) and EDTA plasma (x-axis) samples withdrawn from each of 35 individuals. The line indicates linear regression and the statistic of the fit is indicated.
TRIFMA for MASP-1
Preliminary attempts to construct a sandwich-type assay were non-productive and we thus turned towards an inhibition assay based on inhibition of the binding of anti-MASP-1 antibody to a surface coat of rCCP1-CCP2-SP. Accordingly, decreasing signals were seen when increasing concentration of plasma were applied, as illustrated by the standard curve in Fig. 1b, which was generated by applying serial dilutions of our standard plasma pool. The value for MASP-1 content of this pool was estimated at 5·7 µg/ml by comparison with a preparation of pure rCCP1-CCP2-SP. Figure 1b shows a dilution curve of this reagent next to the dilution curve of the standard serum.
A number of dilution buffers were assessed. The most consistent results for plasma and serum were obtained with the complex assay buffer composition detailed in Materials and methods. This is a high-ionic-strength calcium-containing buffer (the high ionic strength lowers the background in the assay but also prevents coagulation if diluting, e.g. EDTA or citrate plasma in a calcium-containing buffer) with proteins added to reduce background signals.
We found a 60-fold dilution to be suitable for plasma samples to be assayed for MASP-1. For routine analyses, three internal controls were added to each assay plate. The means and interassay coefficients of variation (CVs), determined from 10 individual assays for the three internal controls, were: 15·5 µg/ml, 7·68 µg/ml, 3·72 µg/ml and 11%, 13% and 8%, respectively. The sensitivity of the assay, i.e. the concentration yielding a signal two standard deviations (SDs) above the background, was 20 ng/ml in the well, i.e. corresponding to plasma with 1·2 µg/ml when the 60-fold dilution was used. This is considerably below the lowest value encountered in the cohort of 105 blood donors, as described below.
While dose-related signals were seen after adding rCCP1-CCP2-SP, signals comparable to background were seen when rMAp44 or rMASP-3 were added instead (not shown). The selectivity was also confirmed by adding each of these three proteins to plasma before dilution for the MASP-1 assay. Only the addition of rCCP1-CCP2-SP gave an additive response.
MASP-1 concentrations in healthy blood donors
Plasma from 105 blood donors were analysed in order to determine the normal variation in MASP-1 and the results are shown in Fig. 1c. The levels of MASP-1 were not distributed normally, but were distributed log-normally, and Fig. 1d illustrates the normal distribution of the log-transformed values. The median was 10·7 µg/ml (quartile range 8·5–12·6 µg/ml), mean 11·1 µg/ml, with a minimal value of 4·2 µg/ml and a maximal value of 29·8 µg/ml.
In three healthy individuals we compared the levels obtained when testing serum, EDTA, citrate and heparin plasma taken consecutively from the same person. Figure 2a shows that for all three individuals comparable values were seen in serum and citrate plasma, whereas heparin plasma showed higher values (mean 153%; range 137–168%) than serum. Slightly lower values were seen in EDTA plasma compared to serum. A possible difference between serum and EDTA plasma levels was studied further by comparing the values of corresponding serum and EDTA plasma samples from 35 normal healthy individuals. While there was excellent correlation (r = 0·83, P < 0·0001), the serum values (mean, 14·1 µg/ml) are, on average, 1·5 times higher than the EDTA plasma values (mean, 9·4 µg/ml) (Fig. 2b).
Further specificity of assay and size of MASP-1 in serum
Proteins in a serum sample were separated by GPC and the fractions were tested for MASP-1 content. When fractionation was performed at a physiological salt concentration in a calcium-containing Tris buffer we found the MASP-1 to be present in a major symmetrical peak (Fig. 3a) eluting at 11–14 ml, with the highest concentration at 12·5 ml at an estimated apparent Mr of approximately 600 kDa. This could represent MASP-1 in complex with MBL, H-ficolin and L-ficolin, as these molecules elute in the same range. These recognition molecules all elute over several fractions, but only peak positions are indicated on the figure. When we fractionated serum in a buffer known to dissociate MBL/MASP complexes (i.e. containing EDTA and high salt concentration), we found MASP-1 to elute after 16 ml at a position corresponding to ∼75 kDa (Fig. 3b). This could represent the polypeptide chain of MASP-1 (theoretically, 77 kDa based on amino acid composition only).
Fig. 3.
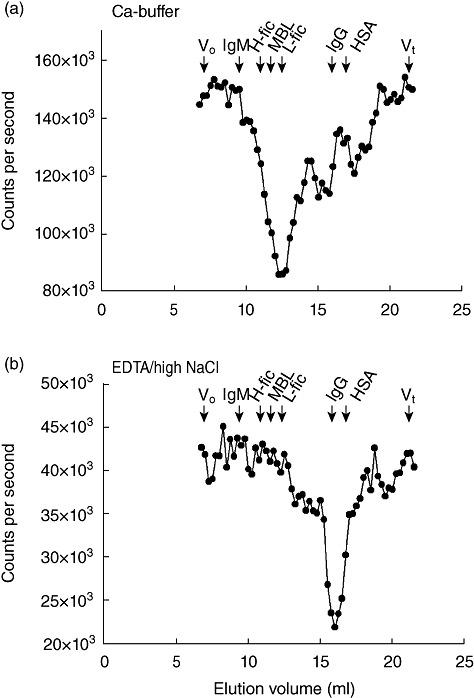
Mannan-binding lectin (MBL)-associated serine protease-1 (MASP-1) in serum analysed by gel permeation chromatography. N-hydroxysulphosuccinimide (NHS) (50 µl) was passed through a Superose 6 column in (a) isotonic buffer with calcium ions or in (b) a buffer with 1 M NaCl and ethylenediamine tetraacetic acid (EDTA). Fractions were collected and analysed for MASP-1 content (●) by time-resolved immunofluorimetric assay (TRIFMA). Arrows indicate the elution volume of immunoglobulin (Ig)M (970 kDa), IgG (150 kDa) and human serum albumin (HSA, 67 kDa). The elution positions of MBL and H-ficolin and L-ficolin are also indicated. The experiment was repeated twice with similar results. Elution volumes are indicated on the x-axis.
MASP-1 in sequential samples
The concentration of MASP-1 in sequential samples obtained from four apparently healthy individuals during a 50-day period was evaluated. As evident from Fig. 4, we found the MASP-1 level to be stable during this time-period. We saw a variation of approximately 25%, i.e. mean of percentage of highest versus lowest levels in the individual during this period for these four individuals were 30, 32, 20 and 18%.
Fig. 4.
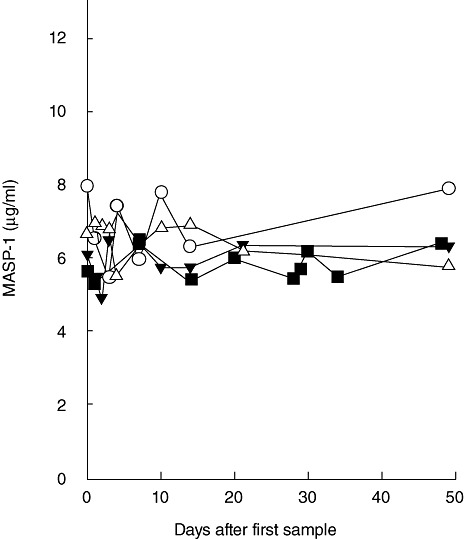
Intra-individual variation of mannan-binding lectin (MBL)-associated serine protease-1 (MASP-1) over time. The concentration of MASP-1 was estimated in plasma samples obtained over a 50-day period from four individuals.
Ontogeny
Samples obtained from 14 cord blood samples and from corresponding sequential samples throughout the first year of life (6, 9 and 12 months) were analysed for MASP-1 level. Figure 5 illustrates that in three of the infants hardly any change was seen from birth until 1 year of age, whereas in the 11 others we saw an increase from birth to the 6-month sample, and no further increase during the next 6 months. Overall, we found a ×1·6 increase from first-day sample and the sample taken at 12 months, indicating that newborns have near-adult levels at birth.
Fig. 5.
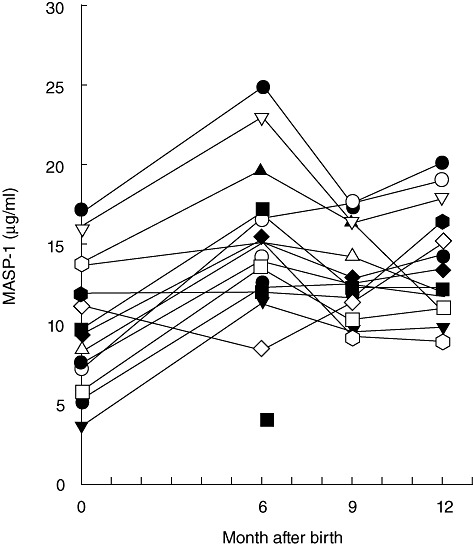
Ontogeny of mannan-binding lectin (MBL)-associated serine protease-1 (MASP-1). The concentration of MASP-1 was estimated in sequential samples from 14 term infants during their first year of life. The values at zero are the estimates in serum from blood taken from the umbilical cord.
Acute-phase response
As an example of an acute-phase reaction we tested sequential serum samples obtained from six patients operated for colorectal cancer (first sample taken before initiation of operation). Previously, these samples were tested for the classical acute-phase proteins interleukin (IL)-6 and C-reactive protein (CRP) and were also tested for MBL and MASP-2 [29], MASP-3 and MAp44 [21] and M-ficolin [24]. We selected samples from six patients with a low pre-operation CRP level, a high post-operation rise in CRP and a drop to near CRP baseline at the latest samples taken. The CRP response is depicted in Fig. 6 on the right-hand y-axis and the values for MASP-1 on the left-hand y-axis. The MASP-1 response is quite varied. Following operation, we saw a drop in MASP-1 level in the patients, reaching a level of a mean of 71%, varying between 43 and 90% of samples taken before operation. The drop was already seen in the first sample taken after operation, i.e. after 12 h (for three cases a slightly lower level was seen in the next sample after 24 h), and thus we do not know if even lower levels were reached before this. Importantly, this drop happens at the same time that the increase is seen in CRP. The drop in MASP-1 levels is followed by an increase with a mean of 189%, varying between 106 and 302%, compared to the pre-operation sample, and between 177 and 435% when compared with the sample with the lowest level. The increase peaked in all cases except one after the CRP levels dropped to lower levels.
Fig. 6.
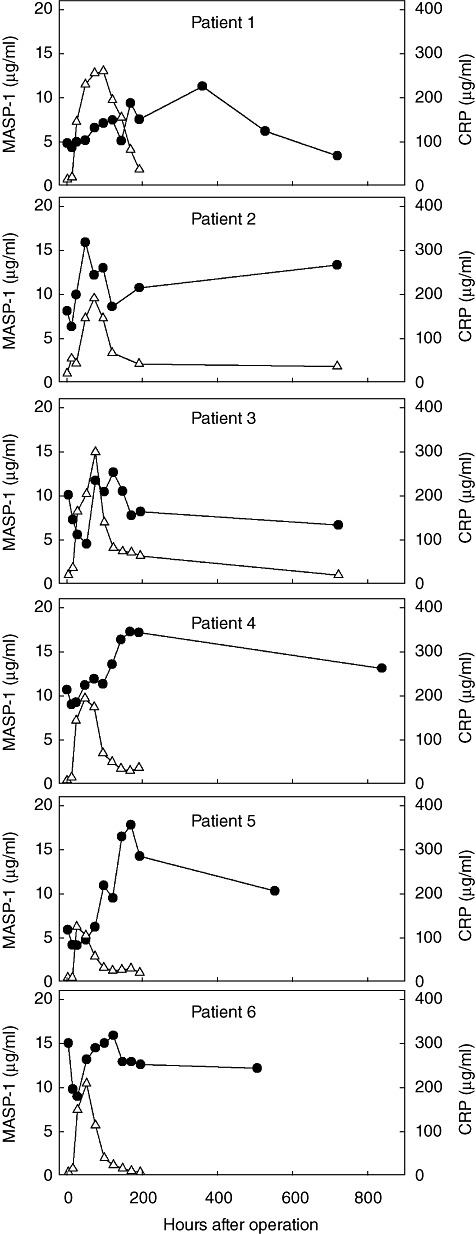
Acute-phase response. Mannan-binding lectin (MBL)-associated serine protease-1 (MASP-1) levels were measured in samples taken sequentially from six patients undergoing surgery for colorectal carcinoma. Day 0 is a sample taken just before operation. Each of the six graphs illustrates the results from one patient. The C-reactive protein (CRP) levels in the samples are also given (right-hand axis). MASP-1 concentrations are given as filled circles and the CRP levels as open triangles.
Correlation between levels of MASP-1, MASP-3 and MAp44
MASP-3 and MAp44 are encoded by the same gene (MASP1) as MASP-1 and share large parts of the polypeptide chain [25]. We have measured the level of MASP-3 and MAp44 previously in the normal blood donors presented here, and the individual levels of all three proteins are illustrated in Fig. 7. MASP-1 and MAp44 may be correlated weakly positively (Fig. 7b), but analysis of association of the data using a two-tailed Spearman non-parametric test show no obvious associations, considering P-values < 5% as significant [P-value and coefficient of correlation; MASP-1 versus MASP-3, 0·15 (−0·14), MASP-1 versus MAp44, 0·11 (0·16), MASP-3 versus MAp44, 0·11 (0·16)].
Fig. 7.
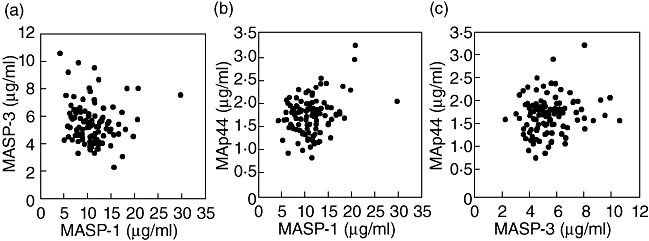
Comparison of the serum levels of the three proteins encoded by the MASP1 gene. The three graphs compare the levels of: (a) mannan-binding lectin (MBL)-associated serine protease-1 (MASP-1) (x-axis) and MASP-3 (y-axis), (b) MASP-1 (x-axis) and MAp44 (y-axis) and (c) MASP-3 (x-axis) and MAp44 (y-axis). The drawn lines indicate the linear regression fit. The rs value is the non-parametric Spearman correlation coefficient. Note differences in range of axes.
Discussion
The complement system is a powerful component of the innate immune system. It is clearly involved in a number of anti-microbial processes but, as discussed in recent reviews, it is also a potentially very harmful inflammatory element [1]–[3]. There is thus a need for sensitive and robust assays enabling the determination of the concentrations of factors of the complement system in various body fluids.
Initiation of complement activation happens via three different pathways, i.e. the alternative, classical and lectin pathways. The composition of the two first pathways have long been established, whereas for the lectin pathway new members have been added during recent years [4],[10]. In the present report we extend our previous studies of the lectin pathway of the complement system and provide serum concentrations for the last of the known lectin pathway components, namely that of MASP-1.
A rat anti-human MASP-1 antibody was obtained after immunization with a peptide corresponding to the C-terminal part of MASP-1. The MASP-1 assay described in this report exploits the binding of this antibody to microtitre wells coated with recombinant protein representing the last three C-terminal domains of MASP-1. MASP-1 in samples competes with this interaction and the level of inhibition seen is thus a measure of the MASP-1 content of the sample. In principle, such an inhibition assay is dependent only on the number of exposed epitopes and is not influenced by oligomerization of the antigen or whether the antigen is in complex with other proteins. After examining several buffer compositions, we arrived at one with high salt concentration and calcium. The specificity of the assay was corroborated experimentally (see below).
We found a median of 11 µg MASP-1/ml serum in the cohort of 105 Caucasian adult blood donors. Terai et al. [30] reported the results obtained with an assay using a biotinylated anti-A-chain antibody (mab 1E2) for development in an assay where the capture antibody was another anti-A chain antibody (mab 2B11). As MASP-1, MASP-3 and MAp44 share the sequence detected by both these antibodies this assay should, in principle, detect all three proteins of these (the latter two had not been discovered at the time when that report was published) with equal sensitivity. Examining 1063 normal sera from Japanese donors, they reported a mean concentration (of MASP-1 + MASP-3 + MAp44) of 6·27 µg/ml serum [30]. We have recently measured the concentrations of MASP-3 and MAp44, which are listed in Table 1. Disregarding possible ethnic differences, the discrepancy is likely to be due to the calibration of the assays against different materials.
Table 1.
The serum concentrations of proteins of the lectin pathway of the complement system.
| Name | Weight of chain (kDa) | Weight of multimers (kDa) | Measured conc. (µg/ml) | Conc. (nM) | Conc. of multimers (nM) | Relative conc. | |
|---|---|---|---|---|---|---|---|
| Pattern recognition molecules (dodecamers) | MBL | 24 | 288 | 1·1 | 48 | 4 | 0·07 |
| H-ficolin | 31 | 372 | 19·5 | 624 | 52 | 0·87 | |
| L-ficolin | 31 | 372 | 3·4 | 109 | 9 | 0·15 | |
| M-ficolin | 32 | 384 | 1·4 | 48 | 4 | 0·07 | |
| Associated molecules (dimers) | MASP-1 | 77 | 154 | 11·0 | 143 | 72 | 1 |
| MASP-2 | 73 | 146 | 0·4 | 6 | 3 | 0·04 | |
| MASP-3 | 79 | 158 | 5·0 | 63 | 32 | 0·44 | |
| MAp44 | 41 | 82 | 1·7 | 41 | 21 | 0·29 | |
| MAp19 | 18 | 36 | 0·4 | 22 | 11 | 0·15 |
Calculated based on the non-modified amino acid content of the mature polypeptide chain. The isoform 1 of H-ficolin and isoform a of L-ficolin was used for these two proteins. Dodecamers is assumed for the recognition molecules and dimers for the mannan-binding lectin (MBL)-associated serine proteases (MASPs) and MBL-associated proteins (Maps). The relative concentration of MASP-1 dimers is set to 1.
We found that all the MASP-1 is found in large complexes at sizes indicating an association with MBL and ficolins, suggesting that most MASP-1 is associated with these recognition molecules, and possibly also other proteins. When we disrupted the interaction with EDTA and high NaCl concentration we found MASP-1 to elute at a position corresponding to the single polypeptide chain, indicating that the complexes are indeed dissociated. The indicated size must be used with caution, as the estimate may be affected by glycosylations and rely further on the relative shapes of the protein under study compared with the standard proteins used for calibration. The finding of all of MASP-1 in large complexes is still in line with the earlier suggestion, at a time when ficolin-MASP interactions were not known by us [27] and others [30], that much of the MASPs and MAps in serum are not associated with MBL.
From birth at term and during the following 3 months there was an increase in MASP-1, but in general a level quite similar to the level after 12 months, and indeed adult levels, were seen (Fig. 5). None were below 3 µg/ml at delivery. This indicates that whatever the function of MASP-1, one may regard the newborn as probably having sufficient quantities.
An issue when comparing samples between different groups of patients is the possible variation of the parameters over time. In general, measurements on samples obtained sequentially from four apparently healthy volunteers through a 50-day period showed only minor variations (Fig. 4). This stable level makes it possible to compare MASP-1 concentrations in samples taken at various time-points, although the situation may be different in some patient populations. Conversely, measurements on samples retrieved during an acute-phase response, induced by a major operation, showed that MASP-1 was rapidly down-regulated and subsequently up-regulated for some time following the operation (Fig. 6). The increase happened slowly, roughly 3 days after the peak of the CRP response, and reached levels only approximately twice that of the pre-operation sample. We do not know if the colon cancer by itself has an influence on the pre-operation MASP-1 levels, and it is possible that a greater response may be induced by infections. A possible acute-phase response must thus be taken into account when studying data sets from patients.
A puzzling early finding was that the levels of MASP-1 determined in heparin plasma were higher than in the corresponding serum, citrate plasma or EDTA plasma (Fig. 2). We can offer no explanation for this observation, but it may have to do with interference by the interaction of enzyme inhibitors in serum because, e.g. anti-thrombin-III in complex with heparin is known to bind and inhibit MASP-1 much better than without heparin [13]. For comparison of samples in routine analyses it is thus important to not compare heparin plasma values directly with serum values. A much smaller, but significant, difference between serum and EDTA plasma levels was also indicated.
We did not see a strong correlation between serum levels of MASP-1, MASP-3 and MAp44 (Fig. 7). Intuitively, one might expect to find a positive correlation between MASP-1, MASP-3 and MAp44 levels in serum due to common transcriptional regulation, but this may well be countered by the negative correlating effects of alternative splicing and its regulation [25]. Similarly, one might expect to find a positive correlation between MASP-1 and members of the MBL/ficolin family due to their association and presumable stabilizing carrier effect. We also found a weak negative correlation of MASP-1 levels and MBL levels in the cohort examined, and a weak positive correlation of MASP-1 and MASP-2 (not shown). However, this picture may be greatly complicated by the interaction of the five different MASPs/MAps with the four recognition molecules. Dissecting the intricacies of individual versus concerted regulation of these components and their interactions within each individual is an overwhelming task. One interesting question that may be addressed in this study, however, is the total stoichiometry between MASP/MAp dimers and PRM binding sites for such dimers. In this respect, the level of MASP-1 is the last piece in this puzzle. In Table 1 we have provided calculations of the concentration of the MASPs and MAps and the recognition molecules of the lectin pathway. The MASPs and MAps are believed to form homodimers. The molecular concentration of MASP-1 dimers (72 nM) is approximately two to three times higher than MASP-3 and MAp44 dimers and 24 times higher than MASP-2 dimers (Table 1). In comparison, the dimer MASP-1 concentration equals the molecular concentration of H-ficolin but is 18 times higher than the MBL and M-ficolin concentration and eight times higher than the L-ficolin concentration. Recently, collectin-kidney 1 (CL-K1 or collectin11) was shown to interact with MASP-3 and/or MASP-1 and is found at 340 ng/ml [31] or 2·1 µg/ml [32] in serum, i.e. roughly 4 nM dodecamers assuming 1 µg/ml. The total concentration of dimers of MASPs and MAps is equal to 140 nM compared to the 70 nM of the assumed dodecameric recognition molecules. This indicates that, at least on average, a balanced concentration exists in serum. Notably, each MASP/MAp dimer may exhibit an intrinsic (perhaps sterically determined) affinity for a particular PRM and/or a particular oligomerization state of this PRM. The comparatively simplistic calculations presented here cannot account for this. Furthermore, our use of means/medians determined in a cohort of 105 donors may mask great independent interindividual variations in each parameter.
It is our hope that the availability of an assay for MASP-1 may further our understanding of the biological role of MASP-1 and should permit detailed studies of selected patient populations.
Acknowledgments
This work was supported by Novo Nordisk Foundation and by The Danish Council for Independent Research, Medical Sciences.
Disclosure
None of the authors has any conflict of interest related to this manuscript.
References
- 1.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11:785–97. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Le Friec G, Kemper C. Complement: coming full circle. Arch Immunol Ther Exp. 2009;57:393–407. doi: 10.1007/s00005-009-0047-4. [DOI] [PubMed] [Google Scholar]
- 3.Walport MJ. Complement. Second of two parts. N Engl J Med. 2001;344:1140–44. doi: 10.1056/NEJM200104123441506. [DOI] [PubMed] [Google Scholar]
- 4.Thiel S. Complement activating soluble pattern recognition molecules with collagen-like regions, mannan-binding lectin, ficolins and associated proteins. Mol Immunol. 2007;44:3875–88. doi: 10.1016/j.molimm.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Matsushita M, Endo Y, Fujita T. Cutting edge: complement activating complex of ficolin and mannose-binding lectin-associated serine protease. J Immunol. 2000;164:2281–4. doi: 10.4049/jimmunol.164.5.2281. [DOI] [PubMed] [Google Scholar]
- 6.Garlatti V, Belloy N, Martin L, et al. Structural insights into the innate immune recognition specificities of L- and H-ficolins. EMBO J. 2007;26:623–33. doi: 10.1038/sj.emboj.7601500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thiel S, Vorup-Jensen T, Stover CM, et al. A second serine protease associated with mannan-binding lectin that activates complement. Nature. 1997;386:506–10. doi: 10.1038/386506a0. [DOI] [PubMed] [Google Scholar]
- 8.Matsushita M, Thiel S, Jensenius JC, Terai I, Fujita T. Proteolytic activities of two types of mannose-binding lectin associated serine protease. J Immunol. 2000;165:2637–42. doi: 10.4049/jimmunol.165.5.2637. [DOI] [PubMed] [Google Scholar]
- 9.Kocsis A, Kékesi KA, Szász R, et al. Selective inhibition of the lectin pathway of complement with phage display selected peptides against mannose-binding lectin-associated serine protease (MASP)-1 and -2: significant contribution of MASP-1 to lectin pathway activation. J Immunol. 2010;185:4169–78. doi: 10.4049/jimmunol.1001819. [DOI] [PubMed] [Google Scholar]
- 10.Degn SE, Hansen AG, Steffensen R, Jacobsen C, Jensenius JC, Thiel S. MAp44, a human protein associated with pattern recognition molecules of the complement system and regulating the lectin pathway of complement activation. J Immunol. 2009;183:7371–8. doi: 10.4049/jimmunol.0902388. [DOI] [PubMed] [Google Scholar]
- 11.Degn SE, Thiel S, Nielsen O, Hansen AG, Steffensen R, Jensenius JC. MAp19, the alternative splice product of the MASP2 gene. J Immunol Methods. 2011;373:89–101. doi: 10.1016/j.jim.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takahashi M, Ishida Y, Iwaki D, et al. Essential role of mannose-binding lectin associated serine protease-1 in activation of the complement factor D. J Exp Med. 2010;207:29–37. doi: 10.1084/jem.20090633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dobó J, Harmat V, Beinrohr L, Sebestyén E, Závodszky P, Gál P. MASP-1, a promiscuous complement protease: structure of its catalytic region reveals the basis of its broad specificity. J Immunol. 2009;183:1207–14. doi: 10.4049/jimmunol.0901141. [DOI] [PubMed] [Google Scholar]
- 14.Iwaki D, Kanno K, Takahashi M, Endo Y, Matsushita M, Fujita T. The role of mannose-binding lectin-associated serine protease-3 in activation of the alternative complement pathway. J Immunol. 2011;187:3751–58. doi: 10.4049/jimmunol.1100280. [DOI] [PubMed] [Google Scholar]
- 15.Krarup A, Gulla KC, Gál P, Hajela K, Sim RB. The action of MBL-associated serine protease 1 (MASP1) on factor XIII and fibrinogen. Biochim Biophys Acta. 2008;1784:1294–300. doi: 10.1016/j.bbapap.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 16.Megyeri M, Makó V, Beinrohr L, et al. Complement and endothelial PAR4 activation is a link between activated human endothelial cells: complement protease MASP-1. J Immunol. 2009;183:3409–16. doi: 10.4049/jimmunol.0900879. [DOI] [PubMed] [Google Scholar]
- 17.Sirmaci A, Walsh T, Akay H, et al. MASP1 mutations in patients with facial, umbilical, coccygeal, and auditory findings of Carnevale, Malpuech, OSA, and Michels syndromes. Am J Hum Genet. 2010;87:679–86. doi: 10.1016/j.ajhg.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rooryck C, Diaz-Font A, Osborn DP, et al. Mutations in lectin complement pathway genes COLEC11 and MASP1 cause 3MC syndrome. Nat Genet. 2011;43:197–203. doi: 10.1038/ng.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Super M, Lu J, Thiel S, Levinsky RT, Turner MW. Association of low levels of mannan-binding protein with a common defect of opsonisation. Lancet. 1989;334:1236–39. doi: 10.1016/s0140-6736(89)91849-7. [DOI] [PubMed] [Google Scholar]
- 20.Stengaard-Pedersen K, Thiel S, Gadjeva M, et al. Inherited deficiency of mannan-binding lectin-associated serine protease 2. N Engl J Med. 2003;349:554–60. doi: 10.1056/NEJMoa022836. [DOI] [PubMed] [Google Scholar]
- 21.Degn SE, Jensen L, Gál P, et al. Biological variations of MASP-3 and MAp44, two splice products of the MASP1 gene involved in regulation of the complement system. J Immunol Methods. 2010;361:37–50. doi: 10.1016/j.jim.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 22.Moller-Kristensen M, Jensenius JC, Jensen L, et al. Levels of mannan-binding lectin-associated serine protease-2 in healthy individuals. J Immunol Methods. 2003;282:159–67. doi: 10.1016/j.jim.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 23.Krarup A, Sørensen UB, Matsushita M, Jensenius JC, Thiel S. Effect of capsulation of opportunistic pathogenic bacteria on binding of the pattern recognition molecules mannan-binding lectin, L-ficolin, and H-ficolin. Infect Immun. 2005;73:1052–60. doi: 10.1128/IAI.73.2.1052-1060.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wittenborn T, Thiel S, Jensen L, Nielsen HJ, Jensenius JC. Characteristics and biological variations of M-ficolin, a pattern recognition molecule, in plasma. J Innate Immun. 2010;2:167–80. doi: 10.1159/000218324. [DOI] [PubMed] [Google Scholar]
- 25.Degn SE, Jensenius JC, Thiel S. Disease-causing mutations in genes of the complement system. Am J Hum Genet. 2011;88:689–705. doi: 10.1016/j.ajhg.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guesdon JL, Ternynck T, Avrameas S. The use of avidin–biotin interaction in immunoenzymatic techniques. J Histochem Cytochem. 1979;27:1131–9. doi: 10.1177/27.8.90074. [DOI] [PubMed] [Google Scholar]
- 27.Thiel S, Petersen SV, Vorup-Jensen T, et al. Interaction of C1q and mannan-binding lectin (MBL) with C1r, C1s, MBL-associated serine proteases 1 and 2, and the MBL-associated protein MAp19. J Immunol. 2000;165:878–87. doi: 10.4049/jimmunol.165.2.878. [DOI] [PubMed] [Google Scholar]
- 28.Thiel S, Bjerke T, Hansen D, Poulsen LK, Schiøtz PO, Jensenius JC. Ontogeny of human mannan-binding protein, a lectin of the innate immune system. Pediatr Allergy Immunol. 1995;6:20–3. doi: 10.1111/j.1399-3038.1995.tb00252.x. [DOI] [PubMed] [Google Scholar]
- 29.Ytting H, Christensen IJ, Basse L, et al. Influence of major surgery on the mannan-binding lectin pathway of innate immunity. Clin Exp Immunol. 2006;144:239–46. doi: 10.1111/j.1365-2249.2006.03068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Terai I, Kobayashi K, Matsushita M, Fujita T. Human serum mannose-binding lectin (MBL)-associated serine protease-1 (MASP-1): determination of levels in body fluids and identification of two forms in serum. Clin Exp Immunol. 1997;110:317–23. doi: 10.1111/j.1365-2249.1997.tb08334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoshizaki T, Ohtani K, Motomura W, et al. Comparison of human blood concentrations of collectin kidney 1 (CL-K1) and mannan-binding lectin (MBL) Biochemistry. 2011;151:57–64. doi: 10.1093/jb/mvr114. [DOI] [PubMed] [Google Scholar]
- 32.Hansen S, Selman L, Palaniyar N, et al. Collectin 11 (CL-11, CL-K1) is a MASP-1/3-associated plasma collectin with microbial-binding activity. J Immunol. 2010;185:6096–104. doi: 10.4049/jimmunol.1002185. [DOI] [PubMed] [Google Scholar]


