Abstract
Although the hallmark of primary biliary cirrhosis (PBC) is the presence of anti-mitochondrial antibodies (AMA), a significant number of patients have anti-nuclear antibodies (ANA) directed primarily against two nuclear proteins, gp210 and sp100. In PBC, there are considerable data on the specificity of these anti-nuclear antibodies as well as suggestive evidence that antibodies to gp210 predict a poor outcome. However, a further understanding of the significance of these autoantibodies has been hampered by limitations in accessing human subjects in a preclinical or early asymptomatic stage. To overcome this limitation, we have taken advantage of transgenic mice with abrogated transforming growth factor-β signalling in T cells (dnTGF-βRII) that develop histological features of PBC as well as the same AMA specificity. We studied these mice for serum ANA, including specific autoantibodies against gp210 and sp100. We further examined sera from dnTGF-βRII mice with concurrent deletions of the genes encoding interleukin (IL)-12p35, IL-12p40, IL-23p19, IL-17, IL-6, interferon (IFN)-γ or tumour necrosis factor (TNF)-α. Sera from all the dnTGF-βRII mouse lines contained antibodies against gp210 and sp100. Of significance, mice with germline deletions of the genes encoding IL-12p40, IL-23p19, IL-17, IL-6 and TNF-α had significantly lower titres of anti-gp210 antibodies. These results provide a platform to dissect the mechanisms of gp210 and sp100 autoantibody production in dnTGF-βRII mice as well as to study the possible role of ANA in the pathophysiology of PBC.
Keywords: AMA, ANA, autoantibodies, cytokines, gp210
Introduction
More than 90% of patients with primary biliary cirrhosis (PBC) have serum anti-mitochondrial antibodies (AMA), which react most frequently with an epitope on the E2 subunit of the pyruvate dehydrogenase enzyme complex (PDC-E2) [1]. In addition, almost 50% [2] of patients with PBC have anti-nuclear antibodies (ANA). These primarily recognize a nuclear pore membrane glycoprotein gp210, various proteins of nuclear bodies, prototypically sp100 [3], and centromeric proteins. Autoantibodies against a nuclear envelope protein in PBC were first suspected based on observations by indirect immunofluorescence (IIF) microscopy that sera from a subset of patients label the nuclear periphery with a nuclear rim pattern, and recognize a polypeptide with an apparent molecular mass of approximately 200 kDa [4]–[7]. In 1990, Courvalin et al. [8] identified the autoantigen as gp210. Most anti-gp210 antibodies recognize a stretch of only 15 amino acids in the protein's carboxyl-terminal ‘tail’ that faces the nuclear pore complex [9]. In 1987, autoantibodies from patients with PBC that produced a fine speckled multiple nuclear dot pattern when examined by IIF microscopy were shown to recognize a protein with an apparent molecular mass of approximately 100 kDa (sp100) [10]. Subsequently, other nuclear dot proteins were identified as autoantigens in PBC, and the prototypic sp100 was characterized by complementary DNA cloning [11]. Anti-sp100 antibodies recognize at least three non-overlapping domains of the protein and two stretches of 16–20 amino acids may contain the predominant autoepitopes [12],[13]. Antibodies against gp210 and sp100 are highly specific for PBC and are found very rarely in other diseases [3], whereas the specificity of anti-centromere antibodies for PBC is confounded by the association with the limited form of systemic sclerosis, a disease known to associate with PBC [14].
The value of anti-gp210 and anti-sp100 in the diagnosis of PBC is being recognized increasingly [15], but the influence of these autoantibodies on pathophysiology and disease outcome remains unclear. In early studies, anti-gp210 antibodies were not associated with particular clinical features, having only a weak association with concurrent arthritis in one case [4],[16]. In subsequent studies, anti-gp210 antibodies were found to be associated with more advanced liver disease and poorer outcomes [17]–[21]. However, these clinical studies were necessarily retrospective, because of limited access to subjects at preclinical or early asymptomatic stages of PBC. Furthermore, the possible role of gp210 and sp100 autoantibodies in pathogenesis has been difficult to ascertain because of problems associated with accessing human tissue samples. To overcome these limitations of human clinical studies, we have turned to a murine model of PBC [22], which is a transgenic mouse expressing a dominant-negative form of transforming growth factor (TGF)-βeta receptor type II (dnTGF-βRII) under the control of a CD4 promoter and lacking the CD8 silencer. These mice have disrupted TGF-β signalling in T cells and are deficient in activities dependent upon regulatory T cells and develop mononuclear cell infiltration in multiple organs [23]. Notably, these mice develop lymphocytic cell infiltration of liver portal tracts with associated bile duct injury, and also AMA directed to the mitochondrial autoantigens specific to PBC [24]. However, these mice have not been examined for autoantibodies against gp210 and sp100, the major nuclear autoantigens specific to PBC.
We now demonstrate that dnTGF-βRII mice contain serum autoantibodies against both gp210 and sp100, even when crossed to mice with germline deletions of the genes encoding the proinflammatory cytokines, interleukin (IL)-12, IL-23, IL-17, IL-6, interferon (IFN)-γ or tumour necrosis factor (TNF)-α. However, dnTGF-βRII mice lacking IL-12p40, IL-23p19, IL-17, IL-6 or TNF-α have lower titres of anti-gp210 antibodies. These results justify the use of mouse models to study the significance of gp210 and sp100 autoantibodies in PBC, and demonstrate that generation of particular ANA may be down-modulated by deprivation of IL-23, IL-17, IL-6 and TNF-α signalling.
Materials and methods
Mice
The dnTGF-βRII mice, developed originally by R. A. Flavell [23], were bred onto the C57BL/6 (B6) background at the University of California, Davis animal facility. B6·129S1-Il12atm1Jm/J (IL-12p35−/−), B6·129S1-Il12btm1Jm/J (IL-12p40−/−), B6·129S6-Il6tm1Kopf/J (IL-6−/−), B6·129S7-Ifngtm1Ts/J (IFN-γ−/−) and B6·129S-Tnftm1Gkl/J (TNF-α−/−) mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). IL-17−/− mice were obtained from Yoichiro Iwakura (University of Tokyo, Tokyo, Japan). IL-23p19−/− mice were generously provided by Frederic J. de Sauvage [25]. To generate dnTGF-βRII/IL-12p35−/− mice, IL-12p35−/− mice were mated with dnTGF-βRII mice to obtain dnTGF-βRII/IL-12p35+/− mice and the male offspring were subsequently back-crossed onto female IL-12p35−/− mice. Similarly, other dnTGF-βRII mouse lines were generated by back-crossing female IL-12p40−/−, IL-23p19−/−, IL-6−/−, IL-17−/−, IFN-γ−/− and TNF-α−/− mice to male dnTGF-βRII/IL-12p40+/−, dnTGF-βRII/IL-23p19+/−, dnTGF-βRII/IL-6+/−, dnTGF-βRII/IL-17+/−, dnTGF-βRII/IFN-γ+/− and dnTGF-βRII/TNF-α+/−, respectively. Genotypes of mice were confirmed by polymerase chain reactions using genomic DNA. Mice were fed the sterile rodent Helicobacter medicated dosing system diet (Bio-Serv, Frenchtown, NJ, USA) and maintained in individually ventilated cages under specific pathogen-free conditions. Experiments were performed with approval from the UC Davis Institutional Animal Care and Use Committee.
IIF microscopy
Serum samples were diluted with phosphate-buffered saline (PBS) pH 7·4 at a 1:100 ratio. A total of 25 µl of diluted sera was dispensed into each well on the Hep-2 substrate slide (NOVA Lite HEp-2 ANA; Inova Diagnostics, San Diego, CA, USA). The slides were incubated at room temperature for 1 h and then washed with PBS. Secondary antibodies (Alexa-488-conjugated goat anti-mouse immunoglobulin (Ig)G; Invitrogen, Carlsbad, CA, USA) in a volume of 25 µl/well were then added at a predetermined optimum dilution of 1:400. The slides were incubated at room temperature for 30 min and then washed with PBS. After coverslips were applied with mounting media (ProLong Gold AntIIFde Reagent with 4′,6-diamidino-2-phenylindole; Invitrogen), the slides were observed by using a confocal microscope (Zeiss LSM 700; Carl Zeiss Microscopy, Thornwood, NY, USA).
Enzyme-linked immunosorbent assay (ELISA)
To detect antibodies against PDC-E2, 96-well plates were coated with a recombinant human PDC-E2 glutathione-S-transferase fusion protein in coating buffer at a concentration of 5 µg/well. Plates were incubated at 4°C overnight and blocked with PBS containing 3% milk at room temperature for 1 h. To detect antibodies against gp210 and sp100, QUANTA Lite gp210/sp100 (Inova Diagnostics) was used; 96-well plates were precoated with purified peptides that are identified as dominant epitopes of the gp210/sp100 protein [16],[26]–[28]. Serum samples were diluted with PBS containing 3% milk at a 1:250 ratio for detection of anti-PDC-E2 antibodies or with horseradish peroxidase (HRP) sample diluent (Inova Diagnostics) at 1:50–1:100 for detection of anti-gp210 and anti-sp100 antibodies. A total of 100 µl of diluted serum was dispensed into each well. The plates were incubated at room temperature for 1 h and then washed with PBS containing 0·05% Tween-20 (PBS-T). Secondary antibodies in a volume of 100 µl/well (HRP-conjugated goat anti-mouse IgG, IgA and IgM; Zymed, San Diego, CA, USA) were then added at a predetermined optimum dilution of 1:3000. Plates were incubated at room temperature for 1 h and then washed with PBS-T. Solutions A and B of BD OptEIA (BD Biosciences, Franklin Lakes, NJ, USA) were mixed at a 1:1 ratio and then added to the wells as substrate. Plates were incubated in the dark for colour development. Sulphuric acid (2N) was added to the wells to stop the reaction. Optical density (OD) was measured using an ELISA plate reader at 450 nm. The antibody potency for each sample is expressed in semi-quantitative units (ELISA units), using a calibrator as an index. The ELISA unit for each sample was calculated as follows: the OD of the sample divided by the OD of the calibrator and then multiplied by the number of units assigned to the calibrator. Both positive and negative control sera were used for standardization. Mouse sera with ELISA units greater than the mean ± 2 standard deviations (s.d.) of the negative control samples were considered positive.
Expression of data and statistical analysis
Data are presented as means ± s.d. Statistical analysis was performed using Prism software (Graphpad Software, La Jolla, CA, USA). Differences between groups were tested by one-way analysis of variance followed by Dunnett's multiple comparison test. P-values less than 0·05 were considered statistically significant.
Results
Detection of ANA by IIF
The presence of ANA and AMA was first detected by IIF on HEp-2 cells (Fig. 1). Serum from C57BL/6 mice was used as negative control, as these mice have the same genetic background as dnTGF-βRII mice. Staining characteristics of AMA were observed in all the dnTGF-βRII murine models of PBC, including those with deletions of IL-12p35, IL-12p40, IL-23p19, IL-17, IL-6, IFN-γ or TNF-α (Fig. 1). However, the nuclear staining revealed three distinct patterns depending on which cytokine gene was deleted. The multiple nuclear dot pattern was observed with sera from most of the mouse lines, including dnTGF-βRII, dnTGF-βRII/IL-12p35−/−, dnTGF-βRII/IL-12p40−/−, dnTGF-βRII/IL-23p19−/− and dnTGF-βRII/IFN-γ−/− mice (Fig. 1, pattern I). However, nuclear periphery (rim-like) staining was only clearly noticeable in dnTGF-βRII/IL-17−/− mice (Fig. 1, pattern II). Nuclear fluorescence with sera from dnTGF-βRII/IL-6−/− or dnTGF-βRII/TNF-α−/− mice was much weaker (Fig. 1, pattern III), suggesting that the ANA titres were lower than in sera from the other dnTGF-βRII mouse lines. Centromeric, homogeneous and nucleolar staining patterns were not observed with any of the serum samples.
Fig. 1.
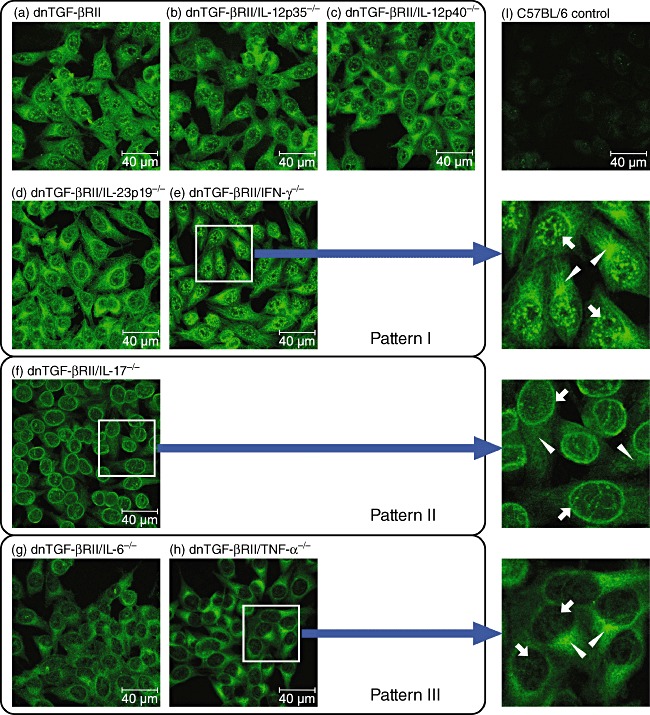
Detection of anti-nuclear antibodies (ANA) and anti-mitochondrial antibodies (AMA) by indirect immunofluorescence (IIF). Photomicrographs reflect staining patterns of serum samples (1:100 dilution) from (a) dominant-negative form of transforming growth factor (TGF)-βeta receptor type II (dnTGF-βRII), (b) dnTGF-βRII/interleukin (IL)-12p35−/−, (c) dnTGF-βRII/IL-12p40−/−, (d) dnTGF-βRII/IL-23p19−/−, (e) dnTGF-βRII/interferon (IFN)-γ−/−, (f) dnTGF-βRII/IL-17−/−, (g) dnTGF-βRII/IL-6−/−, (h) dnTGF-βRII/tumour necrosis factor (TNF)-α−/− and (i) C57BL/6 control mice. Mitochondrial staining was observed in all the dn TGF-βRII mice (white triangles), whereas three major nuclear staining patterns, I, II and III, were observed (white arrows). The most representative images are shown for each mouse strain (n ≥ 4). Size reference bars = 40 µm.
Analysis of ANA by ELISA
Sometimes IIF cannot detect specific ANA patterns because of a co-existing high cytoplasmic fluorescence signal resulting from serum AMA. Therefore, we used ELISA to further identify serum ANA against gp210 and sp100 (Table 1). The ELISA assays were developed based on previous molecular studies that used truncated peptides to identify predominant epitopes of gp210/sp100. The antigen used by the gp210 kit includes a stretch of 15 amino acids at the carboxyl-terminal cytoplasmic tail of the gp210 protein that can be recognized by PBC sera [9]. The antigen used by the sp100 kit includes several amino acid fragments located within the 240–474 region of sp100, which has been identified as the most antigenic region of the sp100 protein [26],[29],[30]. Sera from 75–100% of the mice in each of the dnTGF-βRII lines had detectable anti-gp210 antibodies. The percentages of sera from mice with detectable anti-sp100 were also fairly high, but varied more between the different strains, from 63 to 100%.
Table 1.
Detection of antibodies to gp210 and sp100 by enzyme-linked immunosorbent assay (ELISA)†.
| ANA | |||||
|---|---|---|---|---|---|
| Mouse | Age (week) | n | Anti-GP210 | Anti-SP100 | |
| Control | C57BL/6 | 12–24 | 26 | 0/26 | 0/26 |
| Murine models of PBC | dnTGF-βRII | 24 | 21 | 21/21 | 21/21 |
| dnTGF-βRII/IL-12p35−/− | 24 | 16 | 16/16 | 15/16 | |
| dnTGF-βRII/IFN-γ−/− | 16 | 4 | 4/4 | 4/4 | |
| dnTGF-βRII/IL-12p40−/− | 24 | 12 | 11/12 | 9/12 | |
| dnTGF-βRII/IL-23p19−/− | 24 | 13 | 13/13 | 13/13 | |
| dnTGF-βRII/IL-17−/− | 24 | 16 | 16/16 | 16/16 | |
| dnTGF-βRII/IL-6−/− | 24 | 19 | 18/19 | 12/19 | |
| dnTGF-βRII/TNF-α−/− | 12 | 4 | 3/4 | 3/4 | |
Data are presented as number of positive sera/total number of sera studied. ANA: anti-nuclear antibodies; dnTGF-βRII: dominant-negative form of transforming growth factor (TGF)-βeta receptor type II; IL: interleukin; IFN: interferon; TNF: tumour necrosis factor; PBC: primary biliary cirrhosis.
Deletion of the genes encoding IL-6 and TNF-α from dnTGF-βRII mice resulted in a significant decrease in serum titres of anti-gp210 antibodies (Fig. 2a), consistent with results using IIF to detect ANA. Deletion of the genes encoding IL-p40, IL-23p19 and IL-17 also resulted in significantly lower titres of serum anti-gp210 antibodies, even though some degree of nuclear fluorescence was still observed on IIF, suggesting that other ANA may be present in sera from these mouse lines. Deletion of the genes encoding IL-12p35 and IFN-γ had no effects on the titres of serum anti-gp210 antibodies in dnTGF-βRII mice. There was no significant difference in the titres of serum anti-sp100 antibodies between dnTGF-βRII mice with or without deletions of the cytokine genes (Fig. 2b). These results suggest that in dnTGF-βRII mice genetic ablation of IL-12p40, IL-23p19, IL-17, IL-6 and TNF-α could influence the titres of serum anti-gp210, but not anti-sp100 antibodies.
Fig. 2.
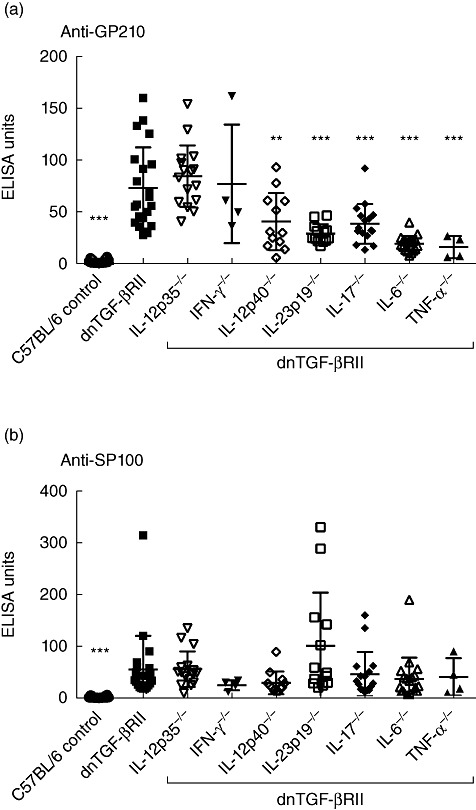
Serum anti-gp210 (a) and anti-sp100 (b) antibodies in C57BL/6 (n = 26), dominant-negative form of transforming growth factor (TGF)-βeta receptor type II (dnTGF-βRII) (n = 21), dnTGF-βRII/interleukin (IL)-12p35−/− (n = 16), dnTGF-βRII/interferon (IFN)-γ−/− (n = 4), dnTGF-βRII/IL-12p40−/− (n = 12), dnTGF-βRII/IL-23p19−/− (n = 13), dnTGF-βRII/IL-17−/− (n = 16), dnTGF-βRII/IL-6−/− (n = 19) and dnTGF-βRII/tumour necrosis factor (TNF)-α−/− (n = 4) mice. Samples are from 24-week-old mice, except for C57BL/6 (age 12–24 weeks), dnTGF-βRII/IFN-γ−/− (age 16 weeks) and dnTGF-βRII/TNF-α−/− (age 12 weeks). Statistical comparisons for every other line are to dnTGF-βRII mice (***P < 0·001; **P < 0·01; *P < 0·05).
All the sera studied by ELISA from dnTGF-βRII mouse lines had antibodies against PDC-E2, the titres of which were increased with deletion of the genes encoding IL-12p35 and IL-23p19 (Table 2). None of the alterations of the cytokine genes increased the titres of anti-gp210 or anti-sp100 ANA. Deletion of the gene for IL-6 decreased the titres of both AMA and anti-gp210 antibodies, suggesting that IL-6 plays a common role in their generation in dnTGF-βRII mice.
Table 2.
Effects of cytokine deletion on serum AMA (anti-PDC-E2) and ANA (anti-gp210 and anti-sp100) in dnTGF-βRII mice†.
| AMA | ANA | |||
|---|---|---|---|---|
| Mouse | Anti-PDC-E2 | Anti-GP210 | Anti-SP100 | |
| dnTGF-βRII | IL-12p35−/− | ↑*** | – | – |
| IFN-γ−/− | – | – | – | |
| IL-12p40−/− | – | ↓** | – | |
| IL-23p19−/− | ↑* | ↓*** | – | |
| IL-17−/− | – | ↓*** | – | |
| IL-6−/− | ↓** | ↓*** | – | |
| TNF-α−/− | – | ↓*** | – | |
Upward arrows indicate increases, downward arrows decreases and ‘–’ no significant change compared to dnTGF-βRII mice
P < 0·001
P < 0·01
P < 0·05).
AMA: anti-mitochondrial antibodies; ANA: anti-nuclear antibodies; dnTGF-βRII: dominant-negative form of transforming growth factor (TGF)-βeta receptor type II; IL: interleukin; IFN: interferon; TNF: tumour necrosis factor.
Discussion
We have reported previously that dnTGF-βRII mice have disease features that resemble human PBC histologically and serologically, notably lymphocytic infiltration of liver portal tracts with damaged biliary ductules and AMA directed specifically against PDC-E2 [24]. The aim of this study was to examine this model, and mice with additional germline deletions of select inflammatory cytokines, for the presence of autoantibodies against the known PBC-specific nuclear autoantigens, gp210 and sp100. There is an 87% amino acid sequence identity between human and mouse gp210. The sp100 superfamily domain is also highly conserved between human and mouse sp100. We demonstrate that dnTGF-βRII mice not only produced AMA, but also ANA, with unique specificity against nuclear pore protein gp210 and the nuclear dot protein sp100, as occurs in PBC. The inference is that blockage of TGF-β and impaired regulatory T cell activity account for the development of both AMA and ANA caused by the loss of B cell and T cell tolerance to particular self-antigens. There are several key phenotypical features of human PBC in the dnTGF-βRII mouse, including autoantibodies to gp210, sp100 and mitochondrial autoantigens, as well as lymphocytic cell infiltrates of small bile ducts. The data reported herein implicate cytokines in playing a distinct role in the production of autoantibodies in this model. For example, ablation of IL-12p40 resulted in significantly lower serum anti-gp210 titres, but had no effect on AMA titres.
Our laboratory has reported that blockage of the IL-12p40 but not the IFN-γ signalling pathway reduced dramatically the histological features of PBC and significantly decreased titres of intrahepatic proinflammatory cytokines in dnTGF-βRII/IL-12p40−/− mice, but did not affect serum AMA titres compared to dnTGF-βRII controls [31]. It is well established that IFN-γ and IL-12 secreted by immune cells play important roles in autoimmune inflammatory responses [32]. IL-12 is a heterodimeric cytokine comprised of products of two separate genes encoding IL-12A (p35) and IL-12B (p40). It stimulates the growth and function of T cells and natural killer cells which, in turn, produce IFN-γ and TNF-α[33]. In this study, the deletion of the genes for IL-12p40, but not IL-12p35 or IFN-γ, resulted in significantly lower serum anti-gp210 titres compared to dnTGF-βRII mice, suggesting that IL-12 and IFN-γ may not modulate anti-gp210 production. IL-17, which is produced by T helper type 17 (Th17) cells, is also one of the inflammatory cytokines increased in human autoimmune diseases [34],[35]. The expansion of Th17 cells is mediated by IL-23 [36], a heterodimeric cytokine consisting of IL-12p40 and IL-23p19. In addition to IL-23 and IL-17, IL-6 along with TGF-β also directs the differentiation of Th17 cells from naive CD4 T cells [37]. Deletion of those cytokines that are associated closely with Th17 cells led to significantly lower serum anti-gp210 titres compared to dnTGF-βRII mice, suggesting that Th17 cells orchestrate anti-gp210 generation. However, this pattern was not apparent in AMA production (Table 2).
IL-6 is one of the proinflammatory cytokines that is elevated in both the serum and the liver in PBC [38],[39]. Additionally, increased levels of TNF-α have been suggested to correlate with more advanced progression of PBC [40]. In the present study, anti-gp210 antibodies were reduced significantly in both dnTGF-βRII/IL-6−/− and dnTGF-βRII/TNF-α−/− mice compared to dnTGF-βRII controls. However, our previous study in dnTGF-βRII/IL-6−/− mice showed that blocking IL-6 signalling exacerbated liver disease significantly, whereas there was substantial improvement in the concurrent inflammatory bowel disease [41]. This occurred despite production of lower titres of AMA and anti-gp210 antibodies, which is attributed to the essential role of IL-6 on B cell differentiation and activation to produce immunoglobulins [42],[43]. Notably, depletion of B cells with loss of immunoglobulin dnTGF-βRII mice still caused more severe histological autoimmune cholangitis [44]. This suggests that autoreactive B cells and their autoantibodies are not of major importance in the histological severity of cholangitis in dnTGF-βRII mice.
Serum autoantibodies have been demonstrated to be a powerful diagnostic tool in liver autoimmune diseases [45]. Several studies have addressed the diagnostic value of PBC-specific ANA as surrogate serological makers when AMA is undetectable [16],[20],[46]–[48]. IIF and ELISA are the two usual methods to detect the presence of these PBC-specific ANA in clinical studies [17],[20],[46],[49]–[52]. However, the diagnostic results from IIF and ELISA do not always correspond perfectly [20],[46],[50],[52], perhaps because concomitant AMA or other ANA contribute to fluorescent reactivity on cellular substrates. The inconsistency between IIF and ELISA could also be attributed to the truncated protein fragments used in ELISA, which are portions of a full-length gp210/sp100 protein in IIF. It is claimed [53],[54], but not established rigorously, that PBC patients have multiple types of ANA, such as anti-chromatin, anti-dsDNA, anti-ssDNA, anti-histone, anti-Scl-70, anti-Sm, anti-Ro (SS-A), anti-La (SS-B) and anti-RNP, observed more usually in autoimmune hepatitis, systemic lupus erythematosus and other autoimmune diseases. While these may occur infrequently, the only other relatively common ANA specificity in PBC is anti-centromere. Although anti-gp210 and anti-sp100 antibodies most frequently yield nuclear rim/periphery and multiple nuclear dot staining patterns for ANA, our IIF and ELISA results did not correspond fully. Because a single staining pattern on IIF could be attributed to multiple ANA, ELISA, which is more antigen-specific, may be a more effective way to identify and quantify specific ANA in our animal models and in human subjects.
Only a few studies have addressed the significance of ANA on disease outcome or the role of the antigens they recognize in PBC. Although, in PBC, a positive correlation between gp210 expression in biliary epithelial cells of small bile ducts and the degree of portal inflammation has been reported [55], there is still a lack of laboratory data on how or why ANA are generated. Using the mouse models of PBC developed in our laboratory could help to answer these questions. Our data also emphasize the remarkable specificity of ANA in humans and corresponding mouse models of PBC and encourage us to further dissect the mechanisms that lead to the appearance of this repertoire of autoreactivity.
Disclosure
The authors declare no conflict of interest.
References
- 1.Kaplan MM, Gershwin ME. Primary biliary cirrhosis. N Engl J Med. 2005;353:1261–73. doi: 10.1056/NEJMra043898. [DOI] [PubMed] [Google Scholar]
- 2.Rose NR, Mackay IR. The autoimmune diseases. 4th edn. St Louis, MO/London: Elsevier Academic Press; 2006. [Google Scholar]
- 3.Worman HJ, Courvalin JC. Antinuclear antibodies specific for primary biliary cirrhosis. Autoimmun Rev. 2003;2:211–17. doi: 10.1016/s1568-9972(03)00013-2. [DOI] [PubMed] [Google Scholar]
- 4.Lassoued K, Brenard R, Degos F, et al. Antinuclear antibodies directed to a 200-kilodalton polypeptide of the nuclear envelope in primary biliary cirrhosis. A clinical and immunological study of a series of 150 patients with primary biliary cirrhosis. Gastroenterology. 1990;99:181–6. doi: 10.1016/0016-5085(90)91246-3. [DOI] [PubMed] [Google Scholar]
- 5.Lozano F, Pares A, Borche L, et al. Autoantibodies against nuclear envelope-associated proteins in primary biliary cirrhosis. Hepatology. 1988;8:930–8. doi: 10.1002/hep.1840080438. [DOI] [PubMed] [Google Scholar]
- 6.Lassoued K, Guilly MN, Andre C, et al. Autoantibodies to 200 kD polypeptide(s) of the nuclear envelope: a new serologic marker of primary biliary cirrhosis. Clin Exp Immunol. 1988;74:283–8. [PMC free article] [PubMed] [Google Scholar]
- 7.Ruffatti A, Arslan P, Floreani A, et al. Nuclear membrane-staining antinuclear antibody in patients with primary biliary cirrhosis. J Clin Immunol. 1985;5:357–61. doi: 10.1007/BF00918255. [DOI] [PubMed] [Google Scholar]
- 8.Courvalin JC, Lassoued K, Bartnik E, Blobel G, Wozniak RW. The 210-kD nuclear envelope polypeptide recognized by human autoantibodies in primary biliary cirrhosis is the major glycoprotein of the nuclear pore. J Clin Invest. 1990;86:279–85. doi: 10.1172/JCI114696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nickowitz RE, Worman HJ. Autoantibodies from patients with primary biliary cirrhosis recognize a restricted region within the cytoplasmic tail of nuclear pore membrane glycoprotein Gp210. J Exp Med. 1993;178:2237–42. doi: 10.1084/jem.178.6.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szostecki C, Krippner H, Penner E, Bautz FA. Autoimmune sera recognize a 100 kD nuclear protein antigen (sp-100) Clin Exp Immunol. 1987;68:108–16. [PMC free article] [PubMed] [Google Scholar]
- 11.Szostecki C, Guldner HH, Netter HJ, Will H. Isolation and characterization of cDNA encoding a human nuclear antigen predominantly recognized by autoantibodies from patients with primary biliary cirrhosis. J Immunol. 1990;145:4338–47. [PubMed] [Google Scholar]
- 12.Selmi C, Mackay IR, Gershwin ME. The autoimmunity of primary biliary cirrhosis and the clonal selection theory. Immunol Cell Biol. 2011;89:70–80. doi: 10.1038/icb.2010.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blüthner M, Schäfer C, Schneider C, Bautz FA. Identification of major linear epitopes on the sp100 nuclear PBC autoantigen by the gene-fragment phage-display technology. Autoimmunity. 1999;29:33–42. doi: 10.3109/08916939908995970. [DOI] [PubMed] [Google Scholar]
- 14.Cavazzana I, Ceribelli A, Taraborelli M, et al. Primary biliary cirrhosis-related autoantibodies in a large cohort of Italian patients with systemic sclerosis. J Rheumatol. 2011;38:2180–5. doi: 10.3899/jrheum.110167. [DOI] [PubMed] [Google Scholar]
- 15.Liu H, Norman GL, Shums Z, et al. PBC screen: an IgG/IgA dual isotype ELISA detecting multiple mitochondrial and nuclear autoantibodies specific for primary biliary cirrhosis. J Autoimmun. 2010;35:436–42. doi: 10.1016/j.jaut.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 16.Nickowitz RE, Wozniak RW, Schaffner F, Worman HJ. Autoantibodies against integral membrane proteins of the nuclear envelope in patients with primary biliary cirrhosis. Gastroenterology. 1994;106:193–9. doi: 10.1016/s0016-5085(94)95333-3. [DOI] [PubMed] [Google Scholar]
- 17.Nakamura M, Kondo H, Mori T, et al. Anti-gp210 and anti-centromere antibodies are different risk factors for the progression of primary biliary cirrhosis. Hepatology. 2007;45:118–27. doi: 10.1002/hep.21472. [DOI] [PubMed] [Google Scholar]
- 18.Wesierska-Gadek J, Penner E, Battezzati PM, et al. Correlation of initial autoantibody profile and clinical outcome in primary biliary cirrhosis. Hepatology. 2006;43:1135–44. doi: 10.1002/hep.21172. [DOI] [PubMed] [Google Scholar]
- 19.Itoh S, Ichida T, Yoshida T, et al. Autoantibodies against a 210 kDa glycoprotein of the nuclear pore complex as a prognostic marker in patients with primary biliary cirrhosis. J Gastro Hepatol. 1998;13:257–65. doi: 10.1111/j.1440-1746.1998.01553.x. [DOI] [PubMed] [Google Scholar]
- 20.Muratori P, Muratori L, Ferrari R, et al. Characterization and clinical impact of antinuclear antibodies in primary biliary cirrhosis. Am J Gastroenterol. 2003;98:431–7. doi: 10.1111/j.1572-0241.2003.07257.x. [DOI] [PubMed] [Google Scholar]
- 21.Invernizzi P, Podda M, Battezzati PM, et al. Autoantibodies against nuclear pore complexes are associated with more active and severe liver disease in primary biliary cirrhosis. J Hepatol. 2001;34:366–72. doi: 10.1016/s0168-8278(00)00040-4. [DOI] [PubMed] [Google Scholar]
- 22.Ueno Y, Ambrosini YM, Moritoki Y, Ridgway WM, Gershwin ME. Murine models of autoimmune cholangitis. Curr Opin Gastroenterol. 2010;26:274–9. doi: 10.1097/MOG.0b013e32833755aa. [DOI] [PubMed] [Google Scholar]
- 23.Gorelik L, Flavell RA. Abrogation of TGFbeta signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 2000;12:171–81. doi: 10.1016/s1074-7613(00)80170-3. [DOI] [PubMed] [Google Scholar]
- 24.Oertelt S, Lian Z-X, Cheng C-M, et al. Anti-mitochondrial antibodies and primary biliary cirrhosis in TGF-beta receptor II dominant-negative mice. J Immunol. 2006;177:1655–60. doi: 10.4049/jimmunol.177.3.1655. [DOI] [PubMed] [Google Scholar]
- 25.Ghilardi N, Kljavin N, Chen Q, Lucas S. Compromised humoral and delayed-type hypersensitivity responses in IL-23-deficient mice. J Immunol. 2004;172:2827–33. doi: 10.4049/jimmunol.172.5.2827. [DOI] [PubMed] [Google Scholar]
- 26.Züchner D, Sternsdorf T, Szostecki C, Heathcote EJ, Cauch-Dudek K, Will H. Prevalence, kinetics, and therapeutic modulation of autoantibodies against Sp100 and promyelocytic leukemia protein in a large cohort of patients with primary biliary cirrhosis. Hepatology. 1997;26:1123–30. doi: 10.1002/hep.510260506. [DOI] [PubMed] [Google Scholar]
- 27.Bandin O, Courvalin JC, Poupon R, Dubel L, Homberg JC, Johanet C. Specificity and sensitivity of gp210 autoantibodies detected using an enzyme-linked immunosorbent assay and a synthetic polypeptide in the diagnosis of primary biliary cirrhosis. Hepatology. 1996;23:1020–4. doi: 10.1002/hep.510230512. [DOI] [PubMed] [Google Scholar]
- 28.Tartakovsky F. Detection of Gp210 autoantibodies in primary biliary cirrhosis using a recombinant protein containing the predominant autoepitope. Hepatology. 1995;21:495–500. [PubMed] [Google Scholar]
- 29.Manuel Lucena J, Montes Cano M, Luis Caro J, et al. Comparison of two ELISA assays for anti-Sp100 determination. Ann NY Acad Sci. 2007;1109:203–11. doi: 10.1196/annals.1398.024. [DOI] [PubMed] [Google Scholar]
- 30.Szostecki C, Will H, Netter HJ, Guldner HH. Autoantibodies to the nuclear Sp100 protein in primary biliary cirrhosis and associated diseases: epitope specificity and immunoglobulin class distribution. Scand J Immunol. 1992;36:555–64. doi: 10.1111/j.1365-3083.1992.tb03224.x. [DOI] [PubMed] [Google Scholar]
- 31.Yoshida K, Yang G-X, Zhang W, et al. Deletion of interleukin-12p40 suppresses autoimmune cholangitis in dominant negative transforming growth factor beta receptor type II mice. Hepatology. 2009;50:1494–500. doi: 10.1002/hep.23132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dardalhon V, Korn T, Kuchroo VK, Anderson AC. Role of Th1 and Th17 cells in organ-specific autoimmunity. J Autoimmun. 2008;31:252–6. doi: 10.1016/j.jaut.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hunter CA. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat Rev Immunol. 2005;5:521–31. doi: 10.1038/nri1648. [DOI] [PubMed] [Google Scholar]
- 34.Matusevicius D, Kivisäkk P, He B, et al. Interleukin-17 mRNA expression in blood and CSF mononuclear cells is augmented in multiple sclerosis. Mult Scler. 1999;5:101–4. doi: 10.1177/135245859900500206. [DOI] [PubMed] [Google Scholar]
- 35.Aarvak T, Chabaud M, Miossec P. IL-17 is produced by some proinflammatory Th1/Th0 cells but not by Th2 cells. J Immunol. 1999;162:1246–51. [PubMed] [Google Scholar]
- 36.Kikly K, Liu L, Na S, Sedgwick JD. The IL-23/Th(17) axis: therapeutic targets for autoimmune inflammation. Curr Opin Immunol. 2006;18:670–5. doi: 10.1016/j.coi.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 37.Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 38.Barak V, Selmi C, Schlesinger M, et al. Serum inflammatory cytokines, complement components, and soluble interleukin 2 receptor in primary biliary cirrhosis. J Autoimmun. 2009;33:178–82. doi: 10.1016/j.jaut.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 39.Nagano T, Yamamoto K, Matsumoto S, et al. Cytokine profile in the liver of primary biliary cirrhosis. J Clin Immunol. 1999;19:422–7. doi: 10.1023/a:1020511002025. [DOI] [PubMed] [Google Scholar]
- 40.Tanaka A, Quaranta S, Mattalia A, et al. The tumor necrosis factor-alpha promoter correlates with progression of primary biliary cirrhosis. J Hepatol. 1999;30:826–9. doi: 10.1016/s0168-8278(99)80135-4. [DOI] [PubMed] [Google Scholar]
- 41.Zhang W, Tsuda M, Yang G-X, et al. Deletion of interleukin-6 in mice with the dominant negative form of transforming growth factor beta receptor II improves colitis but exacerbates autoimmune cholangitis. Hepatology. 2010;52:215–22. doi: 10.1002/hep.23664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muraguchi A, Hirano T, Tang B, et al. The essential role of B cell stimulatory factor 2 (BSF-2/IL-6) for the terminal differentiation of B cells. J Exp Med. 1988;167:332–44. doi: 10.1084/jem.167.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hirano T, Yasukawa K, Harada H, et al. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature. 1986;324:73–6. doi: 10.1038/324073a0. [DOI] [PubMed] [Google Scholar]
- 44.Moritoki Y, Zhang W, Tsuneyama K, et al. B cells suppress the inflammatory response in a mouse model of primary biliary cirrhosis. Gastroenterology. 2009;136:1037–47. doi: 10.1053/j.gastro.2008.11.035. [DOI] [PubMed] [Google Scholar]
- 45.Invernizzi P, Lleo A, Podda M. Interpreting serological tests in diagnosing autoimmune liver diseases. Semin Liver Dis. 2007;27:161–72. doi: 10.1055/s-2007-979469. [DOI] [PubMed] [Google Scholar]
- 46.Granito A, Muratori P, Muratori L, et al. Antinuclear antibodies giving the ‘multiple nuclear dots’ or the ‘rim-like/membranous’ patterns: diagnostic accuracy for primary biliary cirrhosis. Aliment Pharmacol Ther. 2006;24:1575–83. doi: 10.1111/j.1365-2036.2006.03172.x. [DOI] [PubMed] [Google Scholar]
- 47.Invernizzi P, Selmi C, Ranftler C, Podda M, Wesierska-Gadek J. Antinuclear antibodies in primary biliary cirrhosis. Semin Liver Dis. 2005;25:298–310. doi: 10.1055/s-2005-916321. [DOI] [PubMed] [Google Scholar]
- 48.Bogdanos D. Positive markers in AMA-negative PBC. Am J Gastroenterol. 2003;98:241–3. doi: 10.1111/j.1572-0241.2003.07270.x. [DOI] [PubMed] [Google Scholar]
- 49.Muratori P, Granito A, Ferri S, et al. Multiple nuclear dots and rim-like/membranous IgG isotypes in primary biliary cirrhosis. Autoimmunity. 2009;42:224–7. doi: 10.1080/08916930802709133. [DOI] [PubMed] [Google Scholar]
- 50.Muratori L, Granito A, Muratori P, Pappas G, Bianchi FB. Antimitochondrial antibodies and other antibodies in primary biliary cirrhosis: diagnostic and prognostic value. Clin Liver Dis. 2008;12:vii, 261–76. doi: 10.1016/j.cld.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 51.Rigopoulou EI, Davies ET, Pares A, et al. Prevalence and clinical significance of isotype specific antinuclear antibodies in primary biliary cirrhosis. Gut. 2005;54:528–32. doi: 10.1136/gut.2003.036558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Muratori P, Muratori L, Cassani F, et al. Anti-multiple nuclear dots (anti-MND) and anti-SP100 antibodies in hepatic and rheumatological disorders. Clin Exp Immunol. 2002;127:172–5. doi: 10.1046/j.1365-2249.2002.01719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hu C-J, Zhang F-C, Li Y-Z, Zhang X. Primary biliary cirrhosis: what do autoantibodies tell us? World J Gastroenterol. 2010;16:3616–29. doi: 10.3748/wjg.v16.i29.3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Czaja AJ. Autoantibodies as prognostic markers in autoimmune liver disease. Dig Dis Sci. 2010;55:2144–61. doi: 10.1007/s10620-010-1268-4. [DOI] [PubMed] [Google Scholar]
- 55.Nakamura M, Takii Y, Ito M, et al. Increased expression of nuclear envelope gp210 antigen in small bile ducts in primary biliary cirrhosis. J Autoimmun. 2006;26:138–45. doi: 10.1016/j.jaut.2005.10.007. [DOI] [PubMed] [Google Scholar]


