Abstract
Major histocompatibility complex (MHC) class I-restricted T cell epitopes are generated mainly by the immunoproteasome in antigen-presenting cells. Therefore, inhibition of activity of this proteolytic complex molecule is thought to be a potential treatment for cell-mediated autoimmune diseases. We therefore studied the efficacy of an immunoproteasome inhibitor, ONX 0914 (formerly PR-957), for the treatment of autoimmune thyroid diseases, including cell-mediated Hashimoto's thyroiditis and autoantibody-mediated Graves' hyperthyroidism using mouse models. Our data show that ONX 0914 was effective prophylactically and therapeutically at suppressing the degree of intrathyroidal lymphocyte infiltration and, to a lesser degree, the titres of anti-thyroglobulin autoantibodies in non-obese diabetic (NOD)-H2h4 mice, an iodine-induced autoimmune thyroiditis model. It also inhibited differentiation of T cells to T helper type 1 (Th1) and Th17 cells, effector T cell subsets critical for development of thyroiditis in this mouse strain. In contrast, its effect on the Graves' model was negligible. Although ONX 0914 exerts its immune-suppressive effect through not only suppression of immune proteasome but also other mechanism(s), such as inhibition of T cell differentiation, the present results suggest that the immunoproteasome is a novel drug target in treatment of Hashimoto's thyroiditis in particular and cell-mediated autoimmune diseases in general.
Keywords: Graves' hyperthyroidism, Hashimoto's thyroiditis, immunoproteasome, NOD-H2h4 mouse
Introduction
Graves' disease and Hashimoto's thyroiditis are located at the extreme ends of the autoimmune thyroid disease spectrum [1]. In this regard, Grave's disease is an autoantibody-mediated disease where antigen-presentation by major histocompatibility complex (MHC) class II expressed on antigen-presenting cells (APCs) to T cell receptors (TCRs) on CD4+ T cells is highly crucial for stimulating B cells and inducing the pathogenic stimulatory anti-thyrotrophin receptor (TSHR) autoantibodies (thyroid-stimulating antibody, TSAb). Conversely, Hashimoto's thyroiditis is cell-mediated, where not only antigen-presentation by MHC class II but also antigen presentation by MHC class I on APCs to TCRs on CD8+ T cells is relevant to the generation of antigen-specific cytotoxic T lymphocytes. Thus, inhibition of antigen presentation by MHC class I may, in theory, be efficacious for the treatment of cell-mediated autoimmune diseases such as Hashimoto's thyroiditis.
Multiple aspects of biological processes are regulated by the ubiquitin–proteasome system [2],[3]. The 26S (or constitutive) proteasome is expressed in all mammalian cells and comprises a single catalytic 20S proteasome with 19S regulatory particles attached to both ends. The 20S proteasome consists of 28 subunits arranged as a cylindrical particle containing four heteroheptameric rings of α1–7, β1–7β1–7 and α1–7, of which β1, β2 and β5 are proteolytically active with caspase-like, trypsin-like and chymotrypsin-like activities, respectively. This standard type of proteasome degrades various proteins tagged with a polyubiquitin chain. In contrast, immune cells such as lymphocytes, macrophages and dendritic cells tend to express three other subunits, β1i, β2i and β5i, in their 20S proteasome instead of the standard β1, β2 and β5. The resulting proteasome, the immunoproteasome, produces MHC class I-restricted T cell epitopes more efficiently. This processing of antigenic proteins by immunoproteasome in antigen-presenting cells is highly crucial for eliciting efficient cell-mediated immune responses. Indeed, knock-out mice of each subunit have demonstrated altered MHC class I-restricted antigen presentation [4]–[6].
On the basis of these findings, small molecule proteasome inhibitors such as bortezomib have been used recently to treat cell-mediated autoimmune diseases [7]–[11]. However, these chemicals are non-selective; i.e. they inhibit both the constitutive proteasome and immunoproteasome, thus having considerable toxicity. However, a selective inhibitor of immunoproteasome β5i subunit, ONX 0914 (formerly PR-957), has been generated recently [12], and proven to be effective for the treatment of cell-mediated autoimmune diseases including experimental collagen-induced arthritis and dextran sulphate sodium-induced colitis [12],[13].
This study was therefore performed to evaluate and compare the preventive and therapeutic effects of ONX 0914 on Hashimoto's thyroiditis and Graves' hyperthyroidism in mouse models.
Materials and methods
Mice
Non-obese diabetic (NOD)-H2h4 mice were obtained from Jackson Laboratory Inc. (Bar Harbor, ME, USA) and bred in the animal facility at Nagasaki University. BALB/c mice were from Charles River Japan Laboratory Inc. (Tokyo, Japan). Both male and female NOD-H2h4 mice, and female BALB/c mice, were used for the current study. All the mice were kept in specific pathogen-free conditions. Animal care and all experimental procedures were performed in accordance with the Guideline for Animal Experimentation of Nagasaki University, with the approval of the Institutional Animal Care and Use Committee.
Induction of thyroiditis
Six–8-week-old NOD-H2h4 mice were supplied with 0·15% sodium iodine (NaI) in the drinking water. Eight weeks after NaI provision mice were euthanized, and the thyroid glands and blood were harvested to determine the extent of thyroiditis and cytokine mRNA expression and the titres of serum anti-thyroglobulin (Tg) autoantibodies, respectively.
Groups of mice were treated with 2–25 mg/kg ONX 0914 (kindly provided by Onyx Pharmaceuticals, Inc., South San Francisco, CA, USA) dissolved in the vehicle [10% Capisol™ (sulphobutylether betacyclodextrin) and 10 mM citrate buffer, pH 3·5] or with vehicle only for 9 weeks, starting 1 week prior to iodine administration or for 4 weeks following 4 weeks of iodine administration.
Evaluation of thyroiditis
Thyroid tissues were fixed in 10% formalin and embedded in paraffin. Five-µm-thick sections were prepared and stained with haematoxylin and eosin (H&E). Thyroiditis was assessed for extent of lymphocyte infiltration as follows: grade 0, no lymphocytic infiltration; grade 1, less than 10% lymphocytic infiltration of the thyroid; grade 2, 10–30% lymphocytic infiltration; grade 3, 30–50% lymphocytic infiltration; and grade 4, greater than 50% lymphocytic infiltration [14]. The final thyroiditis scores were expressed as means of at least three non-contiguous sections from each thyroid gland.
Enzyme-linked immunosorbent assay (ELISA) assay for anti-Tg autoantibodies
Tg was purified from mouse thyroid glands, as described previously [14]. ELISA wells were coated overnight with 100 µl Tg protein (10 µg/ml) and incubated with mouse sera (1:1000 dilutions). After incubation with horseradish peroxidase-conjugated anti-mouse immunoglobulin (Ig)G (A3673; Sigma, St Louis, MO, USA), colour was developed using orthophenylene diamine and H2O2 as substrate, and the optical density (OD) was read at 492 nm.
Induction of Graves' hyperthyroidism
Construction, amplification and purification of non-replicative recombinant human adenovirus expressing the human TSHR A-subunit (Ad-TSHR289) and determination of the viral particle concentration have been described previously [15],[16]. BALB/c mice were injected intramuscularly into the quadriceps with 100 µl phosphate-buffered saline (PBS) containing 1010 particles of Ad-TSHR289 on two occasions at 3-week intervals. Blood samples and thyroid tissues were obtained 2 weeks after the second immunization.
Thyroxine (T4) and anti-TSHR antibody measurements
Serum-free T4 was measured with a radioimmunoassay kit (DPC free T4 kit; Diagnostic Products, Los Angeles, CA, USA). Anti-TSHR antibodies in mouse sera were determined by flow cytometric assay with Chinese hamster ovary cells stably expressing the full-length hTSHR, as described previously [17]. This assay measures the titres of anti-TSHR antibodies recognizing the native TSHR expressed on the cell surface. The data are expressed as the percentage of mean fluorescence index (MFI) compared to the mean values from control untreated mice. In both assays, the normal range is defined as the mean ± 3 standard deviations (s.d.) of control mice.
Splenocyte culture
Splenocytes from BALB/c mice, obtained 2 weeks after the second immunization, were cultured (triplicate aliquots) at 5 × 105 cells per well in a 96-well round-bottomed culture plate in the presence or absence of 10 µg/ml TSHR289 protein, as described previously [18]. Four days later, the splenocytes were harvested and used for real-time reverse transcription–polymerase chain reaction (RT–PCR), as described below.
Real-time RT–PCR for cytokine expression
RNA was isolated from thyroid lobes of NOD-H2h4 mice or cultured splenocytes as mentioned above using Isogen (Nippon Gene, Tokyo, Japan), and 0·5 µg RNA was reverse-transcribed with SuperScript III (Invitrogen, Carlsbad, CA, USA) in the presence of random hexamers to generate cDNA. Quantitative reverse transcription–PCR (qRT–PCR) was performed using SYBR Premix ExTaq (Takara, Shiga, Japan) and primer pairs for β-actin [CTG AAC CCT AAG GCC AAC CGT G (forward) and GGC ATA CAG GGA CAG CAC AGC C (reverse)]; interferon (IFN)-γ (5′-CAC GGC ACA GTC AAT GAA AG-3′ and 5′-CCT TGC TGT TGC TGA AGA AG-3′); and interleukin-17 (IL-17) (5′-TCC AGA AGG CCC TCA GAC TA-3′ and 5′-CAG TTT GGG ACC CCT TTA C-3′). The cycle threshold values were determined using Thermal Cycler Dice Real-Time System (Takara) and used to calculate the relative expression levels of the target genes normalized against β-actin.
Statistical analysis
All data were analysed by either Student's t-test or χ2 test. A P-value of less than 0·05 was considered statistically significant.
Results
The effect of ONX 0914 on iodine-induced autoimmune thyroiditis in NOD-H2h4 mice
As reported previously [14],[19], iodine administration in the drinking water for 8 weeks induces intrathyroidal lymphocyte infiltration and anti-Tg autoantibodies in NOD-H2h4 mice. Intraperitoneal injection of between 2 and 25 mg/kg doses of ONX 0914 twice a week for 9 weeks starting 1 week prior to iodine administration inhibited the development of thyroiditis in male and female mice in a dose-dependent manner (Fig. 1a). Representative histology of normal thyroid gland (from a control mouse) and grades 2 and 4 thyroiditis (from iodine-treated mice) is shown in Fig. 2a–c, respectively. However, its effect on anti-Tg antibody titres was less evident, with significant suppression observed only with 25 mg/kg ONX 0914 (Fig. 1b).
Fig. 1.
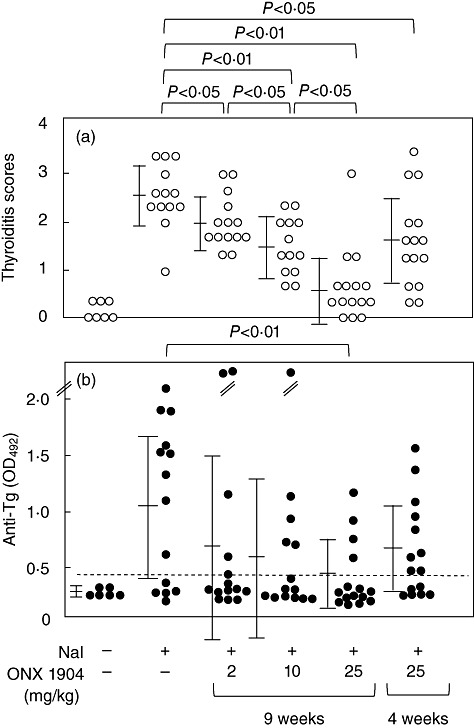
Thyroiditis scores (a) and anti-thyroglobulin (Tg) antibody titres (b) in control, iodine-treated and iodine/ONX 0914-treated mice. The mice were fed with the drinking water containing 0·15% NaI for 8 weeks. Two to 25 mg/kg ONX 0914 was given for 9 weeks, starting 1 week prior to iodine administration (the 9-week group), or for 4 weeks following 4-week administration of iodine (the 4-week group). Thyroid histology was examined with haematoxylin and eosin (H&E) staining to score the extent of intrathyroidal lymphocyte infiltration (see Materials and methods). Data are shown for individual mice, and also as means ± standard deviation. The horizontal broken line designates the normal upper limit.
Fig. 2.
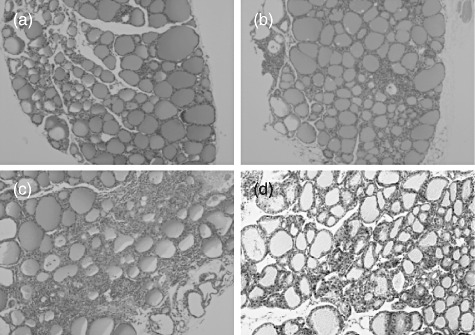
Representative histology of thyroid glands in control and iodine-fed non-obese diabetic (NOD)-H2h4 mice and hyperthyroid BALB/c mice. (a) Normal thyroid histology in a control mouse; (b,c), grades 2 and 4, respectively, thyroiditis in iodine-treated NOD-H2h4 mice, and (d) hyperthyroid BALB/c mice.
RT–PCR analysis of cytokine expression in thyroid glands at the end of the experiments (after 8 weeks' iodine administration) revealed that ONX 0914 treatment lowered intrathyroidal expression of IL-17 and IFN-γ, cytokines critical for thyroiditis development [14],[20] (Fig. 3a,b).
Fig. 3.
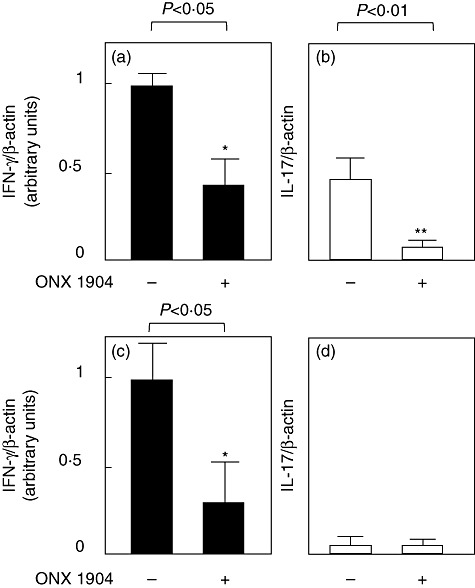
Expression of interferon (IFN)-γ (a,c) and interleukin (IL)-17 mRNAs (b,d) in the thyroid glands of iodine-treated and iodine/ONX 0914-treated non-obese diabetic (NOD)-H2h4 mice (a,b) and in cultured splenocytes from adenovirus-expressing anti-thyrotrophin receptor (Ad-TSHR)-treated and Ad-TSHR/ONX 0914-treated BALB/c mice (c,d). Total RNA was extracted and subjected to reverse transcription–polymerase chain reaction (RT–PCR) to amplify β-actin, IL-17 and interferon (IFN)-γ mRNAs, and expression levels of IL-17 and IFN-γ relative to that of β-actin are shown (see Materials and methods).
To investigate its effect on existing thyroiditis and anti-Tg antibodies, ONX 0914 was given to mice that had already been treated with iodine for 4 weeks, at which time T cells are already primed, and in some mice thyroiditis and anti-Tg antibodies can be seen [14]. As shown in Fig. 1, a significant decrease in thyroiditis scores but not in anti-Tg antibodies was observed in male and female mice treated with 25 mg/kg ONX 0914 for 4 weeks. Indeed, this therapeutic effect was much weaker than that of prophylactic administration.
Thus, ONX 0914 is both preventive and therapeutic in iodine-induced thyroiditis, an effect probably mediated not only by inhibition of immunoproteasome, but also by suppression of Th1 and Th17 differentiation.
The effect of ONX 0914 on Graves' hyperthyroidism in Ad-TSHR A-subunit model
The effect of ONX 0914 was also evaluated in our Graves' model involving immunization of susceptible BALB/c mice with Ad-TSHR289, which efficiently induces hyperthyroidism and TSAb [15], revealing no effect. Thus, up to 25 mg/kg ONX 0914 given for 6 weeks from 1 week before the first immunization did not affect anti-TSHR antibody titres or free T4 levels at all (Fig. 4).
Fig. 4.
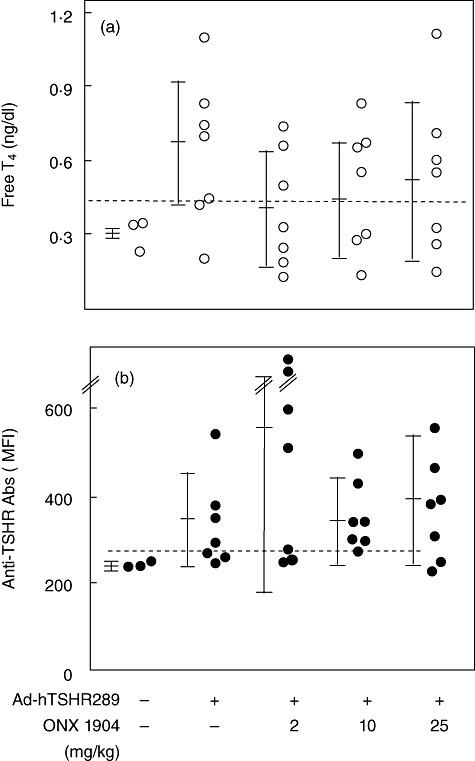
Serum-free T4 concentrations (a) and anti-thyrotrophin receptor (TSHR) antibody titres (b) in BALB/c mice immunized with adenovirus-expressing (Ad)-TSHR289 with/without ONX 0914 treatment (2–25 mg/kg). Free T4 and anti-TSHR antibody levels were determined by radioimmunoassay and flow cytometry, respectively (see Materials and methods). Data are shown for individual mice. Mean ± standard deviation values are shown for each group. The horizontal broken lines designate the normal upper limits.
RT–PCR analysis of cytokine expression in splenocytes that were prepared from immunized and immunized/ONX 0914-treated mice and cultured with TSHR289 protein for 4 days demonstrated a decrease in IFN-γ expression with ONX 0914 treatment. Expression levels of IL-17 were very low in two groups (Fig. 3c,d).
It should be noted that no obvious side effects were observed in the present study. The maximum tolerance dose of ONX 0914 in mice is reported to be 30 mg/kg body weight [12],[13].
Discussion
In this study we studied the preventive and therapeutic potential of an immunoproteasome inhibitor, ONX 0914, for autoimmune thyroid diseases, including Hashimoto' thyroiditis and Graves' disease, using mouse models. The former disease is cell-mediated, while the latter is autoantibody-mediated. Because this compound primarily suppresses MHC class I-mediated, i.e. cell-mediated, autoimmune responses [12], we hypothesized that its efficacy would be higher in Hashimoto's thyroiditis compared to Graves' disease. The present study showed that our assumption was correct. Thus, ONX 0914 suppressed the degree of intrathyroidal lymphocyte infiltration dose-dependently, although its effect on the titres of anti-Tg antibodies was less evident (statistically significant only with the highest dose). A decrease in anti-collagen antibodies has also been reported in a collagen-induced arthritis model [12] (also see below). Because anti-Tg antibody production does not appear to be due to the primary autoimmune reaction, but is rather a secondary response to tissue destruction, decreased Tg antibodies may be attributed to suppressed thyroid destruction. This interpretation may also be applied to decreased anti-collagen antibodies as mentioned above [12].
Graves' hyperthyroidism was not suppressed by this compound as we anticipated but, unexpectedly, a significant decrease in mRNA levels for IFN-γ, a critical cytokine for the pathogenesis [21], was observed in antigen-stimulated splenocytes. Curiously, we have also previously found a discrepancy between disease development and splenocyte production of IFN-γ in this mouse model: Mycobacterium bovis bacillus Calmette–Guérin (BCG) suppressed the development of hyperthyroidism but augmented IFN-γ secretion in T cell recall assay [22]. More study is needed to clarify the significance of IFN-γ in this Graves' model.
Furthermore, our results demonstrate that this compound is not only preventive, but also therapeutic. In general, immune-suppressants that inhibit antigen-presentation are thought to be efficacious at the early stage of immune response, and to be more effective in spontaneous models (including the Hashimoto's model we used here) than inducible models. This is because immune cells are probably challenged continuously by an autoantigen in spontaneous models, while an antigen is given by bolus injection(s) in inducible models.
However, ONX 0914 has multiple functions other than inhibition of immunoproteasome. For example, it inhibits IL-6, IL-23 and tumour necrosis factor (TNF)-α production in a nuclear factor (NF)-κB-independent manner in endotoxin-stimulated monocytes; suppresses IFN-γ and IL-2 release from CD3/CD28-stimulated T cells; blocks T cell differentiation to Th17; suppresses development of collagen antibody-induced arthritis, a T cell-independent disease [12]; and decreases slightly the percentage of peripheral CD11c+ and CD19+ cells [13]. Indeed, suppression of expression of a Th1 cytokine IFN-γ and a Th17 cytokine IL-17 was also observed in Hashimoto's model, suggesting inhibition of Th1 and Th17 differentiation. Although the immune-suppressive mechanism of ONX 0914 is primarily inhibition of expression of MHC class I-restricted epitopes, other functions on multiple immune effector cells (e.g. inhibition of cytokine production, T cell differentiation and/or antibody synthesis) may also be significant. It is also reported that immunoproteasome also plays a role in innate immunity; i.e. it increases production of various cytokines (IFN-γ, IL-16, IL-1β and TNF-α) from dendritic cells [23].
The non-selective proteasome inhibitor bortezomib has been shown previously to be effective at treating some autoimmune diseases such as lupus, arthritis, colitis and autoimmune encephalitis through inhibition of NF-κB, a transcription factor regulating expression of numerous proinflammatory cytokines [7]–[10]. In contrast, the effects of ONX 0914 are independent of NF-κB [12]. This may explain our unpublished data showing that high doses of bortezomib given twice a week for 9 weeks, starting 1 week before iodine administration, was not effective at all on thyroiditis and anti-Tg antibody levels, nor on splenocyte production of IL-17 and IFN-γ in NOD-H2h4 mice.
In contrast, the effect of ONX 0914 on the Graves' model was negligible. Graves' hyperthyroidism involves primarily the generation of stimulatory anti-TSHR antibodies. The effect of ONX 0914 on the production of TSHR antibodies as well as of Tg antibodies (see above) is unremarkable.
The present study, as well as previous studies [6],[12], show clearly the efficacy of an immunoproteasome inhibitor ONX 0914 in the treatment of cell-mediated autoimmune diseases, suggesting that the immunoproteasome is a novel drug target in the treatment of these types of disease. However, a loss of function mutation in β5i has been identified very recently in joint contracture, muscular atrophy, microcytic anaemia and panniculitis-associated lipodystrophy syndrome and Nakajo–Nishimura syndrome, both autoinflammatory syndromes [24],[25]. Thus, in certain circumstances, decreased activity of immunoproteasome might lead to aberrantly activated immune responses. Further studies are necessary to study in more detail the role(s) played by immunoproteasome in the pathogenesis of autoimmune diseases.
Acknowledgments
We thank Onyx Pharmaceuticals, Inc. for kindly providing ONX 0914.
Disclosure
The authors have nothing to disclose.
References
- 1.Braverman LE. Wermer and Ingbar's The thyroid. Philadelphia, PA: Lippincott, Williams & Wilkins; 2000. [Google Scholar]
- 2.Tanaka K, Kasahara M. The MHC class I ligand-generating system: roles of immunoproteasomes and the interferon-gamma-inducible proteasome activator PA28. Immunol Rev. 1998;163:161–76. doi: 10.1111/j.1600-065x.1998.tb01195.x. [DOI] [PubMed] [Google Scholar]
- 3.Groettrup M, Kirk CJ, Basler M. Proteasomes in immune cells: more than peptide producers? Nat Rev Immunol. 2010;10:73–8. doi: 10.1038/nri2687. [DOI] [PubMed] [Google Scholar]
- 4.Fehling HJ, Swat W, Laplace C, et al. MHC class I expression in mice lacking the proteasome subunit LMP-7. Science. 1994;265:1234–7. doi: 10.1126/science.8066463. [DOI] [PubMed] [Google Scholar]
- 5.Van Kaer L, Ashton-Rickardt PG, Eichelberger M, et al. Altered peptidase and viral-specific T cell response in LMP2 mutant mice. Immunity. 1994;1:533–41. doi: 10.1016/1074-7613(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 6.Basler M, Moebius J, Elenich L, Groettrup M, Monaco JJ. An altered T cell repertoire in MECL-1-deficient mice. J Immunol. 2006;176:6665–72. doi: 10.4049/jimmunol.176.11.6665. [DOI] [PubMed] [Google Scholar]
- 7.Conner EM, Brand S, Davis JM, et al. Proteasome inhibition attenuates nitric oxide synthase expression, VCAM-1 transcription and the development of chronic colitis. J Pharmacol Exp Ther. 1997;282:1615–22. [PubMed] [Google Scholar]
- 8.Fissolo N, Kraus M, Reich M, et al. Dual inhibition of proteasomal and lysosomal proteolysis ameliorates autoimmune central nervous system inflammation. Eur J Immunol. 2008;38:2401–11. doi: 10.1002/eji.200838413. [DOI] [PubMed] [Google Scholar]
- 9.Lee SW, Kim JH, Park YB, Lee SK. Bortezomib attenuates murine collagen-induced arthritis. Ann Rheum Dis. 2009;68:1761–7. doi: 10.1136/ard.2008.097709. [DOI] [PubMed] [Google Scholar]
- 10.Neubert K, Meister S, Moser K, et al. The proteasome inhibitor bortezomib depletes plasma cells and protects mice with lupus-like disease from nephritis. Nat Med. 2008;14:748–55. doi: 10.1038/nm1763. [DOI] [PubMed] [Google Scholar]
- 11.Inoue S, Nakase H, Matsuura M, et al. The effect of proteasome inhibitor MG132 on experimental inflammatory bowel disease. Clin Exp Immunol. 2009;156:172–82. doi: 10.1111/j.1365-2249.2008.03872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muchamuel T, Basler M, Aujay MA, et al. A selective inhibitor of the immunoproteasome subunit LMP7 blocks cytokine production and attenuates progression of experimental arthritis. Nat Med. 2009;15:781–7. doi: 10.1038/nm.1978. [DOI] [PubMed] [Google Scholar]
- 13.Basler M, Dajee M, Moll C, Groettrup M, Kirk CJ. Prevention of experimental colitis by a selective inhibitor of the immunoproteasome. J Immunol. 2010;185:634–41. doi: 10.4049/jimmunol.0903182. [DOI] [PubMed] [Google Scholar]
- 14.Horie I, Abiru N, Nagayama Y, et al. T helper type 17 immune response plays an indispensable role for development of iodine-induced autoimmune thyroiditis in nonobese diabetic-H2h4 mice. Endocrinology. 2009;150:5135–42. doi: 10.1210/en.2009-0434. [DOI] [PubMed] [Google Scholar]
- 15.Chen CR, Pichurin P, Nagayama Y, Latrofa F, Rapoport B, McLachlan SM. The thyrotropin receptor autoantigen in Graves disease is the culprit as well as the victim. J Clin Invest. 2003;111:1897–904. doi: 10.1172/JCI17069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagayama Y, Kita-Furuyama M, Ando T, et al. A novel murine model of Graves' hyperthyroidism with intramuscular injection of adenovirus expressing the thyrotropin receptor. J Immunol. 2002;168:2789–94. doi: 10.4049/jimmunol.168.6.2789. [DOI] [PubMed] [Google Scholar]
- 17.Saitoh O, Abiru N, Nakahara M, Nagayama Y. CD8+CD122+ T cells, a newly identified regulatory T subset, negatively regulate Graves' hyperthyroidism in a murine model. Endocrinology. 2007;148:6040–6. doi: 10.1210/en.2007-0300. [DOI] [PubMed] [Google Scholar]
- 18.Nagayama Y, Mizuguchi H, Hayakawa T, Niwa M, McLachlan SM, Rapoport B. Prevention of autoantibody-mediated Graves'-like hyperthyroidism in mice with IL-4, a Th2 cytokine. J Immunol. 2003;170:3522–7. doi: 10.4049/jimmunol.170.7.3522. [DOI] [PubMed] [Google Scholar]
- 19.Horie I, Abiru N, Sakamoto H, Iwakura Y, Nagayama Y. Induction of autoimmune thyroiditis by depletion of CD4+CD25+ regulatory T cells in thyroiditis-resistant IL-17, but not interferon-{gamma} receptor, knockout nonobese diabetic-H2h4 mice. Endocrinology. 2011;152:4448–54. doi: 10.1210/en.2011-1356. [DOI] [PubMed] [Google Scholar]
- 20.Yu S, Sharp GC, Braley-Mullen H. Dual roles for IFN-gamma, but not for IL-4, in spontaneous autoimmune thyroiditis in NOD.H-2h4 mice. J Immunol. 2002;169:3999–4007. doi: 10.4049/jimmunol.169.7.3999. [DOI] [PubMed] [Google Scholar]
- 21.McLachlan SM, Nagayama Y, Rapoport B. Insight into Graves' hyperthyroidism from animal models. Endocr Rev. 2005;26:800–32. doi: 10.1210/er.2004-0023. [DOI] [PubMed] [Google Scholar]
- 22.Nagayama Y, McLachlan SM, Rapoport B, Oishi K. Graves' hyperthyroidism and the hygiene hypothesis in a mouse model. Endocrinology. 2004;145:5075–9. doi: 10.1210/en.2004-0683. [DOI] [PubMed] [Google Scholar]
- 23.Hensley SE, Zanker D, Dolan BP, et al. Unexpected role for the immunoproteasome subunit LMP2 in antiviral humoral and innate immune responses. J Immunol. 2010;184:4115–22. doi: 10.4049/jimmunol.0903003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arima K, Kinoshita A, Mishima H, et al. Proteasome assembly defect due to a proteasome subunit beta type 8 (PSMB8) mutation causes the autoinflammatory disorder, Nakajo–Nishimura syndrome. Proc Natl Acad Sci USA. 2011;106:14914–19. doi: 10.1073/pnas.1106015108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agarwal AK, Xing C, DeMartino GN, et al. PSMB8 encoding the beta5i proteasome subunit is mutated in joint contractures, muscle atrophy, microcytic anemia, and panniculitis-induced lipodystrophy syndrome. Am J Hum Genet. 2010;87:866–72. doi: 10.1016/j.ajhg.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]


