Abstract
Hev b 13 is an allergenic esterase obtained from the rubber tree Hevea brasiliensis, which has been shown recently to induce human monocytes to release interleukin (IL)-10 in vitro, and to exert a potent anti-inflammatory effect in vivo. Moreover, Hev b 13 has been shown to reduce clinical signs of inflammation and also histological damage to the distal colon of mice with 2,4,6-trinitrobenze sulphonic acid (TNBS)-induced colitis after its oral administration. The aim of this study was to investigate the effect of Hev b 13 on human mononuclear cells, as well as its therapeutic use in the methylated bovine serum albumin (mBSA) model of antigen-induced arthritis. Five days before the intra-articular challenge, and daily thereafter for 8 days, Hev b 13 was administered by oral gavage. In mice treated with a dose of 0·5 mg/kg of Hev b 13, the severity of oedema, leucocyte infiltration, pannus formation and cartilage erosion were reduced significantly. These findings underscore the anti-inflammatory activity suggested previously for Hev b 13, an activity speculated to be related to its interaction with monocytes/macrophages and the consequent stimulation of IL-10 release and reduction of tumour necrosis factor (TNF) release. The study also opens a wide range of possible applications in the field of immune-mediated inflammatory diseases.
Keywords: arthritis, Hev b 13, interleukin-10, methylated bovine serum albumin, tumour necrosis factor
Introduction
The early nodule-specific protein homologue, or Hev b 13, is a 391-amino acid glycoprotein obtained from the rubber tree Hevea brasiliensis. The protein is allergenic, recognized by immunoglobulin E in sera from latex-allergic patients, and possesses lipase and esterase activities, which might be associated with plant defence [1],[2].
Hev b 13 has been shown recently to strongly induce human mononuclear cells to release interleukin (IL)-10, an immunoregulatory cytokine, in vitro[3]. Moreover, in an experimental mouse model of Crohn's disease, oral administration of Hev b 13 was shown to have potent anti-inflammatory activity [4]. However, in-vivo distribution of the macromolecule after oral administration is unknown in this model.
IL-10 is known to exert anti-inflammatory effects predominantly through suppression of the macrophage function and inhibition of proinflammatory cytokine production [5]–[10]. IL-10 has also been implicated as an important immunoregulator in the cytokine network involved in rheumatoid arthritis (RA), because the neutralization of endogenously produced IL-10 in diseased synovial membrane cultures resulted in increased levels of tumour necrosis factor (TNF)-α and IL-1β[11]. Furthermore, a recent meta-analysis has indicated an association between IL-10 promoter polymorphisms and susceptibility to RA [12]. This systemic, chronic inflammatory disease affects 0·5–1% of the world's population; its aetiology and pathogenesis show several of the clinical features of an autoimmune process. The syndrome is characterized by pain, stiffness and symmetrical synovitis of diarthrodial joints, which leads to articular damage and functional decline, as well as substantial comorbidity in the cardiovascular, neurological and metabolic systems [13].
Administration of anti-IL-10 antibodies also accelerated the onset and increased the severity of arthritis in a mouse model of type II collagen-induced arthritis (CIA) [14],[15], whereas immune augmentation of this cytokine prevents disease expression and development in CIA [15],[16].
Nevertheless, only limited efficacy was observed when IL-10 was administered systemically to RA patients [17],[18]. Current therapies for RA rely on disease-modifying anti-rheumatic drugs (DMARDs), such as methotrexate and sulphasalazine, and biological agents [mainly tumour necrosis factor (TNF) inhibitors]; however, these approaches are associated with only partial clinical benefit, as well as toxic adverse effects and high costs. Therefore, despite the remarkable advances made in recent years, there is still much progress to be made with regard to treatment for RA [7],[19].
Due to its potent stimulation of IL-10 production and anti-inflammatory activity we hypothesized that Hev b 13 may be a preventative for RA, and may improve joint inflammation. Given this background, we determined to examine the efficacy of Hev b 13-treatment in antigen-induced arthritis (AIA). AIA is a T helper type 1 (Th1)-mediated experimental model of joint disease that follows intra-articular injection of protein antigen [e.g. methylated bovine serum albumin (mBSA)] into the knee joints of previously immunized animals [20]. The histopathological appearance of AIA resembles RA, with synovial hyperplasia, perivascular infiltration of lymphocytes and plasma cells, lymphoid follicles, pannus and cartilage erosions [21]. In this study, we therefore investigated the effect of Hev b 13 on human mononuclear cells and its potential therapeutic use in the mBSA-induced model of arthritis.
Materials and methods
Reagents
Histopaque-1077, RPMI-1640 medium, complete and incomplete Freund's adjuvants and mBSA were purchased from Sigma-Aldrich Brasil Ltda (São Paulo, SP, Brazil). Fetal bovine serum and phytohaemagglutinin were purchased from Invitrogen Brasil Ltda (São Paulo, SP, Brazil). BD OptEIATM human interferon (IFN)-γ, TNF, IL-6, IL-1β and transforming growth factor (TGF)-β1 enzyme-linked immunosorbent assay (ELISA) kits were purchased from BD-Brasil (São Paulo, SP, Brazil). [3H]-Thymidine (10 Ci/mmol) and the liquid scintillation cocktail OptiPhase HiSafe 3 were purchased from PerkinElmer do Brasil Ltda (São Paulo, SP, Brazil).
Obtaining Hev b 13
Hev b 13 was purified as described previously [3]. Briefly, latex extracted from H. brasiliensis trees (clone RRIM 600) was coagulated, and the serum so obtained applied to a diethylaminoethyl (DEAE) cellulose column. The protein was purified through two further chromatographic steps (on Asahipak ES-502NP and Sephadex G-50 columns), after which its identity was determined by peptide mass fingerprinting and amino-terminal protein sequencing.
Human peripheral blood mononuclear cell (PBMC) culture
The experimental protocol was approved by the Research Ethics Committee (HCRP process no. 9575/2010), in compliance with ICH-GCP guidelines, as well as with Resolution 196/96 of the National Health Council (BRA). Informed consent was signed by healthy blood donors and investigators prior to beginning the study.
Mononuclear cells (1 × 106/ml) were isolated from whole venous blood by Ficoll-Hypaque density gradient centrifugation, according to procedures adapted from Böyum [22] and as described elsewhere [3]. The cells were resuspended in RPMI-1640 medium with 10% fetal bovine serum, seeded in 96-well plates in order to assess cytokine production. To this end, cells were stimulated in triplicate with three different concentrations (1, 10 and 100 µg/ml) of Hev b 13, both in the presence and absence of phytohaemagglutinin (PHA; 20 µg/ml). After 72 h of incubation at 37°C in a saturated humid environment with 5% CO2, supernatants were collected and cytokine levels assessed by commercial ELISA kits.
Cell proliferation was also quantified by measuring the incorporation of [3H]-thymidine (0·5 µCi/well) for an additional 16-h period [23].
Antigen-induced arthritis in mice
Animals
The animal study protocols (no. 030/2010) were approved by the Commission of Ethics in Animal Research of Faculty of Medicine of Ribeirao Preto, University of São Paulo, according to governmental guidelines for animal care and experimentation.
Pathogen-free male BALB/c mice, weighing approximately 25 g, were obtained from the Animal Centre of University of São Paulo. They were housed under controlled temperature (25°C), humidity (55%) and a 12-h light/dark cycle, and were given free access to a diet of regular laboratory chow and water.
Induction of arthritis and study protocol
Antigen-induced arthritis was established as described previously, with some modifications [5]. Briefly, mice were injected subcutaneously at the back of the neck with 200 µl of an emulsion containing 500 µg of mBSA in phosphate-buffered saline (PBS) and an equal volume of Freund's complete adjuvant. Booster injections of 500 µg of mBSA in Freund's incomplete adjuvant were administered subcutaneously in each side of the flank, after 7 days and again after 14 days. Control, sham-immunized mice received injections of the same emulsions, but without mBSA. Arthritis was induced on day 21 by an intra-articular injection of 50 µg of mBSA in 10 µl of PBS into the right knee. The left knee, into which 10 µl of PBS was injected, was used as a control.
To assess whether early administration of Hev b 13 protects against development of arthritis, mice receiving antigen emulsions were randomized to receive water or Hev b 13. Purified Hev b 13 was administered using oral gavage (0·5 mg/kg/day) from day 17 after the first immunization to the end of the experimental period. Mice were euthanized at days 4, 8 or 13 after the intra-articular challenge.
Macroscopic assessment of arthritis severity
Mice were euthanized by cervical dislocation, and the skin of the knee joint was removed. Swelling severity was compared between the two knees and expressed as a clinical score, ranging from 1 to 5, where 1 = no difference between mBSA-injected knee and the PBS-injected control knee, 2 = slight discoloration of the mBSA-injected knee, 3 = discoloration of the mBSA-injected knee and mild lateral swelling, 4 = discoloration of the mBSA-injected knee and moderate lateral swelling and 5 = discoloration of the mBSA-injected knee to the point where the ligament is no longer visible and severe lateral swelling [24]. All scoring was performed by one investigator (J. C. N.) without knowledge of the treatment protocols.
Histological assessment of arthritis severity
The knee joints were dissected and fixed in 3·7% buffered formaldehyde. Fixed tissues were decalcified in formic acid–sodium citrate reagent [25], dehydrated and embedded in paraffin. Six-µm sections were deparaffinized with xylene and stained with Masson's trichrome, using routine methods. All tissues were examined in a blinded fashion.
Statistical analysis
A mixed-effects linear regression model, followed by an orthogonal contrast post-hoc test, was used to compare levels of cytokines released in response to different levels of stimulation. For evaluation of clinical score, Kruskal–Wallis and Dunn's post-hoc test were used. Statistical analyses were performed with sas version 9 software (SAS Institute, Inc., Cary, NC, USA).
Results
Hev b 13 decreases TNF release without altering cell proliferation
The effect of Hev b 13 on human PBMCs was investigated by measuring cell proliferation and cytokine release. The effect of this stimulus was also evaluated in a T lymphocyte-enriched population, where PHA had been added as a specific mitogen.
Figure 1a shows that proliferation rates remain unchanged after Hev b 13 treatments. The levels of IFN-γ in culture supernatants were also not altered (Fig. 1b). It is noteworthy, however, that the PHA-stimulation markedly increased the release of this cytokine.
Fig. 1.
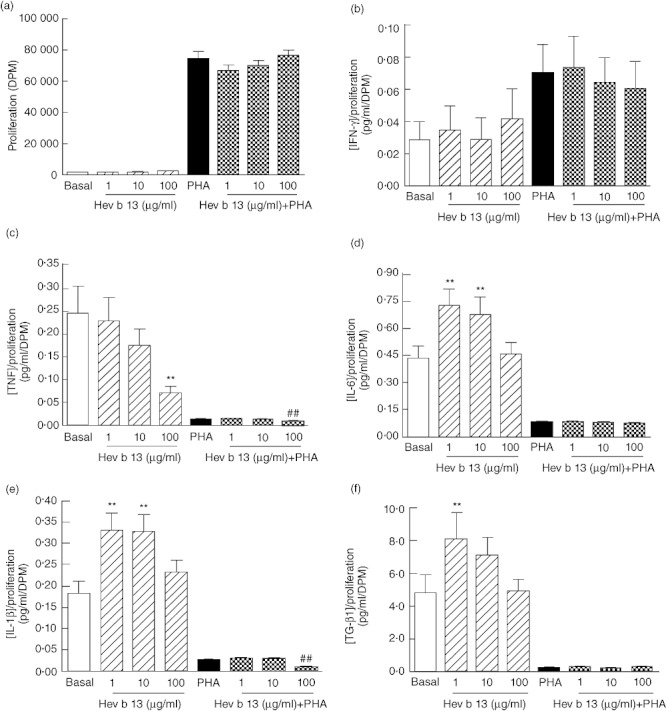
Effects of Hev b 13 on human peripheral blood mononuclear cells. After 72 h of culture in the absence or presence of phytohaemagglutinin (PHA; 20 µg/ml), supernatants were collected and tested for the indicated cytokines. An additional 16-h labelling period with [3H]-thymidine was used to assess proliferation. Cytokine concentration values were normalized to cell proliferation rates. (a) Proliferation rates of cells treated with Hev b 13 in the absence and presence of PHA. Relative levels of (b) interferon (IFN)-γ, (c) tumour necrosis factor (TNF), (d) interleukin (IL)-6, (e) IL-1β and (f) transforming growth factor (TGF)-β1 in the supernatants of these cells. Data are expressed as means ± standard error of the mean (n = 10 individuals per group). **P < 0·01, compared to basal levels (non-stimulated group); ##P < 0·01, compared to PHA-stimulated cells.
Conversely, levels of TNF (Fig. 1c) decreased in a dose-dependent manner in both PHA-stimulated and unstimulated cells, reaching a 3·5-fold decrease with 100 µg/ml of Hev b 13 and a 1·6-fold decrease with 100 µg/ml of Hev b 13 + PHA compared to basal and PHA levels, respectively.
IL-6 (Fig. 1d) and IL-1β (Fig. 1e) levels followed a similar profile in the absence of PHA, with the lower doses of Hev b 13 stimulating an increase in cytokine release and the highest dose returning the concentration of these cytokines to basal levels. As shown in Fig. 1e, a 2·6-fold decrease in IL-1β concentration occurred at 100 µg/ml of Hev b 13 in the presence of the mitogen.
TGF-β1 levels (Fig. 1f) were increased significantly by the 1 µg/ml dose of Hev b 13, but also decreased to basal concentrations as the protein dose escalated. In an enriched population, however, none of the tested doses over-stimulated cytokine release.
Hev b 13 attenuates severity and improves histological outcome in mBSA-induced arthritis
Previously immunized mice which were subjected to an intra-articular injection of mBSA developed arthritic changes in the challenged knee joint, while the contralateral joint showed no swelling or other pathology. The clinical signs of inflammation reached a peak on day 8, and were relieved almost entirely by day 13 (data not shown).
For treatment, a daily oral dose of 0·5 mg/kg of Hev b 13 was chosen after a similar preliminary study, in which doses of 0·5 and 5·0 mg/kg of FrHb 3, the fraction derived from the first step in the production of purified Hev b 13, had been administered successfully (results not shown). As illustrated in Fig. 2, the severity of swelling and discoloration of mBSA-injected knees was markedly higher among water-treated mBSA control mice than among Hev b 13-treated mice. It was noteworthy that no adverse effects, clinical or behavioural, were observed in animals treated with Hev b 13, suggesting that this particular dose is probably not toxic in vivo.
Fig. 2.
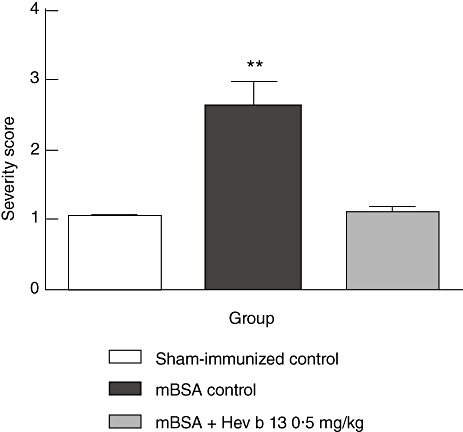
Effect of Hev b 13 treatment on severity score of mice with methylated bovine serum albumin (mBSA)-induced arthritis. Mice were immunized on days 0, 7 and 14 and challenged intra-articularly on day 21. The daily oral treatment was initiated on day 17. Severity score was determined 8 days following intra-articular injections. Data are expressed as mean ± standard error of the mean (n = 10–11 mice per group). **P < 0·01 versus both other groups.
Histological examination of the knee joints of mice with mBSA-induced arthritis revealed severe inflammation and destruction. As shown in Fig. 3, tissue sections from this group showed synovial hyperplasia, pannus formation and cartilage and bone erosion, accompanied by massive immune cell infiltration (involving mainly lymphocytes and plasma cells) into the synovial membrane and joint space. When mice were treated with Hev b 13, a striking improvement in these morphological changes became apparent, with a significant reduction in the inflammatory activity and leucocyte infiltration (Fig. 3).
Fig. 3.
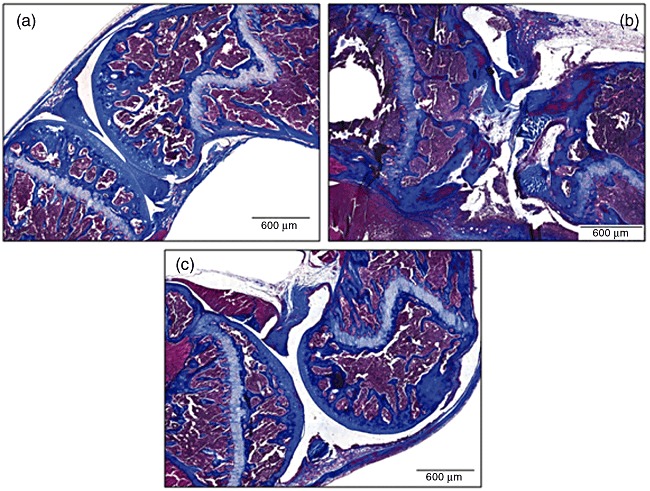
Photomicrographs of representative histological sections from the knee joints of mice subjected to the methylated bovine serum albumin (mBSA)-induced model of arthritis. Mice were immunized on days 0, 7 and 14 and challenged intra-articularly on day 21. The daily oral treatment was initiated on day 17, and euthanasia was performed 8 days following intra-articular injection. (a) Sham-immunized control mice. (b) Mice with mBSA-induced arthritis, which received only water treatment. (c) Mice with mBSA-induced arthritis treated with 0·5 mg/kg of Hev b 13. All slides were stained with Masson's trichrome (original magnification ×72).
Discussion
In the present study we demonstrate, for the first time, that the early oral administration of Hev b 13 protects mice effectively against the development of inflammation in the mBSA-induced arthritis model, a well-established experimental model that resembles the histopathology of human RA. After 13 days of treatment with Hev b 13 at a daily dose of 0·5 mg/kg (12 nmol/kg/day), mice showed significantly reduced oedema, leucocyte infiltration, cartilage disruption and fibrovascular ingrowth. These findings underscore the potent anti-inflammatory effect suggested previously for Hev b 13 [4], and indicate that the active compound enters the circulatory system.
The mechanism of action for Hev b 13 is speculated to be related to its interaction with immune cells. In a previous report, we showed that Hev b 13 stimulates human monocytes to release high levels of IL-10 in vitro[3]. By analysing a panel of cytokines, the present study revealed a major decrease in TNF levels when PBMCs were cultured in the presence of 100 µg/ml of Hev b 13, which was followed numerically by a reduction in the IL-1β levels in supernatants from T lymphocyte-enriched cell populations. Whether this immunomodulation is translated into an effective prevention mechanism for both inflammatory diseases studied [trinitrobenzene sulphonic acid (TNBS)-induced colitis [4] and mBSA-induced arthritis] remains to be determined.
Whether or not the diminished TNF levels are the consequence of IL-10 augmentation by Hev b13 treatment, reduction of TNF remains a powerful method for controlling inflammation. Results from clinical trials with anti-TNF agents confirm the biological relevance of TNF to the pathogenesis of chronic non-infectious inflammation of joints, skin and gut, and places it in the centre of many converging disease pathways [26]. TNF transduces signals ranging from cellular activation and proliferation to cytotoxicity and apoptosis. Its effector functions include the ability to induce the production of other proinflammatory cytokines (including IL-1 and IL-6) and chemokines, which leads ultimately to leucocyte accumulation in the inflamed tissue [7].
This study indicates conclusively that Hev b 13 has potential as an anti-arthritic agent. The results generated in this and our previous study [3] suggest that the therapeutic mechanism of Hev b13 may involve stimulation of monocytes/macrophages to increase release of IL-10 and decrease release of TNF. This work also highlights some of many possible applications for Hev b 13, or a fragment of this protein, in the field of immune-mediated inflammatory diseases.
Acknowledgments
The authors thank Professor Amilton Antunes Barreira (FMRP-USP) for his permission to use the facilities and equipment of the Laboratory of Neurosciences, CEMEQ for statistical analysis, and FAEPA (Foundation for the Support of Instruction, Research and Treatment) and FINEP (Research and Projects Financing through Pele Nova Biotecnologia S/A) for providing financial support. L. B. Teixeira also thanks CAPES (Coordination for the Improvement of Higher Education Personnel) for a PhD fellowship.
Disclosure
None of the authors has conflicts of interest to declare.
References
- 1.Arif SA, Hamilton RG, Yusof F, et al. Isolation and characterization of the early nodule-specific protein homologue (Hev b 13), an allergenic lipolytic esterase from Hevea brasiliensis latex. J Biol Chem. 2004;279:23933–41. doi: 10.1074/jbc.M309800200. [DOI] [PubMed] [Google Scholar]
- 2.Palosuo T, Lehto M, Kotovuori A, et al. Latex allergy: low prevalence of immunoglobulin E to highly purified proteins Hev b 2 and Hev b 13. Clin Exp Allergy. 2007;37:1502–11. doi: 10.1111/j.1365-2222.2007.02810.x. [DOI] [PubMed] [Google Scholar]
- 3.Teixeira LB, Pitz HS, Epifânio VLAA, Lachat JJ, Foss NT, Coutinho-Netto J. Allergenic esterase Hev b 13 induces interleukin-10 release by human mononuclear cells. Phytochemistry. (in press) [Google Scholar]
- 4.Teixeira LB, Epifânio VLAA, Lachat JJ, Foss NT, Coutinho-Netto J. Oral treatment with Hev b 13 improves experimental colitis in mice. Clin Exp Immunol. doi: 10.1111/j.1365-2249.2012.04589.x. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Waal Malefyt R, Haanen J, Spits H, et al. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med. 1991;174:915–24. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209–20. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fiorentino DF, Zlotnik A, Vieira P, et al. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991;146:3444–51. [PubMed] [Google Scholar]
- 8.Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, O'Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991;147:3815–22. [PubMed] [Google Scholar]
- 9.Ding L, Shevach EM. IL-10 inhibits mitogen-induced T cell proliferation by selectively inhibiting macrophage costimulatory function. J Immunol. 1992;148:133–9. [PubMed] [Google Scholar]
- 10.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 11.Katsikis PD, Chu CQ, Brennan FM, Maini RN, Feldmann M. Immunoregulatory role of interleukin 10 in rheumatoid arthritis. J Exp Med. 1994;179:1517–27. doi: 10.1084/jem.179.5.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee YH, Bae SC, Choi SJ, Ji JD, Song GG. Associations between interleukin-10 polymorphisms and susceptibility to rheumatoid arthritis: a meta-analysis. Mol Biol Rep. 2012;39:81–7. doi: 10.1007/s11033-011-0712-7. [DOI] [PubMed] [Google Scholar]
- 13.Brennan FM, McInnes IB. Evidence that cytokines play a role in rheumatoid arthritis. J Clin Invest. 2008;118:3537–45. doi: 10.1172/JCI36389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kasama T, Strieter RM, Lukacs NW, Lincoln PM, Burdick MD, Kunkel SL. Interleukin-10 expression and chemokine regulation during the evolution of murine type II collagen-induced arthritis. J Clin Invest. 1995;95:2868–76. doi: 10.1172/JCI117993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joosten LA, Lubberts E, Durez P, et al. Role of interleukin-4 and interleukin-10 in murine collagen-induced arthritis: protective effect of interleukin-4 and interleukin-10 treatment on cartilage destruction. Arthritis Rheum. 1997;40:249–60. doi: 10.1002/art.1780400209. [DOI] [PubMed] [Google Scholar]
- 16.Apparailly F, Verwaerde C, Jacquet C, Auriault C, Sany J, Jorgensen C. Adenovirus-mediated transfer of viral IL-10 gene inhibits murine collagen-induced arthritis. J Immunol. 1998;160:5213–20. [PubMed] [Google Scholar]
- 17.Keystone E, Wherry J, Grint P. IL-10 as a therapeutic strategy in the treatment of rheumatoid arthritis. Rheum Dis Clin North Am. 1998;24:629–39. doi: 10.1016/s0889-857x(05)70030-2. [DOI] [PubMed] [Google Scholar]
- 18.Narula K. Interleukin-10: a therapeutic cytokine for chronic inflammatory diseases. Curr Opin Anti-inflamm Immunomod Investig Drugs. 2000;2:307–13. [Google Scholar]
- 19.Scott DL, Wolfe F, Huizinga TWJ. Rheumatoid arthritis. Lancet. 2010;376:1094–108. doi: 10.1016/S0140-6736(10)60826-4. [DOI] [PubMed] [Google Scholar]
- 20.Brackertz D, Mitchell GF, Mackay IR. Antigen-induced arthritis in mice. I. Induction of arthritis in various strains of mice. Arthritis Rheum. 1977;20:841–50. doi: 10.1002/art.1780200314. [DOI] [PubMed] [Google Scholar]
- 21.Williams RO. Rodent models of arthritis. Clin Exp Immunol. 1998;114:330–2. doi: 10.1046/j.1365-2249.1998.00785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Böyum A. Isolation of mononuclear cells and granulocytes from human blood: isolation of mononuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- 23.Foss NT, Foss-Freitas MC, Ferreira MA, Cardili RN, Barbosa CM, Foss MC. Impaired cytokine production by peripheral blood mononuclear cells in type 1 diabetic patients. Diabetes Metab. 2007;33:439–43. doi: 10.1016/j.diabet.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 24.Martin E, Capini C, Duggan E, et al. Antigen-specific suppression of established arthritis in mice by dendritic cells deficient in NF-κB. Arthritis Rheum. 2007;56:2255–66. doi: 10.1002/art.22655. [DOI] [PubMed] [Google Scholar]
- 25.Morse A. Formic acid–sodium citrate decalcification and butyl alcohol dehydration of teeth and bones for sectioning in paraffin. J Dent Res. 1945;24:143–53. [Google Scholar]
- 26.Sfikakis PP. The first decade of biologic TNF antagonists in clinical practice: lessons learned, unsolved issues and future directions. Curr Dir Autoimmun. 2010;11:180–210. doi: 10.1159/000289205. [DOI] [PubMed] [Google Scholar]


