Abstract
The co-stimulatory molecule CD137 (4-1BB) plays a crucial role in the development and persistence of asthma, characterized by eosinophilic airway inflammation, mucus hypersecretion, airway hyperreactivity, increased T helper type 2 (Th2) cytokine production and serum immunoglobulin (Ig)E levels. We have shown previously that application of an agonistic CD137 monoclonal antibody (mAb) prevented and even reversed an already established asthma phenotype. In the current study we investigated whether deficiency of the CD137/CD137L pathway affects the development of allergic airway inflammation or the opposite immune reaction of respiratory tolerance. CD137−/− and wild-type (WT) mice were sensitized and challenged with the model allergen ovalbumin (OVA) and analysed for the presence of allergic disease parameters (allergy protocol). Some animals were tolerized by mucosal application of OVA prior to transferring the animals to the allergy protocol to analyse the effect of CD137 loss on tolerance induction (tolerance protocol). Eosinophilic airway inflammation, mucus hypersecretion, Th2 cytokine production and elevated allergen-specific serum IgE levels were increased equally in CD137−/− and WT mice. Induction of tolerance resulted in comparable protection from the development of an allergic phenotype in both mouse strains. In addition, no significant differences could be identified in CD4+, CD8+ and forkhead box protein 3 (FoxP3+) regulatory T cells, supporting the conclusion that CD137−/− mice show equal Th2-mediated immune responses compared to WT mice. Taken together, CD137−/− mice and WT mice develop the same phenotype in a murine model of Th2-mediated allergic airway inflammation and respiratory tolerance.
Keywords: allergic airway inflammation, CD137 (4-1BB), co-stimulation, murine asthma model, respiratory tolerance
Introduction
The prevalence of allergic diseases, including asthma, rhinitis and atopic dermatitis, has increased continuously over the last decades, especially in western populations [1]. Atopic asthma is characterized by eosinophilic airway inflammation and mucus hypersecretion, airway hyperreactivity and elevated serum immunoglobulin (Ig)E levels. It is associated strongly, but not exclusively, with the overproduction of T helper type 2 (Th2) cytokines. However, the majority of the human population has achieved immunological tolerance against common allergens protecting against the development of allergic diseases.
Antigen-specific activation of naive T cells is the initial step in both protective tolerance induction and Th2-polarized immune reactions against allergens. In addition to signals from the T cell receptor (TCR), a co-stimulatory signal, which can be provided by various receptor–ligand-interaction pairs, is crucial for optimal T cell activation. The co-stimulatory molecule CD137 belongs to the tumour necrosis factor receptor (TNFR) family and is expressed on activated CD4+ and CD8+ T cells [2] and various non-T cells [3], including B cells [4], natural killer (NK) cells [5] or dendritic cells (DCs) [6]. Several studies provide evidence that cross-linking of CD137 on T cells either with its naturally occurring ligand (CD137L) or by agonistic anti-CD137 monoclonal antibody (mAb) exerts various forms of immune activation both in vitro and in vivo[7]–[10]. In-vivo stimulation of CD137 resulted in rejection of tumours [11],[12], cardiac allograft and skin transplants [13],[14], inhibition of graft-versus-host disease (GVHD) [15] or autoimmune responses [16],[17] and promotion of viral defence [18].
After the generation of CD137-deficient (CD137−/−) mice, the role of the CD137/CD137L pathway in T cell immunity was studied further [19]. T cells derived from CD137−/− mice showed enhanced proliferation, whereas their capacity for secretion of cytokines interleukin (IL)-2, IL-4 and interferon (IFN)-γ was diminished [19]. The frequency and function of NK and NK T cells was reduced in CD137−/− mice. However, the influence of CD137 deficiency on maturation or steady-state CD4+ and CD8+ T cell populations has not yet been reported [20]. So far, CD137−/− mice have not been analysed in allergic airway disease models. In this regard, we and others have shown a critical role of CD137 in the immune response of allergic asthma [21]–[23]. Stimulation with agonistic anti-CD137 mAb not only prevented, but even reversed the complete asthma phenotype mediated partly by IFN-γ-producing CD8+ T cells [21]. In the present study, we followed a contrasting approach and investigated the effect of CD137 deficiency in the same OVA-based asthma model published previously [21] by comparative analysis of CD137−/− and wild-type (WT) mice.
We were further interested in whether the absence of CD137 influences the establishment of respiratory tolerance, because several co-stimulatory molecules, including CD134 (OX-40), cytotoxic T lymphocyte antigen (CTLA)-4 and inducible co-stimulator (ICOS), have been shown to play a role in regulatory T cell (Treg) function and are thus implicated to be involved in the development and maintenance of tolerance [24],[25]. CD137 is expressed constitutively on murine Tregs, whereas in humans CD137 is up-regulated rapidly on natural and inducible Tregs. The exact importance of CD137 in Tregs remains controversial, but an increasing body of evidence points towards a critical role for Treg expansion, survival and function [24],[26],[27]. However, so far the role of CD137/CD137L pathway in the context of development and maintenance of respiratory tolerance is uncertain. Therefore, aside from the classical OVA-based sensitization and challenge protocol, we compared WT and CD137−/− mice which were additionally tolerized with OVA prior to sensitization. Here we describe for the first time the phenotype of CD137−/− mice in a model of allergic airway inflammation as well as respiratory tolerance.
Materials and methods
Mice
CD137−/− mice were provided by Professor Dr Robert Mittler from Emory University (Atlanta, GA, USA). C57BL/6J control mice were purchased from Charles River (Sulzfeld, Germany). The animal protocols were constructed according to institutional and state guidelines and approved by the local animal welfare committee. Eight to 10-week-old age- and sex-matched mice were immunized with ovalbumin [OVA, lipopolysaccharide (LPS) content-reduced <10 EU/mg protein, as described previously [28]] using two protocols (Fig. 1), as follows.
Fig. 1.
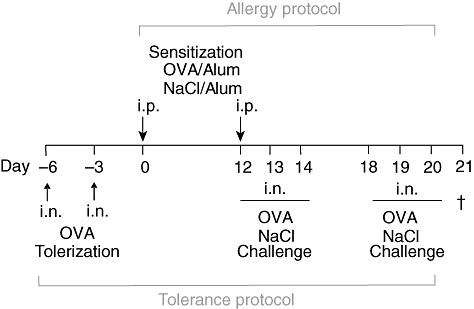
Immunization protocols used for tolerization, sensitization and challenge of wild-type (WT) C57BL6/J and CD137−/− mice with the model allergen ovalbumin (OVA). Mice were treated twice intranasally (i.n.) with 500 µg OVA to induce respiratory tolerance (TOL). Next, tolerized mice and positive control group (OVA) mice were sensitized with 20 µg OVA/Alum intraperitoneally (i.p.) on days 0 and 12; afterwards mice underwent six i.n. OVA applications separated into two challenge periods on 3 consecutive days (12–14 and 18–20). Control mice (Alum) received i.p. injections of 0·9% NaCl mixed with adjuvant and i.n. challenges of 0·9% NaCl. Mice were killed 24 h after last local OVA provocation on day 21 and analysed with regard to the development of allergic airway disease phenotype.
Allergy protocol
Mice were sensitized twice intraperitoneally (i.p.) with OVA (20 µg in 200 µl 0·9% NaCl) in adjuvant (Imject Alum®, PerbioScience, Bonn, Germany) followed by six challenges in which 20 µg OVA in 40 µl of 0·9% NaCl was given intranasally (i.n.) (allergy protocol). Control mice (Alum) received injections and challenges of 0·9% NaCl. Mice were killed 24 h after the last local allergen provocation.
Tolerance protocol
To induce respiratory tolerance, WT and CD137−/− mice were pretreated twice i.n. with 500 µg OVA in 40 µl of 0·9% NaCl. Thereafter, mice underwent allergy protocol as described above.
Bronchoalveolar lavage fluid (BALF)
BALF from each individual mouse was obtained by flushing the lungs with PBS/2 mM ethylenediamine tetraacetic acid (EDTA); the total number and differentiation of BALF cells were then determined as described previously [28].
Lung histology
Lung histological sections and computer-based quantification of the degree of pulmonary inflammation [haematoxylin and eosin (H&E)] and mucus production [periodic acid-Schiff (PAS)] were performed as described previously [28].
OVA-specific IgE, IgG1 and IgG2a
Serum levels of OVA-specific IgE, IgG1 and IgG2a were measured by enzyme-linked immunosorbent assay (ELISA), according to standard protocol. IgE concentrations (pg/ml) were calculated using a standard curve for mouse anti-OVA IgE (AbD Serotec, Oxford, UK). Data for IgG1 and IgG2a are presented as titre values derived from analysis of optical density (OD) values versus factors of serum dilution series using a logarithmic curve-fitting model.
In vitro cytokine production and proliferation
Spleen and bronchial lymph node (bLN) isolated cells were restimulated in vitro with OVA (200 µg/ml) in RPMI-1640 containing 10% fetal calf serum (FCS), 100 U/ml penicillin and 100 µg/ml streptomycin. Cytokines (IL-4, IL-5, IL-13, IFN-γ) were measured in supernatants after 3 days using DuoSet ELISA kits (R&D Systems, Minneapolis, MN, USA), according to the manufacturer's instructions. Cell cultures were pulsed with 3[H]-thymidine and incorporated activity was measured in a Betaplate scintillation counter.
Flow cytometry
Single-cell suspensions from spleen, lung and bLN were incubated with fluorescently labelled antibodies for 20 min at 4°C in phosphate-buffered saline (PBS)/0·5% bovine serum albumin (BSA). Intracellular staining of forkhead box protein 3 (FoxP3) was performed using the eBioscience kit, according to the manufacturer's instructions. Briefly, cells were surface-stained, fixed and incubated with antibody to FoxP3 for 30 min at 4°C. Data were collected on a flow cytometer FACS Canto II (BD Biosciences, Mountain View, CA, USA) and analysed using FlowJo (Treestar Inc., Ashland, OR, USA) software. Absolute cell numbers were calculated based on relative percentages obtained from FACS analysis.
Antibodies
Anti-murine antibodies used in this study included: CD4 [phycoerythrin (PE), RM4-5], CD8 [peridinin chlorophyll (PerCP-Cy5·5, 53-6·7], CD25 (PE-Cy7, PC61) from BD Biosciences (Mountain View, CA, USA) and FoxP3 [allophycocyanin (APC), FJK-16s] from eBioscience (San Diego, CA, USA).
Statistical analysis
Statistical analyses were performed using GraphPad Prism (La Jolla, CA, USA). Significance between two groups, e.g. WT OVA versus CD137−/− OVA, was estimated using the Mann–Whitney U-test. P-values ≤ 0·05 were considered significant (*) and ≤0·01 as highly significant (**).
Results
CD137 deficiency has no effect on development of allergic airway inflammation
We analysed comparatively CD137−/−versus WT mice in our asthma model [21],[28],[29] to examine whether the loss of CD137 expression affects the development of Th2-cell driven airway inflammation. Using the allergy protocol (Fig. 1), we first investigated eosinophilic lung infiltration by BALF analysis. Both OVA-sensitized and challenged CD137−/− and WT mice showed increased total cell counts (Fig. 2b) along with a high proportion of eosinophils (Fig. 2c). Other BALF cell subtypes such as macrophages and neutrophils also did not differ between OVA-immunized WT and CD137−/− mice. Next, we examined lung sections with regard to airway inflammation and mucus production (Fig. 3). Comparable to WT mice, CD137−/− immunized mice showed severe pulmonary inflammation with perivascular and peribronchial cell infiltrates and swelling of airway epithelium (H&E staining; Fig. 3a, right panel). Furthermore, we detected mucus hypersecretion and goblet cell hyperplasia using PAS staining of lung slices (Fig. 3a, left panel) in OVA-treated WT mice, which was similarly detectable in the CD137−/− immunized group. The histological pathology findings were confirmed by computer-assisted analysis of lung sections using an objective, investigator-independent software based on morphometric image analysis (Fig. 3b) without revealing any significant differences between the two mouse strains.
Fig. 2.
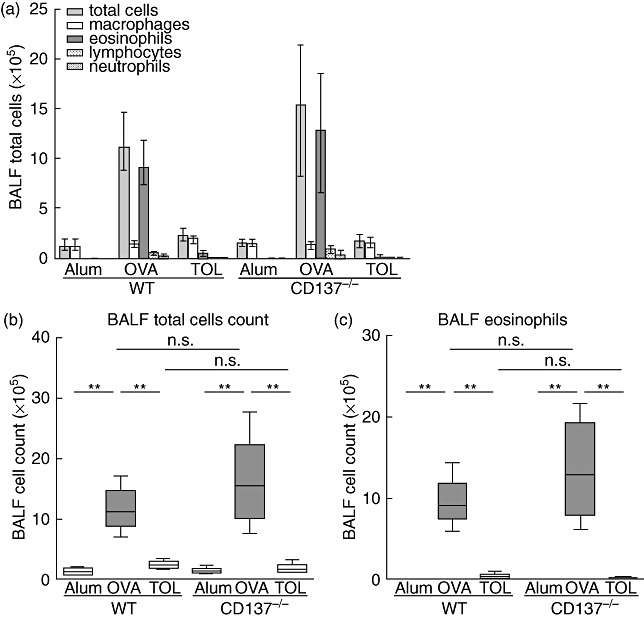
Bronchoalveolar lavage fluid (BALF) analysis of wild-type (WT) and CD137−/− mice. Mice were immunized with ovalbumin (OVA) according to the protocols described in Fig. 1. BALF was obtained from each individual mouse to determine total cell count and BALF cell differentials on cytospins. Enhanced total cell counts (a,b) and eosinophilic inflammation (a,c) were observed in OVA-sensitized and challenged WT and CD137−/− mice. In contrast, tolerized mice showed low total BALF cell count and eosinophil counts comparable with control animals. Data from one representative of three independent experiments are presented as median ± interquartile range (a) or whiskers dot-plots (b; c), n ≥ 5 animals per group; **P ≤ 0·01, not significant (n.s.) P > 0·05; Mann–Whitney U-test.
Fig. 3.
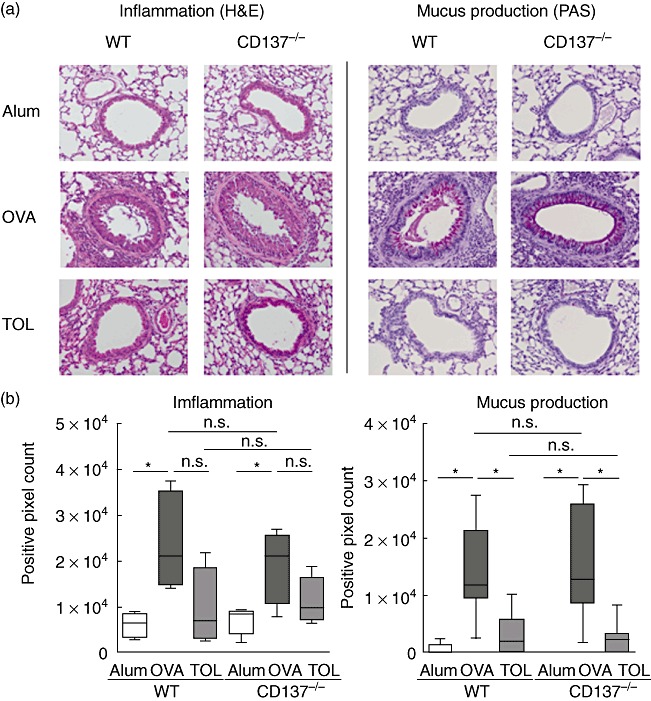
Histological staining of lung specimens of wild-type (WT) and CD137−/− mice. (a) Representative lung sections stained with haematoxylin and eosin (H&E) for detection of airway inflammation (left panel) and periodic acid-Schiff (PAS) for analysis of mucus secretion (right panel). Magnification: ×200 for all photographs. (b) Computer-assisted quantification of staining patterns using an image analysing software. WT ovalbumin (OVA) and CD137−/− OVA groups showed increased airway inflammation together with strong mucus production and goblet cell hyperplasia compared to controls. Tolerization prior to OVA sensitization of both WT and CD137−/− mice reduced levels of inflammation and mucus secretion efficiently. Data are illustrated as whiskers box-plots; n ≥ 5 animals per group; *P ≤ 0·05, not significant (n.s.) P > 0·05; Mann–Whitney U-test.
Absence of CD137 has no impact on IgE serum levels, in vitro lymphocyte proliferation and Th2 cytokine production
Elevated serum levels of allergen-specific IgE and IgG1 in mice are typical features of Th2-linked immune reactions, whereas IgG2a in mice is associated with Th1 immune responses. Hence, we determined allergen-specific Ig levels in sera of immunized mice by ELISA (Fig. 4). Comparable to WT mice, sensitization and challenge of CD137−/− mice resulted in significantly enhanced OVA-specific IgE and IgG1 levels; in contrast, in the corresponding non-immunized controls IgE and IgG1 levels were very low to undetectable (**P ≤ 0·01). We did not identify significant changes between OVA-specific IgE, IgG1 and IgG2a serum levels of the WT and CD137−/− OVA-immunized groups. Next, we assessed lymphocyte proliferation after in vitro OVA restimulation using the 3[H]-thymidine incorporation assay. Lymphocytes derived from the spleen (Fig. 5a) or bLNs (data not shown) of OVA-sensitized and challenged WT or CD137−/− mice showed equally enhanced proliferation, while lymphocytes isolated from controls proliferated only slightly. In addition, we determined cytokine production in supernatants of lymphocyte cell cultures by ELISA. Th2 cytokines IL-5 and IL-13 were increased markedly in cell cultures of both OVA-immunized CD137−/− and WT mice compared to controls (**P ≤ 0·01) (Fig. 5b), but no significant differences were observed between IL-5 and IL-13 production in spleen cell cultures derived from CD137−/−versus WT mice that underwent the allergy protocol. Th2 cytokine IL-4 and IFN-γ, as signs of the Th1 response, were very low (<50 pg/ml) to undetectable (data not shown).
Fig. 4.
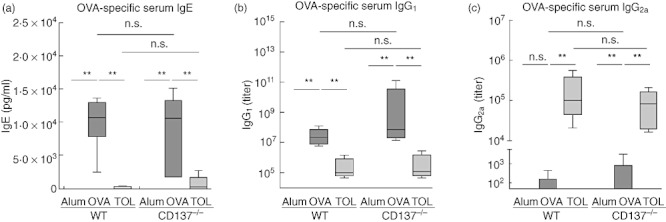
Ovalbumin (OVA)-specific immunoglobulin (Ig) levels in sera of wild-type (WT) and CD137−/− mice. Mice were immunized with OVA according to the protocols described in Fig. 1. Serum from each individual mouse was obtained on day 21 and OVA-specific IgE (a), IgG1 (b) and IgG2a (c) serum levels were measured by sandwich enzyme-linked immunosorbent assay (ELISA). Data are presented as whiskers box-plots. One representative of three independent experiments is shown; n ≥ 5 animals per group; **P ≤ 0·01, not significant (n.s.) P > 0·05; Mann–Whitney U-test.
Fig. 5.
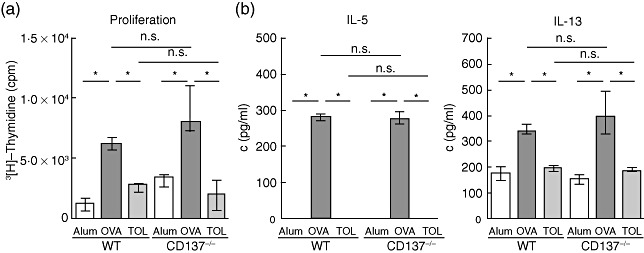
3[H]-Thymidine-proliferation assay and T helper type 2 (Th2) cytokine enzyme-linked immunosorbent assay (ELISA) of in vitro restimulated lymphocytes isolated from wild-type (WT) and CD137−/− mice. Mice were immunized with ovalbumin (OVA) according to the protocols described in Fig. 1. Lymphocytes isolated from pooled spleen derived from WT and CD137−/− mice were restimulated with 200 µg/ml OVA in 96-well tissue-culture plates for 48 h, pulsed with 1 µCi tritiated thymidine per well for 14 h, and thereafter the incorporated activity was measured in a betaplate scintillation counter (a). Supernatants were analysed by ELISA with regard to the production of the Th2 cytokines Interleukin (IL)-5 and IL-13 (b). Data are presented as median ± interquartile range. One representative of three independent experiments is shown; n ≥ 5 animals per group; **P ≤ 0·01, *P ≤ 0·05, not significant (n.s.) P > 0·05; Mann–Whitney U-test.
CD137 is dispensable for the development of respiratory tolerance
As demonstrated above, we observed similar allergic parameters in CD137−/− and WT mice after OVA sensitization and challenge, demonstrating that CD137 is not required for the development of a Th2-dominated allergic phenotype. Furthermore, we were interested in whether CD137 co-stimulation is involved in respiratory tolerance induction. Hence, mice were tolerized via mucosal application of OVA before sensitization (Fig. 1, tolerance protocol). Consistent with previous studies [28],[30], tolerized WT mice (WT TOL) showed reduced signs of allergic airway disease and resembled the control group (WT Alum). CD137−/− mice were equally protected: we did not detect any significant differences with regard to total BALF cell count and eosinophilia (Fig. 2b,c) or pulmonary inflammation and mucus production (Fig. 3). Furthermore OVA-specific IgE, IgG1 and IgG2a serum levels (Fig. 4), in vitro proliferation and Th2 cytokine production were equivalent (Fig. 5a,b). To summarize, all measured parameters were comparable in tolerized wild-type and CD137−/− mice, suggesting that loss of CD137 is not critical for respiratory tolerance induction in our model.
No differences in T cell subsets in CD137−/−versus WT mice
We determined T cell subsets via flow cytometry in spleen and lungs from individual WT and CD137−/− mice on day 21 of the immunization protocols (Fig. 1). Similarly, we found significantly elevated percentages and numbers of CD4+ T cells in lung of OVA-immunized WT and CD137−/− mice (Fig. 6b); in parallel, we observed a slight trend towards reduced proportions of splenic CD4+ T cells after sensitization and challenge (Fig. 6a). With regard to CD8+ T cell frequency, we detected no significant differences after immunization. Again, CD137−/− mice had comparable percentages and absolute numbers in spleen and lung to the WT groups independent of the immunization protocol used. Analysis of Treg (CD4+FoxP3+) cells revealed significantly enhanced percentages in lung (Fig. 6b) of both OVA-immunized mice strains, whereas we did not observe this increase in spleen (Fig. 6a). Tolerization resulted in a significantly decreased proportion of Tregs in CD137−/− mice, whereas in WT mice this reduction did not reach significance due to an outlier, while a trend was detectable. In addition, we analysed pooled bLN fractions for T cell subsets without detecting any differences (data not shown). In summary, no significant differences were identified in CD4+, CD8+ and FoxP3+ Tregs in CD137−/− mice compared with WT mice; these results support our conclusion that CD137−/− mice show an equal Th2-mediated immune response.
Fig. 6.
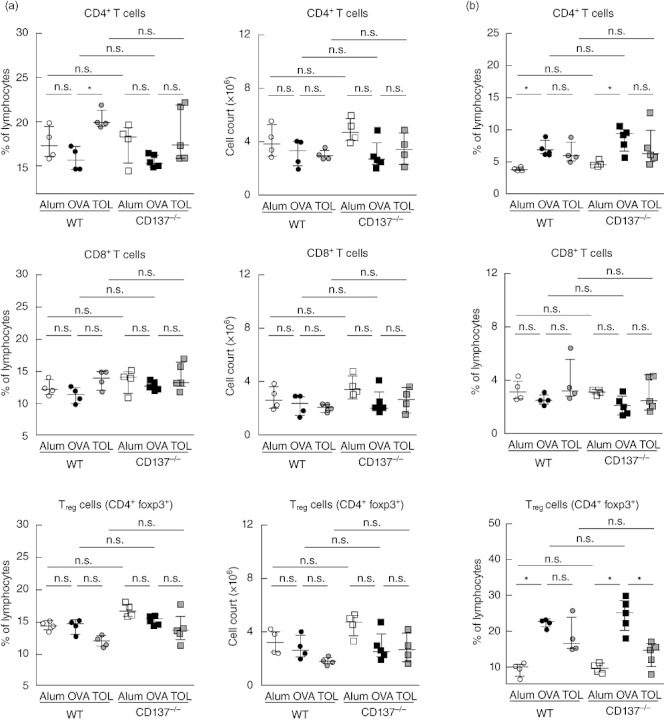
Comparative FACS analysis of T cell subsets isolated from spleen and lung of wild-type (WT) and CD137−/− mice. Mice were immunized with ovalbumin (OVA) according to the protocols described in Fig. 1. Lymphocyte fractions freshly isolated from individual spleen (a) or individual lung (b) derived from WT and CD137−/− mice on day 21 were determined using flow cytometry. Percentages and total cell counts of CD4+, CD8+ and regulatory T cells (Treg) [CD4+forkhead box protein 3 (FoxP3+)] cells from one experiment are presented as scatter-plots (median ± interquartile range); n ≥ 4 animals per group; *P ≤ 0·05, not significant (n.s.) P > 0·05; Mann–Whitney U-test.
Discussion
In our previous work we have shown that administration of an agonistic CD137 mAb inhibited the development of asthma and, moreover, was even capable of reversing established airway hyperreactivity (AHR), eosinophilic airway inflammation and production of allergen-specific IgE in our murine asthma model [21]. Similarly, in a model of atopic conjunctivitis, stimulation of CD137 before or after sensitization inhibited the development of allergic disease [31]. Based on these findings, showing a strong effect of CD137 receptor stimulation in Th2 cell-mediated diseases, we expected differences when we compared WT and CD137−/− mice in our asthma model. However, in contrast to our expectation, the absence of CD137 signalling did not affect the development of allergic asthma; WT and CD137−/− mice developed comparably strong airway eosinophilic inflammation, mucus hypersecretion and enhanced OVA-specific serum IgE levels.
The finding that CD137 stimulation via an agonistic mAb had significant effects on the manifestation of allergic parameters [21], whereas missing CD137 signalling did not affect the generation of an allergic phenotype in our model, is difficult to interpret. The potent effect of the CD137 agonistic mAb was associated with reduced production of Th2 cytokines, while secretion of IFN-γ was increased strongly. IFN-γ is one of the main inhibitors of Th2 cell development and cytokine production which play a crucial role in the development and persistence of allergic asthma. Depletion of CD8+ T cells or blockade of IFN-γ partly abolished the protective effect of CD137 agonistic mAb treatment, indicating that this observation was mediated by IFN-γ-secreting CD8+ T cells [21]. This effect is absent in CD137−/− mice, which show comparable Th2 cytokine levels and CD4+ as well as CD8+ T cell frequencies compared to WT mice. In contrast to CD137 triggering the development of Th2 cytokine-producing cells is not affected in CD137−/− mice in our model, which might partly explain the missing difference between WT and CD137−/− mice in our allergic asthma model.
Previous reports also show that lack of CD137 signalling does not mandatorily exert opposite results compared with stimulation of this receptor. For instance, treatment with CD137 agonistic mAbs has been shown to exert powerful anti-cancer effects in tumour models, while CD137−/− mice were remarkably resistant to tumour growth [5],[7],[11]. Follow-up studies demonstrated that CD137 signalling regulates the balance between CD8+ T cells and NK cells via modulation of IFN-γ production. Whereas both CD8+ T cells and NK cells are essentially required to suppress tumour growth in CD137−/− mice, the therapeutic effect of CD137 mAb treatment is dependent mainly on CD8+ T cell-mediated anti-tumour immunity [32]. In addition to tumour models, mice lacking CD137 receptor or CD137 ligand expression have been studied in models of infection and autoimmune disorders [2],[7]. Given the key role of CD8+ T cells in controlling viral infection and the potent CD8+ T cell-inducing effect of agonistic CD137 mAb, CD137 triggering as a strategy to enhance the anti-viral response showed therapeutic potential. Conversely, even in the absence of CD137 expression, anti-viral immunity seems to be functional, as CD137−/− mice showed reduced severity in a herpetic stromal keratitis (HSK) model [33]. With regard to bacterial infection, CD137−/− mice showed lower mortality in a model of polymicrobial sepsis induced by caecal ligation and puncture [34]. In comparison to WT controls, CD137−/− mice exhibited higher numbers of macrophages and neutrophils accomplished with better bacterial clearance and enhanced survival in this infection model. Similar results were observed after treatment with blocking anti-CD137L mAb, whereas the administration of CD137 agonistic mAb aggravated polymicrobial sepsis and decreased survival of WT mice [34]. Treatment with agonistic CD137 mAb has been demonstrated to efficiently prevent or even reverse autoimmune responses in murine studies, including models for lupus, rheumatoid arthritis and experimental autoimmune encephalomyelitis [35]–[37]. Analysis of CD137−/− mice with regard to autoimmune disorders revealed a divergent outcome. Jeon et al. showed that CD137 gene deletion results in the improvement of atherosclerosis in hyperlipidaemic mice [38]. However, lprl CD137−/− mice show increased immune activation and develop a dramatic autoimmune phenotype leading to early mortality in a lupus model [39]. Recently, it has been demonstrated that CD137 deficiency protects against obesity-induced inflammation and metabolic disorders [40].
In general, CD137−/− mice show no defect in T cell development, as percentages of CD4+ and CD8+ T cells in spleen and thymus were similar to WT mice under steady-state conditions [19]. In vitro stimulation of CD137−/− lymphocytes with anti-CD3 or mitogens revealed an increased proliferation relative to WT cells [19]. The observed hyperreactivity of cells from CD137−/− mice did not correlate with IL-2 secretion. Besides decreased IL-2 levels, the capacity for IL-4 and IFN-γ production was also diminished in CD137−/− cell cultures. In contrast to this unspecific stimulation, we did not detect significant differences in the proliferation of CD137−/− T cells when antigen-specific stimulation with OVA was used. Lee et al. reported enhanced CD4+ T cell responsiveness to protein antigen in CD137−/− mice [41]. Using a model of subcutaneous immunization with OVA together with adjuvant this group showed that CD137−/− mice generated higher primary CD4+ T cell effector responses compared to WT mice and showed enhanced OVA-specific Th1 and Th2 cytokine levels in the spleen, depending on the adjuvant used. Based on this study, CD137 seems to be involved in priming and might play a role in limiting the early expansion of CD4+ T cells at the initial stage of immune response to protein antigen. In line with our observations, this study demonstrates that CD137−/− mice are not compromised in their capacity to elicit CD4+ T cell-mediated immune responses. Similar to our results, Lee et al. could not detect a difference with regard to the IgG1 response, suggesting that even in the absence of CD137 signalling, T cell-dependent humoral antibody responses to protein antigen develop normally [41]. However, in contrast to this study, we did not observe a strong increase in Th2 cytokine levels in splenocyte cell cultures of CD137−/− OVA group compared with WT OVA. Whereas Lee et al. applied OVA subcutaneously only once to study initial T cell priming, we immunized WT and CD137−/− mice twice i.p. with OVA and aluminium hydroxide as adjuvant, followed by six i.n. challenge periods. Therefore, the differences seen between these studies might be explained by the prolonged immunization protocol including OVA challenge periods to induce local recall response in our model. Whether CD137 plays a distinct role in priming versus recall responses in OVA-based models needs to be investigated further. It is possible that experimental models without the powerful effect of aluminium hydroxide as adjuvant could reveal minor changes between WT and CD137−/− mice that may be underestimated in our acute model based on OVA/Alum sensitization. Thus, testing of CD137−/− mice in another asthma protocol, i.e. with a weaker immunization protocol or with house dust mite as model allergen, could be a future perspective.
Another possible explanation for the missing phenotype of CD137−/− mice with regard to asthma is that the missing CD137/CD137L co-stimulation might be compensated by other co-stimulatory signalling pathways, as we have shown previously for CD30 and CD134 (OX40) in a chronic asthma model [42]. CD30, another co-stimulatory molecule of the TNFR superfamily, proved to be crucial for the development of asthma in an acute model [29] while, in contrast, we did not see differences between CD30−/− and WT mice in the chronic model [42]. We demonstrated that reduced expression of OX40 on T cells in the acute model and up-regulation in the chronic model indirectly supported a compensatory role of OX40 for CD30 signalling. Similarly, application of agonistic anti-OX40 mAb restored the asthma phenotype in CD30−/− mice in the acute model, whereas chronic airway inflammation was reduced in the presence of an inhibitory anti-OX40 ligand mAb. Therefore, it is possible that in CD137−/− mice the role of CD137 signalling is compensated likewise by other co-stimulatory pathways. To confirm this possible explanation we are now analysing the complex expression pattern of other co-stimulatory molecules in CD137−/− mice.
Co-stimulation is not only relevant for the generation of effector T cell responses; several co-stimulatory molecules, including CD134 (OX-40), CTLA-4 and ICOS, have been indicated to also contribute to tolerance mechanisms mediated by Tregs[24],[25]. CD137 expression has been found on Tregs and CD137 signalling has been shown to promote proliferation and survival of Tregsin vitro[26],[27]. In a murine model of diabetes, treatment with anti-CD137 mAb increased Treg numbers significantly, which mediated protective effects after adoptive transfer into non-obese diabetic–severe combined immunodeficiency (NOD–SCID) recipients [17]. In contrast, other studies have pointed towards a negative effect of CD137 stimulation on Treg induction or activity. Choi et al. demonstrated that CD137 signalling neutralizes the suppressive function of Tregsin vitro and in vivo[43]. Another study suggests that CD137 signalling is not important for Treg function, as Tregs isolated from CD137−/− mice prevented colitis pathology efficiently in a CD4+ T cell transfer model to SCID mice [44]. So far, the exact importance of the CD137/CD137L pathway for Treg function or generation of respiratory tolerance in vivo has not been studied. Therefore, we also investigated whether CD137 might play an immune regulatory role in vivo. CD137 deficiency had no impact on respiratory tolerance induction in our model, as CD137−/− mice were protected equally from the development of allergic parameters compared to WT mice by mucosal antigen application prior to sensitization. We could not detect changes in Treg frequencies between WT and CD137−/− mice. Thus, the lack of CD137 seems not to inhibit Treg development or function in our model.
Taken together, our results demonstrate that loss of CD137/CD137L signalling neither affects the generation of Th2-mediated allergic airway inflammation nor influences the induction of respiratory tolerance in our murine model. While the current study investigated the role of CD137 in a murine model of allergic asthma, there are only limited data on CD137 function in the human system with regard to allergic, Th2-mediated immune responses: CD137 expression has been detected on eosinophils and associated with apoptosis of eosinophils [45]. Moreover, CD137 expression has been reported on T cells infiltrating the conjunctival stroma in patients with severe allergic conjunctivitis compared with controls [46]. Thus, future studies are required to elucidate the exact role of CD137 signalling in allergic diseases in humans.
Acknowledgments
This study was supported by the German Research Foundation [Research Training Group GRK 1441 ‘Allergic response in lung and skin’; SFB 578 (TP14) ‘Immune reactions of the lung in infection and allergy’]. We thank Professor Dr Robert Mittler from Emory University, School of Medicine, Atlanta (USA) for kindly providing CD137−/− mice and Jana Bergmann, Björn Zweigait and Christin Albrecht for excellent technical assistance.
Disclosure
The authors declare no financial or commercial conflict of interest.
References
- 1.Pearce N, Ait-Khaled N, Beasley R, et al. Worldwide trends in the prevalence of asthma symptoms: phase III of the International Study of Asthma and Allergies in Childhood (ISAAC) Thorax. 2007;62:758–66. doi: 10.1136/thx.2006.070169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Croft M. The role of TNF superfamily members in T-cell function and diseases. Nat Rev Immunol. 2009;9:271–85. doi: 10.1038/nri2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vinay DS, Kwon BS. 4-1BB signaling beyond T cells. Cell Mol Immunol. 2011;8:281–4. doi: 10.1038/cmi.2010.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pollok KE, Kim YJ, Hurtado J, Zhou Z, Kim KK, Kwon BS. 4-1BB T-cell antigen binds to mature B cells and macrophages, and costimulates anti-mu-primed splenic B cells. Eur J Immunol. 1994;24:367–74. doi: 10.1002/eji.1830240215. [DOI] [PubMed] [Google Scholar]
- 5.Melero I, Johnston JV, Shufford WW, Mittler RS, Chen L. NK1.1 cells express 4-1BB (CDw137) costimulatory molecule and are required for tumor immunity elicited by anti-4-1BB monoclonal antibodies. Cell Immunol. 1998;190:167–72. doi: 10.1006/cimm.1998.1396. [DOI] [PubMed] [Google Scholar]
- 6.Futagawa T, Akiba H, Kodama T, et al. Expression and function of 4-1BB and 4-1BB ligand on murine dendritic cells. Int Immunol. 2002;14:275–86. doi: 10.1093/intimm/14.3.275. [DOI] [PubMed] [Google Scholar]
- 7.Wang C, Lin GH, McPherson AJ, Watts TH. Immune regulation by 4-1BB and 4-1BBL: complexities and challenges. Immunol Rev. 2009;229:192–215. doi: 10.1111/j.1600-065X.2009.00765.x. [DOI] [PubMed] [Google Scholar]
- 8.Croft M. Costimulation of T cells by OX40, 4-1BB, and CD27. Cytokine Growth Factor Rev. 2003;14:265–73. doi: 10.1016/s1359-6101(03)00025-x. [DOI] [PubMed] [Google Scholar]
- 9.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 10.Vinay DS, Kwon BS. Role of 4-1BB in immune responses. Semin Immunol. 1998;10:481–9. doi: 10.1006/smim.1998.0157. [DOI] [PubMed] [Google Scholar]
- 11.Melero I, Shuford WW, Newby SA, et al. Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nat Med. 1997;3:682–5. doi: 10.1038/nm0697-682. [DOI] [PubMed] [Google Scholar]
- 12.Wilcox RA, Flies DB, Zhu G, et al. Provision of antigen and CD137 signaling breaks immunological ignorance, promoting regression of poorly immunogenic tumors. J Clin Invest. 2002;109:651–9. doi: 10.1172/JCI14184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeBenedette MA, Wen T, Bachmann MF, et al. Analysis of 4-1BB ligand (4-1BBL)-deficient mice and of mice lacking both 4-1BBL and CD28 reveals a role for 4-1BBL in skin allograft rejection and in the cytotoxic T cell response to influenza virus. J Immunol. 1999;163:4833–41. [PubMed] [Google Scholar]
- 14.Shuford WW, Klussman K, Tritchler DD, et al. 4-1BB costimulatory signals preferentially induce CD8+ T cell proliferation and lead to the amplification in vivo of cytotoxic T cell responses. J Exp Med. 1997;186:47–55. doi: 10.1084/jem.186.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blazar BR, Kwon BS, Panoskaltsis-Mortari A, Kwak KB, Peschon JJ, Taylor PA. Ligation of 4-1BB (CDw137) regulates graft-versus-host disease, graft-versus-leukemia, and graft rejection in allogeneic bone marrow transplant recipients. J Immunol. 2001;166:3174–83. doi: 10.4049/jimmunol.166.5.3174. [DOI] [PubMed] [Google Scholar]
- 16.Foell JL, Diez-Mendiondo BI, Diez OH, et al. Engagement of the CD137 (4-1BB) costimulatory molecule inhibits and reverses the autoimmune process in collagen-induced arthritis and establishes lasting disease resistance. Immunology. 2004;113:89–98. doi: 10.1111/j.1365-2567.2004.01952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Irie J, Wu Y, Kachapati K, Mittler RS, Ridgway WM. Modulating protective and pathogenic CD4+ subsets via CD137 in type 1 diabetes. Diabetes. 2007;56:186–96. doi: 10.2337/db06-0793. [DOI] [PubMed] [Google Scholar]
- 18.Halstead ES, Mueller YM, Altman JD, Katsikis PD. In vivo stimulation of CD137 broadens primary antiviral CD8+ T cell responses. Nat Immunol. 2002;3:536–41. doi: 10.1038/ni798. [DOI] [PubMed] [Google Scholar]
- 19.Kwon BS, Hurtado JC, Lee ZH, et al. Immune responses in 4-1BB (CD137)-deficient mice. J Immunol. 2002;168:5483–90. doi: 10.4049/jimmunol.168.11.5483. [DOI] [PubMed] [Google Scholar]
- 20.Vinay DS, Choi BK, Bae JS, Kim WY, Gebhardt BM, Kwon BS. CD137-deficient mice have reduced NK/NKT cell numbers and function, are resistant to lipopolysaccharide-induced shock syndromes, and have lower IL-4 responses. J Immunol. 2004;173:4218–29. doi: 10.4049/jimmunol.173.6.4218. [DOI] [PubMed] [Google Scholar]
- 21.Polte T, Foell J, Werner C, et al. CD137-mediated immunotherapy for allergic asthma. J Clin Invest. 2006;116:1025–36. doi: 10.1172/JCI23792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cho YS, Kwon B, Lee TH, et al. 4-1 BB stimulation inhibits allergen-specific immunoglobulin E production and airway hyper-reactivity but partially suppresses bronchial eosinophilic inflammation in a mouse asthma model. Clin Exp Allergy. 2006;36:377–85. doi: 10.1111/j.1365-2222.2006.02445.x. [DOI] [PubMed] [Google Scholar]
- 23.Sun Y, Blink SE, Liu W, et al. Inhibition of Th2-mediated allergic airway inflammatory disease by CD137 costimulation. J Immunol. 2006;177:814–21. doi: 10.4049/jimmunol.177.2.814. [DOI] [PubMed] [Google Scholar]
- 24.So T, Lee SW, Croft M. Immune regulation and control of regulatory T cells by OX40 and 4-1BB. Cytokine Growth Factor Rev. 2008;19:253–62. doi: 10.1016/j.cytogfr.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burmeister Y, Lischke T, Dahler AC, et al. ICOS controls the pool size of effector-memory and regulatory T cells. J Immunol. 2008;180:774–82. doi: 10.4049/jimmunol.180.2.774. [DOI] [PubMed] [Google Scholar]
- 26.Zheng G, Wang B, Chen A. The 4-1BB costimulation augments the proliferation of CD4+CD25+ regulatory T cells. J Immunol. 2004;173:2428–34. doi: 10.4049/jimmunol.173.4.2428. [DOI] [PubMed] [Google Scholar]
- 27.Zhang P, Gao F, Wang Q, et al. Agonistic anti-4-1BB antibody promotes the expansion of natural regulatory T cells while maintaining Foxp3 expression. Scand J Immunol. 2007;66:435–40. doi: 10.1111/j.1365-3083.2007.01994.x. [DOI] [PubMed] [Google Scholar]
- 28.Behrendt AK, Hansen G. CD27 costimulation is not critical for the development of asthma and respiratory tolerance in a murine model. Immunol Lett. 2010;133:19–27. doi: 10.1016/j.imlet.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 29.Polte T, Behrendt AK, Hansen G. Direct evidence for a critical role of CD30 in the development of allergic asthma. J Allergy Clin Immunol. 2006;118:942–8. doi: 10.1016/j.jaci.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 30.Polte T, Hennig C, Hansen G. Allergy prevention starts before conception: maternofetal transfer of tolerance protects against the development of asthma. J Allergy Clin Immunol. 2008;122:1022–30 e5. doi: 10.1016/j.jaci.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 31.Fukushima A, Yamaguchi T, Ishida W, et al. Engagement of 4-1BB inhibits the development of experimental allergic conjunctivitis in mice. J Immunol. 2005;175:4897–903. doi: 10.4049/jimmunol.175.8.4897. [DOI] [PubMed] [Google Scholar]
- 32.Choi BK, Kim YH, Kim CH, et al. Peripheral 4-1BB signaling negatively regulates NK cell development through IFN-gamma. J Immunol. 2010;185:1404–11. doi: 10.4049/jimmunol.1000850. [DOI] [PubMed] [Google Scholar]
- 33.Seo SK, Park HY, Choi JH, et al. Blocking 4-1BB/4-1BB ligand interactions prevents herpetic stromal keratitis. J Immunol. 2003;171:576–83. doi: 10.4049/jimmunol.171.2.576. [DOI] [PubMed] [Google Scholar]
- 34.Nguyen QT, Ju SA, Park SM, et al. Blockade of CD137 signaling counteracts polymicrobial sepsis induced by cecal ligation and puncture. Infect Immun. 2009;77:3932–8. doi: 10.1128/IAI.00407-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Foell J, McCausland M, Burch J, et al. CD137-mediated T cell co-stimulation terminates existing autoimmune disease in SLE-prone NZB/NZW F1 mice. Ann NY Acad Sci. 2003;987:230–5. doi: 10.1111/j.1749-6632.2003.tb06052.x. [DOI] [PubMed] [Google Scholar]
- 36.Seo SK, Choi JH, Kim YH, et al. 4-1BB-mediated immunotherapy of rheumatoid arthritis. Nat Med. 2004;10:1088–94. doi: 10.1038/nm1107. [DOI] [PubMed] [Google Scholar]
- 37.Sun Y, Lin X, Chen HM, et al. Administration of agonistic anti-4-1BB monoclonal antibody leads to the amelioration of experimental autoimmune encephalomyelitis. J Immunol. 2002;168:1457–65. doi: 10.4049/jimmunol.168.3.1457. [DOI] [PubMed] [Google Scholar]
- 38.Jeon HJ, Choi JH, Jung IH, et al. CD137 (4-1BB) deficiency reduces atherosclerosis in hyperlipidemic mice. Circulation. 2010;121:1124–33. doi: 10.1161/CIRCULATIONAHA.109.882704. [DOI] [PubMed] [Google Scholar]
- 39.Vinay DS, Kim JD, Asai T, Choi BK, Kwon BS. Absence of 4 1BB gene function exacerbates lacrimal gland inflammation in autoimmune-prone MRL-Faslpr mice. Invest Ophthalmol Vis Sci. 2007;48:4608–15. doi: 10.1167/iovs.07-0153. [DOI] [PubMed] [Google Scholar]
- 40.Kim CS, Kim JG, Lee BJ, et al. Deficiency for costimulatory receptor 4-1BB protects against obesity-induced inflammation and metabolic disorders. Diabetes. 2011;60:3159–68. doi: 10.2337/db10-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee SW, Vella AT, Kwon BS, Croft M. Enhanced CD4 T cell responsiveness in the absence of 4-1BB. J Immunol. 2005;174:6803–8. doi: 10.4049/jimmunol.174.11.6803. [DOI] [PubMed] [Google Scholar]
- 42.Polte T, Fuchs L, Behrendt AK, Hansen G. Different role of CD30 in the development of acute and chronic airway inflammation in a murine asthma model. Eur J Immunol. 2009;39:1736–42. doi: 10.1002/eji.200839004. [DOI] [PubMed] [Google Scholar]
- 43.Choi BK, Bae JS, Choi EM, et al. 4-1BB-dependent inhibition of immunosuppression by activated CD4+CD25+ T cells. J Leukoc Biol. 2004;75:785–91. doi: 10.1189/jlb.1003491. [DOI] [PubMed] [Google Scholar]
- 44.Maerten P, Kwon BS, Shen C, et al. Involvement of 4-1BB (CD137)-4-1BBligand interaction in the modulation of CD4 T cell-mediated inflammatory colitis. Clin Exp Immunol. 2006;143:228–36. doi: 10.1111/j.1365-2249.2005.02991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heinisch IV, Bizer C, Volgger W, Simon HU. Functional CD137 receptors are expressed by eosinophils from patients with IgE-mediated allergic responses but not by eosinophils from patients with non-IgE-mediated eosinophilic disorders. J Allergy Clin Immunol. 2001;108:21–8. doi: 10.1067/mai.2001.116864. [DOI] [PubMed] [Google Scholar]
- 46.Sumi T, Ishida W, Ebihara N, Fukushima A. T cell-related costimulatory molecules in the conjunctiva of patients with severe allergic conjunctivitis. Cornea. 2010;29:622–7. doi: 10.1097/ICO.0b013e3181c377bd. [DOI] [PubMed] [Google Scholar]


