Abstract
LIGHT [the name of which is derived from ‘homologous to lymphotoxins, exhibits inducible expression, competes with herpes simplex virus glycoprotein D for herpes simplex virus entry mediator (HVEM), and expressed by T lymphocytes’], is a member of the tumour necrosis factor superfamily that is involved in various inflammatory diseases. We aimed to estimate the relevance of plasma LIGHT levels as a biomarker for atopic dermatitis (AD). In order to understand the putative role of LIGHT in AD pathogenesis, we also investigate the effects of LIGHT on a monocytic cell line, human acute monocytic leukaemia cell line (THP-1). We examined plasma LIGHT levels, total serum IgE, serum value of CCL17 and peripheral blood eosinophil counts in patients with AD and healthy subjects. The effects of LIGHT on activation and apoptosis in THP-1 cells were also investigated. The plasma concentrations of LIGHT in AD patients were significantly higher than those in healthy individuals and the concentrations decreased as the symptoms were improved by treatment. The LIGHT plasma concentrations correlated with IgE levels and the Severity Scoring of AD (SCORAD) index. In addition, LIGHT stimulation increased expression of CD86 and induced production of interleukin-1β in THP-1 cells. Apoptosis was inhibited, the Bcl-2 level increased and the caspase-3 level decreased in THP-1 cells stimulated with LIGHT, compared to unstimulated control cells. These results suggest that plasma LIGHT levels may be one of the promising biomarkers for AD.
Keywords: atopic dermatitis, LIGHT, THP-1
Introduction
LIGHT [the name of which is derived from ‘homologous to lymphotoxins, exhibits inducible expression, competes with herpes simplex virus glycoprotein D for herpes simplex virus entry mediator (HVEM), and expressed by T lymphocytes’] is a 29-kDa membrane protein and a member of the tumour necrosis factor (TNF) superfamily [1]. LIGHT is induced upon activation of CD4(+) and CD8(+) T cells, monocytes/macrophages, natural killer cells, immature dendritic cells (DC) and platelets [2]–[6]. Accumulated evidence suggests the involvement of LIGHT in the pathogenesis of inflammatory disorders such as rheumatoid arthritis [7], ankylosing spondylitis [8] and inflammatory bowel diseases [9], and it promises to be one of the biomarkers for these diseases. The line-up of LIGHT-producing cells including T cells, platelets and monocyte/macrophages implies that LIGHT may be one of the biomarkers representing disease activity of inflammatory skin diseases such as atopic dermatitis (AD) [10]–[13]. However, the LIGHT plasma values in patients with AD and their relationship with other markers representing the severity and activity of AD has not yet been investigated.
LIGHT binds mainly to HVEM [14]–[16], a member of the TNF receptor superfamily, and to the lymphotoxin β receptor (LTβR) [5],[17],[18]. HVEM is expressed on T cells, B cells, natural killer cells, monocytes/macrophages, neutrophils and DC [3],[15],[19]. The LTβR is not expressed on primary T cells or monocytes, but is expressed prominently on stromal cells of lymph nodes in the red pulp and along the borders of red and white pulp [18]. It is thus suggested that the LIGHT signal in the inflammatory process is mediated mainly by HVEM. Among HVEM-expressing cells, lymphocytes, monocytes/macrophages and DC are considered to be involved mainly in the pathogenesis of AD when they are activated [10]. LIGHT has been shown to co-stimulate proliferation and activation of T cells and increase their expression of adhesion molecules [19]–[21]. LIGHT and CD40L act synergistically to stimulate proliferation of B cells, whereas LIGHT alone is sufficient to induce immunoglobulin production [22]. In addition, the blockade or deletion of LIGHT signals in mice results in increased susceptibility to Leishmania major[23], suggesting its role in developing DC and/or lymphocyte-mediated immune responses. Although it has been demonstrated that LIGHT induces the production of TNF-α, interleukin (IL)-2, CXC chemokine ligand (CXCL)-8 and chemokine ligand 2 (CCL2) in monocytes/macrophages [6],[7], more detailed information about the effects of LIGHT on the phenotype, function and lifespan of monocytes, which is important in the understanding of the putative role of LIGHT in the pathogenesis of AD, remains scarce.
In this study, we determined the possibility of plasma LIGHT values as a biomarker of AD. We also investigated the effects of LIGHT on human acute monocytic leukaemia cell line (THP-1) by estimating their cytokine production, co-stimulatory molecule expression and survival.
Materials and methods
Subjects
Twenty-nine adult patients (18 men, 11 women; median age: 30 years; range: 17–42 years) with AD were enrolled into the study. Diagnosis of AD was based on the criteria of Hanifin and Rajka [24], and clinical severity was determined using the Severity Scoring of AD (SCORAD) index (median score: 78; range: 6–102). The peripheral eosinophil count (median: 795; range: 180–3707/µl), serum immunoglobulin (Ig)E (median: 10210; range: 819–66900 IU/ml), serum CCL17 (median: 1204; range: 141–3860 pg/ml) and serum lactate dehydrogenase (LDH) (median: 274; range: 150–436 U/l) were also examined in 21 of these patients. Each patient had had a history of AD for more than 3 years. The control group comprised 16 healthy subjects (seven men, nine women; median age: 32 years; range: 21–48 years). All subjects gave written consent; the study was approved by the University Ethics Committee and conducted in accordance with the Declaration of Helsinki.
Reagents and chemicals
Recombinant human LIGHT (rhLIGHT) was purchased from R&D Systems (Minneapolis, MN, USA) and diluted in phosphate-buffered saline (PBS) containing 0·1% bovine serum albumin (BSA). According to the manufacturer, rhLIGHT contains < 100 pg endotoxin contamination per µg protein, as determined by the low-density lipoprotein method. Anti-TR2/HVEM monoclonal antibody (mAb) (clone 122) was obtained from ThermoFisher Scientific (Fremont, CA, USA). The following antibodies were used in the study: anti-human CD86 monoclonal antibody (BD PharMingen, San Diego, CA, USA); phycoerythrin-conjugated anti-human Bcl-2 antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA); and fluorescein isothiocyanate (FITC)-conjugated rabbit anti-active caspase-3 antibody (BD PharMingen). Lipopolysaccharide (LPS) was from Sigma (St Louis, MO, USA). All other reagents were obtained from Sigma unless indicated otherwise.
Cell preparation and cultures
The human monocytic cell line THP-1 was obtained from American Type Culture Collection (Manassas, VA, USA) and maintained in RPMI-1640 medium containing 10% non-heat-inactivated fetal bovine serum (FBS) and 1% antibiotic/anti-mycotic solution (both from Gibco, Carlsbad, CA, USA) at 37°C in 5% CO2. THP-1 cells were resuspended at 1 × 106 cells/ml in RPMI-1640 medium containing 10% heat-inactivated FBS and 1% antibiotic/anti-mycotic solution with or without various concentrations of rhLIGHT or LPS. All working medium contained < 10 pg/ml of LPS, as determined by a limulus amoebocyte lysate test (Sigma).
Flow cytometric analysis
For cell surface staining, cells were processed following standard procedures and analysis was performed on a FACScalibur machine (Becton Dickinson, San Jose, CA, USA). Staining was performed with the appropriate phycoerythrin (PE)-isotype control or CD86 mAb. To analyse the expression of HVEM, the cells were incubated with an antibody specific for HVEM or mouse IgG and then stained with goat anti-mouse IgG FITC (Becton Dickinson). For intracellular detection, cells were fixed for 10 min at room temperature in 4% formaldehyde and then washed twice in PBS supplemented with 1% FBS, 0·01% NaN3 and 0·1% saponin. Then, 5 × 105 cells in 100 µl were stained using antibodies against Bcl-2 and activated caspase-3. Cells were washed once in the same medium and once in PBS–FBS. All data are presented after subtraction of the background from a corresponding isotypic control.
Enzyme-linked immunosorbent assay (ELISA)
Plasma values of human LIGHT and CCL17 were assayed with a commercially available ELISA kit (R&D Systems). The LIGHT detection limit is 1·2 pg/ml. The concentrations of IL-1β, IL-18, IL-6, IL-10 and TNF-α in the culture supernatants of THP-1 cells and monocytes were measured using commercially available ELISA kits (eBiosciences, San Diego, CA, USA).
Cell death assay
To induce apoptosis, the THP-1 cells were washed five times with PBS and then suspended in 96-well plates for 48 h under conditions of serum withdrawal. Human monocytes were cultured in serum-free medium or medium containing anti-CD95/Fas mAb CH11 to induce apoptosis. WST-1 (4-[3-(4-iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1,3-benzene disulphonate) reagent was then added to the wells and incubation was continued for an additional 4 h at 37°C. Following this incubation, the A450 of the cell cultures was measured and cell viability was calculated using the formula: [(Atest − Abackground)/(Acontrol − Abackground)] × 100, where A is the absorbance at A450, background refers to the medium alone and control refers to the untreated cell population.
Statistical analysis
Statistical differences were determined using the Mann–Whitney U-test for comparison of LIGHT values between normal subjects and patients with AD and Wilcoxon's signed-ranks test for the transition of plasma LIGHT levels in association with the treatments. Spearman's rank correlation coefficient analysis was carried out to examine the relationship between the levels of LIGHT in plasma and clinical markers such as total serum IgE levels, serum CCL17, peripheral blood eosinophil counts and SCORAD index. The results of in-vitro study were analysed by Wilcoxon's signed-ranks test. P-values < 0·05 were considered to be statistically significant. Results are expressed as the mean ± standard error of the mean (s.e.m.), unless indicated otherwise.
Results
Increased plasma LIGHT levels in patients with AD
To explore the possibility of LIGHT as a biomarker of AD, we examined the LIGHT levels in plasma from chronic AD patients. As shown in Fig. 1a, LIGHT levels were significantly higher in AD patients (median: 34·5 pg/ml, range: 7·7–323·7 pg/ml, lower quartile: 14·8 pg/ml, upper quartile: 65·4 pg/ml) than in healthy controls (median: 905 pg/ml, range: < 1·2–36·7 pg/ml, lower quartile: < 1·2 pg/ml, upper quartile: 26·3, P < 0·0006).
Fig. 1.
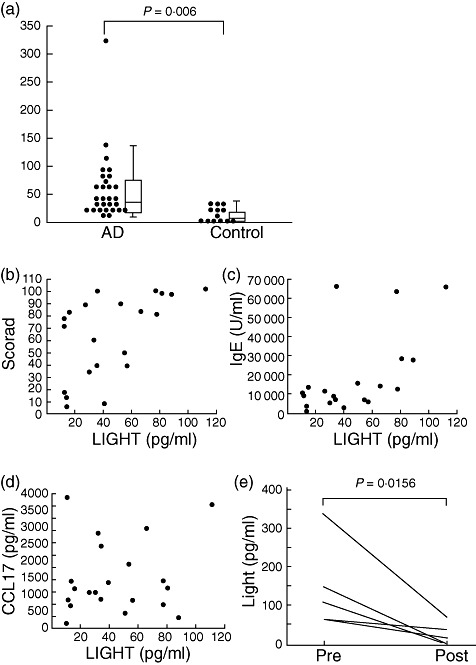
Levels of LIGHT [the name of which is derived from ‘homologous to lymphotoxins, exhibits inducible expression, competes with herpes simplex virus glycoprotein D for herpes simplex virus entry mediator, and expressed by T lymphocytes’] are increased in patients with atopic dermatitis (AD). (a) Plasma levels of LIGHT in patients with AD (n = 29) during excerbation and in healthy subjects (control: n = 16). Correlation of plasma LIGHT and Severity Scoring of AD (SCORAD) (b), serum immunoglobulin (Ig)E (c) and serum CCL17 (d) were assessed in 21 patients. (e) Changes of plasma LIGHT levels before and after treatment in AD patients. Plasma samples were obtained when the eruptions were aggravated (Pre) and after treatment (Post) (n = 5). LIGHT levels were measured by enzyme-linked immunosorbent assay.
Plasma LIGHT levels correlated with the SCORAD index and serum IgE levels (Table 1, Fig. 1b,c). However, there was no significant correlation between the LIGHT levels and plasma LDH levels, CCL17 (Fig. 1d) or peripheral eosinophil counts (Table 1). These findings indicate that elevated LIGHT levels are associated with clinical severity of AD.
Table 1.
Correlation between plasma LIGHT levels and other biomarkers of atopic dermatitis.
| Median value (range) | Correlation r | Analysis P | |
|---|---|---|---|
| Serum IgE (U/ml) | 10 210 (819–67 000) | 0·59 | 0·005 |
| Serum LDH (U/l) | 261 (150–436) | 0·20 | 0·4035 |
| Serum CCL17 (pg/ml) | 1204 (141–3860) | 0·07 | 0·761 |
| Eosinophil (counts/µl) | 795 (180–3707) | 0·05 | 0·830 |
| SCORAD index | 78 (6–102) | 0·57 | 0·007 |
Reference values: serum IgE, <380 U/ml; serum lactate dehydrogenase (LDH), 114–243 U/l; peripheral eosinophil counts, <420/µl; serum CCL17, 0–449 pg/ml. n = 21.
We then analysed whether LIGHT levels changed in association with improvement of eruption in AD. The five AD patients (three men, two women) were treated with conventional therapy such as topical steroids and oral anti-histamines in in- and out-patients. The SCORAD index was improved by approximately 20 points from before-to-after treatment. The LIGHT levels decreased markedly in plasma collected from these patients after treatment compared to that before treatment (Table 2, Fig. 1e). These results suggest that the plasma LIGHT level changes in association with the disease activity of in AD.
Table 2.
Severity Scoring of atopic dermatitis (AD) (SCORAD) index and plasma LIGHT [the name of which is derived from ‘homologous to lymphotoxins, exhibits inducible expression, competes with herpes simplex virus glycoprotein D for herpes simplex virus entry mediator (HVEM), and expressed by T lymphocytes’] levels before to after treatment from the five patients with AD.
| SCORAD index | LIGHT (pg/ml) | |||
|---|---|---|---|---|
| Patient | Pre | Post | Pre | Post |
| 1 | 45 | 20 | 339·3 | 72·3 |
| 2 | 43 | 16 | 107·7 | 2·6 |
| 3 | 49 | 22 | 60·2 | 16·1 |
| 4 | 40 | 15 | 65·4 | 35·0 |
| 5 | 90 | 35 | 148·0 | 2·6 |
LIGHT alters the phenotype and function of THP-1cells
To examine the effct of LIGHT on the phenotype, cytokine-producing capacity and lifespan of monocytes, we used the human monocytic cell line THP-1, which also expressed HVEM on flow cytometry (Fig. 2a).
Fig. 2.
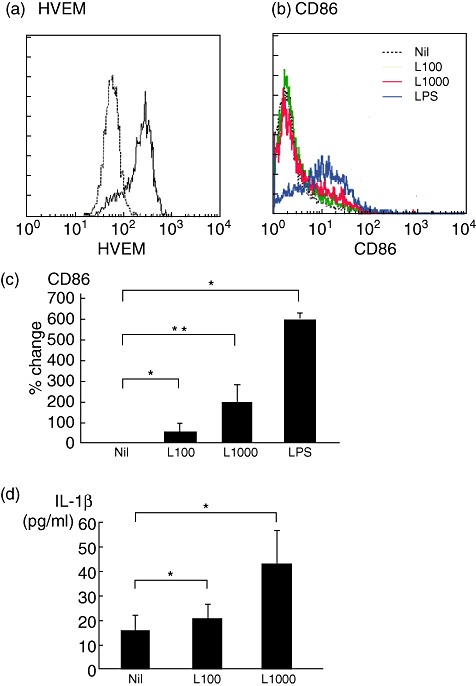
LIGHT [the name of which is derived from ‘homologous to lymphotoxins, exhibits inducible expression, competes with herpes simplex virus glycoprotein D for herpes simplex virus entry mediator, and expressed by T lymphocytes’] alters the phenotype and function of human acute monocytic leukaemia cell line (THP-1) cells. (a) Expression of HVEM in THP-1 cells. THP-1 cells were stained with fluorescein isothiocyanate (FITC)-conjugated anti-HVEM antibody (solid line) or isotype-matched control (dotted line) and expression of HVEM was analysed by cell flow cytometry. The representative date of three similar experiments are shown. (b) Phenotype and function of THP-1 cells incubated with or without 100 ng/ml (L100) or 1000 ng/ml (L1000) of recombinant human LIGHT, or 100 ng/ml of lipopolysaccharide (LPS) as a positive control. Representative examples of CD86 expression determined by flow cytometry. (c) CD86 expression on THP-1 cells, as revealed by the mean ± standard error of the mean (s.e.m.) (error bars) of six to seven independent experiments. (d) Interleukin (IL)-1β concentration in cell culture supernatants measured by enzyme-linked immunosorbent assay. The data are presented as the mean ± s.e.m. (error bars) of six to seven independent experiments. *P < 0·05 compared with control.
The cells were incubated for 48 h in the presence or absence of 100 ng/ml or 1000 ng/ml of rhLIGHT, or 100 ng/ml of LPS as a positive control. The expression of CD86 on THP-1 cells, which is known to be up-regulated in association with their activation, was then determined. Treatment with rhLIGHT induced a significant increase in expression of CD86 on the surface of THP-1 cells (Fig. 2b,c). Culture supernatants were collected and measurement of cytokine concentrations by ELISA showed that LIGHT significantly enhanced the production of IL-1β by THP-1 cells (Fig. 2d). The LIGHT effects were weaker than those induced by LPS. Neither IL-6, TNF-α, IL-10 nor IL-18 was detected in the supernatant of THP-1 with LIGHT-treated cells (data not shown).
LIGHT prevents apoptosis of THP-1 cells
Next, we examined whether apoptosis of THP-1 cells was inhibited by LIGHT. In an initial experiment to induce apoptosis, THP-1 cells were cultured for 48 h without serum in the presence of different concentrations of LIGHT and the proportion of apoptotic cells was assessed by WST-1 assay, as reported previously [25]. As shown in Fig. 3a, LIGHT inhibited apoptosis in a dose-dependent manner. LIGHT 1000 ng/ml reduced apoptosis of THP-1 cells by 77%. The effect of LIGHT on monocyte survival was weaker than those induced by LPS.
Fig. 3.
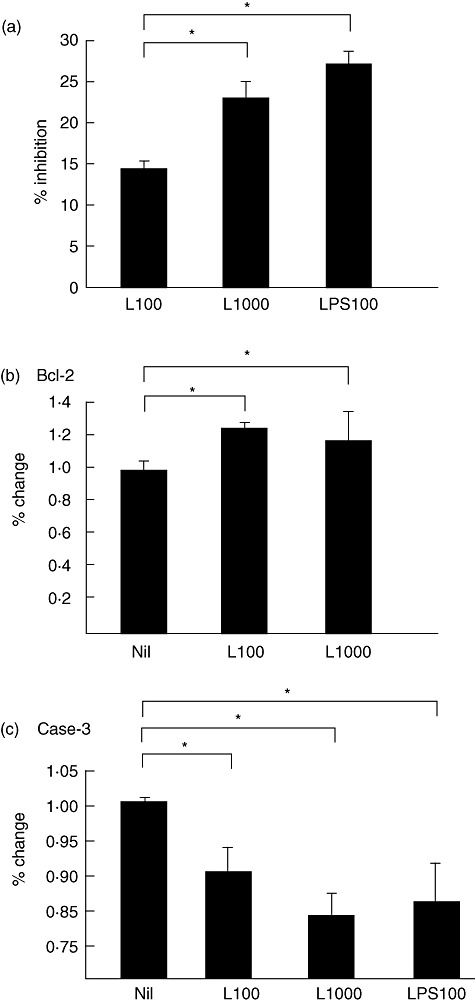
(a) Apoptosis of human acute monocytic leukaemia cell line (THP-1) cells incubated with or without 100 ng/ml (L100) or 1000 ng/ml (L1000) of recombinant human LIGHT [the name of which is derived from ‘homologous to lymphotoxins, exhibits inducible expression, competes with herpes simplex virus glycoprotein D for herpes simplex virus entry mediator, and expressed by T lymphocytes’], or 100 ng/ml of lipopolysaccharide (LPS) as a positive control. Apoptosis was measured by a WST-1 assay for cells incubated with culture medium without serum. Effects of LIGHT on Bcl-2 (b) and activated caspase-3 (c) expression in THP-1 cells incubated with or without 100 ng/ml (L100) or 1000 ng/ml (L1000) of rhLIGHT, or 100 ng/ml of LPS. All data were obtained by flow cytometry. The data are presented as the mean ± standard error of the mean (error bars) of six independent experiments. *P < 0·05 compared with control.
Effect of LIGHT on Bcl-2 and activated caspase-3 expression in THP-1 cells
The Bcl-2 family of proteins plays a role in apoptosis regulation [26],[27]. Therefore, we examined whether expression of Bcl-2 proteins in THP-1 cells was affected by LIGHT. THP-1 was cultured for 48 h without serum in the presence of different concentrations of LIGHT and intracellular expression of Bcl-2 was analysed by flow cytometry. As shown in Fig. 3b, LIGHT enhanced the expression of Bcl-2 in THP-1 cells. Caspases also play a role in many forms of apoptosis [28], and a central role in the execution of apoptosis has been shown for caspase-3. Therefore, we also examined whether the expression of active caspase-3 was affected by LIGHT. THP-1 cells were cultured for 48 h without serum in the presence of different concentrations of LIGHT and intracellular expression of caspase-3 was analysed by flow cytometry. As shown in Fig. 3c, LIGHT reduced the expression of caspase-3 in THP-1 cells.
Discussion
In this study, we show that the plasma concentrations of LIGHT in AD patients were higher than those in healthy volunteers. The plasma LIGHT levels showed a correlation with IgE. Furthermore, the LIGHT levels showed a significant correlation with the SCORAD index, which agrees with the result that treatment of AD decreased the LIGHT concentration. However, in our study plasma LIGHT levels did not correlate significantly with serum CCL17 levels, which are considered to reflect the degree of local inflammatory reaction; i.e., disease activity of AD [29]. Conversely, SCORAD represents the clinical state of patients with AD; i.e., disease severity of AD. To calculate SCORAD, the area of eruptions, intensity of eruptions, including erythema, oedema/papule, oozing/crust, excoriation, lichenification, and xerosis, pruritus and insomnia are evaluated. The discrepancy between their correlations regarding plasma LIGHT levels may be explained by the difference in major sources of cells producing LIGHT and CCL17. It has been demonstrated previously that keratinocytes, T cells and DC are major sources of CCL17 in AD patients [29], whereas LIGHT is induced upon activation of T cells, monocytes/macrophages, natural killer cells, immature DC and platelets [2]–[6]. Scratching because of severe itch often results in excoriation and haemorrhaging at the lesion in AD, which activate platelets [30]. In chronic eruption such as lichenification, monocytes are activated, prolonging their lifespan by escaping apoptosis and infiltrating the inflammatory site [31]. Activation of platelets and monocytes/macrophages in patients with AD [30] may thus contribute much more to increased levels of plasma LIGHT than serum CCL17 values. Interestingly, itch, excoriation and lichenification are all included in computing SCORAD, while they do not always seem to represent the degree of local inflammatory reaction.
In this study, LIGHT stimulation increased the expression of CD86 and induced the production of IL-1β in THP-1 cells. These results agree with previous observations showing the proinflammatory property of LIGHT [6],[7]. Interestingly, activated monocyte/macrophages show increased production of LIGHT and enhanced expression of HVEM, suggesting that monocytes/macrophages could be activated in an autocrine manner [7]. It may thus be speculated that LIGHT could activate monocytes in the circulation and skin lesions and be involved in the inflammatory process in AD.
Conversely, a recent report showed that HVEM-over-expressing DC ameliorated autoimmune myocarditis by producing a regulatory cytokine, IL-10, which had further effects on the induction of IL-10-producing CD4(+) T cells [32], indicating that the LIGHT–HVEM pathway is involved in immune regulation. Although treatment of monocytes and THP-1 cells with LIGHT did not induce IL-10 production in our study (data not shown), the capability of LIGHT to regulate immune responses should be estimated in further investigations.
Unstimulated monocytes undergo apoptosis within approximately 24 h and maintain homeostasis in tissues, whereas apoptosis is decreased in monocytes stimulated with cytokines and cross-linked with CD40L and FcεRI [33]. Such monocytes can survive for several months and remain in an inflamed site, leading to establishment of chronic inflammation. In this study, apoptosis of THP-1 cells was inhibited after treatment with LIGHT, with a corresponding increase in Bcl-2, an anti-apoptotic protein and a decrease in caspase-3, a pro-apoptotic protein. In inflammatory diseases, LIGHT produced by activated T cells, platelets and monocytes/macrophages would prolong the lifespan of monocytes, which may contribute to chronic inflammation. Monocytes differentiate into macrophages or DC, depending on the surrounding microenvironment, and these cells modify the disease conditions according to their respective characteristics. Therefore, it is of interest to investigate the effect of LIGHT on the differentiation of monocytes.
As mentioned above, LIGHT induces proliferation and immunoglobulin production [22]. B cells class-switched by T helper type 2 (Th2) cytokines to produce IgE are stimulated by LIGHT to produce large amounts of IgE, which may account for the correlation of plasma concentration of LIGHT with the IgE level. In addition, LIGHT–HVEM stimulation induces co-stimulation of T cells, particularly CD8(+) T cells [4],[34],[35]. Recently, it has been shown that CD8(+) T cells are recruited early to allergen exposure sites in atopy patch-test reactions in patients with AD [13], suggesting an early and critical role for CD8(+) T cells in the development of AD lesions and a possible involvement of LIGHT in this process by co-stimulation of CD8(+) T cells.
Recently, LIGHT-transgenic mice have been shown to display elevated serum levels of triglyceride and cholesterol even on a normal diet, which is exaggerated further when the mice were placed on a high-fat, high-cholesterol diet [35]. Kusunoki et al. reported that childhood obesity has positive associations with prevalence and AD severity [36]. Although information about neither body weight nor serum lipid values are available in our patients, it is interesting to estimate the relationship between plasma LIGHT values and body mass index or serum lipid values.
In conclusion, we propose that plasma LIGHT levels may be a promising biomarker for AD. The precise role of the LIGHT–HVEM pathway in the pathogenesis of AD should be explored further.
Acknowledgments
This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture Sports, Science and Health and Labor Science Research Grants for Research on Allergic Disease and Immunology from the Ministry of Health, Labor and Welfare of Japan (N. K.).
Disclosure
The authors declare that there is no conflict of interest.
References
- 1.Marui DN, Ebner R, Montgomery RI, et al. LIGHT, a new member of the TNF superfamily, and lymphotoxin alpha are ligands for herpes virus entry mediator. Immunity. 1998;8:21–30. doi: 10.1016/s1074-7613(00)80455-0. [DOI] [PubMed] [Google Scholar]
- 2.Harrop JA, McDonnell PC, Brigham-Burke M, et al. Herpes virus entry mediator ligand (HVEM-L), a novel ligand for HVEM/TR2, stimulates proliferation of T cells and inhibits HT29 cell growth. J Biol Chem. 1998;273:27548–56. doi: 10.1074/jbc.273.42.27548. [DOI] [PubMed] [Google Scholar]
- 3.Morel Y, Schiano de Colella JM, Harrop J, et al. Reciprocal expression of the TNF family receptor herpes virus entry mediator and its ligand LIGHT on activated T cells: LIGHT down-regulates its own receptor. J Immunol. 2000;165:4397–404. doi: 10.4049/jimmunol.165.8.4397. [DOI] [PubMed] [Google Scholar]
- 4.Tamada K, Shimozaki K, Chapoval AI, et al. LIGHT, a TNF-like molecule, costimulates T cell proliferation and is required for dendritic cell-mediated allogeneic T cell response. J Immunol. 2000;164:4105–10. doi: 10.4049/jimmunol.164.8.4105. [DOI] [PubMed] [Google Scholar]
- 5.Granger SW, Rickert S. LIGHT–HVEM signaling and the regulation of T cell-mediated immunity. Cytokine Growth Factor Rev. 2003;14:289–96. doi: 10.1016/s1359-6101(03)00031-5. [DOI] [PubMed] [Google Scholar]
- 6.Otterdal K, Smith C, Oie E, et al. Platelet-derived LIGHT induces inflammatory responses in endothelial cells and monocytes. Blood. 2006;108:928–35. doi: 10.1182/blood-2005-09-010629. [DOI] [PubMed] [Google Scholar]
- 7.Kim WJ, Kang YJ, Koh EM, Ahn KS, Cha HS, Lee WH. LIGHT is involved in the pathogenesis of rheumatoid arthritis by inducing the expression of pro-inflammatory cytokines and MMP-9 in macrophages. Immunology. 2005;114:272–9. doi: 10.1111/j.1365-2567.2004.02004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haroon N, Tsui FW, O'Shea FD, et al. From gene expression to serum proteins: biomarker discovery in ankylosing spondylitis. Ann Rheum Dis. 2010;69:297–300. doi: 10.1136/ard.2008.102277. [DOI] [PubMed] [Google Scholar]
- 9.Wang YG, Kim KD, Wang J, Yu P, Fu YX. Stimulating lymphotoxin beta receptor on the dendritic cells is critical for their homeostasis and expansion. J Immunol. 2005;175:6997–7002. doi: 10.4049/jimmunol.175.10.6997. [DOI] [PubMed] [Google Scholar]
- 10.Bieber T. Atopic dermatitis. Ann Dermatol. 2010;22:125–34. doi: 10.5021/ad.2010.22.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakazawa M, Sugi N, Kawaguchi H, Ishii N, Nakajima H, Minami M. Predominance of type 2 cytokine-producing CD4+ and CD8+ cells in patients with atopic dermatitis. J Allergy Clin Immunol. 1997;99:673–82. doi: 10.1016/s0091-6749(97)70030-7. [DOI] [PubMed] [Google Scholar]
- 12.Koga C, Kabashima K, Shiraishi N, Kobayashi M, Tokura Y. Possible pathogenic role of Th17 cells for atopic dermatitis. J Invest Dermatol. 2008;128:2625–30. doi: 10.1038/jid.2008.111. [DOI] [PubMed] [Google Scholar]
- 13.Hennino A, Jean-Decoster C, Giordano-Labadie F, et al. CD8+ T cells are recruited early to allergen exposure sites in atopy patch test reactions in human atopic dermatitis. J Allergy Clin Immunol. 2011;127:1064–7. doi: 10.1016/j.jaci.2010.11.022. [DOI] [PubMed] [Google Scholar]
- 14.Kwon BS, Tan KB, Ni J, et al. A newly identified member of the tumor necrosis factor receptor superfamily with a wide tissue distribution and involvement in lymphocyte activation. J Biol Chem. 1997;272:14272–6. doi: 10.1074/jbc.272.22.14272. [DOI] [PubMed] [Google Scholar]
- 15.Harrop JA, Reddy M, Dede K, et al. Antibodies to TR2 (herpes virus entry mediator), a new member of the TNF receptor superfamily, block T cell proliferation, expression of activation markers, and production of cytokines. J Immunol. 1998;161:1786–94. [PubMed] [Google Scholar]
- 16.Jung HW, La SJ, Kim JY, et al. High levels of soluble herpes virus entry mediator in sera of patients with allergic and autoimmune diseases. Exp Mol Med. 2003;35:501–8. doi: 10.1038/emm.2003.65. [DOI] [PubMed] [Google Scholar]
- 17.Crowe PD, VanArsdale TL, Walter BN, et al. A lymphotoxin-beta-specific receptor. Science. 1994;264:707–10. doi: 10.1126/science.8171323. [DOI] [PubMed] [Google Scholar]
- 18.Murphy M, Walter BN, Pike-Nobile L, et al. Expression of the lymphotoxin beta receptor on follicular stromal cells in human lymphoid tissues. Cell Death Differ. 1998;5:497–505. doi: 10.1038/sj.cdd.4400374. [DOI] [PubMed] [Google Scholar]
- 19.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 20.Murphy KM, Nelson CA, Sedý JR. Balancing co-stimulation and inhibition with BTLA and HVEM. Nat Rev Immunol. 2006;6:671–81. doi: 10.1038/nri1917. [DOI] [PubMed] [Google Scholar]
- 21.Ware CF. Network communications: lymphotoxins, LIGHT, and TNF. Annu Rev Immunol. 2005;23:787–819. doi: 10.1146/annurev.immunol.23.021704.115719. [DOI] [PubMed] [Google Scholar]
- 22.Duhen T, Pasero C, Mallet F, Barbarat B, Olive D, Costello RT. LIGHT costimulates CD40 triggering and induces immunoglobulin secretion; a novel key partner in T cell-dependent B cell terminal differentiation. Eur J Immunol. 2004;34:3534–41. doi: 10.1002/eji.200425598. [DOI] [PubMed] [Google Scholar]
- 23.Xu G, Liu D, Okwor I, et al. LIGHT is critical for IL-12 production by dendritic cells, optimal CD4+ Th1 cell response, and resistance to Leishmania major. J Immunol. 2007;179:6901–9. doi: 10.4049/jimmunol.179.10.6901. [DOI] [PubMed] [Google Scholar]
- 24.Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol. 1980;92:44–7. [Google Scholar]
- 25.Nara K, Kusafuka T, Yoneda A, Oue T, Sangkhathat S, Fukuzawa M. Silencing of MYCN by RNA interference induces growth inhibition, apoptotic activity and cell diffrentiation in a neuro blastoma cell line with MYCN amplication. Int J Oncol. 2007;30:1189–96. [PubMed] [Google Scholar]
- 26.Lagasse E, Weissman IL. Enforced expression of Bcl-2 in monocytes rescues macrophages and partially reverses osteopetrosis in op/op mice. Cell. 1997;89:1021–31. doi: 10.1016/s0092-8674(00)80290-1. [DOI] [PubMed] [Google Scholar]
- 27.Yang E, Korsmeyer SJ. Molecular thanatopsis: a discourse on the BCL2 family and cell death. Blood. 1996;88:386–401. [PubMed] [Google Scholar]
- 28.Green DR. Apoptotic pathways:the roads to ruin. Cell. 1998;94:695–8. doi: 10.1016/s0092-8674(00)81728-6. [DOI] [PubMed] [Google Scholar]
- 29.Kakinuma T, Nakamura K, Wakugawa M, et al. Thymus and activation-regulated chemokine in atopic dermatitis: serum thymus and activation-regulated chemokine level is closely related with disease activity. J Allergy Clin Immunol. 2001;107:535–41. doi: 10.1067/mai.2001.113237. [DOI] [PubMed] [Google Scholar]
- 30.Katoh N. Platelets as versatile regulators of cutaneous inflammation. J Dermatol Sci. 2009;53:89–95. doi: 10.1016/j.jdermsci.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 31.Bratton DL, Hamid Q, Boguniewicz M, Doherty DE, Kailey JM, Leung DY. Granulocyte macrophage colony-stimulating factor contributes to enhanced monocytes survival in chronic atopic dermatitis. J Clin Invest. 1995;95:211–18. doi: 10.1172/JCI117642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cai G, Wang H, Qin Q, et al. Amelioration of myocarditis by HVEM-overexpressing dendritic cells through induction of IL-10-producing cells. Cardiovasc Res. 2009;84:425–33. doi: 10.1093/cvr/cvp219. [DOI] [PubMed] [Google Scholar]
- 33.Katoh N, Kraft S, Wessendorf JH, Bieber T. The high-affinity IgE receptor (FcεRI) blocks apoptosis in normal human monocytes. J Clin Invest. 2000;105:183–90. doi: 10.1172/JCI6895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schneider K, Potter KG, Ware CF. Lymphotoxin and LIGHT signaling pathways and target genes. Immunol Rev. 2004;202:49–66. doi: 10.1111/j.0105-2896.2004.00206.x. [DOI] [PubMed] [Google Scholar]
- 35.Lo JC, Wang Y, Tumanov AV, et al. Lymphotoxin receptor-dependent control of lipid homeostasis. Science. 2007;316:285–8. doi: 10.1126/science.1137221. [DOI] [PubMed] [Google Scholar]
- 36.Kusunoki T, Morimoto T, Nishikomori R, et al. Obesity and the prevalence of allergic diseases in schoolchildren. Pediatr Allergy Immunol. 2008;19:527–34. doi: 10.1111/j.1399-3038.2007.00686.x. [DOI] [PubMed] [Google Scholar]


