Abstract
OTHER THEMES PUBLISHED IN THIS IMMUNOLOGY IN THE CLINIC REVIEW SERIES
Metabolic diseases, host responses, cancer, autoinflammatory diseases, allergy.
Thymus dysfunction, especially immune suppression, is frequently associated with various virus infections. Whether viruses may disturb the thymus function and play a role in the pathogenesis of autoimmune diseases is an open issue. Enteroviruses, especially Coxsackievirus B4 (CV-B4), have been largely suggested as potential inducers or aggravating factors of type 1 diabetes (T1D) pathogenesis in genetically predisposed individuals. Several pathogenic mechanisms of enterovirus-induced T1D have been suggested. One of these mechanisms is the impairment of central self-tolerance due to viral infections. Coxsackievirus-B4 is able to infect murine thymus in vitro and in vivo and to infect human thymus in vitro. Thymic epithelial cells and thymocytes are targets of infection with this virus, and several abnormalities, especially disturbance of maturation/differentiation processes, were observed. Altogether, these data suggest that CV-B infection of thymus may be involved in the pathogenesis of T1D. Further investigations are needed to explore this hypothesis.
Keywords: Coxsackievirus B4, human, mouse, thymus, type 1 diabetes
Introduction
Infection of the thymus with viruses is an issue that has been addressed but has been poorly investigated, except in the case of human immunodeficiency virus (HIV) infection [1]. As well as HIV, other viruses can infect the thymus which may have consequences on the architecture and functions of that organ. Marked abnormalities of the thymus and its functions have been reported in the course of viral infections, although the presence of viruses in the thymus has not been evidenced [2].
The thymus is a major part of the immune system, therefore infection of that organ with a virus can facilitate immune tolerance towards viral antigens, and thus may greatly influence the outcome of the infection, with persistence of the virus in the host [3],[4]. Thymus being the central site for self-tolerance establishment, it cannot be discounted that a viral infection may lead to thymus dysfunction resulting in disturbed self-tolerance, possibly involved in autoimmune pathogenic processes.
Type 1 diabetes (T1D), also termed ‘juvenile diabetes’ or ‘insulin-dependent diabetes’, which is due to selective destruction and/or impairment of pancreatic insulin-producing β cells, is accompanied frequently by severe complications, and is becoming a huge public health problem as its incidence is in continual and dramatic increase all over the world [5],[6].
The relationship between enteroviruses, especially type B Coxsackieviruses (CV-B) and T1D, in genetically predisposed individuals has been highlighted largely through epidemiological studies [7]–[11]. Several mechanisms not mutually exclusive have been suggested to elucidate the viral pathogenesis of T1D [11],[12]. One of the possible mechanisms is the disturbance of central tolerance as a result of the infection of thymus with viruses.
Viral infections can disturb the thymus
Clinical evidence and experimental findings show that viral infections are responsible for thymus abnormalities and dysfunctions, although in some cases the organ has not been reached by the infectious agent.
HIV
HIV, belonging to the Retroviridae family, is the virus that has been associated most frequently with thymus disorders in the literature.
HIV has been detected in thymuses of infected individuals [13]–[16]. In addition, human intrathymic T cell precursors and their progeny, representing many stages of T cell ontogeny, have been demonstrated to be susceptible to HIV-1 infection in vitro[17]. The chimeric severe combined immunodeficiency–human (SCID-hu) xenograft mouse model (bearing human T cells derived from transplantation of human thymic fragments and liver tissue under the renal capsule) showed that human thymocytes are also susceptible to HIV infection in vivo[18],[19]. Moreover, it is likely that HIV infects the thymic microenvironment, as marked disruptions and significant viral loads have been observed in the thymic compartment in HIV-infected SCID-hu mice [19], a finding that was confirmed later by the demonstration of infected thymic dendritic cells [20]. Whether or not the thymic epithelium is also infected by this virus is an issue that deserves further investigation. Indeed, thymic epithelial cells (TEC) were shown previously to contain HIV RNA in human autopsy samples and also in the SCID-hu mouse model, although productive infection of these cells could not be demonstrated definitively [14],[19]. Furthermore, the same SCID-hu mouse model showed degenerating TEC even without detection of HIV in these cells [19]. The relevance of TEC infection lies in the fact that these cells play a critical role in the differentiation of T cell precursors, providing a microenvironment with a unique capacity to generate functional and self-tolerant T cells [21],[22].
HIV infection is generally accompanied by several cytological and histological abnormalities in the thymus network. Indeed, autopsy and biopsy studies have demonstrated that the thymus in HIV-infected individuals is abnormal, with marked involution, effacement of the medulla and cortex, depletion of epithelial elements, thymocyte apoptosis, variable degrees of plasma cell infiltration and fibrosis and absence of Hassall's corpuscles [2],[14],[23],[24]. Studies using the SCID-hu mouse showed similar abnormalities [19].
Damage to the thymic epithelium may alter the thymic microenvironment and contribute to the immune suppression observed in acquired immune deficiency syndrome (AIDS) patients and models. Importantly, it has been observed that thymic epithelial fragments from AIDS children arrest T cell differentiation of normal bone marrow-derived CD34+ stem cells in vitro[25]. Similarly, HIV-1 infection has been shown to interrupt thymopoiesis in vivo in the SCID-hu mouse model [26].
The thymus releases mature lymphocytes into the periphery of the immune system. This function can be evaluated through analysis of recent thymic emigrants (RTEs) [27], that themselves can be estimated by the presence of T cell receptor excision circles (Trecs), circular DNA fragments derived from the rearrangement of TCR genes, that remain within RTEs [28]. Trec analysis in HIV and simian immunodefiency virus (SIV) infections revealed decreased numbers of Trec+ T lymphocytes in the peripheral blood compared with uninfected individuals [29],[30]. Interestingly, specific highly active anti-retroviral therapy seems to correct this defect in AIDS patients [31].
Another important feature is that the thymic secretory function is also affected in HIV-infected individuals, as the blood levels of thymic peptides are abnormal [23]. For example, thymosin α1 levels are elevated in many patients with AIDS, especially in the early stages [23],[32]. In contrast, a consistent and long-term diminution of thymulin secretion has been documented in AIDS patients, in terms of both serum levels and intrathymic contents of the hormone [24],[33],[34].
Hepatitis viruses
It is known that mouse hepatitis viruses (MHV), which are members of the Coronaviridae family, show a tropism to thymic stromal cells [35] and T lymphocytes [36]. Otherwise, thymus involution was described in MHV-A59-infected BALB/c mice [37]. That involution was characterized by a severe transient atrophy resulting from apoptosis of immature CD4+CD8+ T cells that might be caused by infection of a small proportion of TEC.
Marked thymic involution characterized by striking diminution of thymus weight and cellularity was also observed in CBA mice infected intraperitoneally with MHV-3, together with a significant decrease in thymocyte subpopulations and significant numbers of apoptotic cells [38].
In humans, Trec quantification revealed an impairment of RTEs, reflecting a thymic dysfunction in hepatitis C virus (HCV)-infected patients [39].
Measles
Measles, a member of the Paramyxoviridae family, is generally followed by immune suppression with transient lymphopenia and impaired cell-mediated immunity [40],[41]. Impaired thymic function seems to contribute to measles virus-induced immune suppression. Indeed, measles virus infects TEC and monocytes in the thymus of humans and monkeys [42],[43], leading to a decrease in the size of the thymic cortex [44],[45]. In human thymic implants in SCID-hu mice, measles virus infection of TEC causes thymocyte apoptosis [46]. Measles virus replication in human TEC in vitro results in terminal differentiation and apoptosis [47]. Surprisingly, with regard to thymic output, an increase in TREC+ CD4+ T cells has been reported in measles virus-infected children despite severe lymphopenia [48].
Cytomegalovirus (CMV)
Infections with CMV (belonging to the Herpesviridae family) are also immunosuppressive, resulting in poor cellular responses from cultured blood leucocytes, low CD4/CD8 ratios and potential secondary infections [49].
At the thymic level, CMV infection in the SCID-Hu mouse results in high and persistent viral replication in the thymus. The majority of virus-infected cells were localized in the thymic medulla and immunofluorescence analysis identified TEC rather than any haematopoietic cell population as the principal hosts for viral replication [50].
Infection of BALB/c mice with murine (M)CMV decreased the numbers of cells recovered from the thymus by 80–90% after 4–7 days, although fewer than 0·001% were infected productively with the virus. A loss of cortical thymocytes was evident in histological sections and correlated with depletion of CD4+CD8+ cells [51].
Rabies virus
Suppression of cell-mediated immunity is also a common feature of rabies virus infection [52],[53]. This phenomenon relies essentially upon thymocyte apoptosis and thymus atrophy (despite no evidence of virus infection), as observed in numerous studies carried out in mice [52],[54]–[56].
Altogether, these data show that viruses belonging to various families can infect the thymus in vivo and in vitro. Clearly, viruses can impair thymus functions significantly.
Thymus and T1D
Like any autoimmune disease, T1D results from self-tolerance breakdown. Self-tolerance establishment is initiated at the central level within the thymus. Thus, it cannot be excluded that disturbance in thymic architecture and/or function may play a role in the development of autoimmune processes. At the peripheral level, self-tolerance is based on regulatory T cells (Treg), a specialized subset of T cells whose functions include the suppression of autoreactive T cells.
In the case of T1D, pancreatic islet β cells are targeted selectively by the autoimmune destruction process, meaning that there is a defect in the recognition of islet β cell antigens. Anomalies in Treg cells functions and numbers have been associated with autoimmunity towards islet β cells and are thought to play a role in the progression of T1D [57]. At the thymic level, this defect can arise from several aberrations encountered during T cell education through positive and negative selection.
Alteration of the repertoire affecting positive selection
During positive selection, the newly rearranged TCRs expressed on developing thymocytes interact with MHC molecules on cortical TEC; thus, any anomaly in MHC and/or TEC may lead to aberrant positive selection. Diabetes-associated MHC class II molecules [I-Ag7 in non-obese diabetic (NOD) mice and human leucocyte antigen (HLA)-DQ8 in humans] mediate the selection of a large number of autoreactive T lymphocytes with strong affinity for islet β cell autoantigens [58]. Interestingly, the grafting of purified TEC from embryos of NOD mice to newborn C57BL/6 nude mice results in the development of insulitis, suggesting a functional anomaly in TEC from NOD mice cells [59].
Defect of negative selection
During negative selection, developing T cells interact with thymic epithelium- and bone marrow-derived antigen-presenting cells (APCs), in particular thymic medullary dendritic cells. Thus, aberrant negative selection results essentially from anomalies affecting thymic APCs.
Like the majority of ubiquitous or organ-specific autoantigens, several islet β cell antigens involved in T1D, such as glutamic acid decarboxylase (GAD) and proteins of the insulin family, are expressed promiscuously in the thymus to be presented to thymocytes during education [60],[61]. The decreased expression of these antigens can disturb the negative selection of autoreactive T lymphocytes, which may predispose to the development of autoimmunity. In humans, susceptibility to T1D is associated with a polymorphism in the 5′ region of the insulin gene, which influences the rate of expression of peptides derived from insulin by APCs in the thymus. The protective allele is associated with a high level of thymic expression of insulin and the susceptibility allele to a low level [61]. NOD mice which express neither the pro-insulin 2 nor the islet-cell antigen 69 (ICA69) in the thymus develop diabetes rapidly [62],[63], as in BioBreeding Diabetes Prone (BBDP) rats, which do not express type 2 insulin-like growth factor (Igf2) in thymus [64]. Furthermore, depletion of Ins2 expression in medullary TEC is sufficient to break central tolerance and induce anti-insulin autoimmunity and rapid diabetes onset in mouse [65].
Interestingly, intrathymic transplantation of pancreatic islet cells reduces autoimmunity towards β cells and prevents diabetes development in NOD/Lt mice [66]. Thus, the thymus could also play a role in acquired tolerance and may be a potential candidate in the therapeutics of autoimmune diseases.
Negative selection might also be affected owing to antigen-processing defects. A defect of peptide presentation can result from the weak affinity of TCR for unstable MHC–peptide complexes and/or from a defect in antigen processing by proteases of thymic APCs [58],[67].
Major defects in the architecture of the thymic stroma found in animal models of diabetes are also thought to contribute to a defect in negative selection [58],[67]. In NOD mice, for example, medullar TEC are present in the cortex, and large areas devoid of TEC and expression of MHC molecules are observed in the thymus [68]. Multiple thymocyte migration-related abnormalities have also been observed in the NOD mouse thymus [69].
Because negative selection is based on the apoptosis of autoreactive T lymphocytes, it is possible that a defect of apoptotic factors and/or a genetically determined resistance of thymocytes to apoptosis (as described in the NOD mouse [70]) can contribute to impaired negative selection [58],[67].
Thymus and Coxsackievirus B4
Epidemiological studies have clearly shown an association between enterovirus infections, especially CV-B and T1D, and strongly support the role of these viruses as potential triggers of that disease in genetically predisposed individuals [7]–[10]. Experimental investigations suggest that several pathogenic mechanisms of CV-B4 infection may be involved in the impairment of pancreatic β cells [7]–[10]. Our group has investigated the hypothesis of virus-induced disturbance of thymus in the development of autoimmunity against these cells (see Fig. 1).
Fig. 1.
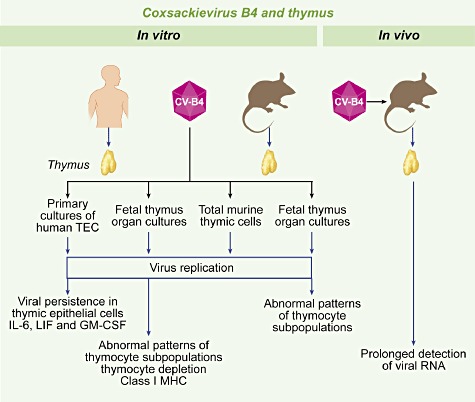
Coxsackievirus B4 (CV-B4) and thymus. Overview of experimental studies performed in vitro in human and mouse systems and in vivo in mice. Thymus fragments, obtained from children undergoing corrective cardiovascular surgery for congenital cardiopathies, were processed for isolating thymic epithelial cells (TEC). Human and mouse fetal thymus organ cultures were performed and total thymocytes, isolated from mouse thymus, were cultured. CV-B4 can infect thymocytes and TEC, which results in abnormal patterns of thymocyte populations, overproduction of cytokines and increased expression of class I major histocompatibility complex (MHC). The virus can replicate and persist in TEC. Oral inoculation of CV-B4 to 3–4-week-old Swiss albino mice results in persistence of viral RNA (up to 70 days) in thymus.
It was observed that both CV-B4 diabetogenic (E2) and prototype (JVB) strains can replicate and persist in human TEC in vitro with increased production of interleukin (IL)-6, leucocyte migration inhibition factor (LIF) and granulocyte–macrophage colony-stimulating factor (GM-CSF) [71]. In fragments of human fetal thymus, the virus principally infects CD4+CD8+ immature thymocytes and induces increased expression of MHC class I molecules and a severe thymocyte depletion [72].
Because CV-B4 was also able to infect TEC and immature thymocytes, it was hypothesized that the virus was potentially susceptible to modulate the thymic function. To explore this hypothesis more effectively, and due to the difficulty of undertaking experiments in the human system, further studies were performed in a murine model.
It was demonstrated that the diabetogenic strain CV-B4 E2 can reach the thymus in vivo in the course of a systemic infection of outbred Swiss albino mice inoculated through the oral route, the natural contamination route in humans [73]. The infection was characterized by a prolonged detection [until 70 days post-infection (p.i.)] of viral RNA by reverse transcription–polymerase chain reaction (RT–PCR) in the thymus.
When primary cultures of total murine thymic cells were inoculated with CV-B4 E2 and CV-B4 JVB, both viral strains infected and replicated in these cells, as attested by the detection of intracellular negative-strand viral RNA and release of infectious particles in culture supernatants [74]. These findings suggest that thymic cells can play a role in virus dissemination, and therefore in the pathophysiology of CV-B4 infections.
The infection of murine fetal thymus organ cultures was then investigated [75]. It was shown that CV-B4 E2 could replicate within this system, as attested by the detection of intracellular negative-stranded viral RNA by real-time quantitative RT–PCR and infectious particles in culture supernatants. As evidenced by flow cytometry analysis, CV-B4 E2 lead to abnormal patterns of thymocyte populations: a marked increase in the percentages of CD4-CD8-, CD4+ and CD8+ cells and a decrease in the percentage of CD4+CD8+ cells.
The increased proportion of CD4-CD8- thymic T cells observed in vitro is reminiscent of previous data obtained in vivo in a murine model of CV-B4 E2-induced T1D [76]. Indeed, in that study the virus, inoculated through the intraperitoneal route, was cleared rapidly from the thymus but led to a significant increase in CD4-CD8- thymic T cells preceeding the onset of hyperglycaemia.
Conclusion and perspectives: thymus, enterovirus and the pathogenesis of T1D
CV-B4 infection of the thymus has been described in human tissue in vitro, and in mice in vivo and in vitro, and the infection results in the disturbance of T cell differentiation/maturation processes [71]–[76].
The role of alterations in T lymphocyte subsets in the development of T1D cannot be excluded in so far as they have been observed already in NOD mice [77], in BB rats [78] and also in diabetic patients [79],[80]. Whether enterovirus-induced disturbances of thymic cells can play a role in T1D pathogenesis by impairing T cell differentiation and/or central self-tolerance establishment should be investigated further in experimental models in vitro and/or in vivo.
For a clearer understanding of the complex interplay between enterovirus and the thymus in the viral pathogenesis of T1D, the link remains to be made between thymus infection and the development of the disease in human beings. Interestingly, in a recent study macrophages infected with an enterovirus (poliovirus) were evidenced in thymus of some patients with myasthenia gravis, suggesting a viral contribution to the intrathymic alterations leading to the disease [81]. Furthermore, CV-A and CV-B have already been found in human perinatal and neonatal thymus in favour of vertical transmission of the viral infection [82],[83]. Whether enteroviruses are present in the thymus of patients with T1D or patients in the preclinical stages of the disease merits further study.
In T1D, the tolerance of immune system towards β cells is disturbed at the peripheral level through Treg dysfunction [57]. A disturbance of tolerance at the central level through the infection of thymus with enteroviruses cannot be discarded, and could play a role in the pathogenesis of T1D (see Fig. 2).
Fig. 2.
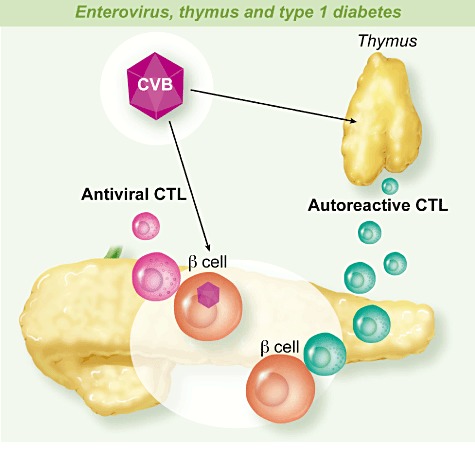
Enterovirus, thymus and type 1 diabetes. Enteroviruses especially coxsackievirus B (CVB) infect β cells in pancreas islets. Persistent and/or successive infections of β cells results in activation of the innate immune system, influenced by the genetic background, followed by activation of the adaptive immune response which produces anti-viral cytolytic T lymphocytes (CTL) able to damage infected β cells that release self-antigens. In this context, β cell antigens activate anti-β cell autoreactive CTL through bystander activation and molecular mimicry. Enterovirus infections of the thymus can disturb the function of that organ involved in the tolerance to self-antigens, which may result in the production of autoreactive CTL that will be activated in the context of the response to the infection of β cells with enteroviruses.
The potential role of thymus dysfunction in the pathogenesis of T1D opens the possibility of targeting this organ for preventive and therapeutic strategies. Indeed, there are increasing promising insights towards intrathymic manipulation. On the basis of the close homology and cross-tolerance between insulin, the primary T1D autoantigen and Igf2, the dominant thymic self-antigen of the insulin family, a novel type of vaccination, so-called ‘negative/tolerogenic selfvaccination’, is currently being developed for the prevention and cure of T1D [84]. Conversely, intrathymic manipulation also offers a potential way of enhancing the ability of T cells to control infection by increasing the numbers of positively selected thymocytes able to recognize a given molecule of the corresponding infectious agent. This concept of ‘thymic vaccination’ is based on the fact that slightly altered viral peptides bearing lower affinity to the corresponding TCR, rather than to the natural cognate ligand, may induce positive selection of this molecule when injected intrathymically, leading to antigen-specific T cell export from the thymus [2],[85],[86].
Acknowledgments
The studies conducted by the authors were supported by a grant from OSEO, EU FP6 Integrated Project EURO-THYMAIDE (contract LSHB-CT-2003-503410), a grant from Nord-Pas-de-Calais Région (ArCir convention 2004/018), CHRU Lille, the ministère de l'Education nationale de la recherche et de la technologie, Université de Lille 2, France, the Ministère de la Recherche Scientifique, de la Technologie et du Développement des Compétences (LR99ES27), Tunisia and the comité mixte de coopération universitaire franco-tunisien (CMCU 04/G0810), with grants from Egide, Paris. Didier Hober was Fondation pour la Recherche Médicale 2008 prize winner and is a member of the Virus in Diabetes International Study group (VIDIS group).
Disclosure
None.
References
- 1.Ye P, Kirschner DE, Kourtis AP. The thymus during HIV disease: role in pathogenesis and in immune recovery. Curr HIV Res. 2004;2:177–83. doi: 10.2174/1570162043484898. [DOI] [PubMed] [Google Scholar]
- 2.Savino W. The thymus is a common target organ in infectious diseases. PLoS Pathog. 2006;2:e62. doi: 10.1371/journal.ppat.0020062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.King CC, Jamieson BD, Reddy K, et al. Viral infection of the thymus. J Virol. 1992;66:3155–60. doi: 10.1128/jvi.66.5.3155-3160.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Korostoff JM, Nakada MT, Faas SJ, et al. Neonatal exposure to thymotropic gross murine leukemia virus induces virus specific immunologic nonresponsiveness. J Exp Med. 1990;172:1765–75. doi: 10.1084/jem.172.6.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sabin MA, Cameron FJ, Werther GA. Type 1 diabetes – still the commonest form of diabetes in children. Aust Fam Physician. 2009;38:695–7. [PubMed] [Google Scholar]
- 6.DIAMOND Project Group. Incidence and trends of childhood type 1 diabetes worldwide 1990–1999. Diabet Med. 2006;23:857–66. doi: 10.1111/j.1464-5491.2006.01925.x. [DOI] [PubMed] [Google Scholar]
- 7.Jaïdane H, Hober D. Role of coxsackievirus B4 in the pathogenesis of type 1 diabetes. Diabetes Metab. 2008;34:537–48. doi: 10.1016/j.diabet.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 8.Jaïdane H, Sauter P, Sane F, et al. Enteroviruses and type 1 diabetes: towards a better understanding of the relationship. Rev Med Virol. 2010;20:265–80. doi: 10.1002/rmv.647. [DOI] [PubMed] [Google Scholar]
- 9.Yeung WC, Rawlinson WD, Craig ME. Enterovirus infection and type 1 diabetes mellitus: systematic review and meta-analysis of observational molecular studies. BMJ. 2011;342:d35. doi: 10.1136/bmj.d35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hober D, Sane F. Enteroviruses and type 1 diabetes. BMJ. 2011;342:c7072. doi: 10.1136/bmj.c7072. [DOI] [PubMed] [Google Scholar]
- 11.Coppieters KT, Wiberg A, Tracy SM, von Herrath MG. Immunology in the clinic review series; focus on type 1 diabetes and viruses: the role of viruses in type 1 diabetes: a difficult dilemma. Clin Exp Immunol. 2012;168:5–11. doi: 10.1111/j.1365-2249.2011.04554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lind M, Hühn MH, Flodström-Tullberg M. Immunology in the clinic review series; focus on type 1 diabetes and viruses: the innate immune response to enteroviruses and its possible role in regulating type 1 diabetes. Clin Exp Immunol. 2012;168:30–8. doi: 10.1111/j.1365-2249.2011.04557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joshi VV, Oleske JM, Saad S, et al. Thymus biopsy in children with acquired immunodeficiency syndrome. Arch Pathol Lab Med. 1986;110:837–42. [PubMed] [Google Scholar]
- 14.Schuurman HJ, Krone WJA, Broekhuizen R, et al. The thymus in acquired immune deficiency syndrome: comparison with other types of immunodeficiency diseases, and presence of components of human immunodeficiency virus type 1. Am J Pathol. 1989;134:1329–38. [PMC free article] [PubMed] [Google Scholar]
- 15.Papiernik M, Brossard Y, Mulliez N, et al. Thymic abnormalities in fetuses aborted from human immunodeficiency virus type 1 seropositive women. Pediatrics. 1992;89:297–301. [PubMed] [Google Scholar]
- 16.Scinicariello F, Kourtis AP, Nesheim S, et al. Limited evolution of human immunodeficiency virus type 1 in the thymus of a perinatally infected child. Clin Infect Dis. 2010;50:726–32. doi: 10.1086/650453. [DOI] [PubMed] [Google Scholar]
- 17.Schnittman SM, Denning SM, Greenhouse JJ, et al. Evidence for susceptibility of intrathymic T-cell precursors and their progeny carrying T-cell antigen receptor phenotypes TCR alpha beta + and TCR gamma delta + to human immunodeficiency virus infection: a mechanism for CD4+ (T4) lymphocyte depletion. Proc Natl Acad Sci USA. 1990;87:7727–31. doi: 10.1073/pnas.87.19.7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Namikawa R, Kaneshima H, Lieberman M, et al. Infection of the SCID-hu mouse by HIV-1. Science. 1988;242:1684–6. doi: 10.1126/science.3201256. [DOI] [PubMed] [Google Scholar]
- 19.Stanley SK, McCune JM, Kaneshima H, et al. Human immunodeficiency virus infection of the human thymus and disruption of the thymic microenvironment in the SCID-hu mouse. J Exp Med. 1993;178:1151–63. doi: 10.1084/jem.178.4.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmitt N, Nugeyre MT, Scott-Algara D, et al. Differential susceptibility of human thymic dendritic cell subsets to X4 and R5 HIV-1 infection. AIDS. 2006;20:533–42. doi: 10.1097/01.aids.0000210607.63138.bc. [DOI] [PubMed] [Google Scholar]
- 21.Bonomo A, Matzinger P. Thymus epithelium induces tissue-specific tolerance. J Exp Med. 1993;177:1153–64. doi: 10.1084/jem.177.4.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boyd RL, Tucek CL, Godfrey DI, et al. The thymic microenvironment. Immunol Today. 1993;14:445–59. doi: 10.1016/0167-5699(93)90248-J. [DOI] [PubMed] [Google Scholar]
- 23.Davis AE., Jr The histopathological changes in the thymus gland in the acquired immune deficiency syndrome. Ann NY Acad Sci. 1984;437:493–502. doi: 10.1111/j.1749-6632.1984.tb37173.x. [DOI] [PubMed] [Google Scholar]
- 24.Savino W, Dardenne M, Marche C, et al. Thymic epithelium in AIDS: an immunohistologic study. Am J Pathol. 1986;122:302–7. [PMC free article] [PubMed] [Google Scholar]
- 25.Ruiz ME, Freeman J, Bouhasin JD, et al. Arrest of in vitro T cell differentiation of normal bone marrow-derived CD34+ stem cells with thymic epithelial fragments from children with AIDS. Stem Cells. 1996;14:533–47. doi: 10.1002/stem.140533. [DOI] [PubMed] [Google Scholar]
- 26.Jenkins M, Hanley MB, Moreno MB, et al. Human immunodeficiency virus-1 infection interrupts thymopoiesis and multilineage hematopoiesis in vivo. Blood. 1998;91:2672–8. [PubMed] [Google Scholar]
- 27.Savino W, Mendes-da-Cruz DA, Silva JS, et al. Intrathymic T cell migration: a combinatorial interplay of extracellular matrix and chemokines? Trends Immunol. 2002;23:305–13. doi: 10.1016/s1471-4906(02)02224-x. [DOI] [PubMed] [Google Scholar]
- 28.Geenen V, Poulin JF, Dion ML, et al. Quantification of T cell receptor rearrangement excision circles to estimate thymic function: an important new tool for endocrine-immune physiology. J Endocrinol. 2003;176:305–11. doi: 10.1677/joe.0.1760305. [DOI] [PubMed] [Google Scholar]
- 29.Douek DC, Betts MR, Hill BJ, et al. Evidence for increased T cell turnover and decreased thymic output in HIV infection. J Immunol. 2001;167:6663–8. doi: 10.4049/jimmunol.167.11.6663. [DOI] [PubMed] [Google Scholar]
- 30.Sodora DL, Milush JM, Ware F, et al. Decreased levels of recent thymic emigrants in peripheral blood of simian immunodeficiency virus-infected macaques correlates with alterations within the thymus. J Virol. 2002;76:9981–90. doi: 10.1128/JVI.76.19.9981-9990.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diaz M, Douek DC, Valdez H, et al. T cells containing T cell receptor excision circles are inversely related to HIV replication and are selectively and rapidly released into circulation with antiretroviral treatment. AIDS. 2003;17:1145–9. doi: 10.1097/00002030-200305230-00005. [DOI] [PubMed] [Google Scholar]
- 32.Hersh EM, Rios A, Mansell WA, et al. Elevated serum thymosin α1 levels associated with evidence of immune dysregulation in male homosexuals with a history of infectious disease or Kaposi's sarcoma. N Engl J Med. 1983;308:45–6. doi: 10.1056/nejm198301063080113. [DOI] [PubMed] [Google Scholar]
- 33.Dardenne M, Bach V, Safai B. Low serum thymic hormone levels in patients with acquired immunodeficiency syndrome. N Engl J Med. 1983;309:48–9. doi: 10.1056/NEJM198307073090112. [DOI] [PubMed] [Google Scholar]
- 34.Incefy GS, Pahwa S, Pahwa R, et al. Low circulating thymulin-like activity in children with AIDS and AIDS-related complex. AIDS Res. 1986;2:109–16. doi: 10.1089/aid.1.1986.2.109. [DOI] [PubMed] [Google Scholar]
- 35.Lamontagne L, Jolicoeur P. Low-virulent mouse hepatitis viruses exhibiting various tropisms in macrophages, T and B cell subpopulations and thymic stromal cells. Lab Anim Sci. 1994;44:17–24. [PubMed] [Google Scholar]
- 36.Jolicoeur P, Lamontagne L. Impaired T and B cell subpopulations involved in a chronic disease induced by mouse hepatitis virus type 3. J Immunol. 1994;153:1318–27. [PubMed] [Google Scholar]
- 37.Godfraind C, Holmes KV, Coutelier JP. Thymus involution induced by mouse hepatitis virus A59 in BALB/c mice. J Virol. 1995;69:6541–7. doi: 10.1128/jvi.69.10.6541-6547.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verinaud L, Da Cruz-Höfling MA, Sakurada JK, et al. Immunodepression induced by Trypanosoma cruzi and mouse hepatitis virus type 3 is associated with thymus apoptosis. Clin Diagn Lab Immunol. 1998;5:186–91. doi: 10.1128/cdli.5.2.186-191.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cianci R, Pinti M, Nasi M, et al. Impairment of recent thymic emigrants in HCV infection. Int J Immunopathol Pharmacol. 2005;18:723–8. doi: 10.1177/039463200501800415. [DOI] [PubMed] [Google Scholar]
- 40.Karp CL, Wysocka M, Wahl LM, et al. Mechanism of suppression of cell-mediated immunity by measles virus. Science. 1996;273:228–31. doi: 10.1126/science.273.5272.228. [DOI] [PubMed] [Google Scholar]
- 41.Griffin DE. Measles virus-induced suppression of immune responses. Immunol Rev. 2010;236:176–89. doi: 10.1111/j.1600-065X.2010.00925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moench TR, Griffin DE, Obriecht CR, et al. Acute measles in patients with and without neurological involvement: distribution of measles virus antigen and RNA. J Infect Dis. 1988;158:433–42. doi: 10.1093/infdis/158.2.433. [DOI] [PubMed] [Google Scholar]
- 43.Yamanouchi K, Chino F, Kobune F, et al. Growth of measles virus in the lymphoid tissues of monkeys. J Infect Dis. 1973;128:795–9. doi: 10.1093/infdis/128.6.795. [DOI] [PubMed] [Google Scholar]
- 44.White RG, Boyd JF. The effect of measles on the thymus and other lymphoid tissues. Clin Exp Immunol. 1973;13:343–57. [PMC free article] [PubMed] [Google Scholar]
- 45.Chino F, Kodama H, Ohkawa T, et al. Alterations of the thymus and peripheral lymphoid tissues in fatal measles. A review of 14 autopsy cases. Acta Pathol. 1979;29:493–507. doi: 10.1111/j.1440-1827.1979.tb00205.x. [DOI] [PubMed] [Google Scholar]
- 46.Auwaerter PG, Kaneshima H, McCune JM, et al. Measles virus infection of thymic epithelium in the SCID-hu mouse leads to thymocyte apoptosis. J Virol. 1996;70:3734–40. doi: 10.1128/jvi.70.6.3734-3740.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Valentin H, Azocar O, Horvat B, et al. Measles virus infection induces terminal differentiation of human thymic epithelial cells. J Virol. 1999;73:2212–22. doi: 10.1128/jvi.73.3.2212-2221.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Permar SR, Moss WJ, Ryon JJ, et al. Increased thymic output during acute measles virus infection. J Virol. 2003;77:7872–9. doi: 10.1128/JVI.77.14.7872-7879.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carney WP, Rubin RH, Hoffman RA, et al. Analysis of T lymphocyte subsets in cytomegalovirus mononucleosis. J Immunol. 1981;126:2114–6. [PubMed] [Google Scholar]
- 50.Mocarski ES, Bonyhadi M, Salimi S, et al. Human cytomegalovirus in a SCID-hu mouse: thymic epithelial cells are prominent targets of viral replication. Proc Natl Acad Sci USA. 1993;90:104–8. doi: 10.1073/pnas.90.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Price P, Olver SD, Gibbons AE, et al. Characterization of thymic involution induced by murine cytomegalovirus infection. Immunol Cell Biol. 1993;71:155–65. doi: 10.1038/icb.1993.18. [DOI] [PubMed] [Google Scholar]
- 52.Hirai K, Kawano H, Mifune K, et al. Suppression of cell-mediated immunity by street rabies virus infection. Microbiol Immunol. 1992;36:1277–90. doi: 10.1111/j.1348-0421.1992.tb02130.x. [DOI] [PubMed] [Google Scholar]
- 53.Lafon M. Evasive strategies in rabies virus infection. Adv Virus Res. 2011;79:33–53. doi: 10.1016/B978-0-12-387040-7.00003-2. [DOI] [PubMed] [Google Scholar]
- 54.Marcovistz R, Bertho AL, Matos DC. Relationship between apoptosis and thymocyte depletion in rabies-infected mice. Braz J Med Biol Res. 1994;27:1599–603. [PubMed] [Google Scholar]
- 55.Cardenas-Palomo LF, de Souza-Matos DC, Chaves-Leal E, et al. Lymphocyte subsets and cell proliferation analysis in rabies-infected mice. J Clin Lab Immunol. 1995;46:49–61. [PubMed] [Google Scholar]
- 56.Kasempimolporn S, Tirawatnapong T, Saengseesom W, et al. Immunosuppression in rabies virus infection mediated by lymphocyte apoptosis. Jpn J Infect Dis. 2001;54:144–7. [PubMed] [Google Scholar]
- 57.Filippi CM, Estes EA, Oldham JE, von Herrath MG. Immunoregulatory mechanisms triggered by viral infections protect from type 1 diabetes in mice. J Clin Invest. 2009;119:1515–23. doi: 10.1172/JCI38503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rosmalen JG, van Ewijk W, Leenen PJ. T-cell education in autoimmune diabetes: teachers and students. Trends Immunol. 2002;23:40–6. doi: 10.1016/s1471-4906(01)02088-9. [DOI] [PubMed] [Google Scholar]
- 59.Thomas-Vaslin V, Damotte D, Coltey M, et al. Abnormal T-cell selection on NOD thymic epithelium is sufficient to induce autoimmune manifestations in C57BL/6 athymic nude mice. Proc Natl Acad Sci U S A. 1997;94:4598–603. doi: 10.1073/pnas.94.9.4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Geenen V, Lefèbvre PJ. The intrathymic expression of insulin-related genes: implications for pathophysiology and prevention of type 1 diabetes. Diabetes Metab Rev. 1998;14:95–103. doi: 10.1002/(sici)1099-0895(199803)14:1<95::aid-dmr200>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 61.Klein L, Kyewski B. ‘Promiscuous’ expression of tissue antigens in the thymus: a key to T-cell tolerance and autoimmunity? J Mol Med. 2000;78:483–94. doi: 10.1007/s001090000146. [DOI] [PubMed] [Google Scholar]
- 62.Thebault-Baumont K, Dubois-Laforgue D, Krief P, et al. Acceleration of type 1 diabetes mellitus in proinsulin 2-deficient NOD mice. J Clin Invest. 2003;111:851–7. doi: 10.1172/JCI16584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mathews CE, Pietropaolo SL, Pietropaolo M. Reduced thymic expression of islet antigen contributes to loss of self-tolerance. Ann NY Acad Sci. 2003;1005:412–7. doi: 10.1196/annals.1288.070. [DOI] [PubMed] [Google Scholar]
- 64.Kecha-Kamoun O, Achour I, Martens H, et al. Thymic expression of insulin-related genes in an animal model of autoimmune type 1 diabetes. Diabetes Metab Res Rev. 2001;17:146–52. doi: 10.1002/dmrr.182. [DOI] [PubMed] [Google Scholar]
- 65.Fan Y, Rudert WA, Grupillo M, et al. Thymus-specific deletion of insulin induces autoimmune diabetes. EMBO J. 2009;28:2812–24. doi: 10.1038/emboj.2009.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gerling IC, Serreze DV, Christianson SW, et al. Intrathymic islet cell transplantation reduces beta-cell autoimmunity and prevents diabetes in NOD/Lt mice. Diabetes. 1992;41:1672–6. doi: 10.2337/diab.41.12.1672. [DOI] [PubMed] [Google Scholar]
- 67.Puissant B. Fonction thymique et autoimmunité[Thymic function and autoimmunity] Rev Med Interne. 2004;25:562–72. doi: 10.1016/j.revmed.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 68.Savino W, Boitard C, Bach JF, et al. Studies on the thymus in nonobese diabetic mouse. I. Changes in the microenvironmental compartments. Lab Invest. 1991;64:405–17. [PubMed] [Google Scholar]
- 69.Mendes-da-Cruz DA, Smaniotto S, Keller AC, et al. Multivectorial abnormal cell migration in the NOD mouse thymus. J Immunol. 2008;180:4639–47. doi: 10.4049/jimmunol.180.7.4639. [DOI] [PubMed] [Google Scholar]
- 70.Lamhamedi-Cherradi SE, Luan JJ, Eloy L, et al. Resistance of T cells to apoptosis in autoimmune diabetic (NOD) mice is increased early in life and is associated with dysregulated expression of Bcl-x. Diabetologia. 1998;41:178–84. doi: 10.1007/s001250050887. [DOI] [PubMed] [Google Scholar]
- 71.Brilot F, Chehadeh W, Charlet-Renard C, et al. Persistent infection of human thymic epithelial cells by coxsackievirus B4. J Virol. 2002;76:5260–5. doi: 10.1128/JVI.76.10.5260-5265.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brilot F, Geenen V, Hober D, Stoddart CA. Coxsackievirus B4 infection of human fetal thymus cells. J Virol. 2004;78:9854–61. doi: 10.1128/JVI.78.18.9854-9861.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jaïdane H, Gharbi J, Lobert PE, et al. Prolonged viral RNA detection in blood and lymphoid tissues from coxsackievirus B4 E2 orally-inoculated Swiss mice. Microbiol Immunol. 2006;50:971–4. doi: 10.1111/j.1348-0421.2006.tb03874.x. [DOI] [PubMed] [Google Scholar]
- 74.Jaïdane H, Gharbi J, Lobert PE, et al. Infection of primary cultures of murine splenic and thymic cells with coxsackievirus B4. Microbiol Immunol. 2008;52:40–6. doi: 10.1111/j.1348-0421.2008.00002.x. [DOI] [PubMed] [Google Scholar]
- 75.Brilot F, Jaïdane H, Geenen V, et al. Coxsackievirus B4 infection of murine foetal thymus organ cultures. J Med Virol. 2008;80:659–66. doi: 10.1002/jmv.21016. [DOI] [PubMed] [Google Scholar]
- 76.Chatterjee NK, Hou J, Dockstader P, et al. Coxsackievirus B4 infection alters thymic, splenic, and peripheral lymphocyte repertoire preceding onset of hyperglycemia in mice. J Med Virol. 1992;38:124–31. doi: 10.1002/jmv.1890380210. [DOI] [PubMed] [Google Scholar]
- 77.Zipris D, Crow AR, Delovitch TL. Altered thymic and peripheral T-lymphocyte repertoire preceding onset of diabetes in NOD mice. Diabetes. 1991;40:429–35. doi: 10.2337/diab.40.4.429. [DOI] [PubMed] [Google Scholar]
- 78.Poussier P, Nakhouda AF, Falk JF, et al. Lymphopenia and abnormal lymphocyte subsets in the BB rat: relationship to the diabetic syndrome. Endocrinology. 1982;110:1825–7. doi: 10.1210/endo-110-5-1825. [DOI] [PubMed] [Google Scholar]
- 79.Johnston C, Alviggi L, Millward BA, et al. Alterations in T lymphocyte subpopulations in type 1 diabetes. Exploration of genetic influence in identical twins. Diabetes. 1988;37:1484–8. doi: 10.2337/diab.37.11.1484. [DOI] [PubMed] [Google Scholar]
- 80.Faustman D, Schoenfeld D, Ziegler R. T lymphocyte changes linked to autoantibodies. Association of insulin autoantibodies with CD4+CD45R+ lymphocyte subpopulation in prediabetic subjects. Diabetes. 1991;40:590–7. doi: 10.2337/diab.40.5.590. [DOI] [PubMed] [Google Scholar]
- 81.Cavalcante P, Barberis M, Cannone M, et al. Detection of poliovirus-infected macrophages in thymus of patients with myasthenia gravis. Neurology. 2010;74:1118–26. doi: 10.1212/WNL.0b013e3181d7d884. [DOI] [PubMed] [Google Scholar]
- 82.Iwasaki T, Monma N, Satodate R, et al. An immunofluorescent study of generalized coxsackie virus B3 infection in a newborn infant. Acta Pathol Jpn. 1985;35:741–8. doi: 10.1111/j.1440-1827.1985.tb00615.x. [DOI] [PubMed] [Google Scholar]
- 83.Lozovskaia LS, Osipov SM, Zubkova IV, et al. Study of vertical transmission of coxsackie group enteroviruses in the etiology of congenital immunodeficiencies. Vopr Virusol. 1997;42:175–9. [PubMed] [Google Scholar]
- 84.Geenen V, Mottet M, Dardenne O, et al. Thymic self-antigens for the design of a negative/tolerogenic self-vaccination against type 1 diabetes. Curr Opin Pharmacol. 2010;10:461–72. doi: 10.1016/j.coph.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 85.Fridkis-Valery M, Reche PA, Reinherz E. Peptide variants of viral CTL epitopes mediate positive selection and emigration of Ag-specific thymocytes in vivo. J Immunol. 2004;173:1140–50. doi: 10.4049/jimmunol.173.2.1140. [DOI] [PubMed] [Google Scholar]
- 86.Fridkis-Valery M, Reinherz E. New approaches to eliciting protective immunity through T cell repertoire manipulation: the concept of thymic vaccnation. Med Immunol. 2004;3:1–10. doi: 10.1186/1476-9433-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


