Abstract
Recently, Sirtuin 1 (SIRT1) has been implicated in the molecular control of ageing and immune response. Although the remodelling of periodontal ligament (PDL) in response to mechanical stress (MS) is mediated by several host factors, including cytokines and chemokines, the transmission of mechanical stimuli into specific cellular activity is still not understood fully. This study aimed to investigate the effects of MS, particularly cyclic strain, on immune response genes, as well as SIRT1 and its signal transduction pathways, in human PDL cells. MS up-regulated the expression of SIRT1 and immune response genes encoding cytokines [tumour necrosis factor (TNF)-α, interleukin (IL)-1β], chemokines [IL-8, monocyte cheoattractant protein (CCL)-20], defensins [human β-defensin (hBD)-2, hBD-3] and Toll-like receptors (TLR-2 and TLR-4) in a force- and time-dependent manner. The SIRT1 inducers resveratrol and isonicotinamide attenuated MS-induced cytokine and chemokine expression, but enhanced the expression of defensins and TLRs. Blockade of SIRT1 activity by the SIRT1 inhibitors sirtinol and nicotinamide and down-regulation of SIRT1 expression by SIRT1 siRNA reduced the stimulatory effects of MS on defensins and TLRs, but increased its effects on cytokines and chemokines. MS induced activation of protein kinase B (Akt), protein kinase C (PKC), nuclear factor (NF)-κB and p38 mitogen-activated protein kinase (MAPK), c-Jun N-terminal kinase (JNK) and extracellular signal-regulated kinase (ERK). Treatment with the anti-oxidants N-acetylcysteine and glutathione inhibited MS-induced reactive oxygen species production and expression of cytokines, chemokines, defensins and TLRs. These results suggest that MS activates human PDL cells to express immune/defence genes encoding cytokines, chemokines, defensins and TLRs via a SIRT1 pathway.
Keywords: immune genes, mechanical stress, periodontal ligament cells, SIRT1
Introduction
Orthodontic tooth movement is achieved by the remodelling of alveolar bone and periodontal ligaments (PDL) in response to mechanical loading [1]. The host response to orthodontic force has been described as an aseptic and transitory inflammation, mediated by a variety of endogenous mediators such as cytokines and chemokines, which are involved in adaptive and innate immunity [2]. Chemokines are a superfamily of small chemotactic cytokines recognized as regulators of inflammatory reactions, and the development of an appropriate immune response by co-ordinating leucocyte recruitment [3].
Mechanical stress (MS) or loading increases the production of chemokines and chemokine receptors, including interleukin (IL)-8 receptor in osteoblasts [4], IL-8 in human periodontal ligament (PDL) cells [5] and IL-11 and IL-8 in human PDL cells [6]. A study has reported recently that chemokines such as monocyte chemoattractant protein (MCP)-1, regulated upon activation normal T cell expressed and secreted (RANTES) and macrophage inflammatory protein (MIP)-2 are up-regulated during rat orthodontic tooth movement [5]. However, an equibiaxial tensile strain of a low magnitude inhibits IL-1β-induced synthesis of IL-1β, IL-6 and IL-8 in PDL cells [7]. Furthermore, Lee et al. [8] reported that compressive stress in PDL cells had no significant effect on IL-8 expression. In vivo, IL-1, IL-6, IL-8, IL-11 and tumour necrosis factor (TNF)-α are produced by inflammatory cells and periodontal tissue cells upon the application of orthodontic force [9]. The mechanisms involved in host immune responses to MS, however, are not completely understood.
One host defence mechanism that involves activation of an innate immune response following exposure to the external environment is the production of defensins, small cationic anti-microbial peptides that are classified into the α- and β-defensin subfamilies [10]. Human β-defensin 1 (hBD-1) is expressed constitutively in epithelial cells, whereas hBD-2 and hBD-3 are expressed inducibly by bacteria, Candida albicans and inflammatory cytokines such as TNF-α and IL-1β[11].
Toll-like receptors (TLRs) are a transmembrane receptor family that plays a pivotal role in the modulation of immune response by recognizing pathogen-associated molecular patterns [12]. This recognition subsequently stimulates a sequence of signalling mechanisms, resulting ultimately in the production of various cytokines that serve as a link between innate and specific immune mechanisms. TLR-2 and TLR-4 are the most well-defined members of the TLR superfamily: TLR-4 recognizes lipopolysaccharides in Gram-negative bacteria, whereas TLR-2 plays a major role in the recognition of various bacterial components such as lipoteichoic acid in Gram-positive bacteria, lipoproteins and peptide glycans [13],[14]. The up-regulation of TLR-2 and/or TLR-4 has been shown in macrophages and gingival fibroblasts of inflamed periodontal tissue [15], which suggests that innate immune responses involving the TLRs as signalling receptors contribute to the inflammatory or immune response of periodontal tissue.
Sirtuin 1 (SIRT1) is the human orthologue of the yeast Sir2 protein, the prototypic class III histone deacetylase. SIRT1 has been shown to play a central role in a variety of cellular processes such as stress resistance, metabolism, differentiation and ageing [16]. We have demonstrated previously that SIRT1 exerts anti-inflammatory effects through the modulation of osteoclastogenic cytokine levels in human PDL cells [17]. Furthermore, SIRT1 has been implicated in the regulation of immune function, as it is expressed at high levels in the thymus, including in CD4+ and CD8+ thymocytes, and knocking out SIRT1 increases sensitivity to ionizing radiation-induced apoptosis [18]. Moreover, treatment of T cells with resveratrol, a SIRT1 activator, suppresses proliferation and cytokine production in vitro[19]. Resveratrol also suppresses immune functions by inducing lymphocyte apoptosis [20]. These results suggest that SIRT1 may be involved in the production of immune defence genes in MS-stimulated PDL cells.
We have reported previously that MS induces inflammatory cytokines including IL-1β, TNF-α and IL-6, as well as defence genes such as haem oxygenase-1 (HO-1), in human dental pulp cells [21]. Recently, we demonstrated that MS modulates odontoblastic/osteoblastic differentiation via modulation of the HO-1 pathway in dental pulp and PDL cells [22],[23]. Although the activation of TLRs and production of anti-microbial peptides, cytokines and chemokines, as well as their receptors, are implicated in innate and adaptive immunity [24], there is little information on the involvement of SIRT1 in MS-induced immune genes of PDL cells. The aim of the present study was to investigate the role of SIRT1 in the effects of MS on the expression of immune response genes in human PDL cells and to identify the underlying mechanisms involved.
Materials and methods
Reagents
Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS) and other tissue culture reagents were purchased from Gibco BRL (Grand Island, NY, USA). Resveratrol and sirtinol were purchased from Sigma-Aldrich (St Louis, MO, USA). Affinity purified polyclonal antibodies against mouse TLR-2, TLR-4, I-κBα, nuclear factor (NF)-κB p65 and β-actin monoclonal antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibodies against phospho-extracellular-regulated kinase (p-ERK), ERK, phospho-p38 (p-p38), p38, phospho- c-Jun N-terminal kinase (p-JNK) and JNK were purchased from Cell Signaling Inc. (Beverly, MA, USA). All other chemicals were obtained from Sigma unless indicated otherwise.
Cell culture
Immortalized hPDL cell lines provided by Dr Takada (Hiroshima University) were cultured in α-minimum essential medium (α-MEM; Invitrogen, Grand Island, NY, USA) with 10% fetal bovine serum (FBS) plus penicillin G solution (10 U/ml) and streptomycin (10 mg/ml) in a humidified atmosphere of 5% CO2 at 37°. Telomerase catalytic subunit hTERT gene-immortalized human periodontal ligament (HPDL) cells were derived by transfecting primary cultured hPDL cells from a healthy premolar extracted for orthodontic treatment, as described previously [25],[26]. These immortalized hPDL cells are similar to those in primary PDL-derived cells, and could be a model for the investigation of factors contributing to inflammation and differentiation of PDL cells [17],[22].
For experiments, the cells were seeded into culture dishes and then cultured in DMEM containing 10% FBS for 3 days until 70% confluent. Subsequently, the cells were exposed to MS. All treatments were performed in triplicate.
Application of MS
Human PDL cells (3 × 105/well) were subcultured into six-well, 35-mm flexible-bottomed Uniflex culture plates with a centrally located rectangular portion (15·25 mm × 24·18 mm) coated with type I collagen designed to provide a uniform uni-axial strain, and subjected to an intermittent deformation of 3, 6, 12 or 15% of maximum stretch for 2·5 s followed by 2·5 s of relaxation (12 cycle/min 24 h) with a Flexercell FX-4000 Strain Unit (Flexcell Corporation, Hillsborough, NC, USA), according to the manufacturer's instructions.
Sirt-1 siRNA transfection
siRNA-annealed oligonucleotide duplexes for SIRT1 (sequence 5′->3′ sense: GAUGAAGUUGACCUCCUCAtt; anti-sense: UGAGGAGGUCAACUUCAUCtt) and negative control (catalogue no. SN-1003) were purchased from Bioneer (Daejeon, South Korea) and PDL cells were transfected using lipofectamine 2000 (Gibco BRL), following the manufacturer's instructions.
RNA isolation and reverse transcription–polymerase chain reaction (RT–PCR)
After applying the MS, total RNA was isolated from the cells using Trizol reagent (Invitrogen Life Technologies, Gaithersburg, MD, USA), according to the manufacturer's instructions. Briefly, 1 µg of RNA isolated from each culture was reverse-transcribed using oligo(dT)15 primers (Roche Diagnostics, Mannheim, Germany) and AccuPower RT PreMix (Bioneer), according to the manufacturer's protocols. An amount of cDNA equivalent to 25 ng of total RNA was then subjected to PCR. The primers used for cDNA amplification are listed in Table 1. PCR products were subjected to electrophoresis on 1·2% agarose gel and were stained with ethidium bromide.
Table 1.
Reverse transcriptase–polymerase chain reaction (RT–PCR) primers and conditions.
| Genes | Primer sequence (5′-3′) | Annealing temp (°C) | Cycle number | Product size (bp) |
|---|---|---|---|---|
| TNF-α | Forward: 5′-CTCTFFCCCAFFCAFTCAGA-3′ | 60 | 35 | 519 |
| Reverse: 5′-GGCGTTTGGGAAGGTTGGAT-3′ | ||||
| IL-1β | Forward: 5′-TGGAGATGACAGTTCAGAAG-3′ | 60 | 35 | 288 |
| Reverse: 5′-GTACTGGTGCCGTTTATGC-3′ | ||||
| IL-8 | Forward: 5′-ATGACTTCCAAGCTGGCCGTGGCT-3′ | 62 | 25 | 289 |
| Reverse: 5′-TCTCAGCCCTCTTCAAAAACTTCTC-3′ | ||||
| CCL-20 | Forward: 5′-ATGTGCTGTACCAAGAGTTTG-3′ | 65 | 35 | 291 |
| Reverse: 5′-TTACATGTTCTTGACTTTTTTACTGAGGAG-3′ | ||||
| hBD-1 | Forward: 5′-CCCAGTTCCTGAAATCCTGA-3′ | 60 | 35 | 215 |
| Reverse: 5′-CAGGTGCCTTGAATTTTGGT-3′ | ||||
| hBD-2 | Forward: 5′-CATGAGGGTCTTGTATCTCCTCT-3′ | 55 | 35 | 201 |
| Reverse: 5′-CCTCCTCATGGCTTTTTGCAGC-3′ | ||||
| hBD-3 | Forward: 5′-AGCCTAGCAGCTATGAGGATC-3′ | 60 | 30 | 208 |
| Reverse: 5′-CTTCGGCAGCATTTTGCGCCA-3′ | ||||
| TLR-2 | Forward: 5′-GTGGCCAGCAGGTTCAGGATG-3′ | 55 | 35 | 641 |
| Reverse: 5′-AGGACTTTATCGCAGCTCTCAG-3′ | ||||
| TLR-4 | Forward: 5′-AAGTGTCTGAACTCCCTCCAGG-3 | 55 | 35 | 278 |
| Reverse: 5′-ATGGTCTTATTCATCTGACAGGTGATA-3 | ||||
| SIRT1 | Forward: 5′-TCAGTGTCATGGTTCCTTTGC-3′ | 57 | 35 | 820 |
| Reverse: 5′-AATCTGCTCCTTTGCCACTCT-3′ | ||||
| GAPDH | Forward: 5′-CGGAGTCAACGGATTTGGTCGTAT-3′ | 62 | 25 | 306 |
| Reverse: 5′-CGGAGTCAACGGATTTGGTCGTAT-3′ |
GAPDH, glyceraldehyde 3-phosphate dehydrogenase; TLR, Toll-like receptor; hBD, human β-defensin; CCL, monocyte chemoattractant protein; IL, interleukin; TNF, tumour necrosis factor; bp, base pairs.
Western blot analysis
An equal volume of ×2 sodium dodecyl sulphide (SDS) sample buffer was added and the samples were then boiled for 5 min. Samples (40 µg) were subjected to electrophoresis on 12% SDS-polyacrylamide gels for 2 h at 20 mA and then transferred onto nitrocellulose. The membrane was incubated for 1 h in 5% (wt/vol) dried milk protein in phosphate-buffered saline (PBS) containing 0·05% (vol/vol) Tween-20, washed in PBS and then incubated for 1 h in the presence of primary antibody (1:1000). The membrane was washed extensively with PBS-T and then incubated with anti-mouse IgG antibody conjugated with horseradish peroxidase (HRP) (1:3000) for 1 h. After extensive washes, immunoreactive bands on the membrane were visualized using chemiluminescent reagents according to the manufacturer's protocol (Amersham-Pharmacia, Piscataway, NJ, USA).
Determination of reactive oxygen species (ROS) production
Cells were seeded at 1·25 × 105 cells/well in α-MEM; 16 h later, medium was replaced and anti-oxidants were pretreated for 2 h and exposed to MS (12%) for 24 h. After the 20 µM dichlorodihydrofluorescein diacetate (DCFH-DA) was added, cells were incubated for an additional 30 min. Cell were then detached from the substrate by trypsinization and analysed immediately by flow cytometry (Becton Dickinson, Franklin Lakes, NJ, USA). Histograms were analysed using CellQuest software and were compared with histograms of untreated control cells.
Immunofluorescence labelling of NF-kB p65
Human PDL cells were seeded into six-well plates at 2 × 105 cells/well and treated as described above. For immunofluorescence labelling, MS-applied cells were fixed in 100% methanol for 30 min and washed three times with PBS. After blocking in 5% bovine serum albumin (BSA) in PBS for 1 h at room temperature or overnight at 4°C, the cells were incubated for 1 h with monoclonal mouse anti-NF-κB p65 antibody (1:100) in PBS containing 0·5% BSA. The cells were incubated with fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG antibody (1:100) after serial washings with PBS. Finally, nuclear DNA was stained by incubating with 300 ng/ml propidium iodide (PI) in PBS at room temperature for 5 min. Fluorescent images were obtained by laser scanning confocal microscopy (DMC, Olympus, Tokyo, Japan).
Statistical analysis
Statistical analyses of the data were performed by one-way analyses of variance (anovas) followed by a multiple-comparison Tukey's test using spss version 12·0 (SPSS GmbH, Munich, Germany). Statistical significance was determined at P < 0·05. The relative intensity of the gel bands was assayed using Quantity-One software (Bio-Rad Co., Hercules, CA, USA), and results were normalized to the mRNA and protein level of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and beta-actin, respectively.
Results
Effects of MS on the expression of SIRT1 and host defence/immune-related molecules
To investigate whether SIRT1 is involved in PDL cell responses to MS, we compared SIRT1 mRNA and protein levels in control and MS-exposed cells (Fig. 1a,b). SIRT1 mRNA expression increased in PDL cells exposed to MS in a time- and force-dependent fashion. mRNA expression peaked in cells exposed to 12% MS for 24 h and remained constant when either the force or time was increased further. In addition to the up-regulation of SIRT1 mRNA expression, we also detected a corresponding increase in SIRT1 protein levels.
Fig. 1.
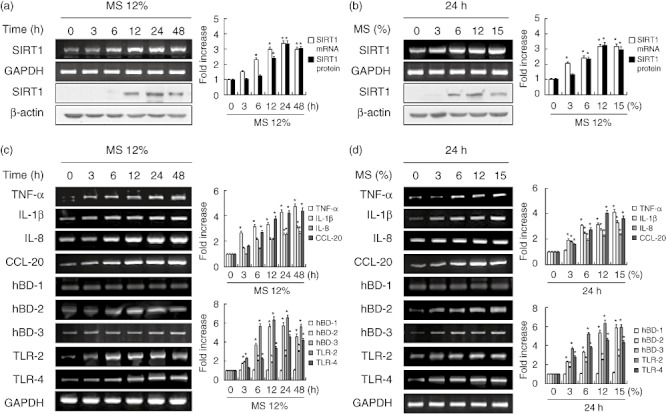
Effects of mechanical stress (MS) on the expression of Sirtuin 1 (SIRT1) and immune response genes in periodontal ligament (PDL) cells. Cells were cultured with or without MS for up to 48 h (a) and under a MS force of 3–15% (b). mRNA and protein expression levels were examined by reverse transcription–polymerase chain reaction (RT–PCR) and Western blotting, respectively. The data are representative of three independent experiments. The bar graph shows the fold increase in protein or mRNA expression compared to control cells. Columns show mean values of triplicate samples and error bars represent the standard deviation. *Statistically significant differences compared with the control, P < 0·05.
To investigate whether MS could induce the expression of host defence or immune effector genes in human PDL cells, cells were stimulated with MS and mRNA expression of genes encoding cytokines (TNF-α and IL-1β), chemokines (IL-8 and CCL-20), anti-microbial peptides (hBD-1, hBD-2 and hBD-3) and pattern recognition receptors (TLR-2 and TLR-4) was measured by RT–PCR (Fig. 1c,d). MS increased the levels of IL-1β, TNF-α, IL-8, CCL-20, hBD-2, hBD-3, TLR-2 and TLR-4 mRNAs in PDL cells in a force- and time-dependent manner. The expression of hBD-1 mRNA did not change in PDL cells exposed to MS. Maximal immune gene induction was observed in cells subjected to 12% MS for 24 h.
Effects of SIRT1 activation and inhibition on MS-induced defence and immune gene expression
Based on these results, we next examined whether the up-regulation of immune and defence gene expression in MS-stimulated cells is mediated by SIRT1. Resveratrol, a well-known SIRT1 activator, up-regulated SIRT1 mRNA and protein levels and enhanced MS-induced expression of the immune genes hBD-2, hBD-3, TLR-2 and TLR-4, but blocked up-regulation of the cytokines and chemokines TNF-α, IL-1β, IL-8 and CCL-20. In contrast, the SIRT1 inhibitor sirtinol attenuated the induction of SIRT1, hBD-2, hBD-3, TLR-2 and TLR-4 expression by MS, but enhanced TNF-α, IL-1β, IL-8 and CCL-20 mRNA expression (Fig. 2a,b).
Fig. 2.
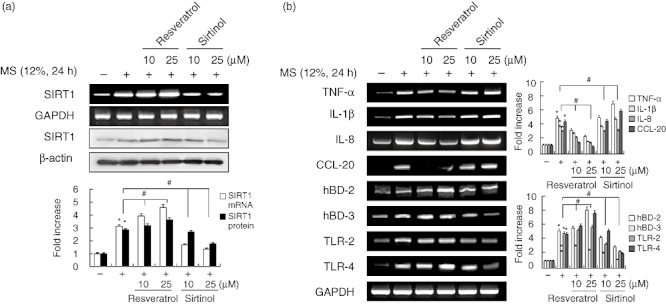
Effects of the Sirtuin 1 (SIRT1) activator resveratrol and SIRT1 inhibitor sirtinol on mechanical stress (MS)-induced immune gene expression in periodontal ligament (PDL) cells. Cells were pretreated with resveratrol or sirtinol for 2 h, and then exposed to MS (12%) for 24 h. mRNA and protein expression were examined by reverse transcription–polymerase chain reaction (RT–PCR) and Western blotting, respectively. The data are representative of three independent experiments. The bar graph shows the fold increase in protein or RNA expression compared to control cells. *Statistically significant differences compared with the control, P < 0·05.
Effects of isonicotinamide and nicotinamide on MS-induced immune gene expression in PDL cells
To extend the investigation of efficacy to other SIRT1 activators and inhibitors, PDL cells were treated with isonicotinamide and nicotinamide. The SIRT1 inducer isonicotinamide increased MS-induced up-regulation of SIRT1, hBD-2, hBD-3, TLR-2 and TLR-4 expression, but attenuated MS-induced TNF-α, IL-1β, IL-8 and CCL-20 expression (Fig. 3a,b). In contrast, pretreatment of PDL cells with nicotinamide, another inhibitor of SIRT1, reduced the induction of SIRT1, hBDs and TLRs expression by MS and increased the induction of cytokine and chemokine expression by MS.
Fig. 3.
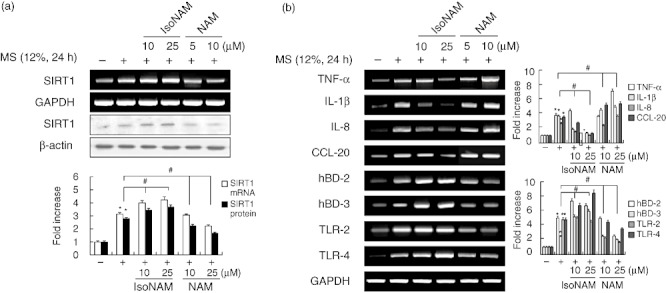
Effects of the Sirtuin 1 (SIRT1) activator isonicotinamide and SIRT1 inhibitor nicotinamide on mechanical stress (MS)-induced immune gene expression in periodontal ligament (PDL) cells. Cells were pretreated with isonicotinamide (IsoNAM) or nicotinamide (NAM) for 2 h, and then exposed to MS (12%) for 24 h. mRNA and protein expression levels were examined by reverse transcription–polymerase chain reaction (RT–PCR) and Western blotting, respectively. The data are representative of three independent experiments. The bar graph shows the fold increase in protein or mRNA expression compared to control cells. *Statistically significant differences compared with the control, P < 0·05.
Effect of silencing SIRT1 on MS-induced immune gene expression
To confirm further the role of SIRT1 in the induction of immune gene expression by MS, we knocked down SIRT1 with a specific siRNA. Transfection of siRNA specific for SIRT1 reduced basal expression of SIRT1 efficiently, as expected, and also reduced SIRT1 expression in the presence of MS (Fig. 4a). Treatment with SIRT1 siRNA abrogated the stimulatory effect of MS on the expression of the immune genes hBD-2, hBD-3, TLR-2 and TLR-4, but increased TNF-α, IL-1β, IL-8 and CCL-20 mRNA levels (Fig. 4b).
Fig. 4.
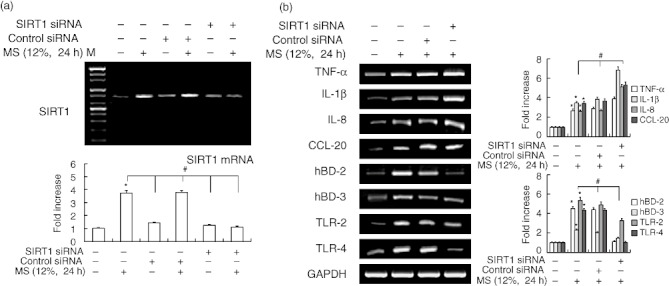
Effect of Sirtuin 1 (SIRT1) siRNA on mechanical stress (MS)-induced immune gene expression in periodontal ligament (PDL) cells. Cells were transfected with control siRNA or SIRT1 siRNA (80 nM), and then exposed to MS (12%) for 24 h. mRNA expression was examined by reverse transcription–polymerase chain reaction (RT–PCR). The data are representative of three independent experiments. The bar graph shows the fold increase in mRNA expression compared to control cells. Columns show mean values of triplicate samples and error bars represent the standard deviation. *Statistically significant differences compared with the control, P < 0·05.
Effects of MS on NF-κB, MAP kinase, PKC and Akt pathways
Because NF-κB activation requires nuclear translocation of the p65 subunit of NF-κB, we examined the effect of MS on the cytosolic and nuclear p65 protein pools by Western blotting. As shown in Fig. 5a, p65 translocated from the cytosol to the nucleus as early as 15 min after MS stimulation, a response that was sustained until 90 min post-stimulation. We also investigated I-κBα degradation and phosphorylation to clarify the mechanism of MS-induced NF-κB activation. Consistent with the observed translocation of the NF-κB subunit, MS induced I-κBα degradation and phosphorylation, as determined by Western blotting. Using confocal microscopy, we monitored the spatial distribution of the p65 subunit of NF-κB. In most of the unstimulated PDL cells, NF-κB was located in the cytoplasm (Fig. 5b, left); in MS-stimulated PDL cells, NF-κB was located in the nuclei (Fig. 5b, right).
Fig. 5.
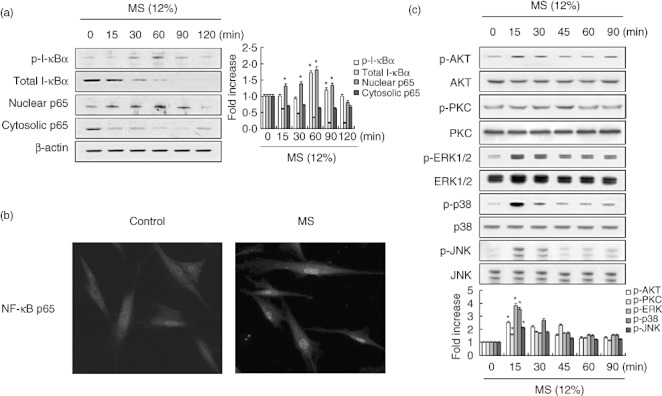
Effect of mechanical stress (MS) on the activation of NF-κB (a, b) and mitogen-activated protein kinase (MAPK), phosphoinositide 3 kinase (PI3K) and protein kinase C (PKC) (c) in periodontal ligament (PDL) cells. Cells were cultured without or with MS (12%) for the indicated time-periods. Cells were analysed by Western blotting (a,c) and confocal microscopy (b). The nuclear translocation of nuclear factor (NF)-κB was detected by indirect immunofluorescence labelling using monoclonal anti-NF-κB antibody followed by fluorescein isothiocyanate (FITC)-conjugated anti-mouse immunoglobulin (magnification ×400). The results are representative of three independent experiments. The bar graph shows the fold increase in protein expression compared to control cells. *Statistically significant differences compared with the control, P < 0·05.
Several studies have shown that the signalling molecules NF-κB, JNK, ERK, p38 mitogen-activated protein kinase (MAPK), protein kinase C (PKC) and protein kinase B (Akt) are involved in the expression of cytokines and chemokines in different cell types [21],[22]. To assess whether MS induces their activation, we next investigated the phosphorylation status of JNK1/2, ERK1/2 and p38 MAPK, PKC and Akt in PDL cells exposed to 12% MS for various periods of time. Figure 5c shows that MS activated Akt, PKC, p38, ERK and JNK significantly, as shown by the increased levels of their phosphorylated forms.
Effects of signal transduction inhibitors on MS-induced immune gene expression
To examine further the signalling pathways involved in MS-induced SIRT1 and immune gene expression, PDL cells were pretreated with various inhibitors of key signalling molecules. The ability of MS to induce the expression of the immune genes encoding IL-1β, TNF-α, IL-8, CCL-20, hBD-2, hBD-3, TLR-2, TLR-4 and SIRT1 was inhibited by the selective p38 inhibitor PD98059, the ERK inhibitor SB203580, the JNK inhibitor SP600125, the phosphoinositide 3 kinase (PI3K) inhibitor LY294002, the NF-κB inhibitor PDTC and the PKC inhibitor Ro-318220 (Fig. 6).
Fig. 6.
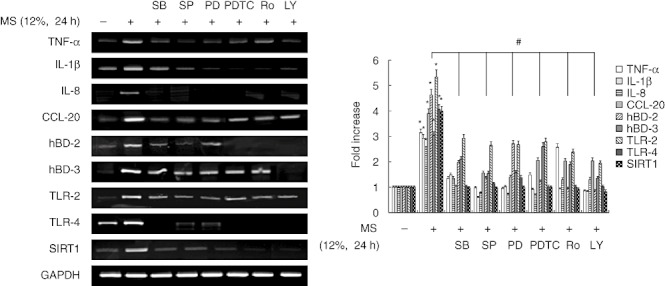
Effects of signal transduction inhibitors on mechanical stress (MS)-induced immune gene expression in periodontal ligament (PDL) cells. Cells were pretreated with the extracellular-regulated kinase (ERK) inhibitor SB203580 (20 µM), the p38 mitogen-activated protein kinase (MAPK) inhibitor PD98059 (20 µM), the c-Jun N-terminal kinase (JNK) inhibitor SP600125 (20 µM), the phosphoinositide 3 kinase (PI3K) inhibitor LY694002 (10 µM), the protein kinase C (PKC) inhibitor Ro-318220 (10 µM) or the nuclear factor (NF)-κB inhibitor pyrrolidine dithiocarbonate (PDTC) (10 µM) for 1 h, and then exposed to MS (12%) for 24 h. mRNA expression was examined by reverse transcription–polymerase chain reaction (RT–PCR). The data are representative of three independent experiments. The bar graph shows the fold increase in mRNA expression compared to control cells. *Statistically significant differences compared with the control, P < 0·05.
Effects of anti-oxidants on MS-induced ROS production and immune gene expression
Because increased ROS production in response to mechanical stress has been described in a variety of cell types [21], we examined ROS production in PDL cells in response to MS by flow cytometry. Exposure to 12% MS for 24 h led to the intracellular accumulation of ROS. Following validation of MS-dependent DCF fluorescence, we tested whether MS-induced ROS production and the expression of SIRT1 and immune response genes could be reduced through ROS inhibition. As shown in Fig. 7a,b, the induction of ROS production and SIRT1 expression by MS was prevented by the anti-oxidants N-acetylcysteine (NAC) and glutathione (GSH). Moreover, NAC and GSH blocked the production of inflammatory cytokines, chemokines, hBDs and TLRs, including IL-1β, TNF-α, IL-8, CCL-20, hBD-2, hBD-3, TLR-2 and TLR-4, in response to MS (Fig. 7c).
Fig. 7.
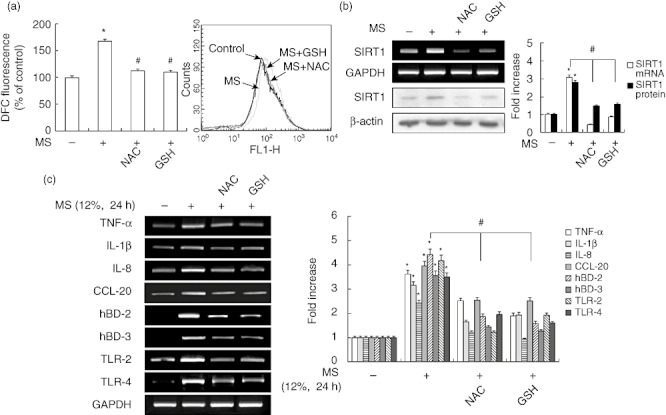
Effects of the anti-oxidants N-acetylcysteine (NAC) and glutathione (GSH) on mechanical stress (MS)-induced immune gene expression and reactive oxygen species (ROS) production in periodontal ligament (PDL) cells. Cells were pretreated with NAC (20 mM) or GSH (5 mM) for 2 h, and then exposed to MS (12%) for 24 h. ROS production was assayed by flow cytometry. mRNA and protein expression levels were examined by reverse transcription–polymerase chain reaction (RT–PCR) and Western blotting, respectively. The bar graph shows the fold increase in protein or mRNA expression compared to control cells. *P < 0·05 versus untreated control; #P < 0·05 versus cells exposed to MS.
Discussion
In this study, we evaluated the inductive effect of cyclic strain or MS on the activity of immune response genes encoding cytokines (IL-1β, TNF-α), chemokines (IL-8, CCL-20), hBDs and TLRs. Our results demonstrate that cyclic MS stimulates the mRNA expression of immune response genes such as IL-1β, TNF-α, IL-8 and CCL20, consistent with the results of previous studies on pulp, PDL cells and osteoblasts [4],[6],[8],[21],[27],[28]. An animal study showed that increased IL-1α and TNF-α expression occurred as early as 24 h after mechanical force application at both compression and tension areas of bone and PDL [29]. In some human studies, IL-1β, IL-6 and TNF-α reached peak levels at 24 h [30],[31]. These results demonstrate that cytokines play a significant role during the early stage of tooth movement, but not during the linear stage. In the present study, expression of cytokines, chemokines, hBDs and TLRs peaked at 24 h in MS-stimulated PDL cells. Therefore, we chose the 24 h time-point for our further studies.
PDL cells are constantly exposed to a variety of physiological and pathological stresses and/or injury, but still generally maintain a healthy balance. Both constitutive (hBD-1) and inducible β-defensins (hBD-2 and hBD-3) are expressed in our PDL cells, suggesting the existence of general and specific innate host defence systems that respond to infection or stress. Dale et al. [32] suggested that oral mucosal cells are in an activated state with respect to hBD-2 expression and that this state contributes to the normal barrier function of the oral epithelium. In contrast, in the epidermis, hBD-2 expression is associated primarily with inflammation and diseased states [10]. In the present study, hBD-2 and hBD-3 were induced by MS, and may be caused in turn by the release of the proinflammatory cytokines IL-1β and TNF-α.
TLRs have been shown to have an affinity for molecules associated with infection and tissue injury. A study has reported recently that in addition to microbial ligands, TLRs have endogenous ligands [33]. Endogenous TLR ligands arising from tissue damage are termed damage-associated molecular patterns (DAMPs), and are becoming increasingly recognized for their role in immune regulation [33]. The results showed clearly that these immune mechanisms also exist in PDL cells, as up-regulation of proinflammatory cytokines, hBDs and TLRs was seen in MS-stimulated cells. Hence, TLR-2 and TLR-4 seem to have numerous ligands, which could explain why DAMPs derived from MS triggered the expression of TLRs and hBDs.
Various studies with different model systems have revealed that stress can either enhance or reduce immune function [34]. It is generally believed that acute and moderate stress can enhance immune function, while chronic stress often results in reduction of immune function and an increase in disease susceptibility [35],[36]. SIRT1 may also play a protective role during times of cellular stress [37]. SIRT1 protein levels in vivo increase with starvation, fasting and calorie restriction, whereas SIRT1 protein decreases with age and senescence [16]. Incubation of PC12 and HEK293 cells in the absence of both serum and glucose induces SIRT1 protein expression through either an increase in transcription [38] or post-transcriptional regulation [39]. In contrast, Nedachi et al. [40] showed that low serum and high glucose represses SIRT1 protein in a mouse myoblast cell line. In this study, we have demonstrated for the first time that both SIRT1 mRNA and protein levels increased significantly in MS-exposed PDL cells. However, because up-regulation of SIRT1 and immune genes occurred in a time-dependent manner that peaked at 24 h of mechanical force, we can rule out the possibility that this response was caused by chronic stress such as serum deprivation. We also found that MS increased cytokines, chemokines, hBDs and TLRs significantly. Chronic stress has a negative impact on immune function, including suppression of innate immunity [36,36]. In addition, we directly compared these MS effects without serum starvation, because serum starvation or serum stimulation can affect SIRT1 protein or mRNA levels [38]–[40].
To assess the role of SIRT1 in host immune defence in PDL cells, we tested the effects of SIRT1 activation, inhibition and gene silencing on the expression of key immune gene markers. Our results indicate that activation of SIRT1 by resveratrol and isonicotinamide in PDL cells increased MS-induced hBD-2, hBD-3, TLR-2 and TLR-4 expression, but reduced MS-induced mRNA expression of cytokines and chemokines (TNF-α, IL-1β, IL-8 and CCL-20). These results are consistent with previous data showing that resveratrol-induced SIRT1 activation and adenoviral-mediated SIRT1 over-expression blocked the expression and release of proinflammatory cytokines in response to environmental stresses [41]–[43]. Furthermore, down-regulation of SIRT1 expression through inhibition of SIRT1 activity using sirtinol and nicotinamide enhanced MS-induced TNF-α, IL-1β, IL-8 and CCL-20 expression, but attenuated MS-induced hBD-2, hBD-3, TLR-2 and TLR-4 expression. As induction of SIRT1 activity by resveratrol and isonicotinamide reversed these effects, the inflammatory and immune effects of MS in PDL cells may be mediated by a SIRT1-dependent pathway. To confirm this suggestion, SIRT1 expression was knocked down by siRNA. Down-regulation of SIRT1 expression by siRNA increased cytokine and chemokine expression in MS-stimulated PDL cells, but reduced hBD and TLR expression. Based on these findings, we propose that SIRT1 is an important target for immune/defence mediators during orthodontic tooth movement.
Regarding the mechanisms of cytokine and chemokine induction, several studies have suggested the involvement of MAPK, NF-κB, PKC and PI3K/Akt pathways [17],[21],[42]. In the present study, MS induced NF-κB activation, as demonstrated by cytosolic I-κBα phosphorylation and degradation, as well as increasing the nuclear expression of p65, the major component of NF-κB. Our results confirmed that MS induced the phosphorylation of p38 MAPK, ERK, JNK, Akt and PKC. In addition, induction of the immune response genes IL-1β, TNF-α, IL-8, CCL-20, hBD-2, hBD-3, TLR-2 and TLR-4 in response to MS was attenuated by selective inhibitors of PI3K, p38, ERK, JNK, PKC and NF-κB (LY294002, SB203580, PD98059, SP600125, Ro-318220 and PDTC, respectively). These results suggest that the immune response effects of MS occur via activation of PI3K, p38, ERK, JNK MAPK, PKC and NF-κB.
The elucidation of a mechanism involving proinflammatory cytokines, chemokines, NF-κB activation and ROS generation is very important in understanding the immune response in MS. TNF-α and IL-1β induce the generation of ROS, primarily by NADPH oxidase, in the membranes of various cell types, including fibroblasts, kidney mesangial cells, endothelial cells and smooth muscle cells [44]. GSH plays an extremely potent role in anti-oxidant defence because it possesses not only direct radical-scavenging ability, but is also an essential component of the glutathione peroxidase system [45]. NAC can react directly with reactive oxygen intermediates and acts as a precursor to glutathione synthesis [46]. In our study, we showed that the anti-oxidants NAC and GSH blocked ROS production and reduced the expression of immune/defence genes, including those encoding IL-1β, TNF-α, IL-8, CCL-20, defensins and TLRs in MS-exposed PDL cells. These results suggest that the cellular event for enhancing cytokines, chemokines, TLRs and defensin signalling triggered by MS is oxidation-dependent.
In conclusion, our data show, for the first time, that MS up-regulates immune response genes encoding cytokines, chemokines, hBDs and TLRs in non-immune PDL cells, and that the SIRT1 pathway is involved strongly in these responses. We also observed that a p38 MAPK-, ERK-, JNK-, PI3K-, PKC- and NF-κB-dependent pathway and an anti-oxidant-sensitive pathway mediate, at least in part, MS-induced immune gene expression. The possible pathways through which MS can modulate immune response are summarized in Fig. 8. A detailed understanding of the mechanotransduction of tooth movement to immune activation, and the inflammatory processes that lead to bone resorption, deposition and remodelling, is required.
Fig. 8.
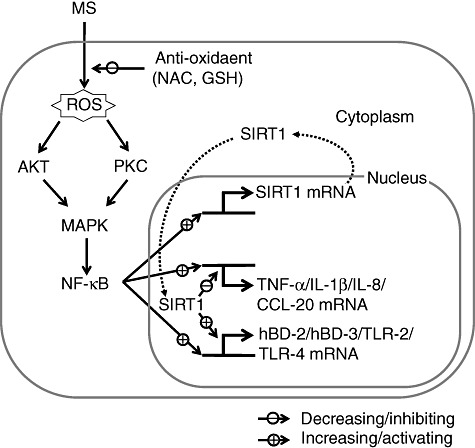
Schematic diagram illustrating the activation of immune genes [human β-defensin (hBD)-2, hBD-3, Toll-like receptor (TLR)-2 and TLR-4], cytokines, and chemokines [tumour necrosis factor (TNF)-α, interleukin (IL)-1β, IL-8 and monocyte chemoattractant protein (CCL)-20] via the Sirtuin 1 (SIRT1) pathway triggered by exposure to mechanical stress (MS) in periodontal ligament (PDL) cells.
Acknowledgments
This work was supported by the Mid-career Researcher Program through National Research Foundation of Korea (NRF) grant funded by the (Ministry of Education, Science and Technology (MEST) (no. 2009-0078526).
Disclosure
The authors declare no financial conflict of interest.
References
- 1.Davidovitch Z. Tooth movement. Crit Rev Oral Biol Med. 1991;2:411–50. doi: 10.1177/10454411910020040101. [DOI] [PubMed] [Google Scholar]
- 2.Tosi MF. Immune responses to infection. J Allergy Clin Immunol. 2005;116:241–9. doi: 10.1016/j.jaci.2005.05.036. [DOI] [PubMed] [Google Scholar]
- 3.Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354:610–21. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- 4.Koyama Y, Mitsui N, Suzuki N, et al. Effect of compressive force on the expression of inflammatory cytokines and their receptors in osteoblastic Saos-2 cells. Arch Oral Biol. 2008;53:488–96. doi: 10.1016/j.archoralbio.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Alhashimi N, Frithiof L, Brudvik P, Bakhiet M. Chemokines are upregulated during orthodontic tooth movement. J Interferon Cytokine Res. 1999;19:1047–52. doi: 10.1089/107999099313271. [DOI] [PubMed] [Google Scholar]
- 6.Li Y, Zheng W, Liu JS, et al. Expression of osteoclastogenesis inducers in a tissue model of periodontal ligament under compression. J Dent Res. 2011;90:115–20. doi: 10.1177/0022034510385237. [DOI] [PubMed] [Google Scholar]
- 7.Long P, Hu J, Piesco N, Buckley M, Agarwal S. Low magnitude of tensile strain inhibits IL-1beta-dependent induction of pro-inflammatory cytokines and induces synthesis of IL-10 in human periodontal ligament cells in vitro. J Dent Res. 2001;80:1416–20. doi: 10.1177/00220345010800050601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee YH, Nahm DS, Jung YK, et al. Differential gene expression of periodontal ligament cells after loading of static compressive force. J Periodontol. 2007;78:446–52. doi: 10.1902/jop.2007.060240. [DOI] [PubMed] [Google Scholar]
- 9.Lee KJ, Park YC, Yu HS, Choi SH, Yoo YJ. Effects of continuous and interrupted orthodontic force on interleukin-1β and prostaglandin E2 production in gingival crevicular fluid. Am J Orthod Dentofacial Orthop. 2004;125:168–77. doi: 10.1016/j.ajodo.2003.03.006. [DOI] [PubMed] [Google Scholar]
- 10.Ali RS, Falconer A, Ikram M, Bissett CE, Cerio R, Quinn AG. Expression of the peptide antibiotics human beta defensin-1 and human beta defensin-2 in normal human skin. J Investig Dermatol. 2001;117:106–11. doi: 10.1046/j.0022-202x.2001.01401.x. [DOI] [PubMed] [Google Scholar]
- 11.Bals R, Wang X, Wu Z, et al. Human beta-defensin 2 is a salt-sensitive peptide antibiotic expressed in human lung. J Clin Invest. 1998;102:874–80. doi: 10.1172/JCI2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–76. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 13.Sha Q, Truong-Tran AQ, Plitt JR, Beck LA, Schleimer RP. Activation of airway epithelial cells by toll-like receptor agonists. Am J Respir Cell Mol Biol. 2004;31:358–64. doi: 10.1165/rcmb.2003-0388OC. [DOI] [PubMed] [Google Scholar]
- 14.Barton GM, Medzhitov R. Toll-like receptor signaling pathways. Science. 2003;300:1524–5. doi: 10.1126/science.1085536. [DOI] [PubMed] [Google Scholar]
- 15.Mori Y, Yoshimura A, Ukai T, Lien E, Espevik T, Hara Y. Immunohistochemical localization of Toll-like receptors 2 and 4 in gingival tissue from patients with periodontitis. Oral Microbiol Immunol. 2003;18:54–8. doi: 10.1034/j.1399-302x.2003.180109.x. [DOI] [PubMed] [Google Scholar]
- 16.Haigis MC, Guarente LP. Mammalian sirtuins-emerging roles in physiology, aging, and calorie restriction. Genes Dev. 2006;20:2913–21. doi: 10.1101/gad.1467506. [DOI] [PubMed] [Google Scholar]
- 17.Kim YS, Lee YM, Park JS, Lee SK, Kim EC. SIRT1 modulates high-mobility group box 1-induced osteoclastogenic cytokines in human periodontal ligament cells. J Cell Biochem. 2010;111:1310–20. doi: 10.1002/jcb.22858. [DOI] [PubMed] [Google Scholar]
- 18.Cheng HL, Mostoslavsky R, Saito S, et al. Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proc Natl Acad Sci USA. 2003;100:10794–9. doi: 10.1073/pnas.1934713100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao X, Xu YX, Janakiraman N, Chapman RA, Gautam SC. Immunomodulatory activity of resveratrol: suppression of lymphocyte proliferation, development of cell-mediated cytotoxicity, and cytokine production. Biochem Pharmacol. 2001;62:1299–308. doi: 10.1016/s0006-2952(01)00775-4. [DOI] [PubMed] [Google Scholar]
- 20.Falchetti R, Fuggetta MP, Lanzilli G, Tricarico M, Ravagnan G. Effects of resveratrol on human immune cell function. Life Sci. 2001;70:81–96. doi: 10.1016/s0024-3205(01)01367-4. [DOI] [PubMed] [Google Scholar]
- 21.Lee SK, Min KS, Kim Y, et al. Mechanical stress activates proinflammatory cytokines and antioxidant defense enzymes in human dental pulp cells. J Endod. 2008;34:1364–9. doi: 10.1016/j.joen.2008.08.024. [DOI] [PubMed] [Google Scholar]
- 22.Cho JH, Lee SK, Lee JW, Kim EC. The role of heme oxygenase-1 in mechanical stress- and lipopolysaccharide-induced osteogenic differentiation in human periodontal ligament cells. Angle Orthod. 2010;80:552–9. doi: 10.2319/091509-520.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee SK, Lee CY, Kook YA, Lee SK, Kim EC. Mechanical stress promotes odontoblastic differentiation via the heme oxygenase-1 pathway in human dental pulp cell line. Life Sci. 2010;86:107–14. doi: 10.1016/j.lfs.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 24.Veerayutthwilai O, Byers MR, Pham TT, Darveau RP, Dale BA. Differential regulation of immune responses by odontoblasts. Oral Microbiol Immunol. 2007;22:5–13. doi: 10.1111/j.1399-302X.2007.00310.x. [DOI] [PubMed] [Google Scholar]
- 25.Kitagawa M, Kudo Y, Iizuka S, et al. Effect of F-spondin on cementoblastic differentiation of human periodontal ligament cells. Biochem Biophys Res Commun. 2006;349:1050–6. doi: 10.1016/j.bbrc.2006.08.142. [DOI] [PubMed] [Google Scholar]
- 26.Kitagawa M, Tahara H, Kitagawa S, et al. Characterization of established cementoblast-like cell lines from human cementum-lining cells in vitro and in vivo. Bone. 2006;39:1035–42. doi: 10.1016/j.bone.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 27.Toms SR, Eberhardt AW. A nonlinear finite element analysis of the periodontal ligament under orthodontic tooth loading. Am J Orthod Dentofacial Orthop. 2003;123:657–65. doi: 10.1016/s0889-5406(03)00164-1. [DOI] [PubMed] [Google Scholar]
- 28.Shimizu N, Yamaguchi M, Goseki T, et al. Cyclic-tension force stimulates interleukin-1α production by human periodontal ligament cells. J Periodontal Res. 1994;29:328–33. doi: 10.1111/j.1600-0765.1994.tb01230.x. [DOI] [PubMed] [Google Scholar]
- 29.Bletsa A, Berggreen E, Brudvik P. Interleukin-1alpha and tumor necrosis factor-alpha expression during the early phases of orthodontic tooth movement in rats. Eur J Oral Sci. 2006;114:423–9. doi: 10.1111/j.1600-0722.2006.00400.x. [DOI] [PubMed] [Google Scholar]
- 30.Ren Y, Hazemeijer H, de Haan B, Qu N, de Vos P. Cytokine profiles in crevicular fluid during orthodontic tooth movement of short and long durations. Periodontology. 2007;78:453–8. doi: 10.1902/jop.2007.060261. [DOI] [PubMed] [Google Scholar]
- 31.Ren Y, Vissink A. Cytokines in crevicular fluid and orthodontic tooth movement. Eur J Oral Sci. 2008;116:89–97. doi: 10.1111/j.1600-0722.2007.00511.x. [DOI] [PubMed] [Google Scholar]
- 32.Dale BA, Kimball JR, Krisanaprakornkit S, et al. Localized antimicrobial peptide expression in human gingiva. J Periodont Res. 2001;36:285–94. doi: 10.1034/j.1600-0765.2001.360503.x. [DOI] [PubMed] [Google Scholar]
- 33.Gordon S. Pattern recognition receptors: doubling up for the innate immune response. Cell. 2002;111:927–30. doi: 10.1016/s0092-8674(02)01201-1. [DOI] [PubMed] [Google Scholar]
- 34.Ader R, Cohen N. Psychoneuroimmunology: conditioning and stress. Annu Rev Psychol. 1993;44:53–85. doi: 10.1146/annurev.ps.44.020193.000413. [DOI] [PubMed] [Google Scholar]
- 35.Dhabhar FS, McEwen BS. Acute stress enhances while chronic stress suppresses cell-mediated immunity in vivo: a potential role for leukocyte trafficking. Brain Behav Immun. 1997;11:286–306. doi: 10.1006/brbi.1997.0508. [DOI] [PubMed] [Google Scholar]
- 36.Shi Y, Devadas S, Greeneltch KM, Yin D, Allan MR, Zhou JN. Stressed to death: implication of lymphocyte apoptosis for psychoneuroimmunology. Brain Behav Immun. 2003;17:S18–S26. doi: 10.1016/s0889-1591(02)00062-4. [DOI] [PubMed] [Google Scholar]
- 37.Kim EJ, Kho JH, Kang MR, Um SJ. Active regulator of SIRT1 cooperates with SIRT1 and facilitates suppression of p53 activity. Mol Cell. 2007;28:277–90. doi: 10.1016/j.molcel.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 38.Nemoto S, Fergusson MM, Finkel T. Nutrient availability regulates SIRT1 through a forkhead-dependent pathway. Science. 2004;306:2105–8. doi: 10.1126/science.1101731. [DOI] [PubMed] [Google Scholar]
- 39.Kanfi Y, Peshti V, Gozlan YM, Rathaus M, Gil R, Cohen HY. Regulation of SIRT1 protein levels by nutrient availability. FEBS Lett. 2008;582:2417–23. doi: 10.1016/j.febslet.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 40.Nedachi T, Kadotani A, Ariga M, Katagiri H, Kanzaki M. Ambient glucose levels qualify the potency of insulin myogenic actions by regulating SIRT1 and FoxO3a in C2C12 myocytes. Am J Physiol Endocrinol Metab. 2008;294:E668–78. doi: 10.1152/ajpendo.00640.2007. [DOI] [PubMed] [Google Scholar]
- 41.Yang SR, Wright J, Bauter M, Seweryniak K, Kode A, Rahman I. Sirtuin regulates cigarette smoke-induced proinflammatory mediator release via RelA/p65 NF-κB in macrophages in vitro and in rat lungs in vivo: implications for chronic inflammation and aging. Am J Physiol Lung Cell Mol Physiol. 2007;292:L567–76. doi: 10.1152/ajplung.00308.2006. [DOI] [PubMed] [Google Scholar]
- 42.Lee JH, Song MY, Song EK, et al. Overexpression of SIRT1 protects pancreatic beta-cells against cytokine toxicity by suppressing the nuclear factor-kappaB signaling pathway. Diabetes. 2009;58:344–51. doi: 10.2337/db07-1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rajendrasozhan S, Yang SR, Kinnula VL, Rahman I. SIRT1, an antiinflammatory and antiaging protein, is decreased in lungs of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177:861–70. doi: 10.1164/rccm.200708-1269OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tolando R, Jovanovic A, Brigelius-Flohe R, Ursini F, Maiorino M. Reactive oxygen species and proinflammatory cytokine signaling in endothelial cells: effect of selenium supplementation. Free Radic Biol Med. 2000;28:979–86. doi: 10.1016/s0891-5849(00)00183-0. [DOI] [PubMed] [Google Scholar]
- 45.Dickinson DA, Forman HJ. Cellular glutathione and thiols metabolism. Biochem Pharmacol. 2002;64:1019–26. doi: 10.1016/s0006-2952(02)01172-3. [DOI] [PubMed] [Google Scholar]
- 46.Blackwell TS, Blackwell TR, Holden EP, Christman BW, Christman JW. In vivo antioxidant treatment suppresses nuclear factor-κB activation and neutrophilic lung inflammation. J Immunol. 1996;157:1630–7. [PubMed] [Google Scholar]


