Abstract
We characterized the underlying mechanisms by which glutathione (GSH)-enhanced natural killer (NK) cells inhibit the growth of Mycobacterium tuberculosis (M. tb) inside human monocytes. We observed that in healthy individuals, treatment of NK cells with N-acetyl cysteine (NAC), a GSH prodrug in conjunction with cytokines such as interleukin (IL)-2 + IL-12, resulted in enhanced expression of NK cytotoxic ligands (FasL and CD40L) with concomitant stasis in the intracellular growth of M. tb. Neutralization of FasL and CD40L in IL-2 + IL-12 + NAC-treated NK cells resulted in abrogation in the growth inhibition of M. tb inside monocytes. Importantly, we observed that the levels of GSH are decreased significantly in NK cells derived from individuals with HIV infection compared to healthy subjects, and this decrease correlated with a several-fold increase in the growth of M. tb inside monocytes. This study describes a novel innate defence mechanism adopted by NK cells to control M. tb infection.
Keywords: glutathione and innate immunity, HIV, monocytes, natural killer cells, tuberculosis
Introduction
Worldwide, tuberculosis (TB) remains the most frequent and important infectious disease causing morbidity and death [1]. One-third of the world's population is latently infected with Mycobacterium tuberculosis (M. tb), the aetiological agent of TB. The World Health Organization (WHO) estimates that about 8–10 million new TB cases occur annually worldwide and the incidence of TB is currently increasing. In this context, TB is in the top three [with malaria and human immunodeficiency virus (HIV)], being the leading causes of death from a single infectious agent, and approximately 2 million deaths are attributable to TB annually [1].
In particular, pulmonary TB, the most common form of TB, is a highly contagious and life-threatening infection. Moreover, enhanced susceptibility to TB in HIV-infected populations is another serious health problem throughout the world [1].
In addition, multi-drug-resistant TB (MDR-TB) has been increasing in incidence in many areas, not only in developing countries but also in industrialized countries, during the past decade [2]. MDR-TB caused by M. tb strains entails extended treatment, expensive and toxic regimens, and higher rates of treatment failure and death. The global resurgence of TB and the rapid emergence of MDR-TB underscore the importance of the development of new anti-TB drugs and new protocols for efficacious clinical control of TB patients using ordinary anti-mycobacterial drugs [2].
Importantly, in 2006 extensively drug-resistant TB (XDR-TB) was reported in all regions of the world and was rapidly classified by WHO as a serious emerging threat to global public health, especially in countries with a high prevalence of HIV infection. XDR-TB is a relatively rare type of MDR-TB and is resistant to almost all drugs used to treat TB [2].
Glutathione (GSH), a principal non-protein thiol, is necessary for maintenance of the intracellular redox state [3],[4]. GSH levels are compromised in individuals with HIV infection in whom the risk of reactivation of latent tuberculosis (LTBI) is several times higher than in young healthy individuals [5]. We have reported previously that GSH facilitates the control of intracellular M. tb growth in both murine and human macrophages. In other words, GSH has direct anti-mycobacterial activity [6]–[8]. These results unfold a novel and potentially important innate defence mechanism adopted by human macrophages to control M. tb infection [6]–[8].
We also demonstrated that GSH activates NK cells to control M. tb infection [9]. These results indicate that GSH not only has direct anti-mycobacterial activity but can also regulate immune cell functions to control M. tb infection. Importantly, we observed that GSH levels are decreased significantly in peripheral blood mononuclear cells (PBMC) and red blood cells (RBC) isolated from both individuals with active tuberculosis (TB) and individuals with HIV infection [10],[11].
The goal of the present study is to characterize in healthy subjects the underlying mechanisms by which GSH, in conjunction with interleukin (IL)-2 + IL-12, enhance the functions of natural killer (NK) cells to inhibit the growth of M. tb inside human monocytes. We quantified the expression of NK cell cytotoxic receptors (NKp30, NKp44 and NKp46), NK activation receptor (NKG2D) and NK cytotoxic ligands (FasL and CD40L) on the NK cell surface and correlated the increased expression of these markers with inhibition of M. tb growth. Furthermore, FasL and CD40L on the NK cell surface were neutralized using neutralizing antibodies, and the effects of blocking these ligands on the intracellular survival of M. tb was tested. Finally, we determined the intracellular levels of GSH in NK cells isolated from healthy individuals and individuals with HIV infection and correlated the differences in GSH levels with the altered viability of M. tb inside human monocytes.
Our results indicate that treatment of NK cells derived from healthy individuals with NAC in combination with IL-2 + IL-12 resulted in control of M. tb infection and the growth inhibitory effects correlated with increased expression of FasL and CD40L on the cell surface of NK cells. Furthermore, NK cells derived from individuals with HIV infection have significantly lower levels of GSH compared to healthy subjects, and this decrease correlated with increased growth of M. tb inside macrophages. Our results indicate a novel pathway by which NK cells control the growth of M. tb inside human monocytes, and this protective innate defence mechanism is somewhat compromised in individuals with HIV infection leading to enhanced susceptibility to M.tb infection.
Materials and methods
Subjects
A total of 23 volunteers (10 healthy subjects and 13 individuals with HIV infection) were recruited for the study. Individuals with HIV infection were recruited from the Foothills acquired inmmune deficiency syndrome (AIDS) project. Healthy subjects without HIV infection or a history of TB were recruited from the university faculty and staff. All HIV-infected volunteers had been diagnosed with HIV-1, were taking some form of anti-retroviral treatment (ART) and had CD4+ T cell counts of between 271 and 1415 cells per mm3. Thirty-five millilitres of blood was drawn once from both healthy volunteers and individuals with HIV infection after obtaining a signed informed consent. All our studies were approved by both the Institutional Review Board and the Institutional Biosafety Committee.
Preparation of bacterial cells for infection
All experiments with M. tb H37Rv were performed in a biosafety level 3 (BSL-3) facility. H37Rv was processed for infection as follows: static cultures of M. tb at their peak logarithmic phase of growth (between 0·5 and 0·8 at A600) were used for infection of monocytes. The bacterial suspension was washed and resuspended in RPMI-1640 (Sigma, St Louis, MO, USA) containing 5% AB serum (Sigma). Bacterial clumps were disaggregated by vortexing five times with 3-mm sterile glass beads. The bacterial suspension was passed through a 5 µm filter (Millipore, Billerica, MA, USA) to remove any further clumps. The total number of organisms in the suspension was ascertained by plating. Processed H37Rv was frozen as stocks at −80°C. At the time of infection, frozen stocks of processed H37Rv were thawed and used for monocyte infection.
Isolation of human monocytes for in vitro M. tb infection studies
Human monocytes were isolated from the blood of healthy volunteers and individuals with HIV infection by density gradient centrifugation using Histopaque (Sigma). Peripheral blood mononuclear cells (PBMCs) (1 × 105/well) were distributed into poly-l-lysine-coated 96-well plates and incubated overnight at 37°C, 5% CO2 to allow monocytes to adhere. Following overnight incubation, non-adherent cells were removed and used for isolation of NK cells. Adherent monocytes (1 × 104/well) were infected with the processed virulent laboratory strain of M. tb, H37Rv at a multiplicity of infection of 10:1.
Purification of NK cells
Autologous CD56+ NK cells were isolated from the non-adherent PBMCs derived from healthy volunteers and individuals with HIV infection by positive selection with magnetic beads conjugated to anti-CD56 using mini-magnetic affinity cell sorting (MACS) columns (Miltenyi, Auburn, CA, USA). The autologous NK-positive cells (95–100% CD56+) were used as effector cells. NK cells isolated from the first five healthy volunteers were used for functional assays (to determine the intracellular viability of M. tb in co-cultures of monocytes and NK cells) and for quantification of the NK cytotoxic receptors (NKp30, NKp44 and NKp46) and ligands (FasL and CD40L). NK cells isolated from the remaining five healthy volunteers were used for neutralization studies (FasL and CD40L) and for measurement of intracellular GSH. NK cells isolated from individuals with HIV infection were used for GSH assays and for the M. tb intracellular survival assays.
Co-incubation of infected monocytes with autologous NK cells from healthy subjects
Prior to co-incubation with monocytes, autologous NK cells were subjected to various treatments such as cytokine stimulation, treatment with a GSH enhancing agent, N-acetyl cysteine (NAC) and treatment with a GSH synthesis inhibitor, buthionine sulphoximine (BSO). Specifically, NK cells were treated as follows: no additives, NAC (20 mM), IL-2 (100 U/ml) + IL-12 (100 U/ml), IL-2 + IL-12 + NAC and BSO (500 µM) for 24 h. Following incubation with stimulants, NK cells were washed with phosphate-buffered saline (PBS), resuspended in fresh RPMI-1640 containing AB serum (without any stimulants) and then added to the infected monocytes. Infected monocyte–NK cell co-cultures were terminated at 1 h and 5 days post-infection to determine the intracellular survival of H37Rv. Infected monocytes cultured in the absence of NK cells were used as negative controls.
Termination of infected monocytes–autologous NK cell co-cultures
Infected monocytes cultured in the presence and absence of NK cells were terminated at 1 h and 5 days post-infection. During termination, supernatants were removed and adherent monocytes were lysed by addition of sterile distilled water. Lysates were plated on 7H11 medium enriched with albumin dextrose complex (ADC) to estimate the extent of H37Rv growth or killing in co-cultures. Cell-free supernatants were plated to determine the extracellular growth of H37Rv.
Quantification of cytotoxic receptors (NKP30, NKP44, NKP46) on NK cells from healthy subjects
The effects of NAC either alone or in combination with IL-2 + IL-12 on up-regulating the expression of NK cell cytotoxic receptors (NKP30, NKP44, NKP46) was determined by staining the cells with phycoerythrin (PE)-labelled monoclonal antibodies against these markers (Miltenyi) and subsequent analysis by flow cytometry. NK cells were treated as follows: sham treatment (RPMI-1640), treatment with freshly prepared NAC (20 mM), IL-2 + IL-12 (ebioscience, San Diego, CA, USA)-treatment, IL-2 + IL-12 + NAC-treatment and BSO (500 µM)-treatment. Twenty-four h after incubation, NK cells were washed with PBS and resuspended in different tubes containing 100 µl of PBS with PE-labelled NKP44, PE-labelled NKP30 or PE-labelled NKP46 and incubated at 4°C for 30 min. Samples were analysed by flow cytometry. Values obtained for different treatments were normalized to control.
Quantification of NK activating receptor (NKG2D) and cytotoxic ligands (FasL and CD40L) on NK cells from healthy subjects
The effects of GSH both alone and in conjunction with cytokines in up-regulating the expressions of NK cell activation receptor (NKG2D) and NK cytotoxic ligands (FasL and CD40L) was determined by staining the cells with PE-labelled monoclonal antibodies against these markers (Miltenyi) and subsequent analysis by flow cytometry. NK cells were treated as follows: mock treated, treatment with 20 mM NAC, treatment with IL-2 + IL-12 and treatment with IL-2 + IL-12 + NAC. Twenty-four h after incubation, NK cells were washed and resuspended in different tubes containing 100 µl of PBS with PE-labelled NKG2D, PE-labelled FasL or fluorescein isothiocyanate (FITC)-labelled CD40L and incubated at 4°C for 30 min. The fluorescence was read using flow cytometry. Values obtained for different treatments were normalized to control.
Neutralization of CD40L and FasL on NK cells from healthy subjects
Neutralization of CD40L and FasL on the NK cell surface was performed to confirm that these cytotoxic ligands are essential for promoting cellular contact between NK cells and monocytes leading to inhibition in the growth of H37Rv. The best-characterized of many known pathways of cytotoxicity induction are mediated by the interaction of FasL with Fas and CD40 with CD40L. NK cells were treated either separately or in combination with blocking antibodies against FasL and CD40L (100 µg/ml). Neutralizing antibodies treated NK cells were added to H37Rv-infected monocytes. Infected cultures were terminated at 1 h and 5 days post-infection to determine the effects of FasL and CD40L neutralization in abrogating the growth inhibition of H37Rv inside human monocytes. Macrophage lysates were plated on 7H11 medium enriched with ADC to estimate the growth or killing of H37Rv.
Assay of GSH levels in NK cells
GSH levels were measured in freshly isolated NK cells from healthy subjects and individuals with HIV infection using an assay kit from Calbiochem (Darmstadt, Germany). NK cells were pelleted by centrifugation and an equal volume of ice-cold 5% metaphosphoric acid (MPA) was added to the pellet. Supernatants collected after centrifugation were analysed for total GSH, as per the manufacturer's instructions. Total GSH in the samples was normalized with protein. Proteins in the samples were estimated by Bradford's method using Bio-Rad reagent (Bio-Rad, Hercules, CA, USA).
Statistical analysis
Statistical analysis of the data was carried out using Statview® and the statistical significance was determined using an unpaired t-test. Differences were considered significant at a level of P < 0·05.
Results
Mycobactericidal assays using monocyte–NK cell co-cultures from healthy individuals
We determined the effects of GSH both alone and in conjunction with IL-2 + IL-12 in enhancing the functions of NK cells to inhibit the intracellular growth of H37Rv inside monocytes by examining the intracellular survival of H37Rv in co-cultures of infected monocytes and NK cells, using a NK cell : monocyte ratio of 10:1. Infected monocytes cultured in the absence of NK cells were included as a negative control. Figure 1a shows the results from studies performed in five healthy subjects. We observed a fourfold increase in the intracellular growth of H37Rv in monocytes cultured in the absence of NK cells in comparison to the monocytes that were co-incubated with NK cells, in which there was only a threefold increase in the growth of H37Rv (Fig. 1). Although the observed growth inhibition of H37Rv was modest, it still highlights the important contribution of NK cells in controlling M. tb infection. Treatment of NK cells with NAC resulted in additional increase in the growth inhibition of H37Rv (only a twofold increase in growth compared to negative control). Further increase in the growth inhibition of intracellular H37Rv was observed when NK cells were treated with IL-2 + IL-12 + NAC. In fact, treatment of NK cells with the combination of IL-2 + IL-12 + NAC resulted in complete stasis in the growth of H37Rv (Fig. 1). Treatment of NK cells with IL-2 + IL-12 did not result in inhibition of H37Rv growth inside monocytes (data not shown). Importantly, co-incubation with BSO-treated NK cells resulted in a maximum and a fivefold increase in the intracellular growth of H37Rv (Fig. 1a). Cell-free supernatants from co-cultures of infected monocytes and NK cells were plated routinely on 7H11 agar to test for extracellular bacterial growth and we did not observe any colonies indicating that there was no lysis of monocytes by NK cells and release of H37Rv into the supernatants. Furthermore, as standard practice the monocyte–NK cell co-cultures were always examined under the microscope prior to termination for cell lysis and we consistently observed intact monolayers of macrophages, further confirming that that the inhibition of M. tb growth is intracellular. The observed growth restriction of M. tb by NK cells occurs at 5 days post-infection. These results provide solid evidence that GSH in conjunction with IL-2 and IL-12 activates NK cells to control M. tb infection. These results led us to examine further the correlation between growth inhibition of M. tb with enhanced expressions of NK cytotoxic receptors, activating receptor and cytotoxic ligands.
Fig. 1.
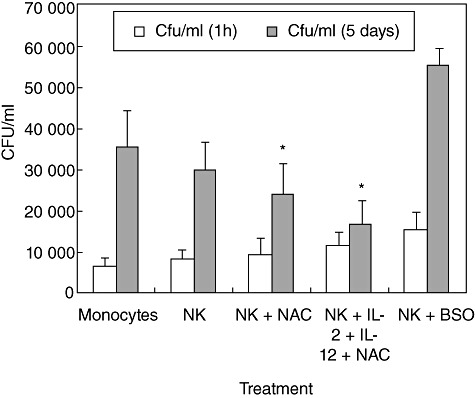
Intracellular survival of H37Rv inside natural killer (NK) cell–monocyte co-cultures from healthy individuals: human monocytes were infected with the processed virulent laboratory strain of Mycobacterium tuberculosis, H37Rv at a multiplicity of infection of 10:1. NK cells were purified using mini-magnetic affinity cell sorting (MACS) column. NK cells were treated as follows: (a) no additives; (b) N-acetyl cysteine (NAC) (20 mM); (c) NAC (20 mM) + interleukin (IL)-2 (100 U/ml) and IL-12 (100 U/ml); and (d) buthionine sulphoximine (BSO) (500 µM) for 24 h. NK cells were washed, resuspended in fresh media and then added to the infected monocytes. Infected monocyte–NK cell co-cultures were terminated at 1 h and 5 days post-infection to determine the intracellular survival of H37Rv inside human monocytes. Monocyte lysates were plated on 7H11 medium enriched with albumin dextrose complex (ADC) to estimate the growth or killing of H37Rv. Results shown are averages from five different experiments performed in triplicate. *Significant difference compared to monocytes cultured in the absence of NK cells.
Expression of NK cytotoxic receptors (NKp44, NKp30, NKp46) on cell surface of NK cells from healthy individuals
We tested the effects of NAC (alone and in combination with IL-2 + IL-12) in up-regulating the NK cell cytotoxic receptors (NKp30, NKp44, NKp46) by staining the cells with immunofluorescence antibodies and detection using flow cytometry. We observed that treatment of NK cells with IL-2 + IL-12 + NAC resulted in an almost threefold increase in the expression of NKp44 and a 1·8-fold increase in NKp30 expression (Fig. 2a,b). Treatment of NK cells with NAC alone resulted in a twofold increase in the expression of NKp44. IL-2 + IL-12 treatment resulted in a twofold increase in the expression of NKp30 and NKp44. We did not observe any changes in the expression of NKp46 during any treatment conditions (Fig. 2c). These results signify the importance of GSH (both alone and in combination with IL-2 + IL-12) in up-regulating the expression of NKp44 and NKp30 on the NK cell surface.
Fig. 2.
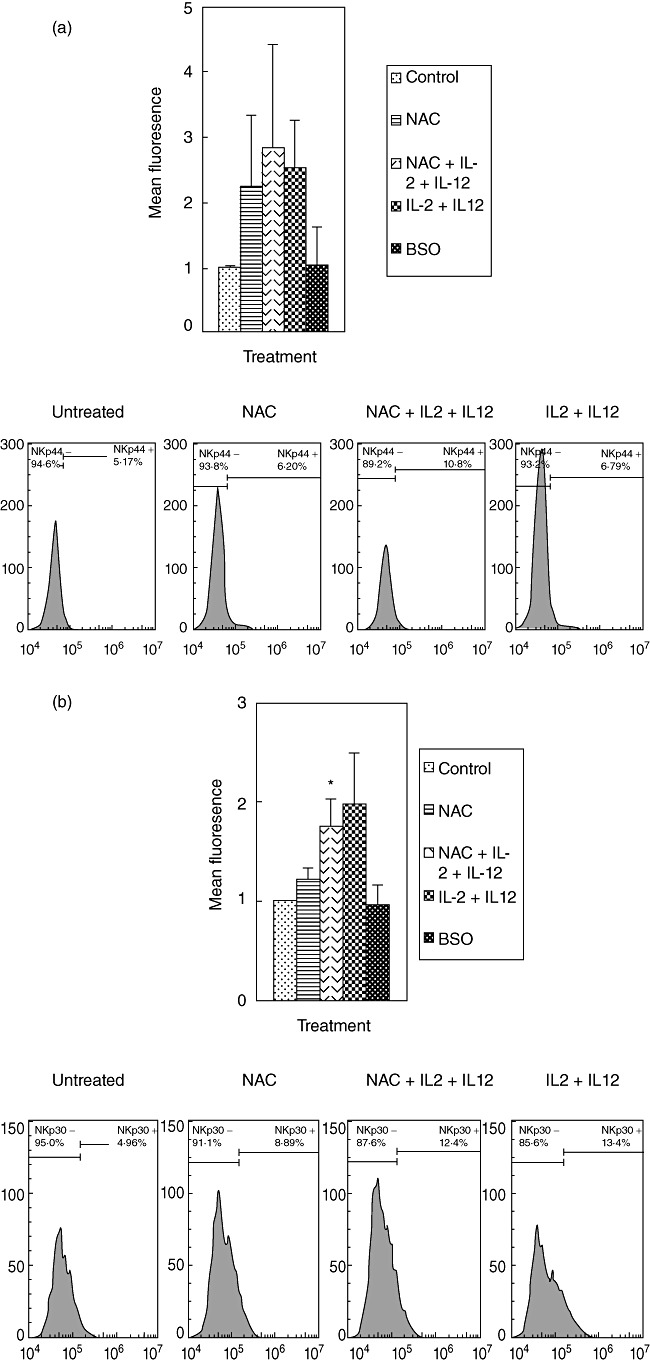
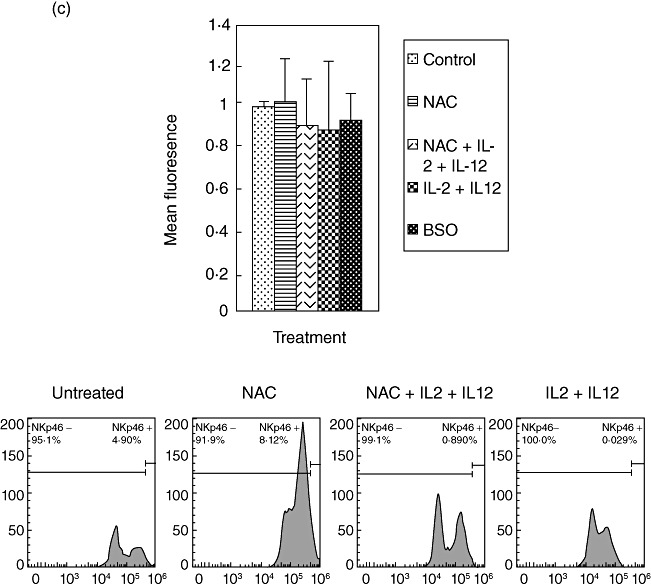
Expression of natural killer (NK) cytotoxic receptors (NKP44, NKP30 and NKP46) on NK cells from healthy individuals. NK cells were treated as follows: no additives, 20 mM of freshly prepared N-acetyl cysteine (NAC), interleukin (IL)-2 (100 U/ml) + IL-12 (100 U/ml), IL-2 + IL-12 + NAC and buthionine sulphoximine (BSO) (500 µM); 24 h after incubation, NK cells were washed and resuspended in 100 µl of phosphate-buffered saline (PBS) containing phycoerythrin (PE)-labelled NKP44 (a), PE-labelled NKP30 (b) and PE-labelled NKP46 (c), incubated at 4°C for 30 min and analysed by flow cytometry. Values obtained from different treatments are normalized to control. Data in (a–c) represent means ± standard error from four different healthy individuals performed in triplicate. Lower panels (a–c) denote representative fluorescence intensity from one experiment.
Expression of NK cell-activating receptor (NKG2D) and cytotoxic ligands (FasL and CD40L) on NK cells derived from healthy individuals
We determined the effects of GSH in conjunction with IL-2 + IL-12 in enhancing the expression of NK cell activating receptor (NKG2D) and cytotoxic ligands (FasL and CD40L) by staining the cells with immunofluorescence antibodies and detection using flow cytometry. We observed that treatment of NK cells with NAC alone as well as NAC in conjunction with IL-2 + IL-12 resulted in increased expressions of NKG2D, CD40L and FasL (Fig. 3a–c). These results signify the importance of GSH (both alone and in combination with IL-2 + IL-12) in up-regulating the expression of activating receptors and cytotoxic ligands. These activating receptors and ligands serve as markers for NK cell activation and regulate the important functions of NK cells, including induction of apoptosis of target cells.
Fig. 3.
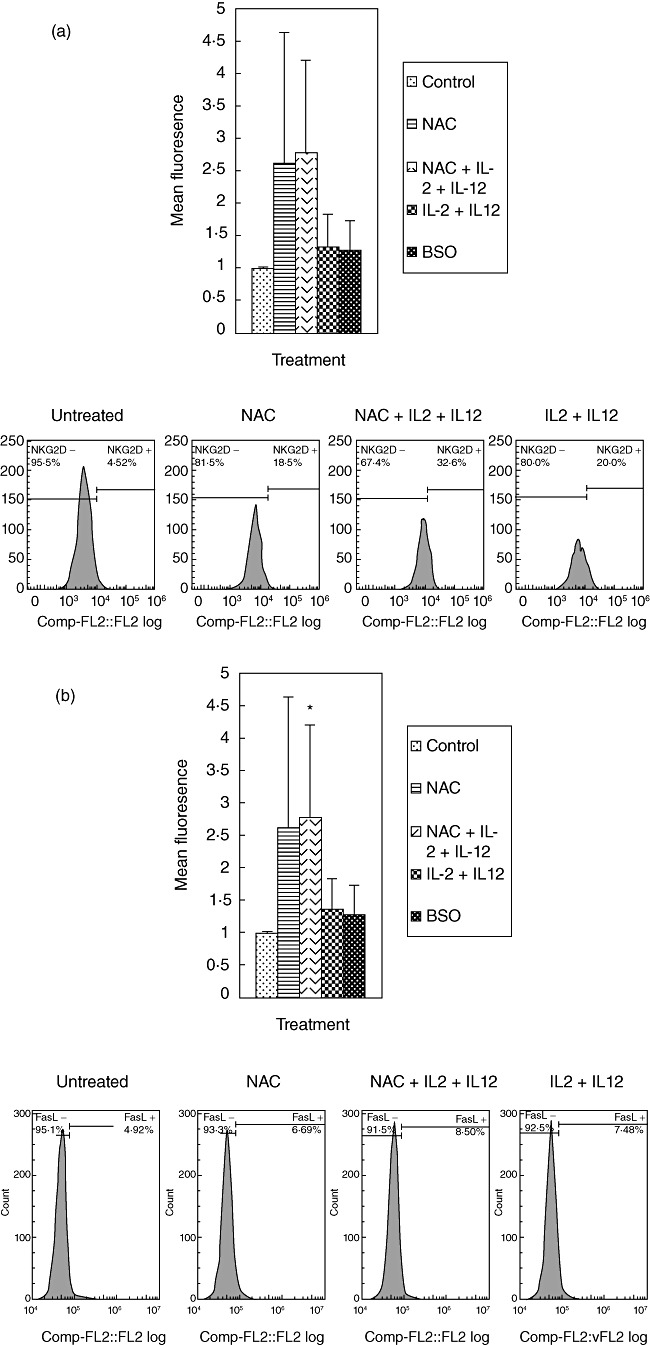
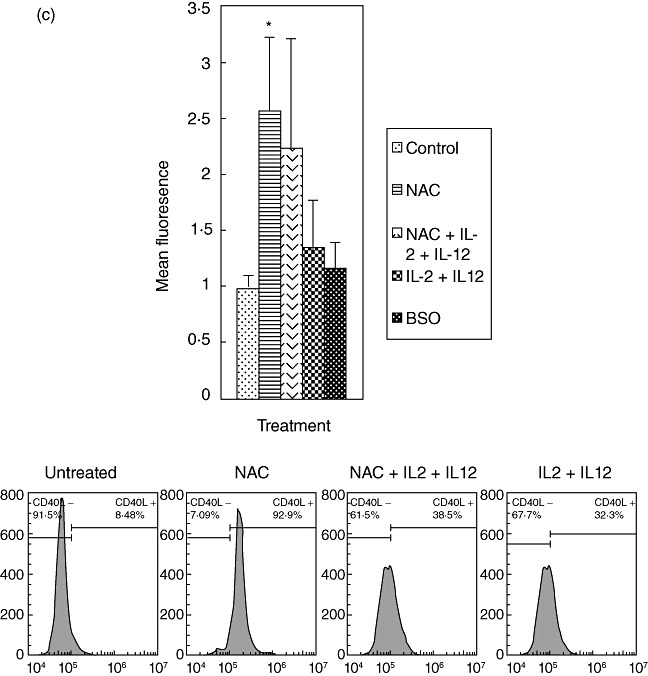
Expression of natural killer (NK) activation receptor (NKG2D) and cytotoxic ligands (FasL and CD40L) on NK cells from healthy individuals. NK cells were treated as follows: no additives, freshly prepared N-acetyl cysteine (NAC) (20 mM), IL-2 (100 U/ml) + IL-12 (100 U/ml), IL-2 + IL-12 + NAC and buthionine sulphoximine (BSO) (500 µM); 24 h after incubation, NK cells were washed and resuspended in 100 µl of phosphate-buffered saline (PBS) containing phycoerythrin (PE)-labelled NKG2D (a), PE-labelled FasL (b) and fluorescein isothiocyanate (FITC)-labelled CD40L (c), incubated at 4°C for 30 min and analysed by flow cytometry. Values obtained from different treatments are normalized to control. Data in (a) and (b) represent means ± standard error (s.e.) from four different healthy individuals performed in triplicate. Data in (c) represent means ± s.e. from six different healthy individuals performed in triplicate. Lower panels (a–c) denote representative fluorescence intensity from one experiment.
Neutralization of CD40L and FasL on NK cells derived from healthy individuals
Because the observed growth inhibition of M. tb is associated with enhanced expression of NK cytotoxic ligands, we confirmed the role of FasL and CD40L in controlling M. tb infection in monocytes by blocking these cytotoxic ligands on NK cell surface using neutralizing antibodies. Consistent with our earlier observations, treatment of NK cells with IL-2 + IL-12 + NAC resulted in stasis in the growth of H37Rv inside monocytes (Fig. 4). Interestingly, neutralization of CD40L resulted in abrogation in the static effect and a threefold increase in the intracellular growth of H37Rv (Fig. 4). Importantly, neutralization of FasL resulted in a fivefold increase in the intracellular growth of H37Rv. Similar to our earlier observations, we observed a four- and fivefold increase in the growth of H37Rv inside monocytes co-cultured with untreated NK cells and monocytes co-cultured with BSO-treated NK cells, respectively (Fig. 4). These results confirm our prediction that growth inhibition of M. tb inside monocytes requires interactions of FasL and CD40L on NK cells with corresponding Fas and CD40 on monocytes and, importantly, GSH in conjunction with cytokines enhance the expressions of these ligands on NK cell surface.
Fig. 4.
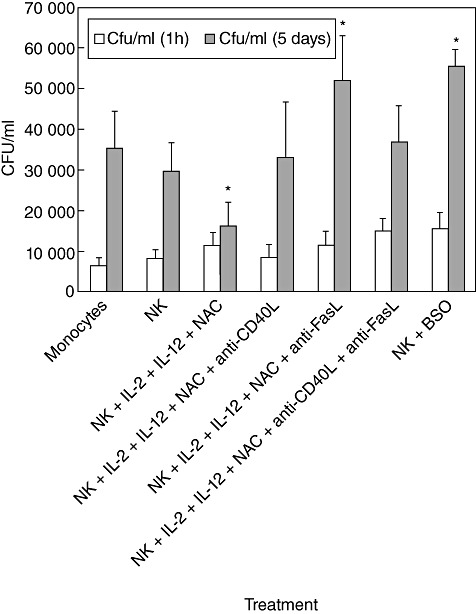
Neutralization of CD40L and FasL in natural killer (NK) cells treated with interleukin (IL)-2 + IL-12 + N-acetyl cysteine (NAC). Human monocytes isolated from healthy individuals were infected with processed H37Rv at a multiplicity of infection of 10:1. NK cells were purified using a mini-magnetic affinity cell sorting (MACS) column. NK cells were treated as follows: (a) no additives; (b) NAC (20 mM) + IL-2 (100 U/ml) and IL-12 (100 U/ml); (c) IL-2 + IL-12 + NAC + anti-FasL (100 µg/ml); (d) IL-2 + IL-12 + NAC + anti-CD40L (100 µg/ml); (e) IL-2 + IL-12 + NAC + anti-FasL + anti-CD40L (100 µg/ml); and (f) buthionine sulphoximine (BSO) (500 mM). Neutralizing antibodies-treated NK cells were added to H37Rv-infected monocytes. Infected cultures were terminated at 1 h and 5 days post-infection to determine the effects of neutralization of FasL and CD40L in abrogating the growth inhibition of H37Rv inside human monocytes. Macrophage lysates were plated on 7H11 medium enriched with albumin dextrose complex (ADC) to estimate the growth or killing of H37Rv. Results shown in are averages from five different experiments performed in triplicate. *Significant difference compared to monocytes cultured in the absence of NK cells.
Assay of GSH levels in NK cells from healthy and HIV-infected subjects
GSH levels were determined in freshly isolated NK cells from five healthy subjects and 10 individuals with HIV infection. We observed that GSH concentrations were significantly lower in NK cells isolated from individuals with HIV infection compared to NK cells from healthy subjects (Fig. 5). Decreased GSH levels in NK cells will impair innate immune responses against M. tb infection.
Fig. 5.
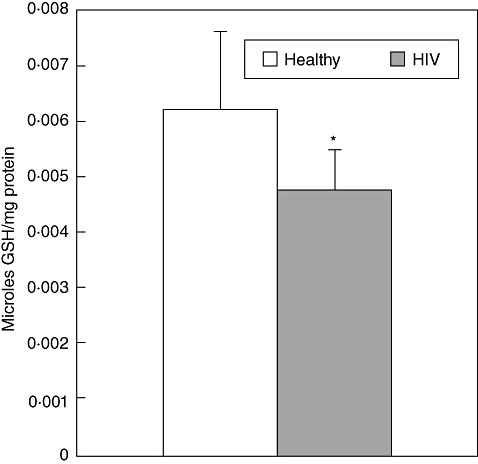
Assay of glutathione (GSH) levels in natural killer (NK) cells isolated from healthy subjects and individuals with human immunodeficiency virus (HIV) infection. Intracellular levels of GSH in NK cell lysates from healthy subjects and individuals with HIV infection were determined by spectrophotometry, using an assay kit from Calbiochem. NK cells (2 × 105/well) lysed with ice-cold 5% metaphosphoric acid (MPA) was added to the pellet. Supernatants were collected after centrifugation and analysed for total GSH as per the manufacturer's instructions. Total GSH in the samples were normalized with protein. Proteins in the samples were estimated by Bradford's method using BioRad reagent. Results shown are averages from experiments performed in five healthy subjects and 10 individuals with HIV infection.
Determination of intracellular viability of M. tb in monocyte–NK cell co-cultures from HIV-positive individuals
The observation that GSH levels are significant decreased in NK cells isolated from individuals with HIV infection led us to test the functional defects of NK cells from this study group. We predicted that augmentation of GSH in NK cells isolated from individuals with HIV infection would enhance their ability to control M. tb infection inside macrophages. We tested our prediction by determining the intracellular survival of H37Rv in co-cultures of monocytes and NK cells from individuals with HIV infection. Results from the experiments performed in seven different individuals revealed a fivefold increase in the growth of H37Rv inside monocytes cultured both in the presence and absence of NK cells (Fig. 6). This is contrast to healthy subjects, where we observed only a fourfold and threefold increase in the growth of H37Rv in monocytes cultured in the absence and presence of NK cells, respectively. These results signify that monocytes and NK cells from individuals with HIV infection are highly permissive to the growth of M. tb. Co-incubation of H37Rv-infected monocytes with NK cells that were treated with NAC+IL-2 + IL-12 resulted in reduction in the fold increases in the growth of M. tb inside monocytes compared to other treatment conditions. There was only a threefold increase in CFU of H37Rv between the initial and final time-point of termination. However, NAC+IL-2 + IL-12-treatment of NK cells did not result in complete stasis in the growth of M. tb as seen in healthy subjects (Fig. 6). Incubation of H37Rv-infected monocytes with BSO-treated NK cells; NAC+IL-2 + IL-12 + anti-FasL-treated NK cells; NAC+IL-2 + IL-12 + anti-CD40L-treated NK cells; NAC+IL-2 + IL-12 + anti-FasL+CD40L-treated NK cells resulted in a sixfold increase in the growth of H37Rv between the initial and final time-point of termination. These results confirm the importance of GSH, FasL and CD40L in inducing growth restriction of M. tb.
Fig. 6.
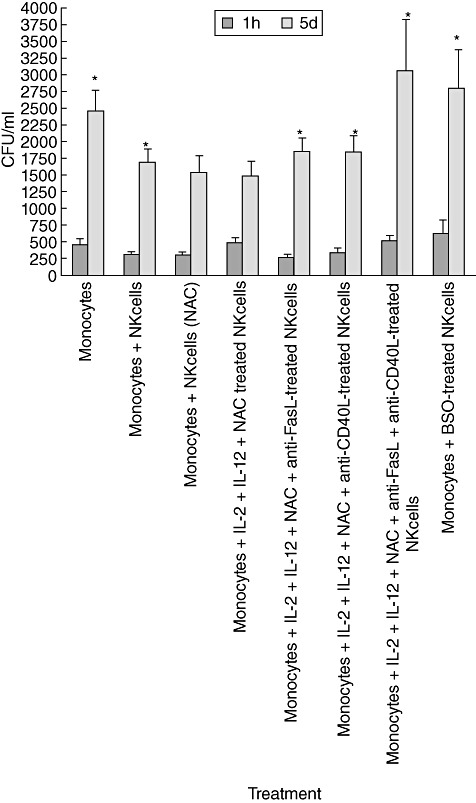
Intracellular survival of H37Rv inside natural killer (NK) cell–monocyte co-cultures from individuals with human immunodeficiency virus (HIV) infection. Human monocytes isolated from individuals with HIV infection were infected in vitro with processed H37Rv at a multiplicity of infection of 10:1. NK cells were purified using a mini-magnetic affinity cell sorting (MACS) column. NK cells were treated as follows: (a) no additives; (b) N-acetyl cysteine (NAC) (20 mM) + interleukin (IL)-2 (100 U/ml) and IL-12 (100 U/ml); (c) IL-2 + IL-12 + NAC + anti-FasL (100 µg/ml); (d) IL-2 + IL-12 + NAC + anti-CD40L (100 µg/ml); (e) IL-2 + IL-12 + NAC + anti-FasL + anti-CD40L (100 µg/ml) and (f) BSO (500 mM). Neutralizing antibodies-treated NK cells were added to H37Rv-infected monocytes. Infected cultures were terminated at 1 h and 5 days post-infection to determine the effects of neutralization of FasL and CD40L in abrogating the growth inhibition of H37Rv inside human monocytes. Macrophage lysates were plated on 7H11 medium enriched with albumin dextrose complex (ADC) to estimate the growth or killing of H37Rv. Results shown are averages from seven different experiments performed in triplicate.
Discussion
Mycobacteria do not synthesize GSH. Rather, they produce mycothiol in millimolar amounts. We have reported that GSH plays an important role in limiting growth of intracellular BCG and H37Rv in both murine and human macrophages [6]–[8]. In other words, GSH has direct anti-mycobacterial activity [7]. Our group is a pioneer in reporting that GSH levels are decreased in individuals with pulmonary TB and in correlating decreased GSH levels with enhanced growth of M. tb and increased production of proinflammatory cytokines [11]. Both macrophages and NK cells play an important role in innate defence against M. tb infection.
NK cells are inflammatory cytokine-producing and/or cytotoxic lymphocytes that comprise a major component of the innate immune system, and are especially responsible for protection against tumorigenisis and viral infection [12]–[15].
In this study, we observed that cytokine stimulation along with GSH enhancement exacerbates the function of NK cells to induce complete stasis in the growth of H37Rv inside human monocytes derived from healthy subjects (Fig. 1). However, this stasis effect is diminished when NK cells were treated with a GSH depleting agent, BSO as evidenced by a fivefold increase in the intracellular growth of H37Rv (Fig. 1).
NK cells bear a variety of cytotoxic receptors that are restricted to NK cells. These include NKp30, NKp44 and NKp46 receptors, the enhanced expression of which is associated with lysis of tumour and microbe-infected cells [14]–[18]. We found that GSH in conjunction with IL-2 + IL-12 enhances the expression of NK-44 and NKP30 on the NK cell surface (Fig. 2).
The destructive potential of NK cells is held in check by the expression of a panel of inhibitory receptors [14]. Of prime importance are the killer-cell immunoglobulin-like receptors (KIR) and CD94/NKG2A receptors that recognize major histocompatibility complex (MHC) class I molecules that tend to be expressed by all normal cells [14]. NK cells express a large variety of activation receptors that mediate the recognition and destruction of aberrant cells [14]. A prime example is NKG2D-DAP10, which recognizes ligands – MICA/B and UBLPs in humans – that tend to be elevated in expression on tumour cells, virally infected cells and other abnormal cells. Many of the ligands recognized by these activation receptors can be expressed by normal cells and, hence, can potentiate autoimmunity [14]. NKG2D-DAP10 can also be expressed by T lymphocytes and, as such, can facilitate several autoimmune diseases [14]. The results of our studies indicate that increasing GSH levels in NK cells from healthy subjects, in both the presence and absence of IL-2 + IL-12, result in up-regulation in the expression of NKG2D (Fig. 3a).
Fas (APO-1/CD95) [19], a member of the TNF receptor family, and its ligand (FasL) [19],[20], play an important role in various processes involving the induction of apoptosis. Binding of FasL to Fas results in the transduction of a signal into the cell, leading to apoptosis [19],[20]. Interestingly, NK cells can express FasL. Studies have shown that that FasL-induced apoptosis of human macrophages is associated with a substantial reduction in the viability of intracellular mycobacteria [20]. A recent study has analysed the expression and functionality of the CD95/CD95L system during acute HIV infection, and its eventual role in PBMC apoptosis [19]. The results of this study indicate that nearly all PBMC, whether CD4 or CD8 T cells, B cells or NK cells, expressed CD95, a key molecule in the process of activation-induced cell death. The reason for this up-regulation is not clearly known. Increased amount of circulating virions and tumour necrosis factor (TNF)-α might be possible reasons for the increased expression of CD95. Interestingly, CD95L, the natural ligand of CD95, was not expressed by cells from patients with acute HIV. These findings indicate that deregulation of the CD95/CD95L system is characteristic of acute HIV infection and may persist for months, even in association with stable reductions in viral load and T cell activation [19].
Engagement of CD40 on antigen-presenting cells is central to the initiation of immune response. NK cells express CD40L (CD154) which interacts with CD40, a 50 kDa membrane glycoprotein expressed on monocytes [21]–[23]. Expression of CD154 in response to Candida albicans, cytomegalovirus or Toxoplasma gondii was impaired in cells from individuals with HIV infection [23]. This defect correlated with decreased production of IL-12 and interferon (IFN)-γ in response to T. gondii. Recombinant CD154 partially restored secretion of IL-12 and IFN-γ in response to T. gondii in cells from patients with HIV infection. Together, defective induction of CD154 is likely to contribute to impaired cell-mediated immunity against opportunistic pathogens in HIV-infected patients [23]. Our results indicate that augmenting GSH levels in NK cells from healthy subjects (both in the presence and absence of IL-2 + IL-12) result in up-regulation in the expressions of both FasL and CD40L (Fig. 3b,c). Importantly, neutralization of FasL and CD40L in IL-2 + IL-12 + NAC-treated NK cells resulted in abrogation in the growth inhibition of H37Rv (Fig. 4).
The ability of GSH in combination with IL-2 + IL-12 to augment the activity of NK cells resulting in the control of M. tb infection inside monocytes in healthy individuals further led us to hypothesize that the levels of GSH will be compromised in NK cells derived from individuals with HIV infection, and this decrease will be accompanied by increased growth of M. tb in co-cultures of monocytes and NK cells. We tested our hypothesis by determining the levels of GSH in NK cells derived from individuals with HIV infection and testing the survival of H37Rv in monocytes cultured in presence of NK cells (both derived from HIV-positive subjects). Our results indicate that individuals with HIV infection have significantly lower levels of GSH in their NK cells in comparison to healthy subjects (Fig. 5). The decrease in GSH levels correlated with increased growth of M. tb inside human macrophages (Fig. 6). Our results signify the importance of GSH in augmenting the functions of NK cells to limit the growth of M. tb inside monocytes and macrophages. In-vitro treatment of NK cells isolated from individuals with HIV infection with NAC + IL-2 + IL-12 resulted in improved control of M. tb infection inside human monocytes (Fig. 6). We also observed a poor uptake of M. tb by monocytes derived from individuals with HIV infection compared to healthy individuals as evidence by one log lower colony counts recovered from the co-cultures of monocytes and NK cells. This reduced uptake of M. tb could be due possibly to impaired phagocytic capacity of monocytes that are derived from HIV-positive individuals.
To summarize, we demonstrate that treatment of NK cells with IL-2 + IL-12 + NAC resulted in inhibition in the growth of H37Rv. This inhibition is accompanied by enhanced expressions of NK activating receptor (NKG2D), cytotoxic receptors (NKP30 and NKP44) and cytotoxic ligands (FasL and CD40L). Our results unveil an important pathway by which cytokines, in conjunction with GSH, enhance the functions of NK cells to control M. tb infection. These studies support our hypothesis that NK cells require adequate GSH levels and cytokine stimulation to effectively carry out its innate defence against M. tb infection in both healthy subjects and individuals with HIV infection.
Acknowledgments
This work is supported by the Potts Memorial Foundation and start-up funds from Western University of Health Sciences. The authors acknowledge Dr Gerald Thrush and Brenda Gonzalez for helpful discussions. We thank the staff at the Western University Medical Center for their help with blood draw.
Disclosure
The authors declare no conflict of interest.
References
- 1.Chiang CY, Centis R, Migliori GB. Drug-resistant tuberculosis: past, present, future. Respirology. 2010;15:413–32. doi: 10.1111/j.1440-1843.2010.01738.x. [DOI] [PubMed] [Google Scholar]
- 2.Dutta NK, Mehra S, Kaushal D. A Mycobacterium tuberculosis sigma factor network responds to cell-envelope damage by the promising anti-mycobacterial thioridazine. PLoS ONE. 2010;5:e10069. doi: 10.1371/journal.pone.0010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deneke SM, Fanburg BL. Regulation of cellular glutathione. Am J Physiol. 1989;257(4 (Pt 1)):L163–73. doi: 10.1152/ajplung.1989.257.4.L163. [DOI] [PubMed] [Google Scholar]
- 4.Griffith OW. Biologic and pharmacologic regulation of mammalian glutathione synthesis. Free Radic Biol Med. 1999;27:922–35. doi: 10.1016/s0891-5849(99)00176-8. [DOI] [PubMed] [Google Scholar]
- 5.Havlir DV, Getahun H, Sanne I, Nunn P. Opportunities and challenges for HIV care in overlapping HIV and TB epidemics. JAMA. 2008;300:423–30. doi: 10.1001/jama.300.4.423. [DOI] [PubMed] [Google Scholar]
- 6.Venketaraman V, Dayaram YK, Amin AG, et al. Role of glutathione in macrophage control of mycobacteria. Infect Immun. 2003;71:1864–71. doi: 10.1128/IAI.71.4.1864-1871.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Venketaraman V, Dayaram YK, Talaue MT, Connell ND. Glutathione and nitrosoglutathione in macrophage defense against M. tuberculosis. Infect Immun. 2005;73:1886–94. doi: 10.1128/IAI.73.3.1886-1889.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dayaram YK, Talaue MT, Connell ND, Venketaraman V. Characterization of a glutathione metabolic mutant of Mycobacterium tuberculosis and its resistance to glutathione and nitrosoglutathione. J Bacteriol. 2006;188:1364–72. doi: 10.1128/JB.188.4.1364-1372.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Millman AC, Salman M, Dayaram YK, Connell ND, Venketaraman V. Natural killer cells, glutathione, cytokines and innate immunity against Mycobacterium tuberculosis. J Interferon Cytokine Res. 2008;28:1–13. doi: 10.1089/jir.2007.0095. [DOI] [PubMed] [Google Scholar]
- 10.Venketaraman V, Rodgers T, Linnares R, et al. Tuberculosis immunity in healthy and HIV-infected subjects. AIDS Res Ther. 2006;3:5. doi: 10.1186/1742-6405-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Venketaraman V, Millman AC, Salman M, et al. Glutathione levels and immune responses in tuberculosis patients. Microb Pathog. 2008;44:255–61. doi: 10.1016/j.micpath.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Yamauchi A, Bloom ET. Requirement of thiol compounds as reducing agents for IL-2-mediated induction of LAK activity and proliferation of human cells. J Immunol. 1993;151:5535–44. [PubMed] [Google Scholar]
- 13.Brill KJ, Li Q, Larkin R, et al. Human natural killer cells mediate killing of intracellular Mycobacterium tuberculosis H37Rv via granule-independent mechanisms. Infect Immun. 2001;69:1755–65. doi: 10.1128/IAI.69.3.1755-1765.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moretta L, Biassoni R, Bottino C, Mingari MC, Moretta A. Human NK-cell receptors. Immunol Today. 2000;21:420–2. doi: 10.1016/s0167-5699(00)01673-x. [DOI] [PubMed] [Google Scholar]
- 15.Sivori S, Vitale M, Morelli L, et al. p46, a novel natural killer cell-specific surface molecule that mediates cell activation. J Exp Med. 1997;186:1129–36. doi: 10.1084/jem.186.7.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vitale M, Bottino C, Sivori S, et al. NKp44, a novel triggering surface molecule specifically expressed by activated natural killer cells, is involved in non-major histocompatibility complex-restricted tumor cell lysis. J Exp Med. 1998;12:2065–72. doi: 10.1084/jem.187.12.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pende D, Parolini S, Pessino A, et al. Identification and molecular characterization of NKp30, a novel triggering receptor involved in natural cytotoxicity mediated by human natural killer cells. J Exp Med. 1999;190:1505–16. doi: 10.1084/jem.190.10.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bermudez LE, Wu M, Young LS. Interleukin-12-stimulated natural killer cells can activate human macrophages to inhibit growth of Mycobacterium avium. Infect Immun. 1995;63:4099–104. doi: 10.1128/iai.63.10.4099-4104.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cossarizza A, Stent G, Mussini C, et al. Deregulation of the CD95/CD95L system in lymphocytes from patients with primary acute HIV infection. AIDS. 2000;14:345–55. doi: 10.1097/00002030-200003100-00007. [DOI] [PubMed] [Google Scholar]
- 20.Oshimi Y, Oda S, Honda Y, Nagata S, Miyazaki S. Involvement of Fas ligand and Fas-mediated pathway in the cytotoxicity of human natural killer cells. J Immunol. 1996;157:2909–15. [PubMed] [Google Scholar]
- 21.Alderson MR, Armitage RJ, Tough TW, Strockbine L, Fanslow WC, Spriggs MK. CD40 expression by human monocytes: regulation by cytokines and activation of monocytes by the ligand for CD40. J Exp Med. 1993;178:669–74. doi: 10.1084/jem.178.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carbone E, Ruggiero G, Terrazzano G, et al. A new mechanism of NK cell cytotoxicity activation: the CD40-CD40 ligand interaction. J Exp Med. 1997;185:2053–60. doi: 10.1084/jem.185.12.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Subauste CS, Wessendarp M, Portilllo JA, et al. Pathogen-specific induction of CD154 is impaired in CD4+ T cells from human immunodeficiency virus-infected patients. J Infect Dis. 2004;189:61–70. doi: 10.1086/380510. [DOI] [PubMed] [Google Scholar]


