Abstract
The development of the human wrist joint has been studied widely, with the main focus on carpal chondrogenesis, ligaments and triangular fibrocartilage. However, there are some discrepancies concerning the origin and morphogenetic time-table of these structures, including nerves, muscles and vascular elements. For this study we used serial sections of 57 human embryonic (n = 30) and fetal (n = 27) specimens from O’Rahilly stages 17–23 and 9–14 weeks, respectively. The following phases in carpal morphogenesis have been established: undifferentiated mesenchyme (stage 17), condensated mesenchyme (stages 18 and 19), pre-chondrogenic (stages 19 and 20) and chondrogenic (stages 21 and over). Carpal chondrification and osteogenic processes are similar, starting with capitate and hamate (stage 19) and ending with pisiform (stage 22). In week 14, a vascular bud penetrates into the lunate cartilaginous mold, early sign of the osteogenic process that will be completed after birth. In stage 18, median, ulnar and radial nerves and thenar eminence appear in the hand plate. In stage 21, there are indications of the interosseous muscles, and in stage 22 flexor digitorum superficialis, flexor digitorum profundus and lumbrical muscles, transverse carpal ligament and collateral ligaments emerge. In stage 23, the articular disc, radiocarpal and ulnocarpal ligaments and deep palmar arterial arch become visible. Radiate carpal and interosseous ligaments appear in week 9, and in week 10, dorsal radiocarpal ligament and articular capsule are evident. Finally, synovial membrane is observed in week 13. We have performed a complete analysis of the morphogenesis of the structures of the human wrist joint. Our results present new data on nervous and arterial elements and provide the basis for further investigations on anatomical pathology, comparative morphology and evolutionary anthropology.
Keywords: carpal, development, embryology, fetuses, morphogenesis, wrist joints
Introduction
The development and morphogenesis of the human wrist joint have been widely studied (Andersen & Bro-Rasmussen, 1961; Gardner, 1963; Lewis, 1970; Oztuna et al. 2003; Lee et al. 2004) but there are no unanimous criteria for the determination of the origin and development of the different elements involved in the organization of this system and no complete study about the morphogenetic time-table in human wrist specimens has been done.
Many studies have been published about distal radius and ulna and carpal chondrification, which has been described classically as a process that starts with capitate (os capitatum) and hamate (os hamatum), and ends with pisiform (os pisiforme) (Gray et al. 1957; Genis, 1970; Hamilton & Mossman, 1975; O’Rahilly & Gardner, 1975; O’Rahilly et al. 1981; Sadler, 2007). Traditionally, a three-phase sequence (undifferentiated condensed mesenchyme, chondrification and ossification) has been accepted in carpal morphogenesis, but some discrepancies have appeared when setting the time-table for these periods. Mesenchymal condensations have been described in specimens with a crown–rump length of 7–12 mm (O’Rahilly et al. 1956) or 10–14 mm (Genis, 1970), and carpal chondrification has been observed first at 13–17 mm (O’Rahilly & Gardner, 1975) or 15–20 mm (Genis, 1970). Ossification centers appear only after birth.
Some studies about the wrist joint showed that the first over sign of joint formation is the appearance of an interzone in the condensed mesenchymal structure (which is the blueprint of the developing skeleton in early embryonic limbs), and that interzone cells have special cytological characteristics that distinguish them from the condensed mesenchyme of neighboring skeletal anlagen (Archer et al. 2003; Pacifici et al. 2006). The interzonal mesenchyme of developing synovial joints becomes trilaminar as a more tenuous intermediate zone appears, splitting the mesenchyme into two dense strata next to the cartilaginous ends of the skeletal elements of the region. As the dense strata of the interzonal mesenchyme also become cartilaginous, subsequent cavitation of the intermediate zone establishes the cavity of the joint. The loose vascularized mesenchyme around the cavity forms the synovial membrane (stratum synoviale) and probably also gives rise to all other intra-articular structures (Gardner, 1963; Genis, 1970; Moore & Persaud, 2000; Moore et al. 2008; Standring, 2008). Some previous studies have shown that the origin of the fibrous capsule (stratum fibrosum) of radiocarpal and intercarpal joints is the interzonal mesenchyme (Keith, 1933; Gardner, 1963; Gardner & O’Rahilly, 1968), whereas other studies have supported an extramesenchymal origin (Haines, 1947).
The triangular fibrocartilage is one of the most analyzed ligamentous structures in the wrist joint because it is frequently involved in wrist pain, especially in the elderly (Mikić, 1978; Cavalcante et al. 2004; Lee et al. 2004; Shigemitsu et al. 2007). Its morphology and development (Palmer & Werner, 1981; Mohiuddin & Janjua, 1982; García-Elias & Domenech-Mateu, 1987; Mérida-Velasco et al. 1996), histological fine structure (Benjamin et al. 1990; Chidgey et al. 1991; Hogikyan & Louis, 1992; Mikić et al. 1992; Nakamura & Yabe, 2000; Nakamura et al. 2001; Nishikawa & Toh, 2002) and immunohistochemical features and regional differences in the content and mobility (Milz et al. 2007; Shigemitsu et al. 2007) have been widely described in the last three decades.
Although numerous authors have been focused on the triangular fibrocartilage, very few studies have been dedicated to other ligamentous structures. The embryonic period has been divided into 23 developmental stages based on external and internal morphological criteria. Stages 10–23 were described in detail by Streeter (1951), stages 1–9 were established by O’Rahilly (1973), and the entire system was revised by O’Rahilly & Müller (1987). The emergence of human wrist joint ligaments starts in stage 22 and their organization is complete by the end of week 14 (Mérida-Velasco et al. 1996). Classically, it has been considered that interosseous intercarpal ligaments (ligamenta intercarpalia interossea) originate from synovial (Andersen & Bro-Rasmussen, 1961) or interzonal mesenchyme (Gardner, 1963; Moore & Persaud, 2000), whereas the rest of the ligaments derive from localized thickenings of the capsule (Gardner et al. 1969; Goss, 1973; Mayfield et al. 1976; Berger & Landsmeer, 1990; Mérida-Velasco et al. 1996; Oztuna et al. 2003; Rouvière & Delmas, 2005).
Several theories have been proposed about the development of the vascular system of the upper limb. Müller (1903) suggested that it results from the remodeling of the complex primitive network, whereas Singer (1933) described a process of sprouting from the axial arterial. Still other authors stated that the definitive arterial pattern takes place by a combined process which begins at stage 12 (Rodriguez-Niedenfuhr et al. 2001). Initially, a capillary plexus enters the limb and in later stages only one trunk, the axial artery which supplies the limb and the terminal capillary plexus. From this axial artery, ulnar (arteria ulnaris) and radial (arteria radialis) arteries appear successively (Rodriguez-Niedenfuhr et al. 2001; Morris et al. 2005; Carlson, 2009).
The arrangement of the muscles of the hand and their innervations has been described (Salsbury, 1937; Lansmeer, 1955; Stack, 1963) and it is generally agreed that the muscles develop in situ and not by migration (Kuczynski, 1972). The original undifferentiated mesenchyme of the hand divides at an early stage into superficial and deep layers. From the superficial layer, three muscle primordia develop: radial, middle and ulnar, giving rise to the abductor pollicis brevis (m. abductor pollicis brevis), flexor digitorum superficialis (m. flexor digitorum superficialis), which extends up towards the forearm to its definitive position (Beatty, 1985), and abductor digiti minimi (m. abductor digiti minimi) muscles, respectively (Bardeen, 1905; Cihák, 1972). The rest of the thenar (eminentia thenaris) and hypothenar (eminentia hypothenaris) eminences derive from the deep layer and develop at a later stage. Interosseous (m. interossei) and a contrahentes muscle are formed from the deep layer. The contrahentes recedes from the ulnar half of the hand and becomes the adductor pollicis muscle (m. adductor pollicis). In stage 23, the classical skeletal muscle striated pattern is completed (Markze, 1971; Cihák, 1972; O’Rahilly & Gardner, 1975). How specific nerves are directed to supply specific muscles is still not fully understood (Al-Qattan et al. 2009). The beginnings of brachial plexus are present in stage 14 (Fritsch, 2003) and the nerves start to enter the limb bud in stage 15 (Rodriguez-Niedenfuhr et al. 2001); however, Al-Qattan et al. (2009) described this latter event in stage 16. The median nerve, the radial (nervus radialis) and the ulnar (nervus ulnaris) nerves enter into the hand plate (Shinohara et al. 1990). In stages 20 and 21, the upper limb nerves were observed in an orientation and arrangement similar to those in the adult, and by the end of stage 23, neural elements of the wrist have achieved their definitive morphology (Lewis, 1902; O’Rahilly & Gardner, 1975; Rodriguez-Niedenfuhr et al. 2001).
The purpose of the present study is (i) to analyze the human wrist joint during the embryonic and early fetal period and determine the sequence of chondrogenic events that take place in the human wrist joint, (ii) to establish the normal pattern of emergence and development of ligaments, fibrous and fibrocartilaginous structures, and (iii) to document the temporal-spatial sequence of the arterial and neuromuscular elements of this region.
Materials and methods
For this study, we used 30 embryos (Table 1) and 27 fetuses (Table 2) from the Embryo Collection of the Department of Anatomy and Human Embryology from the Medical School of the University of Granada and the Department of Morphological Sciences II from the School of Medicine of the Complutense University of Madrid. The crown–rump length of 12–31-mm embryos ranging from O’Rahilly stages 17 to 23, and 33–113-mm crown–rump length fetuses ranging from 9 to 14 weeks of development were analyzed. The descriptions of O’Rahilly stages were summarized with reference to both external and internal development and proposed ages were based on those of O’Rahilly et al. (1981) and O’Rahilly & Müller (1987).
Table 1.
Features and details of human embryos.
| Embryos | Greatest crown–rump length (mm) | Plane of section | O’Rahilly’s stage Ca. | Proposed age (postovulatory days) |
|---|---|---|---|---|
| JD-6 | 12 | Transversal | 17 | 41 |
| JD-5 | 13 | Transversal | 17 | ca. 41 |
| NO | 15 | Transversal | 17 | ca. 41 |
| SG-1 | 15 | Transversal | 17 | ca. 41 |
| GG-1 | 16 | Transversal | 18 | ca. 44 |
| BE-1 | 17 | Transversal | 19 | 47–48 |
| X-12 | 18 | Transversal | 19 | 47–48 |
| EH-19 | 19 | Transversal | 20 | 50–51 |
| JD-7 | 19 | Transversal | 20 | 50–51 |
| BB-5 | 20 | Transversal | 20 | 50–51 |
| JD-2 | 20 | Transversal | 20 | 50–51 |
| PT-9 | 20 | Transversal | 20 | 50–51 |
| R-1 | 21 | Transversal | 21 | ca. 52 |
| MA-7 | 22 | Transversal | 21 | ca. 52 |
| X-6 | 22.5 | Transversal | 22 | ca. 54 |
| PE-8 | 23 | Transversal | 22 | ca. 54 |
| HÁ-2 | 23 | Transversal | 22 | ca. 54 |
| CH-1 | 24 | Transversal | 22 | ca. 54 |
| EA-3 | 24.5 | Sagittal | 22 | ca. 54 |
| BB-4 | 26 | Transversal | 22 | ca. 54 |
| GV-4 | 27 | Transversal | 22 | ca. 54 |
| NA-2 | 27.5 | Transversal | 22 | ca. 54 |
| HE-1 | 28 | Transversal | 23 | 56–57 |
| FA-5 | 28 | Transversal | 23 | 56–57 |
| NA-1 | 29 | Transversal | 23 | 56–57 |
| RI-4 | 29 | Transversal | 23 | 56–57 |
| BB-2 | 30 | Transversal | 23 | 56–57 |
| X-18 | 30 | Transversal | 23 | 56–57 |
| H-23 | 31 | Transversal | 23 | 56–57 |
| X-4 | 31 | Transversal | 23 | 56–57 |
Table 2.
Features and details of human fetuses.
| Fetuses | Crown–rump length (mm) | Plane of section | Weeks of development |
|---|---|---|---|
| H-23 | 33 | Transversal | 9 |
| MA-4 | 35 | Transversal | 9 |
| X-12 | 35 | Transversal | 9 |
| CA-1 | 35 | Transversal | 9 |
| RI-1 | 36 | Transversal | 9 |
| BB-1 | 39 | Transversal | 9 |
| H-19 | 39 | Transversal | 9 |
| GV-3 | 41 | Transversal | 10 |
| AM-1 | 41 | Transversal | 10 |
| PE-7 | 41 | Transversal | 10 |
| ZO-1 | 42 | Transversal | 10 |
| AS-1 | 44 | Transversal | 10 |
| GV-1 | 45 | Transversal | 10 |
| MA-3 | 46 | Transversal | 10 |
| SA-3 | 48 | Transversal | 10 |
| MA-2 | 50 | Transversal | 11 |
| X-8 | 50 | Sagittal | 11 |
| MA-1 | 52 | Transversal | 11 |
| BB-3 | 53 | Transversal | 11 |
| X-11 | 53 | Transversal | 11 |
| H-4 | 62 | Transversal | 12 |
| SA-4 | 63 | Transversal | 12 |
| PE-3 | 70.5 | Transversal | 12 |
| JM-1 | 80 | Transversal | 13 |
| OL-1 | 83 | Transversal | 13 |
| ZO-2 | 102 | Sagittal | 14 |
| BU | 113 | Transversal | 14 |
No information about sex attribution or pathologies was reported in our sample. No individual variations were observed in the specimens analyzed. This study was approved by the Ethics Committee of the Medical Schools of the University of Granada and the Complutense University of Madrid.
Transverse or sagittal serial sections 8–10 μm thick were prepared in 24–72 h 10% formaldehyde fixation for this study. They were dehydrated by growing concentrations of ethanol and cleared with methyl benzoate and benzol, and embedded in ordinary paraffin at 58–60 °C. Harris hematoxylin–eosin (McManus & Mowry, 1968) and Harris hematoxylin–V.O.F. (Gutiérrez et al. 1963) were used for light microscopic study.
Results
Chondrification
In O’Rahilly stage 17, mesenchymal condensations of the future metacarpals (Fig. 1A) and the digital rays can be observed in the hand plate, and in stage 18, carpal chondrogenic differentiation begins. In stage 19, chondrogenesis is evident in several segments of the wrist and hand. In stage 20, capitate bone is the first structure that appears as immature precartilage, and scaphoid (os scaphoideum), lunate (os lunatum), hamate and triquetrum (os triquetrum) bones are noted at stage 21. In stage 22, trapezium (os trapezium) and trapezoid (os trapezoideum) bones are observed (Fig. 1B), with pisiform the latest carpal bone to appear. In stage 23, hamulus (hamulus ossis hamati) appears as a very immature cartilage formation, and the rest of the bone is in a chondrification phase. Its definitive morphology cannot be observed until week 13.
Fig. 1.
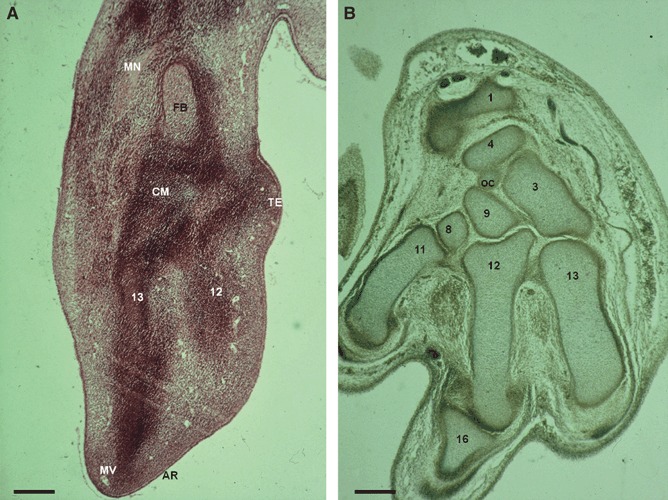
(A) Human embryo: G.G.-1 (16 mm), O’Rahilly stage 18. Transverse section (8 μm thick). AR, apical ridge; CM, carpal mass formed by very condensed mesenchyme; FB, cartilage mold for the future bones of the forearm; MN, median nerve; MV, vascular traces of marginal vein; TE, future thenar eminence. (12) Second metacarpal bone in a very immature chondrogenic phase. (13) Third metacarpal bone in a very immature chondrogenic phase. Scale bar: 125 μm. (B) Human embryo: P.E.-8 (23 mm), O’Rahilly stage 22. Transverse section (8 μm thick). OC, os central. (1) Distal epiphysis of the radius. (3) Capitate bone. (4) Scaphoid. (8) Trapezium bone. (9) Trapezoid bone. (11) First metacarpal bone. (12) Second metacarpal bone. (13) Third metacarpal bone. (16) Phalanx. Scale bar: 125 μm.
A cartilaginous condensation for the future os centrale appears in 23-mm specimens (Fig. 1B). The fusion with the scaphoid starts in stage 23 and is complete in week 9, at the beginning of the fetal period (Fig. 2A).
Fig. 2.
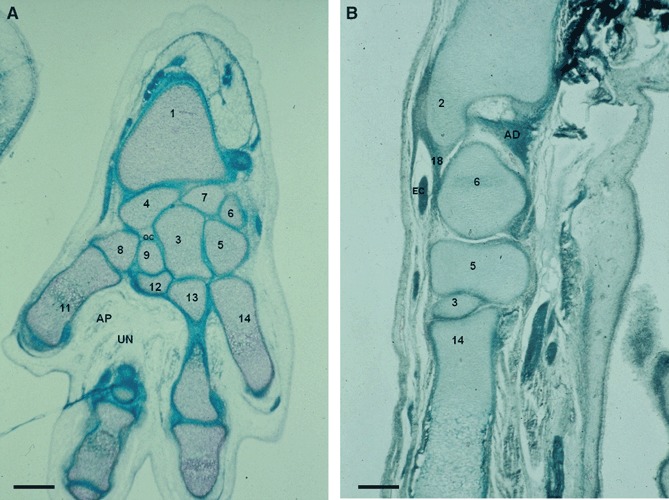
(A) Human fetus: C.A.-1 (35 mm), week 9. Transverse section (10 μm thick). AP, adductor pollicis muscle; OC, os centrale; UN, deep branch of the ulnar nerve. (1) Distal epiphysis of the radius. (3) Capitate. (4) Scaphoid. (5) Hamate. (6) Triquetrum. (7) Lunate. (8) Trapezium. (9) Trapezoid. (11) First metacarpal bone. (12) Second metacarpal bone. (13) Third metacarpal bone. (14) Fourth metacarpal bone. Scale bar: 125 μm. (B) Human fetus: G.V.-1 (45 mm), week 10. Transverse section (10 μm thick). AD, articular disc; EC, extensor carpi ulnaris tendon. (2) Distal epiphysis and styloid process of the ulna. (3) Capitate. (5) Hamate. (6) Triquetrum. (18) Ulnar collateral ligament. (14) Fourth metacarpal bone. Scale bar: 125 μm.
Styloid processes of ulna (processus styloideus ulnae) and radius (processus styloideus radii) chondrifications emerge at stages 20 and 22, respectively, and the ulnar styloid process begins to retreat from the triquetrum after week 11. At the end of the embryonic period (O’Rahilly stage 23), a chondrification center for each carpal element can be observed.
Finally, at week 14, blood vessels appear inside the lunate cartilage mold, as an early sign of the osteogenic process that will be completed after birth (Fig. 3).
Fig. 3.
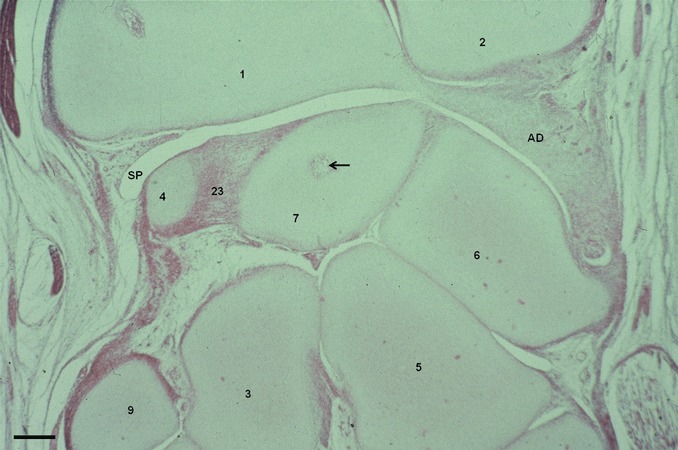
Human fetus Z.O.-2 (102 mm), week 14 fetus. Sagittal section (10 μm thick). Blood vessels emerging inside the lunate cartilage mold (arrow). AD, articular disc; SP, synovial plica. (1) Distal epiphysis of the radius. (2) Distal epiphysis of the ulna. (3) Capitate bone. (4) Scaphoid bone. (5) Hamate bone. (6) Triquetrum bone. (7) Lunate bone. (9) Trapezoid bone. (23) Interosseus ligaments. Scale bar: 300 μm.
Ligaments
Radial and ulnar collateral ligaments emerge in stage 21 and can be clearly depicted in weeks 9 (Fig. 4B) and 10, respectively (Fig. 2B). At the end of the stage 23 we can observe cellular bands corresponding to the interosseous ligaments, and in week 9, they are connecting several carpal bones (between scaphoid-lunate (Fig. 4B) and trapezoid-capitate), and the second and third metacarpal bases. In the last stages of week 11, mesenchymal bridges derived from the primary interzone start the final interosseous ligament organization (lunate-triquetrum, capitate bone-hamate). Finally, at week 14 (Fig. 3), the interosseous ligament morphological development is completed.
Fig. 4.
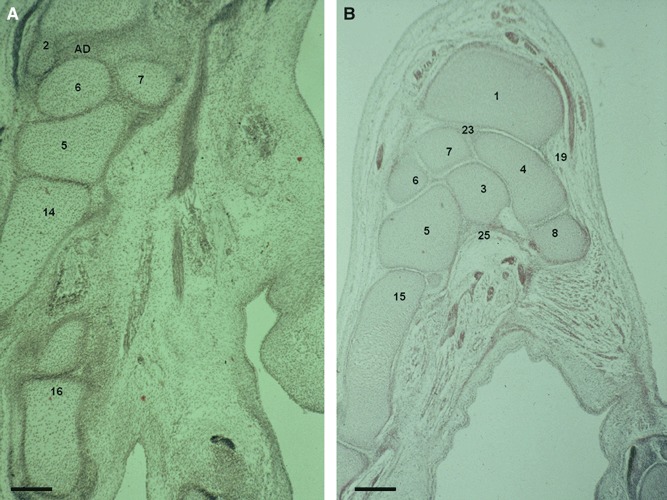
(A) Human embryo: H-23 (31 mm), O’Rahilly stage 23. Transverse section (8 μm thick). AD, articular disc. (2) Distal epiphysis and styloid process of the ulna. (5) Hamate. (6) Triquetrum. (7) Lunate. (14) Fourth metacarpal bone. (16) Phalanges. Scale bar: 300 μm. (B) Human fetus: R.I.-1 (38 mm), week 9. Transverse section (10 μm thick). (1) Distal epiphysis of the radius. (3) Capitate. (4) Scaphoid. (5) Hamate. (6) Triquetrum. (7) Lunate. (8) Trapezium. (15) Fifth metacarpal bone. (19) Radial collateral ligament. (23) Interosseus ligament. (25) Radiate carpal ligament. Scale bar: 125 μm.
Palmar radiocarpal (ligamentum radiocarpale palmare) and ulnocarpal (ligamentum ulnocarpale palmare) ligaments appear in stage 23 (ulnocarpal precedes radiocarpal ligament) and in week 10, dorsal radiocarpal ligament is indicated (Fig. 5A).
Fig. 5.
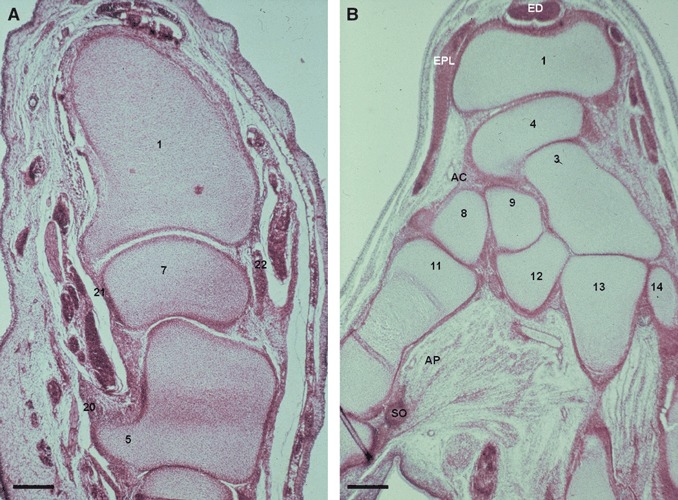
(A) Human fetus: G.V.-1 (45 mm), week 10. Transverse section (8 μm thick). (1) Distal epiphysis of the radius. (5) Hamate bone. (7) Lunate bone. (20) Transverse carpal ligament (flexor retinaculum). (21) Palmar radiocarpal ligament. (22) Dorsal radiocarpal ligament. Scale bar: 125 μm. (B) Human fetus: S.A.-4 (63 mm), week 12. Transversal section (12 μm thick). AC, articular capsule; AP, adductor pollicis muscle; ED, extensor digitorum muscle tendons; EPL, extensor pollicis longus muscle tendon; SO, internal sesamoid ossicle of the first metacarpian. (1) Distal epiphysis of the radius. (3) Capitate bone. (4) Scaphoid bone. (8) Trapezium bone. (9) Trapezoid bone. (11) First metacarpal bone. (12) Second metacarpal bone. (13) Third metacarpal bone. (14) Fourth metacarpal bone. Scale bar: 125 μm.
In stage 22, transverse carpal ligament or flexor retinaculum (retinaculum musculorum flexorum) is observed and in stage 23, this separates the deep branch of ulnar nerve (n. ulnaris) from the median nerve (n. medianus), which is located in the carpal tunnel. Early signs of radiate carpal ligament (carpal tunnel floor) are perceived in week 9 and defined at week 13 (Fig. 6A). In week 10, flexor retinaculum (Fig. 5A) shows a specific corridor for the flexor carpi radialis muscle tendon, and from this week on, all carpal tunnel structures can be observed.
Fig. 6.
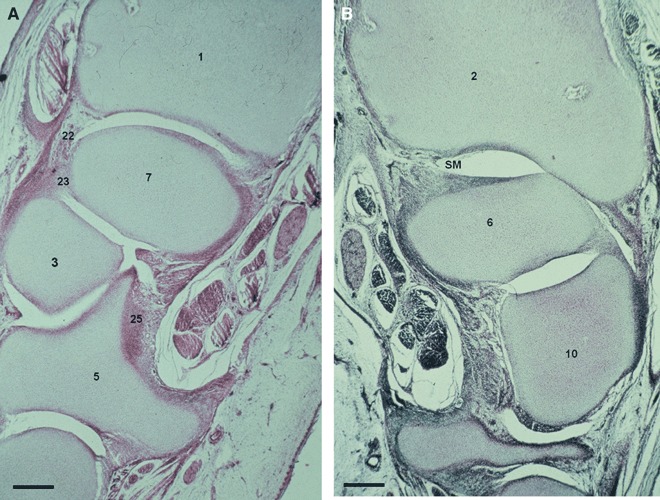
(A) Human fetus O.L.-1 (83 mm), week 13. Transverse section (10 μm thick). (1) Distal epiphysis of the radius. (3) Capitate bone. (5) Hamate bone. (7) Lunate. (22) Dorsal radiocarpal ligament. (23) Interosseus ligament. (25) Radiate carpal ligament. Scale bar: 125 μm. (B) Human fetus O.L.-1 (83 mm), week 13. Transverse section (10 μm thick). SM, cell layer which will differentiate into synovial membrane. (2) Distal epiphysis and styloid process of the ulna. (6) Triquetrum bone. (10) Pisiform bone. Scale bar: 125 μm.
Fibrocartilaginous disc
In stage 21, a thick mesenchymal condensation appears between the distal ulna and the triquetrum (os triquetrum) cartilaginous mold: the future articular disc (discus articularis), which is clearly depicted in stage 23 (Fig. 4A), begins to show its definitive morphology in week 10, and it is completely organized in week 14.
Articular capsule
During week 10, we can discern the organization of the wrist joint fibrous capsule from the intermediate layer of the interzone. In week 12 (Fig. 5B), it is evident that the fibrous capsule, wrist collateral ligaments and radiocarpal ligament and ulnocarpal ligaments come from the interzonal mesenchyme that surrounds the cavity of the joint.
Synovial membrane
Our results allow us to establish that the intermediate layer interzonal mesenchyme participates in synovial membrane formation. We have observed in a 13-week human fetus, subtle cellular bands rising from vascular mesenchyme pouches that cover the inner layer of the fibrous capsule and from which the synovial membrane will originate (Fig. 6B). In week 14, we have observed vascular mesenchyme folds that are bulging into the articular cavity. This is especially evident in the radiocarpal joint (Fig. 3).
Muscles and tendons
At the end of O’Rahilly stage 18 there is an evolution in the future organization of the hand, the presence of the thenar eminence in the palmar side of the thumb oriented to the embryonic side (Fig. 1A). During stage 20, pre-muscular blastemas are differentiating and tendinous formations can be discerned, especially in the thenar region. From stage 21, the future interosseous muscles acquire the structure of striated muscle cell and in O’Rahilly stage 22, flexor digitorum superficialis muscle tendons can be distinguished, while flexor digitorum profundus muscle (m. flexor digitorum profundus) tendons and lumbrical muscle (m. lumbricales) blastemas form a common mass at that level. In stage 23, the classical skeletal striated muscle pattern of the hand is completed.
Extensor digitorum muscle tendons (m. extensor digitorum) can be observed in stage 22, and adductor pollicis muscle (Fig. 7B) and flexor carpi radialis muscle tendons in stage 23. Extensor carpi ulnaris muscle (m. extensor carpi ulnaris) tendon sliding on the ulnar collateral ligament can be clearly seen in week 10 (Fig. 2B). In week 12, the oblique head of the adductor pollicis muscle reaches the internal sesamoid ossicle of the first metacarpal bone (Fig. 5B). In this week, the intrinsic muscle groups of the hand are completely organized.
Fig. 7.
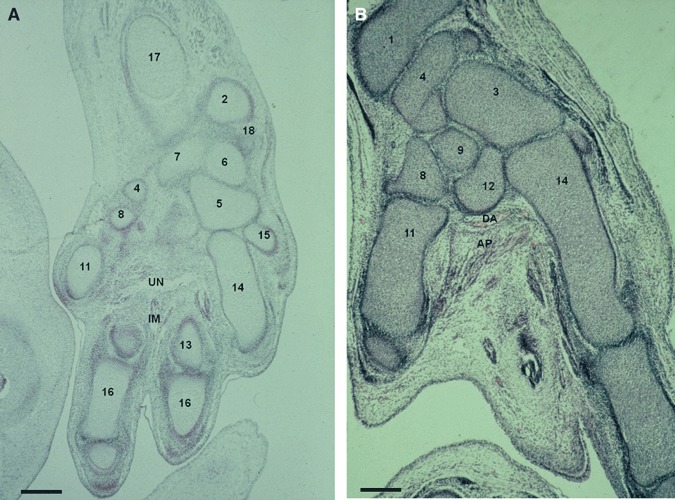
(A) Human embryo: C.H.-1 (24 mm), O’Rahilly stage 22. Transverse-horizontal section (8 μm thick). IM, interosseus muscles; UN, deep branch of the ulnar nerve. (2) Distal epiphysis of the ulna. (4) Scaphoid bone. (5) Hamate bone. (6) Triquetrum bone. (7) Lunate bone. (8) Trapezium bone. (11) First metacarpal bone. (13) Third metacarpal bone. (14) Fourth metacarpal bone. (15) Fifth metacarpal bone. (16) Phalanges. (17) Diaphysis of the radius. (18) Ulnar collateral ligament. Scale bar: 300 μm. (B) Human embryo: H.E.-1 (28 mm), O’Rahilly stage 23 transverse section (8 μm thick). AP. adductor pollicis muscle. (1) Distal epiphysis of the radius. (3) Capitate bone. (4) Scaphoid bone. (8) Trapezium. (9) Trapezoid bone. (11) First metacarpal bone. (12) Second metacarpal bone. (14) Fourth metacarpal bone. Scale bar: 125 μm.
Nerve and blood supply
In O’Rahilly stage 17, the apical ridge and the marginal vein (vena marginalis) can be observed in the distal portion of the upper limb bud, until the end of stage 20. In stage 18, the median nerve (Fig. 1A) and the other main nerve trunks emerge in the hand plate. The median nerve appears next to the transverse carpal ligament in stage 22, and in week 11 it can be observed between flexor retinaculum and flexor digitorum superficialis and profundus muscles. From stage 21, the ulnar nerve crosses transversally on the palmar side and reaches the third and fourth interosseous muscles. In stage 22, the nerve passes down the hamate bone and divides into profound and superficial branches (Fig. 7A). In stage 23, the ulnar nerve, which is separated from the median nerve by the transverse carpal ligament, innervates the adductor pollicis muscle. In this stage, vascular traces of the deep palmar arterial arch (arcus palmaris profundus) (Fig. 7B), which is formed from the terminal part of the radial artery (arteria radialis) with the deep branch of the ulnar artery (arteria ulnaris, ramus profundus), can be observed; this will be completely organized in week 11.
Discussion
We have performed a descriptive analysis of morphogenesis and the morphogenetic time-table of the main structures of the human wrist during the embryonic and early fetal period. Our results add to current knowledge of the early development of the human wrist.
Chondrification
At the end of the sixth week (stage 17), the digital rays can be observed in the hand plate (Moore et al. 2008). According to our results, the carpal chondrification process starts in stage 18 (O’Rahilly & Gardner, 1975; O’Rahilly et al. 1981; Sadler, 2007); this is in contrast to Lewis (1970), who described this event in 19–26, 4-mm embryos.
Our observations agree with classical studies; capitate and hamate are the first chondrogenic centers to appear, and pisiform is the last, in stage 23 (Gray et al. 1957; Pineau & Tardif, 1965; Genis, 1970). In stage 23, hamulus can be observed as immature cartilaginous tissue and its morphological development is completed in week 13.
O’Rahilly et al. (1981) described the emergence of ulnar styloid process chondrification in 7–12-mm specimens. Gray et al. (1957) and O’Rahilly & Gardner (1975) observed both ulnar and radial styloid processes chondrifying in stages 19 and 20, respectively. According to our observations, ulnar and radial styloid processes emerge at stages 20 and 22, respectively. Our findings confirm previous observations by Lewis (1970). This author observed that the ulnar styloid process regresses during the early embryonic period in humans so that it is almost completely excluded from direct participation in the wrist joint after the 60-mm stage. Our confirmation of this result has important implications in evolutionary anthropology, as Lewis noted that this event is shared by all adult humans and other apes, unlike other primates. From a functional viewpoint, the ulna retreat is associated classically with high ranges of forearm rotation, which may facilitate forelimb-dominated behaviors (Lewis, 1970; O’Connor & Rarey, 1979; Sarmiento, 1988).
Classically, it has been considered that a separate cartilaginous condensation for the os centrale appears around the sixth postovulatory week (O’Rahilly & Gardner, 1975) but we did not see this until the end of stage 21. Scaphoid-centrale fusion is shared between African apes and humans, and has been interpreted as an adaptation in knuckle-walkers and an exaptation in hominins, and has been offered as evidence for a knuckle-walking origin of bipedalism (Kivell & Begun, 2007). According to our findings, the incorporation into the scaphoid occurs during the 9th week (O’Rahilly, 1954; Cihák, 1972).
Finally, a vascular bud inside the lunate cartilage mold was detected in one 14-week fetus, an early sign of the osteogenic process that will be completed during the first year of life (Moore et al. 2008).
Ligaments
Concerning the origin of the wrist ligaments, we can conclude that interosseous ligaments derive from interzonal mesenchyme, and the rest of the ligaments are organized as specific condensations of the joint capsule (Mayfield et al. 1976; Mérida-Velasco et al. 1996).
In stage 23, we observed densified cellular bands between cartilaginous molds corresponding with the future interosseous ligaments (Mérida-Velasco et al. 1996). This finding is in contrast to that of O’Rahilly & Gardner (1975), who stated that this event occurred in 21–23- and 16–18-mm embryos, respectively. Berger et al. (1991) and Oztuna et al. (2003) consider that the scapholunate ligament is completely developed in week 10 but according to our results, it cannot be seen until week 11, and will have completed at week 14.
Collateral ligaments can be perceived in stage 21, later than observed by O’Rahilly & Gardner (1975) and earlier than observed by García-Elias & Domenech-Mateu (1987). The presence of the radial collateral ligament is constant in week 9 (Oztuna et al. 2003) and the ulnar collateral ligament can be observed in week 10, deep to the extensor carpi ulnaris muscle tendon. We do not agree with García-Elias & Domenech-Mateu (1987), who did not find evidence of the presence of the ulnar collateral ligament in serial sections of 18 hands (35–115-mm fetuses).
Based in our studies, radiocarpal ligament appears later than ulnocarpal ligament, (O’Rahilly stage 23), in contrast to Gray et al. (1957) and O’Rahilly & Gardner (1975), who observed radiocarpal ligament in earlier stages (21–23 mm and 18–22 mm, respectively) and Lewis (1970), who did not report it until 48-mm. We have not found any evidence of radioscaphoid ligament described by Mayfield et al. (1976) and detailed in fetal wrist with a 17-cm crown–rump length by Berger & Landsmeer (1990).
Fibrocartilaginous disc
The study of the triangular fibrocartilage is very interesting from a morphological and developmental point of view, but it also has an important role in the functional activity of the wrist joint. There has been some controversy concerning the morphology and location of the radioulnar ligament and triangular fibrocartilage. Consistent with previous observations (Mérida-Velasco et al. 1996) we can state that the future triangular disc, which is clear in stage 23, remains as a single formation and shows its definitive organization in week 14. Two histologically different parts on the central and peripheral side of the disc have been described (Mayfield et al. 1976; Mohiuddin & Janjua, 1982; García-Elias & Domenech-Mateu, 1987; Hogikyan & Louis, 1992). The distal side of the disc develops as fibrocartilage (Chidgey et al. 1991) or hyaline-like cartilage (Benjamin et al. 1990; Mikić et al. 1992) and resists the pressure from the ulnar carpus and distortions during rotation (Nakamura et al. 1996, 2001; Milz et al. 2007).
Articular capsule and synovial membrane
We consider that the fibrous capsule derives from the intermediate layer of the interzone (Gardner, 1963; Genis, 1970; Hamilton & Mossman, 1975; Standring, 2008). According to our observations, the organization of the wrist joint common fibrous capsule and ligaments that reinforce this joint cannot be seen until week 10. In the 11th week, the joint cavity was surrounded by the capsule and in week 12 it was evident that the wrist joint capsule, radiocarpal and ulnocarpal collateral ligaments derive from periarticular mesenchyme (Moore et al. 2008; Standring, 2008).
It is not easy to determine the nature of the synovial membrane because of its extraordinary thinness and subtlety. Our observations allow us to state that the intermediate layer of the interzone participates in its formation (Gardner, 1963; Moore & Persaud, 2000; Standring, 2008). In a 13-week human fetus, the synovial membrane is formed by subtle cellular bands ranging from vascular mesenchyme pouches covering the inner layer of the fibrous capsule.
Muscles and tendons
In 17-mm embryos, the muscle blastema in the thenar region differentiates into two layers of premuscle priomordia (Cihák, 1972), but we could not observe it until stage 20. In stage 18, interosseous, flexor digitorum profundus and lumbricals will form from a deep flexor pre-muscle mass that is supplied by the median and ulnar nerves. Interosseous muscles occasionally receive accessory slips from contrahentes muscle (Cihák, 1972), and we can describe them in stage 21 (Lewis, 1902). In stage 19, flexor digitorum profundus independent tendons have formed in the carpal region and the lumbricals have distinct, short fibers ending in tendons that fuse with condensed tissue on digits (Lewis, 1902; Cihák, 1972) but according to our observations, a common premuscle mass is still observed in stage 22.
In stage 19, extensor carpi ulnaris and flexor carpi radialis muscle tendons fuses the fifth and second metacarpals, respectively (Lewis, 1902), and Blechschmidt (1963) stated that in stage 20, extensor digitorum muscle was indicated. We could not observe these muscle tendons until stage 22 (extensor digitorum), stage 23 (flexor carpi radialis) and week 10 (extensor carpi ulnaris). Beatty (1985) described the tendons of flexor digitorum superficialis in the seventh week, in contrast to our observations (O’Rahilly stage 22).
Finally, Cihák (1968) found the contrahentes muscle in early human embryos (19–28 mm crown–rump length), but as the embryos grew (> 30 mm), this muscle disappeared except for a small part that persisted as the adductor pollicis muscle. Based on our observations, this muscle is observed in 28-mm specimens, and will reach the internal sesamoid ossicle of the first metacarpal bone in week 12.
Nerve and blood supply development
O’Rahilly & Gardner (1975) stated that in stages 16 and 17, the median, radial and ulnar nerve entered into the hand plate, but we were not able to see them until stage 18. From stage 21, the wrist and hand nerves represent an orientation and arrangement similar to that in the adult (Shinohara et al. 1990), and in stage 23, the median nerve and the deep branch of the ulnar nerve are separated by the retinaculum flexorum (Mérida-Velasco et al. 1996).
In O’Rahilly stage 17, the apical ridge is still present and the marginal vein (vena marginalis) can be observed in the distal portion of the upper limb bud, until the end of stage 20 (O’Rahilly et al. 1956; Carlsson, 2009). In stage 18, ulnar, median and interosseous arteries have formed (Morris et al. 2005). In stage 19, the palmar arches appear, and these arches and radial artery are well defined in stage 23 (Rodriguez-Niedenfuhr et al. 2001; Morris et al. 2005). We could describe the deep palmar arch appearing in stage 23 but, based on our observations, the definitive morphology of this arterial arch, which crosses the palm deep to the flexor digitorum muscles tendons with the deep branch of the ulnar nerve, could not be observed until week 11.
Concluding remarks
The small degree of variations of each embryo at a given stage allows us to provide a complete description of the morphogenesis and morphogenetic time-table of the human wrist structures. Our results can be used as a basis to explain the role that these structures play in the normal function and pathologies of the human wrist joints, such as those related to the mechanical function (ligaments and triangular fibrocartilage), tendinous and vascular anomalies and nerve entrapment syndromes. Further investigations will be needed to clarify these aspects. The results help in reassessing the timing of fusion of the os centrale as well as the retreat of the ulna from the carpus. Characterizing as accurately as possible the timing of these two major events is of great importance in evolutionary anthropology.
Acknowledgments
The authors would like to thank the Department of Anatomy and Human Embryology of the Medical School, University of Granada and the Department of Morphological Sciences II of the Medical School, Complutense University of Madrid, for providing data on human embryos and fetuses. We also want to thank to Dr. Prof. Juan de Dios García García for all his patience and teaching. Finally, we would like to dedicate this work to Dr. Prof. Luís Álvarez-Guisado, who taught us to be hard workers and honest.
Author contributions
F. Hita: conception and design, images selection and treatment, data analysis and interpretation, manuscript writing. A. Martínez: manuscript writing, image selection and treatment. R. Ortiz: data collection and assembly. O. Caba: data collection and assembly. P. Álvarez: data collection and assembly. J.C Prados: manuscript revision. R. Lomas: Image selection and treatment. A. Aránega: data analysis and interpretation, manuscript revision. I. Sánchez-Montesinos: conception and design, data analysis and interpretation. J.A. Mérida: conception and design, data analysis and interpretation, manuscript revision.
References
- Al-Qattan MM, Yingzi Yang MMBS, Kozin SH. Embryology of the upper limb. J Hand Surg Am. 2009;34A:1340–1350. doi: 10.1016/j.jhsa.2009.06.013. [DOI] [PubMed] [Google Scholar]
- Andersen HF, Bro-Rasmussen F. Histochemical studies on the histogenesis of the joints in human fetuses with special reference to the development of the joint cavities in the hand and foot. Am J Anat. 1961;108:l11–l122. [Google Scholar]
- Archer CW, Dowthwaite GP, Francis-West P. Development of synovial joints. Birth Defects Res. 2003;69:144–155. doi: 10.1002/bdrc.10015. [DOI] [PubMed] [Google Scholar]
- Bardeen CR. Studies of the development of the human skeleton. Am J Anat. 1905;4:265–302. [Google Scholar]
- Beatty E. Upper limb tissue differentiation in the human embryo. Hand Clin. 1985;1:391–403. [PubMed] [Google Scholar]
- Benjamin M, Evans EJ, Pemberton DJ. Histological studies on the triangular fibrocartilage complex of the wrist. J Anat. 1990;172:59–67. [PMC free article] [PubMed] [Google Scholar]
- Berger RA, Landsmeer JM. The palmar radiocarpal ligaments: a study of adult and fetal human wrist joints. J Hand Surg Am. 1990;15:847–854. doi: 10.1016/0363-5023(90)90002-9. [DOI] [PubMed] [Google Scholar]
- Berger RA, Kauer JM, Landsmeer JM. Radioscapholunate ligament: a gross anatomic and histologic study of fetal and adult wrists. J Hand Surg Am. 1991;16:350–355. doi: 10.1016/s0363-5023(10)80125-x. [DOI] [PubMed] [Google Scholar]
- Blechschmidt E. Der menschliche Embryo. Stuttgart: Schattauer-Verlag; 1963. [Google Scholar]
- Carlson BM. Embriología humana y biología del desarrollo. 4th edn. Barcelona: Elsevier; 2009. pp. 212–214. [Google Scholar]
- Cavalcante ML, Rodrigues CJ, Mattar R., Jr Mechanoreceptors and nerve endings of the triangular fibrocartilage in the human wrist. J Hand Surg Am. 2004;29:432–438. doi: 10.1016/j.jhsa.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Chidgey LK, Dell PC, Bittar ES, et al. Histologic anatomy of the triangular fibrocartilage. J Hand Surg Am. 1991;16A:1084–1100. doi: 10.1016/s0363-5023(10)80073-5. [DOI] [PubMed] [Google Scholar]
- Cihák R. Mode of extinction of the contrahentes muscle layer in the embryonal human hand. Folia Morphol. 1968;16:184–194. [PubMed] [Google Scholar]
- Cihák R. Ontogenesis of the skeleton and intrinsic muscles of the human hand and foot. Ergeb Anat Entwicklungsgesch. 1972;46:5–194. [PubMed] [Google Scholar]
- Fritsch E. Development of the human hand: A short, up-to-date overview. Eur Surg. 2003;35:125–128. [Google Scholar]
- García-Elias M, Domenech-Mateu JM. The articular disc of the wrist. Limits and relations. Acta Anat. 1987;128:51–54. doi: 10.1159/000146314. [DOI] [PubMed] [Google Scholar]
- Gardner E. The development and growth of bones and joints. J Bone Joint Surg. 1963;45:856–862. [Google Scholar]
- Gardner E, O’Rahilly R. The early development of the knee joint in staged human embryos. J Anat. 1968;102:289–299. [PMC free article] [PubMed] [Google Scholar]
- Gardner E, Gray DJ, O’Rahilly R. Anatomy. 3rd edn. Philadelphia: W.B. Saunders Co; 1969. pp. 160–163. [Google Scholar]
- Genis JM. Biología del Desarrollo: Fundamentos de Embriología. Barcelona: Espaxs; 1970. [Google Scholar]
- Goss CM. Gray’s Anatomy of the Human Body. 29th edn. Philadelphia: Lea and Febiger; 1973. pp. 333–336. [Google Scholar]
- Gray DJ, Gardner E, O’Rahilly R. The prenatal development of the skeleton and joints of human hand. Am J Anat. 1957;101:169–224. doi: 10.1002/aja.1001010202. [DOI] [PubMed] [Google Scholar]
- Gutiérrez M, Montero C, García JD. Coloración histológica policroma de embriones. Anal Desarrollo. 1963;11:52–56. [Google Scholar]
- Haines RW. The development of joints. J Anat. 1947;81:33–35. [PubMed] [Google Scholar]
- Hamilton WJ, Mossman HW. Embriología Humana. 4th edn. Buenos Aires: Editorial Interamericana; 1975. [Google Scholar]
- Hogikyan JV, Louis DS. Embryologic development and variations in the anatomy of the ulnocarpal ligamentous complex. J Hand Surg Am. 1992;17A:719–723. doi: 10.1016/0363-5023(92)90323-h. [DOI] [PubMed] [Google Scholar]
- Keith A. Human Embryology and Morphology. 5th edn. Baltimore: Williams Wood; 1933. [Google Scholar]
- Kivell TL, Begun DR. Frequency and timing of scaphoid-os centrale fusion in hominoids. J Hum Evol. 2007;52:321–340. doi: 10.1016/j.jhevol.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Kuczynski K. Development of the hand and some anatomical anomalies. Hand. 1972;4:1–10. doi: 10.1016/0072-968x(72)90002-2. [DOI] [PubMed] [Google Scholar]
- Lansmeer JMF. Anatomical and functional investigations on the articulation of the human fingers. Acta Anat Suppl (Basel) 1955;25:1–69. [PubMed] [Google Scholar]
- Lee DH, Dickson KF, Bradley EL. The incidence of wrist interosseus ligament and triangular fibrocartilage articular disc disruptions: a cadaveric study. J Hand Surg Am. 2004;29:676–684. doi: 10.1016/j.jhsa.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Lewis WH. The development of arm in man. Am J Anat. 1902;1:145–184. [Google Scholar]
- Lewis OJ. The development of the human wrist joint during the fetal period. Anat Rec. 1970;166:499–515. doi: 10.1002/ar.1091660308. [DOI] [PubMed] [Google Scholar]
- Markze MW. Origin of the human hand. Am J Phys Anthropol. 1971;34:61–84. doi: 10.1002/ajpa.1330340106. [DOI] [PubMed] [Google Scholar]
- Mayfield JK, Johnson RP, Kilcoyne RF. The ligaments of the human wrist and their functional significance. Anat Rec. 1976;186:417–428. doi: 10.1002/ar.1091860307. [DOI] [PubMed] [Google Scholar]
- Mcmanus JFA, Mowry RW. Técnica Histológica. Madrid: Atika; 1968. [Google Scholar]
- Mérida-Velasco JA, García-García JD, Espín-Ferra J, et al. Development of the human wrist joint ligament. Anat Rec. 1996;245:114–121. doi: 10.1002/(SICI)1097-0185(199605)245:1<114::AID-AR16>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Mikić Z. Age changes in the triangular fibrocartilage of the wrist joint. J Anat. 1978;126:367–384. [PMC free article] [PubMed] [Google Scholar]
- Mikić Z, Somer L, Somer T. Histologic structure of the articular disk of the human distal radioulnar joint. Clin Orthop. 1992;275:29–36. [PubMed] [Google Scholar]
- Milz S, Sicking B, Sprecher CM, et al. An immunohistochemical study of the triangular fibricartilage complex of the wrist: regional variations in cartilage phenotype. J Anat. 2007;211:1–7. doi: 10.1111/j.1469-7580.2007.00742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohiuddin A, Janjua MZ. Form and function of the radioulnar articular disc. Hand. 1982;14:61–66. doi: 10.1016/s0072-968x(82)80044-2. [DOI] [PubMed] [Google Scholar]
- Moore KL, Persaud TVN. Embriologia básica. México: McGraw-Hill Interamericana–Koogan; 2000. [Google Scholar]
- Moore KL, Keith L, Persaud TV. Embriología Clínica. 8th edn. Barcelona: Elsevier; 2008. [Google Scholar]
- Morris LG, Rowe NM, Delacure MD. Superficial dorsal artery of the forearm: case report and review of the literature. Ann Plast Surg. 2005;55:538–541. doi: 10.1097/01.sap.0000181357.29775.7c. [DOI] [PubMed] [Google Scholar]
- Müller E. Beiträge zur Morphologie des Gefässystems. I. Die Armarterien des Menschen. Anat Hefte. 1903;22:377–575. [Google Scholar]
- Nakamura T, Yabe Y. Histological anatomy of the triangular fibrocartilage complex of the human wrist. Ann Anat. 2000;182:567–572. doi: 10.1016/S0940-9602(00)80106-5. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Yabe Y, Horiuchi Y. Functional anatomy of the triangular fibrocartilage complex. J Hand Surg Br. 1996;21B:581–586. doi: 10.1016/s0266-7681(96)80135-5. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Takayama S, Horiuchi Y, et al. Origins and insertions of the triangular fibrocartilage complex: a histological study. J Hand Surg Br. 2001;26B:446–454. doi: 10.1054/jhsb.2001.0562. [DOI] [PubMed] [Google Scholar]
- Nishikawa S, Toh S. Anatomical study of the carpal attachment of the triangular fibrocartilage complex. J Bone Joint Surg. 2002;84B:1062–1065. doi: 10.1302/0301-620x.84b7.12979. [DOI] [PubMed] [Google Scholar]
- O’Connor BL, Rarey KE. Normal amplitudes of radioulnar pronation and supination in several genera of anthropoid primates. Am J Phys Anthropol. 1979;51:39–44. [Google Scholar]
- O’Rahilly R. The prenatal development of the human centrale. Anat Rec. 1954;118:334–335. [Google Scholar]
- O’Rahilly R, Gardner E. The timing and sequence of events in the development of the limbs in the human embryo. Anat Embryol. 1975;148:1–23. doi: 10.1007/BF00315559. [DOI] [PubMed] [Google Scholar]
- O’Rahilly R, Müller F. Developmental Stages in Human Embryos. Washington, DC: Carnegie Institution of Washington; 1987. [Google Scholar]
- O’Rahilly R, Tucker JA. The early development of the larynx in staged human embryos. I. Embryos of the first five weeks (to stage 15) Ann Otol Rhinol Laryngol. 1973;82:1–27. doi: 10.1177/000348947308200502. [DOI] [PubMed] [Google Scholar]
- O’Rahilly R, Gardner E, Gray DJ. The ectodermal thickening and ridge in the limbs of staged human embryos. J Embryol Exp Morphol. 1956;4:254–264. [Google Scholar]
- O’Rahilly R, Bossy J, Muller F. Introduction to the study of embryonic stages in man. Bull Assoc Anat (Nancy) 1981;65:141–236. [PubMed] [Google Scholar]
- Oztuna V, Coşkun B, Polat A, et al. The development of the wrist joint in the fetal period. Acta Orthop Traumatol Turc. 2003;37:254–260. [PubMed] [Google Scholar]
- Pacifici M, Koyama E, Shibukawa Y, et al. Cellular and molecular mechanisms of synovial joint and articular cartilage formation. Ann N Y Acad Sci. 2008;1068:74–86. doi: 10.1196/annals.1346.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer AK, Werner FW. The triangular fibrocartilage complex of the wrist. Anatomy and function. J Hand Surg Am. 1981;6:153–162. doi: 10.1016/s0363-5023(81)80170-0. [DOI] [PubMed] [Google Scholar]
- Pineau H, Tardif F. Morphogénese du carpe. Bull Assoc Anat (Nancy) 1965;127:1282–1291. [Google Scholar]
- Rodriguez-Niedenfuhr M, Burton GJ, Deu J, et al. Development of the arterial pattern in the upper limb of staged human embryos: normal development and anatomic variations. J Anat. 2001;199:407–417. doi: 10.1046/j.1469-7580.2001.19940407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouvière H, Delmas A. Anatomía Humana. Descriptiva, Topográfica y Funcional. 11th edn. Vol. 3. Barcelona: Masson; 2005. pp. 79–83. [Google Scholar]
- Sadler TW. Langman. Embriología Médica con Orientación Clínica. 10th edn. Buenos Aires: Editorial Médica Panamericana; 2007. [Google Scholar]
- Salsbury CR. The interosseous muscles of the hand. J Anat. 1937;71:395–403. [PMC free article] [PubMed] [Google Scholar]
- Sarmiento EE. Anatomy of the hominoid wrist joint: its evolutionary and functional implications. Int J Primatol. 1988;9:281–345. [Google Scholar]
- Shigemitsu T, Tobe M, Mizutani K, et al. Innervation of the triangular fibrocartilage complex of the human wrist: quantitative immunohistochemical study. Anat Sci Int. 2007;82:127–132. doi: 10.1111/j.1447-073X.2007.00173.x. [DOI] [PubMed] [Google Scholar]
- Shinohara H, Naora H, Hashimoto R, et al. Development of the innervation pattern in the upper limb of staged human embryos. Acta Anat (Basel) 1990;138:265–269. doi: 10.1159/000146950. [DOI] [PubMed] [Google Scholar]
- Singer E. Embryological pattern persisting in the arteries of the arm. Anat Rec. 1933;55:403–409. [Google Scholar]
- Stack HG. A study of muscle function in the fingers. Ann R Coll Surg Engl. 1963;33:307–322. [PMC free article] [PubMed] [Google Scholar]
- Standring S. Gray’s Anatomy: The Anatomical Basis of Clinical Practice. 40th edn. Madrid: Churchill Livingstone; 2008. [Google Scholar]
- Streeter GL. Developmental Horizons in Human Embryos. Age Groups XI to XXIII. Embryology Reprint Volume II. Washington, DC: Carnegie Institution; 1951. [Google Scholar]


