Abstract
Ciliary neurotrophic factor (CNTF) is a potent survival molecule for a large number of neuronal and glial cells in culture; its expression in glial cells is strongly upregulated after a variety of nerve tissue injuries. Exogenously administered CNTF produces an anorectic effect via activation of hypothalamic neurons and stimulates neurogenesis in mouse hypothalamus. To determine whether CNTF is produced endogenously in the hypothalamus, we sought cellular sources and examined their distribution in adult mouse hypothalamus by immunohistochemistry. CNTF immunoreactivity (IR) was predominantly detected in the ependymal layer throughout the rostrocaudal extension of the third ventricle, where numerous ependymocytes and tanycytes exhibited specific staining. Some astrocytes in the grey matter of the anterior hypothalamus and in the median eminence of the hypothalamic tuberal region were also positive. Stimulation of cells bearing CNTF receptor α (CNTFRα) induces specific activation of the signal transducer and activator of transcription 3 (STAT3) signalling system. Treatment with recombinant CNTF and detection of the nuclear expression of phospho-STAT3 (P-STAT3) showed that CNTF-producing ependymal cells and tanycytes were intermingled with, or very close to, P-STAT3-positive, CNTFRα-bearing cells. A fraction of CNTF-producing ependymal cells and tanycytes and some median eminence astrocytes also exhibited P-STAT3 IR. Thus, in normal adult mice the ependyma of the third ventricle is both a source of and a target for CNTF, which may play hitherto unknown roles in hypothalamic function in physiological conditions.
Keywords: CNTF receptor, confocal microscopy, cytokines, ependymal cells, immunohistochemistry, STAT3, tanycytes
Introduction
Ciliary neurotrophic factor (CNTF) is a member of the interleukin (IL)-6 cytokine family, a group of structurally related cytokines also including leukaemia inhibitory factor (LIF), IL-11, oncostatin M, cardiotrophin, and recently identified members such as cardiotrophin-like cytokine and neuropoietin (Bauer et al. 2007). CNTF was originally described as a growth factor supporting the survival of chick ciliary ganglion neurons (Adler et al. 1979), and was later proved to be a trophic molecule for a wide variety of motor and sensory neurons in the central and peripheral nervous systems (Sendtner et al. 1994; Sleeman et al. 2000). CNTF is a 200-amino acid peptide with an approximate molecular weight of 23 kDa. In rodents CNTF mRNA is not detectable by Northern blotting or polymerase chain reaction analysis during embryo development, or immediately after birth, but its synthesis begins and gradually increases in the nervous system during postnatal development (Stockli et al. 1989, 1991). In adult rats and mice CNTF has been localized by in situ hybridization and immunohistochemistry primarily in glial cell types, its expression being strongest in peripheral nerve Schwann cells and in cerebral white matter astrocytes (Stockli et al. 1991; Guthrie et al. 1997; Dallner et al. 2002). Importantly, rapid CNTF upregulation is seen in grey matter astrocytes after lesion or deafferentation (Guthrie et al. 1997; Lee et al. 1997; Watt et al. 2006). While the biological effects of the post-injury CNTF increase are still unknown, these data are consistent with a possible in vivo CNTF-dependent, neurotrophic and neuroprotective effect on damaged neurons. Thus, CNTF administration has been proposed as a therapeutic strategy to reduce the neuron loss resulting from a variety of traumatic and genetic lesions.
CNTF binds to a three-part receptor complex (CNTFR) consisting of the ligand-specific binding subunit receptor α (CNTFRα), which is attached to the cell membrane by a glycosylphosphatidylinositol linkage, and the signal-transducing subunits gp130 and LIF receptor b (LIFRb; Davis et al. 1993; Ip et al. 1993). CNTF binding to CNTFRα triggers gp130 and LIFRb heterodimerization, giving rise to an active trimeric receptor complex at the cell membrane (Simi & Ibanez, 2010); the receptor complex activates the Janus family of tyrosine kinases (Jak1/Jak2), thereby leading to tyrosine phosphorylation, dimerization and nuclear translocation of signal transducers and activators of transcription (STATs), mainly STAT3. Phosphorylated STAT3 (P-STAT3) dimers bind to specific response elements in DNA promoter regions to activate the transcription of target genes. CNTF injected intraperitoneally or intravenously rapidly reaches distinctive areas of the brain parenchyma by diffusing through the circumventricular organs, which lack the blood–brain barrier. Furthermore, by crossing the blood–cerebrospinal fluid barrier, it diffuses through the brain ventricular system reaching the ependymal wall of the brain ventricles and possibly the adjacent parenchyma up to a certain depth. It has also been suggested that CNTF can cross the blood–brain barrier in some areas of the brain (Poduslo & Curran, 1996), although the notion remains controversial (Lambert et al. 2001). As a consequence, detection of nuclear P-STAT3 immunoreactivity (IR) after systemic CNTF treatment has been proposed as a reliable neuroanatomical tool for functional mapping of central CNTF actions and the characterization of, at least some, CNTF-responsive, CNTFRα-bearing cells (MacLennan et al. 2000). By this approach CNTF has been shown to induce expression of P-STAT3 and SOCS-3 (suppressor of cytokine signalling-3, which provides an intracellular negative loop to dampen or switch off cytokine stimulation) in cells within and adjacent to the circumventricular organs, in the ependymal layer lining the brain ventricular system, in the meninges and in some perivascular cells (Bjørbæk et al. 1999; Lambert et al. 2001; Anderson et al. 2003; Kelly et al. 2004). Systemic CNTF administration also activates STAT3 signalling in hypothalamic arcuate nucleus neurons, where CNTF action on proopiomelanocortin-expressing neurons is required for its anorectic action (Lambert et al. 2001; Janoschek et al. 2006). Besides this effect on food intake, prolonged systemic treatment of adult mice with rat recombinant CNTF has been reported to stimulate neurogenesis in the hypothalamus (Kokoeva et al. 2005, 2007). These data suggest that several hypothalamic structures located along the midline (circumventricular organs, ependyma and arcuate nucleus) respond to CNTF, which in turn is involved in important processes, such as food intake regulation and adult neurogenesis. This immunohistochemical study was designed to assess the presence and distribution of CNTF in adult mouse hypothalamus in normal conditions. In addition, immunodetection of P-STAT3 in CNTF-injected mice enabled comparison of the distribution of cells producing CNTF and those responding to CNTF, i.e. cells bearing the functional CNTF receptor.
Materials and methods
Animals, CNTF treatment and tissue processing
Adult male Swiss CD-1 mice purchased from Charles River Laboratories (Calco, Italy) were housed in plastic cages in constant environmental conditions (12 h light/dark cycle at 22 °C) with ad libitum access to food and water. Handling was limited to cage cleaning. All efforts were made to minimize animal suffering and to reduce the number of animals used. Experiments were carried out in accordance with EC Council Directive 86/609/EEC of 24 November 1986.
For Western blotting experiments animals were decapitated, the brain was rapidly removed, snap-frozen in liquid nitrogen and stored at −80 °C. For immunohistochemistry, animals were anaesthetized with 100 mg kg−1 ketamine (Ketavet, Farm. Gellini, Aprilia, Italy) in combination with 10 mg kg−1 xylazine (Rompum, Bayer AG, Leverkusen, Germany), and perfused transcardially with 4% paraformaldehyde in 0.1 m phosphate buffer (PB), pH 7.4. Brains were carefully removed from the skull, postfixed with the same fixative solution for 24 h at 4 °C and washed in PB. Free-floating 40-μm-thick coronal sections of the entire brain from the septum to the mammillary body of the hypothalamus were obtained with a Leica VT1200S vibratome (Leica Microsystems, Vienna, Austria) and kept in phosphate-buffered saline (PBS), pH 7.4, at 4 °C until use in immunohistochemical experiments. Adjacent brain sections were used to identify the exact location of individual hypothalamic nuclei and areas by Nissl staining (Paxinos & Franklin, 2001).
Some mice received a single intraperitoneal injection (3 mg kg−1 of body weight) of recombinant rat CNTF (R&D Systems, Minneapolis, MN, USA) or pyrogen-free saline (controls). The volumes of CNTF or vehicle ranged from 180 to 220 μL according to body weight; injections were performed with Hamilton syringes. Forty-five minutes later, treated and control mice were anaesthetized and processed for immunohistochemistry as described above.
Primary antibodies
The following primary antibodies were used for this study: anti-CNTF polyclonal goat serum (R&D Systems; cat # AF-557-NA); anti-CNTF polyclonal chicken serum (Promega, Madison, Wisconsin, USA; cat # G1631); anti-CNTFRα polyclonal goat serum (R&D Systems; cat # AF-559-NA); anti-vimentin polyclonal goat serum (Santa Cruz Biotech., Santa Cruz, CA, USA; cat # sc-7557); anti-glial fibrillary acidic protein (GFAP) monoclonal mouse antibody (Sigma, St Louis, MO, USA; cat # G3893); and anti-phospho-specific-(Tyr705)-STAT3 polyclonal rabbit serum (Cell Signaling Technology, Beverly, MA, USA; cat # 9131).
Western blotting analysis
Crude extracts of mouse brains were prepared from −80 °C frozen material by disruption in liquid nitrogen until samples were in the form of a fine powder, which was suspended in PBS and further treated by sonication at 4 °C. Samples were centrifuged and the supernatants recovered. Protein concentrations were measured by the Coomassie Brilliant Blue method (Bradford, 1976) using bovine serum albumin (BSA) as a standard. Samples were then denatured as described by Laemmli (1970) and proteins separated using 12% sodium dodecyl sulphate (SDS)–polyacrylamide gel electrophoresis and blotted to a PVDF filter (Immobilon Transfer Membranes; Millipore, Billerica, MA, USA) in transfer buffer (CAPS 10 mm, pH 11, and 10% methanol, v/v) at 250 mA for 3 h at 4 °C. To evaluate CNTF IR, filters were treated with a blocking solution consisting of 3% BSA in Tris-buffered saline (TBS; 10 mm Tris-HCl, pH 7.5, 150 mm NaCl, 0.05% Tween 20, 0.2% Triton X-100) overnight at 4 °C to prevent non-specific binding and then incubated with polyclonal goat serum (R&D Systems) or polyclonal chicken serum (Promega), both diluted 1 : 1000 in 3% BSA blocking solution. After extensive washing with TBS and incubation with the horseradish peroxidase (HRP)-conjugated anti-goat IgG secondary antibody (R&D Systems antibody protocol; Sigma-Aldrich) or the HRP-conjugated anti-chicken IgY secondary antibody (Promega antibody protocol), membranes were developed using the SuperSignal West FEMTO chemiluminescent kit (Pierce, Rockford, IL, USA).
Peroxidase immunohistochemistry
Immunohistochemical detection of CNTF was performed according to standard procedures. In brief, free-floating sections were reacted with 0.3% H2O2 (in PBS; 30 min) to block endogenous peroxidase, rinsed with PBS and incubated in a 3% normal serum blocking solution (in PBS; 60 min). Then they were incubated with the specific polyclonal goat serum (R&D Systems; dilution 1 : 100) or the polyclonal chicken serum (Promega; dilution 1 : 200) in PBS, overnight at 4 °C. After a thorough rinse in PBS, sections were incubated in a 1 : 200 v/v biotinylated secondary antibody solution (in PBS; 30 min), then rinsed in PBS and incubated in avidin-biotin peroxidase complex (ABC Elite PK6100, Vector, Burlingame, CA, USA), washed several times in PBS and finally incubated in 3,3′ diaminobenzidine tetrahydrochloride (0.05% in 0.05 m Tris with 0.03% H2O2; 5 min). After immunohistochemical staining, sections were mounted on slides, air-dried, dehydrated in ethanol, cleared with xylene and covered with Entellan. To assess the specificity of the anti-CNTF polyclonal antibodies, additional brain sections were processed as described above with: first, omission of the primary antibody; second, incubation with pre-immune serum; and third, using primary antibodies that had been preabsorbed for 24 h at 4 °C with a fivefold molar excess of purified rat recombinant CNTF.
Immunohistochemical detection of CNTFRα was performed as described above (primary antibody dilution: 1 : 20).
For P-STAT3 immunohistochemical detection unmasking procedures were used (Frontini et al. 2008). Free-floating sections were reacted with 1% NaOH and 1% H2O2 (20 min), 0.3% glycine (10 min) and 0.03% SDS (10 min). After rinsing in PBS, they were blocked with 3% normal goat serum (in 0.2% Triton X-100; 60 min) and incubated with the polyclonal anti-P-STAT3 rabbit serum (dilution 1 : 1000) in PBS, overnight at 4 °C. The next day, the procedure was performed as described above. Staining was not observed when the primary antibody was omitted.
Double-labelling and confocal microscopy
For double-label experiments, free-floating sections were processed according to the P-STAT3 protocol until the primary antibody incubation step. Then they were incubated overnight in a mixture of two primary antibodies: polyclonal chicken anti-CNTF (1 : 50) and polyclonal goat anti-vimentin (1 : 400); polyclonal goat anti-CNTF (1 : 50) and monoclonal mouse anti-GFAP antibody (1 : 1000); or polyclonal rabbit anti-P-STAT3 (1 : 700) and polyclonal goat anti-CNTF (1 : 50). The next day sections were washed twice with PB and incubated in a cocktail of fluorophore-linked secondary antibodies at a dilution of 1 : 100 v/v in PB for 1 h at room temperature. The secondary antibodies were DyLight™649-conjugated anti-goat IgG, DyLight™549-conjugated anti-mouse IgG, DyLight™488-conjugated anti-rabbit IgG and DyLight™549-conjugated anti-chicken IgY (all from Jackson ImmunoResearch, West Grove, PA, USA). Sections were subsequently washed twice with PB, mounted on standard glass slides, air-dried and coverslipped using Vectashield mounting medium (Vector). Sections were viewed under a motorized Leica DM6000 microscope at different magnifications. Fluorescence was detected with a Leica TCS-SL spectral confocal microscope equipped with an Argon and He/Ne mixed gas laser. Fluorophores were excited with the 488 nm, 543 nm and 649 nm lines, and imaged separately. Images (1024 × 1024 pixels) were obtained sequentially from two channels using a confocal pinhole of 1.1200 and stored as TIFF files. Brightness and contrast of the final images were adjusted using the Photoshop 6 software (Adobe Systems, Mountain View, CA, USA).
Results
CNTF IR in mouse hypothalamus
The expression and distribution of CNTF in adult mouse hypothalamus were investigated by immunohistochemistry using two different commercial antibodies, a polyclonal goat (R&D Systems) and a polyclonal chicken (Promega) antibody, raised against recombinant rat CNTF and purified by affinity chromatography. Alignment of Rattus norvegicus (NP_037298) and Mus musculus (NP_740756) CNTF using the multiple sequence alignment software Clustal W version 2.1 showed that rat and mouse CNTF proteins share high sequence homology (95% amino acid identity). Crucially, rat CNTF administered to mice induces the full range of physiological responses observed in rats (Cognet et al. 2004). As a consequence these antibodies, though raised against rat CNTF, have been widely used to detect CNTF in mouse nervous system and peripheral organs by immunohistochemistry (Dallner et al. 2002; Langenhan et al. 2005; Lu et al. 2009; McGregor et al. 2010), and have given negative results in CNTF knockout mice (Langenhan et al. 2005). In the present work the specificity of the two antibodies was further confirmed by Western blotting experiments, which showed that both reliably detected recombinant rat CNTF peptide down to an amount of about 10 ng (Fig. 1). In contrast, Western blotting experiments on protein extracts of adult mouse brain did not show specific bands of the expected molecular weight or of different molecular weights, suggesting that the amount of CNTF found in normal adult mouse brain was simply below the detection threshold of the techniques. This finding agrees with the limited CNTF expression observed in the adult rodent brain. On immunohistochemistry, no significant differences were noted between the two antibodies, except that the polyclonal chicken antibody exhibited a slightly higher background. In addition, double-label experiments using the two anti-CNTF antibodies in the same hypothalamic sections showed complete co-localization. Unless otherwise specified, the images shown in the present paper are thus from sections processed with the polyclonal goat antibody (R&D Systems).
Fig. 1.
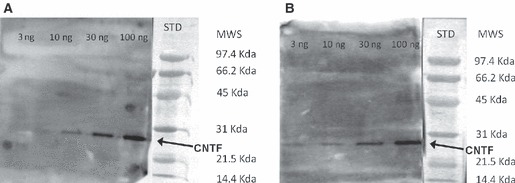
Western blot detection of recombinant rat CNTF. Both the Promega (A) and the R&D Systems (B) antibody detected CNTF down to an amount of about 10 ng. The high level of background in this figure is due to the high concentration of HRP required to increase the sensitivity of the technique to the nanogram range and better to display the absence of non-specific lanes. CNTF, ciliary neurotrophic factor; MWS, molecular weight standard; STD, standards.
As already reported in mouse brain (Dallner et al. 2002), CNTF was strongly expressed in astrocytes scattered in white matter structures, such as the external (Fig. 2A) and internal (Fig. 2B) capsule, the fimbria, the alveus, the optic chiasm and the optic tracts.
Fig. 2.
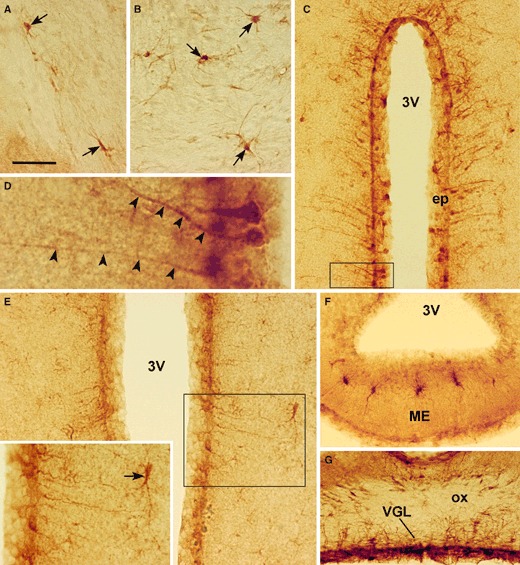
CNTF peroxidase immunohistochemistry in adult mouse brain. Specific staining is detected in astrocytes (arrows) of the external (A) and internal (B) capsule. The ependymal layer (ep) lining the third ventricle (3V) contains positive ependymal cells and tanycytes (C and E). Tanycytes are easily recognized by their long and strongly-stained process (D, arrowheads). A few astrocytes in the hypothalamic parenchyma (inset in E, arrow) and the median eminence (ME, F) are also positive. CNTF IR is also found in the ventral glia limitans (VGL) lining the ventral surface of the brain (G). (D) Enlargement of the framed area in (C). The inset in (E) is the enlargement of the framed area. ox, optic chiasm. Scale bars: 35 μm (A, B and F); 80 μm (C); 14 μm (D); 40 μm (E); 18 μm (inset of E); 50 μm (G).
In the hypothalamus, CNTF IR was seen throughout the rostrocaudal extension of the third ventricle. Rostrally, the vascular organ of the lamina terminalis (VOLT) was positive. In the caudalmost sections, numerous ependymal cells located in the anterior, tuberal and mammillary regions of the third ventricle exhibited specific cytoplasmic staining (Fig. 2C); among them were tanycyte-like cells exhibiting long, strongly stained processes extending to the adjacent grey matter (Fig. 2D). CNTF-positive tanycyte-like cells were scattered among positive and negative ependymal cells in almost all the sections examined. They were particularly numerous in the ventricular wall facing the paraventricular nucleus and in the one located just over the arcuate nucleus and the median eminence, where the ependyma was diffusely positive. Sometimes positive ependymal and tanycyte-like cells also exhibited nuclear staining. In anterior hypothalamus coronal sections grey matter, cells lying a few hundred microns from the ependyma were positive (Fig. 2E); they had a roundish or elongated body with numerous hair-like processes. Such astrocyte-like positive cells were sometimes found in clusters or arranged in short lines. In the coronal sections from the hypothalamic tuberal region, scattered astrocyte-like positive cells were also found in the median eminence (Fig. 2F). The glia limitans on the ventral portion contained CNTF-positive astrocytes (Fig. 2G), as previously described in rat hypothalamus (Watt et al. 2006).
Specific staining was not observed without the primary antibody or after section incubation with non-immune serum. Furthermore, pre-incubation of the antibodies with an excess of recombinant CNTF completely abolished CNTF staining at all sites.
To confirm the phenotype of the CNTF-positive cells in adult mouse hypothalamus, double-label experiments were performed with markers for glial cells. The CNTF-positive tanycyte-like cells also exhibited IR for vimentin (Fig. 3A–C) and GFAP (Fig. 3D–F), two widely used tanycyte markers. The CNTF-positive cells found in the hypothalamic grey matter (Fig. 3G–I) and in the median eminence also contained GFAP, the typical astrocyte marker.
Fig. 3.
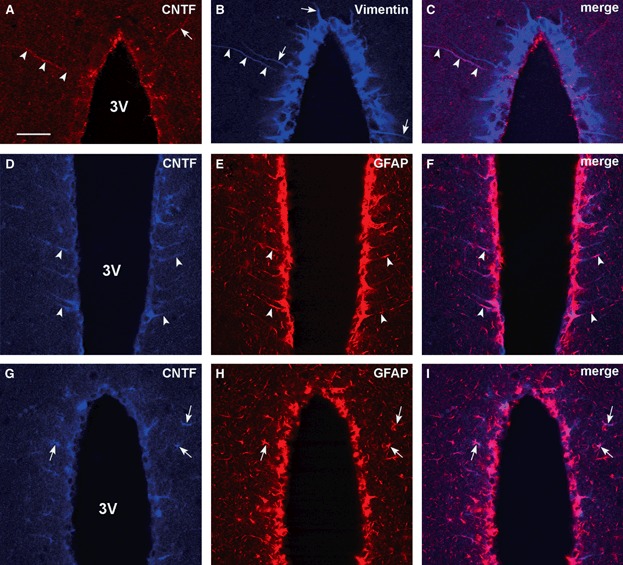
Double-label confocal microscopy in adult mouse hypothalamus. CNTF-positive tanycyte-like cells (A and D, arrowheads) also express vimentin (B and C, arrowheads) and GFAP (E and F, arrowheads). Note one CNTF-positive tanycyte-like cell (A, arrow) that is not positive for vimentin; in turn, some tanycytes exhibiting vimentin IR (B, arrows) are not positive for CNTF. CNTF-positive astrocyte-like cells (G, arrows) are also positive for GFAP (H and I, arrows). In (A), CNTF was detected with the Promega antibody. 3V, third ventricle; CNTF, ciliary neurotrophic factor; GFAP, glial fibrillary acidic protein. Scale bar: 40 μm.
In conclusion, our findings show that CNTF expression in adult mouse hypothalamus, though in no way remarkable quantitatively, is distinctively restricted to specific glial cell types, mostly ependymocytes and tanycytes, as well as to grey matter and median eminence astrocytes.
CNTF-sensitive cells in mouse hypothalamus
The distribution of CNTF-responding cells in the hypothalamus and their spatial relationship with CNTF-producing cells were investigated by immunohistochemical experiments in mice administered intraperitoneal CNTF. In line with previous reports (Lambert et al. 2001; Anderson et al. 2003), systemic injection of recombinant CNTF induced P-STAT3 expression in the brain meninges and in some perivascular cells, in arcuate nucleus neurons, in the ependymal wall of the ventricular system and in the circumventricular organs, such as the VOLT and the median eminence. In the hypothalamus, nuclear staining for P-STAT3 was observed in scattered ependymal cells and tanycytes (Fig. 4A), where also the cytoplasmic process was faintly positive (Fig. 4B). In sections from the hypothalamic tuberal region CNTF induced strong activation of the ependyma facing the arcuate nucleus and the median eminence (Fig. 4C), indicating the presence of highly responsive cells in this portion of the ependyma. In control mice only weak P-STAT3 IR was found in arcuate nucleus neurons (Frontini et al. 2008). Using a polyclonal antibody against CNTFRα, specific staining was detected in meninges, ependymal layers of the lateral and third ventricle, and circumventricular organs (Fig. S1). This staining pattern provided further evidence that the P-STAT3-positive cells found in the injected mice did bear the specific receptor. Double-label experiments in injected mice showed that CNTF-producing ependymal cells and tanycytes were intermingled with CNTF-responsive ependymal cells and tanycytes throughout the third ventricle. Often, CNTF-positive ependymal cells or tanycytes also exhibited P-STAT3 IR (Fig. 4D–F), suggesting that CNTF-producing cells also bear the specific functional receptor. A distinctive, and typical, pattern was seen in the median eminence, where a sizeable population of CNTF-producing cells was concentrated adjacent to the strongly CNTF-responsive ependymal cells, with a considerable degree of overlap (Fig. 5). Grey matter CNTF-producing astrocytes were not positive for P-STAT3, whereas median eminence CNTF-positive astrocytes also exhibited P-STAT3 IR.
Fig. 4.
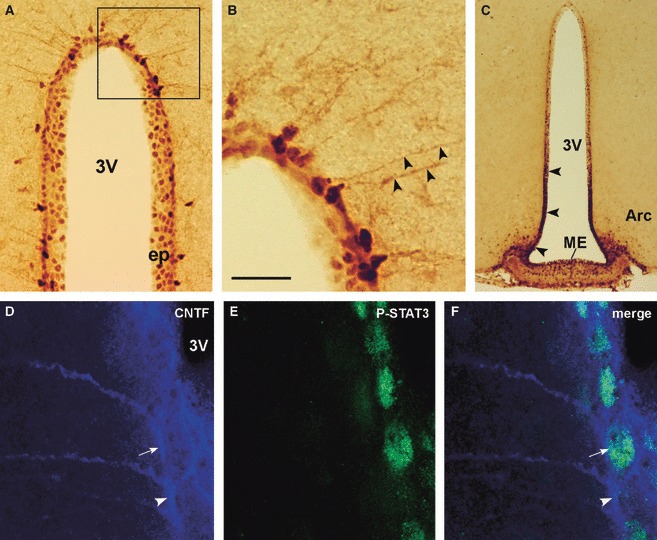
P-STAT3 and CNTF IR in the hypothalamus of CNTF-treated adult mice. By peroxidase immunohistochemistry, staining for P-STAT3 is detected in the nuclei of ependymal cells and tanycytes (A). In the latter, the long process is faintly positive (B, arrowheads). The ependymal layer facing the median eminence (ME) and the arcuate nucleus (Arc, arrowheads) exhibits strong P-STAT3 IR (C). Double-label confocal microscopy (D–F) shows a CNTF-positive tanycyte (arrow) also expressing P-STAT3 located near a CNTF-positive tanycyte (arrowhead) not expressing P-STAT3. (B) Enlargement of the framed area in (A). 3V, third ventricle; CNTF, ciliary neurotrophic factor; ep, ependymal layer; P-STAT3, phospho-signal transducer and activator of transcription 3. Scale bars: 80 μm (A); 20 μm (B); 200 μm (C); 10 μm (D–F).
Fig. 5.
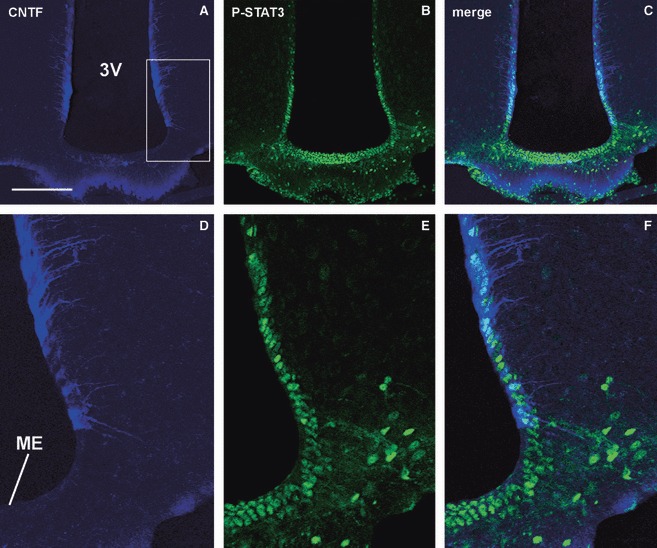
CNTF and P-STAT3 IR in the hypothalamus of CNTF-treated adult mice. A sizeable population of CNTF-positive ependymal cells and tanycytes (A) in a section of the tuberal portion of the hypothalamus is adjacent to and overlaps to a significant extent with the P-STAT3-positive ependyma (B and C). (D–F) Enlargements of the area framed in (A). 3V, third ventricle; CNTF, ciliary neurotrophic factor; ME, median eminence; P-STAT3, phospho-signal transducer and activator of transcription 3. Scale bars: 150 μm (A–C); 50 μm (D and E).
Discussion
CNTF, a potent survival molecule for a variety of embryonic neurons in culture, influences the differentiation of developing neurons and glial cells in vivo. For example, it supports the survival of parasympathetic neurons of the chick ciliary ganglion in vitro (Barbin et al. 1984; Lin et al. 1989; Stockli et al. 1989), it prevents lesion-mediated degeneration of rat motor neurons (Sendtner et al. 1990), it promotes cholinergic differentiation of sympathetic neurons (Ernsberger et al. 1989; Saadat et al. 1989), as well as differentiation of glial progenitors into astrocytes (Hughes et al. 1988; Lillien et al. 1988) and maturation and survival of oligodendrocytes (Louis et al. 1993) in rodents.
Compared with the other growth factors acting on the nervous system, CNTF shows remarkable and distinctive features. First, it is hardly detectable during embryo development and its synthesis begins, or increases greatly, in the nervous system after birth, gradually reaching adult levels during the postnatal period (Stockli et al. 1989, 1991). In addition, its amino acid sequence lacks a signal peptide, suggesting a cytosolic rather than a secretory molecule. As a consequence, a role for it as an extracellular signal in vivo would presumably require its release from healthy cells by an as yet unidentified, non-conventional mechanism; alternatively it could play a role after release from degenerating cells following injury (Guthrie et al. 1997; Lee et al. 1997; Watt et al. 2006). Finally, CNTF expression is profoundly altered after several types of lesion to the central or peripheral nervous systems, where it increases exclusively in glial cells adjacent to the injury site. The notion that it may act as a neurotrophic factor that becomes available in response to nerve damage has led to propose CNTF, or axokine, a CNTF analogue developed for obesity treatment, as a possible therapeutic option to treat a variety of nervous system diseases.
High levels of CNTF mRNA have been detected in the optic nerve and olfactory bulb of adult rat brain using quantitative Northern blotting analysis, intermediate levels being found in the cerebellum and brainstem, and low levels in regions such as hippocampus, striatum, cerebral cortex and septum (Stockli et al. 1991). In situ hybridization and immunohistochemical studies showed that CNTF was predominantly expressed in Schwann cells of the optic and sciatic nerves and in white matter type-1 astrocytes (Dallner et al. 2002). Notably, hybridization was also observed in some areas of the third ventricle ependyma, with stronger labelling in its superior aspect (Guthrie et al. 1997).
In the present paper we showed by immunohistochemistry that CNTF is constitutively expressed predominantly by ependymocytes and tanycytes of the ependymal layer of the third ventricle in mouse hypothalamus and, to a lesser extent, by grey matter and median eminence astrocytes. In addition, CNTF-producing ependymocytes and tanycytes are found adjacent, or in close proximity, to CNTF-responsive ependymocytes and tanycytes. A smaller proportion of CNTF-producing cells is also endowed with CNTFRα, the functional receptor. These distribution patterns strongly suggest that the third ventricle ependyma of normal adult mouse hypothalamus is provided with niches of intermingled CNTF-producing and CNTF-responsive cells that can be involved in CNTF-mediated responses to physiological or pathological stimuli via autocrine and/or paracrine loops. At present the role(s) of CNTF in the ependymal layer of mouse hypothalamus can only be surmised. Nevertheless, CNTF surges detected in the ependymal layer bordering medially the arcuate nucleus may indicate that its effects on proopiomelanocortin-expressing neurons, which are involved in energy balance regulation (Lambert et al. 2001; Janoschek et al. 2006), may also take place in physiological conditions. As suggested for other molecules (Rodrìguez et al. 2005), CNTF could indeed be delivered to arcuate nucleus neurons by the long processes of tanycytes. Another intriguing hypothesis is that CNTF-producing and CNTF-responsive ependymal cells might be involved in the neurogenesis occurring in adult mouse hypothalamus.
In the adult brain, neurogenesis has been most clearly documented in the subventricular zone of the lateral ventricles and in the subgranular zone of the hippocampal formation; CNTF enhances forebrain neurogenesis in adult mice (Emsley & Hagg, 2003). However, recent reports indicate that other brain structures, including the hypothalamus, exhibit neuroproliferative potency. In particular, epidermal growth factor and basic fibroblast growth factor (Xu et al. 2005), insulin-like growth factor I (Perez-Martin et al. 2010), brain-derived neurotrophic factor (Pencea et al. 2001) and, significantly, CNTF (Kokoeva et al. 2005, 2007) have been shown to induce endogenous stem cell proliferation and differentiation into neurons and glial cells in adult rodent hypothalamus. The exact region where hypothalamic stem or progenitor cells originate has not yet been determined. Nevertheless, some data suggest the presence of neurogenic niche-like structures along the third ventricle (Migaud et al. 2010). In addition, administration of the cell proliferation marker bromodeoxyuridine to adult rats followed by labelling of dividing cells a few hours from treatment documented low-level mitotic activity in normal conditions in the ependymal layer of the third ventricle; such activity was greatly increased by systemic administration of basic fibroblast growth factor in ependymal cells and tanycytes (Xu et al. 2005). Finally, tanycytes – remnants of radial glial cells – which have been shown to maintain plastic properties in vitro and in vivo (Rodrìguez et al. 2005), have recently been proposed as possible stem cells in adult mouse circumventricular organs (Bennett et al. 2009).
Intracerebroventricular delivery of CNTF induces cell proliferation in mouse hypothalamus (Kokoeva et al. 2005). The density of proliferating cells was highest in the hypothalamic tuberal region, which contains the arcuate nucleus and the median eminence. Many of these newborn cells differentiated into neurons and some into oligodendrocytes or astrocytes; the newborn neurons differentiated into arcuate nucleus leptin-sensitive neurons, offering a possible explanation for the sustained anorectic effect of CNTF. Our findings, documenting a rich source of CNTF-producing ependymal cells and tanycytes in the hypothalamic tuberal region, lend support to the hypothesis that CNTF-dependent cell proliferation and differentiation may also take place in normal conditions in mouse hypothalamus. In addition, the fact that the CNTF-producing and the CNTF-responsive ependyma were found to be in close proximity suggests that the stem or progenitor cells stimulated by CNTF may be median eminence ependymal cells and/or tanycytes.
In conclusion, our study provides morphological evidence that the ependyma of the third ventricle produces CNTF and also contains its functional receptor, CNTFRα. The close spatial relationship of CNTF-producing and CNTF-responsive cells in the ependymal layer is consistent with the possibility that the mouse ependyma is provided with CNTF-dependent paracrine and/or autocrine loops having an as yet unknown physiological role, which may also be recruited to provide the hypothalamus with protective and compensatory strategies following injury.
Acknowledgments
This work was financed by grants from Università Politecnica delle Marche (Contributi Ricerca Scientifica) and the Italian Ministry of University (PRIN 2007 to AG).
Author contributions
I.S.: performance of experiments, data analysis and interpretation, manuscript writing. M.R.C. and T.L.: performance of experiments and data analysis and interpretation. A.A.: data analysis and interpretation. S.C.: critical revision of the manuscript. A.G.: conception and design, financial support, performance of experiments, data analysis and interpretation, manuscript writing, final approval of the manuscript.
Supporting Information
Fig. S1. P-STAT3 and CNTFRα peroxidaseimmunohistochemistry. In a CNTF-treated mouse P-STAT3 nuclearstaining is detectable in meninges (Men, A), ependyma (ep, C) andmedian eminence (ME, E). In adjacent sections, the same structuresexhibit specific CNTFRα IR (B, D and F). 3V, third ventricle.Scale bars: 15 μm (A and B); 35 μm (C and D);80 μm (E and F).
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
References
- Adler R, Landa KB, Manthorpe M, et al. Cholinergic neuronotrophic factors: intraocular distribution of trophic activity for ciliary neurons. Science. 1979;204:1434–1436. doi: 10.1126/science.451576. [DOI] [PubMed] [Google Scholar]
- Anderson KD, Lambert PD, Corcoran TL, et al. Activation of the hypothalamic arcuate nucleus predicts the anorectic actions of ciliary neurotrophic factor and leptin in intact and gold thioglucose-lesioned mice. J Neuroendocrinol. 2003;15:649–660. doi: 10.1046/j.1365-2826.2003.01043.x. [DOI] [PubMed] [Google Scholar]
- Barbin G, Manthorpe M, Varon S. Purification of the chick eye ciliary neurotrophic factor. J Neurochem. 1984;43:1468–1478. doi: 10.1111/j.1471-4159.1984.tb05410.x. [DOI] [PubMed] [Google Scholar]
- Bauer S, Kerr BJ, Patterson PH. The neuropoietic cytokine family in development, plasticity, disease and injury. Nat Rev Neurosci. 2007;8:221–232. doi: 10.1038/nrn2054. [DOI] [PubMed] [Google Scholar]
- Bennett L, Yang M, Enikolopov G, et al. Circumventricular organs: a novel site of neural stem cells in the adult brain. Mol Cell Neurosci. 2009;41:337–347. doi: 10.1016/j.mcn.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjørbæk C, Elmquist JK, El-Haschimi K, et al. Activation of SOCS-3 messenger ribonucleic acid in the hypothalamus by ciliary neurotrophic factor. Endocrinology. 1999;140:2035–2043. doi: 10.1210/endo.140.5.6736. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cognet I, Guilhot F, Chevalier S, et al. Expression of biologically active mouse ciliary neurotrophic factor (CNTF) and soluble CNTFRalpha in Escherichia coli and characterization of their functional specificities. Eur Cytokine Netw. 2004;15:255–262. [PubMed] [Google Scholar]
- Dallner C, Woods AG, Deller T, et al. CNTF and CNTF receptor alpha are constitutively expressed by astrocytes in the mouse brain. Glia. 2002;37:374–378. [PubMed] [Google Scholar]
- Davis S, Aldrich T, Stahl N, et al. LIFRβ and gp130 as heterodimerizing signal transducers of the tripartite CNTF receptor. Science. 1993;260:1805–1810. doi: 10.1126/science.8390097. [DOI] [PubMed] [Google Scholar]
- Emsley JG, Hagg T. Endogenous and exogenous ciliary neurotrophic factor enhances forebrain neurogenesis in adult mice. Exp Neurol. 2003;183:298–310. doi: 10.1016/s0014-4886(03)00129-8. [DOI] [PubMed] [Google Scholar]
- Ernsberger U, Sendtner M, Rohrer H. Proliferation and differentiation of embryonic chick sympathetic neurons; effects of ciliary neurotrophic factor. Neuron. 1989;2:1275–1284. doi: 10.1016/0896-6273(89)90312-7. [DOI] [PubMed] [Google Scholar]
- Frontini A, Bertolotti P, Tonello C, et al. Leptin-dependent STAT3 phosphorylation in postnatal mouse hypothalamus. Brain Res. 2008;1215:105–115. doi: 10.1016/j.brainres.2008.03.078. [DOI] [PubMed] [Google Scholar]
- Guthrie KM, Woods AG, Nguyen T, et al. Astroglial ciliary neurotrophic factor mRNA expression is increased in fields of axonal sprouting in deafferented hippocampus. J Comp Neurol. 1997;386:137–148. [PubMed] [Google Scholar]
- Hughes SM, Lillien LE, Raff MC, et al. Ciliary neurotrophic factor induces type-2 astrocyte differentiation in culture. Nature. 1988;335:70–73. doi: 10.1038/335070a0. [DOI] [PubMed] [Google Scholar]
- Ip N, McClain J, Barrezueta N, et al. The α component of the CNTF receptor is required for signalling and defines potential CNTF targets in the adult and during development. Neuron. 1993;10:89–102. doi: 10.1016/0896-6273(93)90245-m. [DOI] [PubMed] [Google Scholar]
- Janoschek R, Plum L, Koch L, et al. gp130 signaling in proopiomelanocortin neurons mediates the acute anorectic response to centrally applied ciliary neurotrophic factor. Proc Natl Acad Sci USA. 2006;103:10707–10712. doi: 10.1073/pnas.0600425103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly JF, Elias CF, Lee CE, et al. Ciliary neurotrophic factor and leptin induce distinct patterns of immediate early gene expression in the brain. Diabetes. 2004;53:911–920. doi: 10.2337/diabetes.53.4.911. [DOI] [PubMed] [Google Scholar]
- Kokoeva MV, Yin H, Flier JS. Neurogenesis in the hypothalamus of adult mice: potential role in energy balance. Science. 2005;310:679–683. doi: 10.1126/science.1115360. [DOI] [PubMed] [Google Scholar]
- Kokoeva MV, Yin H, Flier JS. Evidence for constitutive neural cell proliferation in the adult murine hypothalamus. J Comp Neurol. 2007;505:209–220. doi: 10.1002/cne.21492. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lambert PD, Anderson KD, Sleeman MW, et al. Ciliary neurotrophic factor activates leptin-like pathways and reduces body fat, without cachexia or rebound weight gain, even in leptin-resistant obesity. Proc Natl Acad Sci USA. 2001;98:4652–4657. doi: 10.1073/pnas.061034298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenhan T, Sendtner M, Holtmann B, et al. Ciliary neurotrophic factor – immunoreactivity in olfactory sensory neurons. Neuroscience. 2005;134:1179–1194. doi: 10.1016/j.neuroscience.2005.05.017. [DOI] [PubMed] [Google Scholar]
- Lee MY, Deller T, Kirsch M, et al. Differential regulation of ciliary neurotrophic factor (CNTF) and CNTF receptor alpha expression in astrocytes and neurons of the fascia dentata after entorhinal cortex lesion. J Neurosci. 1997;134:1137–1146. doi: 10.1523/JNEUROSCI.17-03-01137.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillien LE, Sendtner M, Rohrer H, et al. Type-2 astrocyte development in rat brain cultures is initiated by a CNTF-like protein produced by type-1 astrocytes. Neuron. 1988;1:485–494. doi: 10.1016/0896-6273(88)90179-1. [DOI] [PubMed] [Google Scholar]
- Lin LFH, Mismer D, Lile JD, et al. Purification, cloning, and expression of ciliary neurotrophic factor (CNTF) Science. 1989;246:1023–1025. doi: 10.1126/science.2587985. [DOI] [PubMed] [Google Scholar]
- Louis JC, Magal E, Takayama S, et al. CNTF protection of oligodendrocytes against natural and tumor necrosis factor-induced death. Science. 1993;259:689–692. doi: 10.1126/science.8430320. [DOI] [PubMed] [Google Scholar]
- Lu Z, Hu X, Zhu C, et al. Overexpression of CNTF in mesenchymal stem cells reduces demyelination and induces clinical recovery in experimental autoimmune encephalomyelitis mice. J Neuroimmunol. 2009;206:58–69. doi: 10.1016/j.jneuroim.2008.10.014. [DOI] [PubMed] [Google Scholar]
- MacLennan AJ, Neitzel KL, Devlin BK, et al. In vivo localization and characterization of functional ciliary neurotrophic factor receptors which utilize JAK-STAT signaling. Neuroscience. 2000;99:761–772. doi: 10.1016/s0306-4522(00)90236-7. [DOI] [PubMed] [Google Scholar]
- McGregor NE, Poulton IJ, Walker EC, et al. Ciliary neurotrophic factor inhibits bone formation and plays a sex-specific role in bone growth and remodelling. Calcif Tissue Int. 2010;86:261–270. doi: 10.1007/s00223-010-9337-4. [DOI] [PubMed] [Google Scholar]
- Migaud M, Batailler M, Segura S, et al. Emerging new sites for adult neurogenesis in the mammalian brain: a comparative study between the hypothalamus and the classical neurogenic zones. Eur J Neurosci. 2010;32:2042–2052. doi: 10.1111/j.1460-9568.2010.07521.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. 2nd edn. San Diego: Academic Press; 2001. [Google Scholar]
- Pencea V, Bingaman KD, Wiegand SJ, et al. Infusion of brain-derived neurotrophic factor into the lateral ventricle of the adult rat leads to new neurons in the parenchyma of the striatum, septum, thalamus, and hypothalamus. J Neurosci. 2001;21:6706–6717. doi: 10.1523/JNEUROSCI.21-17-06706.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Martin M, Cifuentes M, Grondona JM, et al. IGF-I stimulates neurogenesis in the hypothalamus of adult rats. Eur J Neurosci. 2010;31:1533–1548. doi: 10.1111/j.1460-9568.2010.07220.x. [DOI] [PubMed] [Google Scholar]
- Poduslo JF, Curran GL. Permeability at the blood-brain and blood-nerve barriers of the neurotrophic factors: NGF, CNTF, NT-3, BDNF. Mol Brain Res. 1996;36:280–286. doi: 10.1016/0169-328x(95)00250-v. [DOI] [PubMed] [Google Scholar]
- Rodrìguez EM, Blàzquez JL, Pastor FE, et al. Hypothalamic tanycytes: a key component of brain-endocrine interaction. Int Rev Cytol. 2005;247:89–164. doi: 10.1016/S0074-7696(05)47003-5. [DOI] [PubMed] [Google Scholar]
- Saadat S, Sendtner M, Rohrer H. Ciliary neurotrophic factor induces cholinergic differentiation in rat sympathetic neurons in culture. J Cell Biol. 1989;108:1807–1816. doi: 10.1083/jcb.108.5.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sendtner M, Kreutzberg GW, Thoenen H. Ciliary neurotrophic factor prevents the degeneration of motor neurons after axotomy. Nature. 1990;345:440–441. doi: 10.1038/345440a0. [DOI] [PubMed] [Google Scholar]
- Sendtner M, Carrol P, Holtmann B, et al. Ciliary neurotrophic factor. J Neurobiol. 1994;25:1436–1453. doi: 10.1002/neu.480251110. [DOI] [PubMed] [Google Scholar]
- Simi A, Ibanez CF. Assembly and activation of neurotrophic factor receptor complexes. Dev Neurobiol. 2010;70:523–531. doi: 10.1002/dneu.20773. [DOI] [PubMed] [Google Scholar]
- Sleeman MW, Anderson KD, Lambert PD, et al. The ciliary neurotrophic factor and its receptor, CNTFRα. Pharm Acta Helv. 2000;74:265–272. doi: 10.1016/s0031-6865(99)00050-3. [DOI] [PubMed] [Google Scholar]
- Stockli KA, Lottspeich F, Sendtner M, et al. Molecular cloning, expression and regional distribution of rat ciliary neurotrophic factor. Nature. 1989;342:920–923. doi: 10.1038/342920a0. [DOI] [PubMed] [Google Scholar]
- Stockli KA, Lillien LE, Naher-Noé M, et al. Regional distribution, developmental changes, and cellular localization of CNTF-mRNA and protein in the rat brain. J Cell Biol. 1991;115:447–459. doi: 10.1083/jcb.115.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt JA, Bone S, Pressler M, et al. Ciliary neurotrophic factor is expressed in the magnocellular neurosecretory system of the rat in vivo: evidence for injury- and activity-induced upregulation. Exp Neurol. 2006;197:206–214. doi: 10.1016/j.expneurol.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Xu Y, Tamamaki N, Noda T, et al. Neurogenesis in the ependymal layer of the adult 3rd ventricle. Exp Neurol. 2005;192:251–264. doi: 10.1016/j.expneurol.2004.12.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


