Abstract
Determining previous infecting dengue virus (DENV) serotypes has been difficult due to highly cross-reactive immune responses from previous DENV infections. Determining the correlates of serotype-specific immune responses would be crucial in understanding dengue transmission in the community and would also help to determine the correlates of protective immune responses. Therefore, we set out to define highly conserved, serotype-specific regions of the DENVs. Serotype-specific and highly conserved regions of the four DENV serotypes were identified using Basic Local Alignment Search Tool (BLAST) searches and custom perl scripts. Using ex-vivo and cultured enzyme-linked immunospot (ELISPOT) assays, we identified serotype-specific T cell epitopes within the four DENV serotypes in healthy adult donors from Sri Lanka. We identified T cell responses to 19 regions of the four DENV serotypes. Six peptides were from the NS2A region and four peptides were from the NS4A region. All immune donors responded to peptides of at least two DENV serotypes, suggesting that heterologous infection is common in Sri Lanka. Eight of 20 individuals responded to at least two peptides of DENV-4, despite this serotype not being implicated previously in any of the epidemics in Sri Lanka. The use of these regions to determine past and current infecting DENV serotypes will be of value to characterize further the dynamics of silent dengue transmission in the community. In addition, these T cell responses to these regions could be used to characterize DENV serotype-specific immune responses and thus possibly help us to understand the immune correlates of a protective immune response.
Keywords: dengue viruses, highly conserved, non-cross-reactive, serotype-specific, T cells
Introduction
Dengue viral (DENV) infections have become the most important mosquito-borne viral infections in the world, and are one of the major emerging infectious diseases. It is estimated that 2·1 million cases of dengue haemorrhagic fever (DHF)/dengue shock syndrome (DSS) occur every year, resulting in 21 000 deaths [1]. There are four DENV serotypes (DENV1–4), which are closely related. Initial infection with a particular serotype is known as primary infection, which is usually asymptomatic or results in mild disease manifestations. Subsequent infection with other serotypes (secondary dengue infections) may lead to severe disease which manifests in the form of DHF/DSS [2]. However, the majority of both primary and secondary dengue infections (DI) result in asymptomatic/mild clinical disease and are therefore undetected.
The reasons as to why severe DI occurs in only some individuals are not clear. However, studies have suggested that immunopathological [2], host-genetic [3],[4] and viral factors [5] all contribute to the occurrence of severe disease. The cross-reactive nature of the T cell epitopes identified so far has hampered the study of DENV serotype-specific responses and how they evolve over time. As it has been suggested that memory T cell responses to the previous infecting DENV serotype could determine the outcome of subsequent infections [6], it is important to study serotype-specific immune responses in both acute and past DI. Due to the cross-reactive nature of both T cell and antibody responses, it has been difficult to determine the number and serotype of previous infecting DENVs [6]–[8], and thus their influence in subsequent acute DIs. A large number of both CD4+ and CD8+ T cell epitopes have been identified from patients with acute DI and healthy volunteers who received live attenuated monovalent dengue vaccines [9],[10] which have been shown to be highly cross-reactive [8],[11]–[13]. Although some serotype-specific T cell epitopes have also been identified, all such T cell epitopes identified so far show >55% homology between the four DENV serotypes, and therefore could not be considered highly specific [7].
The majority of individuals infected with the dengue virus do not develop a severe immunopathology. Therefore, it is possible that the DV-specific memory T cell repertoire in individuals who have experienced mild/asymptomatic DI is different to those who have experienced severe DIs. Identification of serotype-specific T cell responses would enable us to determine whether the number of past infecting DENVs, the sequence of infection with different serotypes and the quality and quantity of serotype-specific T cell responses for past DIs influence the outcome of subsequent acute DIs. Identification of DENV-specific memory T cell responses in such individuals with past asymptomatic/mild infection would help us to determine the correlates of protective immunity.
The predominant circulating DENV serotypes in a given community is determined by detection of the virus in acutely unwell patients who present with symptoms suggestive of DI to health-care facilities. However, the virus serotypes/genotypes causing ‘silent’ DI could be different to those causing more serious infection, and therefore may not reflect the true nature of virus transmission dynamics in the community. Furthermore, in order to define accurately the epidemiology of past and present DIs, it would be advantageous to have an assay that can distinguish infections reliably between particular DENV serotypes. Furthermore, such an assay would contribute to our understanding of correlates of serotype-specific protective immune responses without potential confounding factors associated with cross-reactive T cell responses. Lastly, such data may be of value in future vaccine development, as they would provide information of immunogenic regions that are serotype-specific, thus minimizing risks associated with possible immune enhancement. Therefore, we proceeded to identify serotype specific T cell epitopes in highly conserved regions of the four DENV serotypes in naturally exposed healthy DENV-immune donors from Sri Lanka. We found that individuals with previous DI had a high frequency of memory T cell responses to serotype-specific conserved peptides of DENV, and that many individuals responded to peptides of DENV-4. However, DENV-4 has been thought previously to be responsible for only <5% of all acute DIs in Sri Lanka [14],[15]. These data show that determining T cell responses to these serotype-specific and non-cross-reactive peptides can be used as a valuable tool in studying the epidemiology of DIs.
Methods
Subjects
The study participants consisted of 24 healthy seropositive and five dengue-seronegative adults from Sri Lanka. Two individuals had DHF in the past and the others had not had a clinically diagnosed DI. The mean age of the seropositive donors was 34 years (range 25–59 years). Ethical approval was granted by the Ethical Review Committee of the University of Sri Jayawardanapura, Sri Lanka and the Oxfordshire Ethics committee of the University of Oxford. Informed written consent was obtained from all study participants. Peripheral blood mononuclear cells (PBMC) were obtained from fresh heparinized blood by Ficoll-Hypaque density gradient centrifugation. They were then resuspended in RPMI-1640 plus 10% fetal calf serum (FCS) for ex-vivo enzyme-linked immunospot (ELISPOT) assays and ex-vivo intracellular cytokine staining (ICS) assays and in RPMI-1640 plus 10% human serum for cell cultures.
Identification and synthesis of non-cross-reactive, conserved regions in the dengue virus
Full-length or near full-length polyprotein sequences for all DENV serotypes (taxonomy i.d. 12637) were downloaded from NCBI (http://www.ncbi.nlm.nih.gov/). The protein sequences were used to construct two Basic Local Alignment Search Tool (BLAST) databases [16] for each serotype. One contained only the serotype-specific proteins and a second contained all proteins from the flaviviridae (taxonomy i.d. 11050) excluding that serotype's proteins. A series of BLAST searches and subsequent analyses using custom perl scripts were used to identify regions of the polyprotein sequence that were unique to a given serotype and a conserved within that serotype. Conservation of polyprotein regions across members of the serogroups was confirmed using FUZZPRO searches [17] with a maximum of five mismatches. Using this approach, 19 serotype-specific conserved regions were identified across all DENV serotypes.
For identified regions of the DENVs, 35 20-mer peptides overlapping by 10 amino acids were synthesized for DENV-2 and DENV-3, 23 20-mer peptides for DENV-1 and 28 20-mer peptides for DENV-4. All peptides that were more than 20 aa long, shown in Table 1, were made into 20-mers which overlap by 10 aa. Synthesis was performed in-house in an automated synthesizer using 9-fluorenylmethyloxycarbonyl (Fmoc) chemistry. The purity of the peptides was determined to be greater than 90% by high-pressure liquid chromatography analysis and mass spectrometry. The source region sequences for the four DENVs are listed in Table 1.
Table 1.
The highly conserved and non-cross-reactive regions of the four dengue viruses that were identified.
| Virus serotype | Serotype-specific, non-cross-reactive and highly conserved regions |
|---|---|
| 1 | LKTEVTNPAV |
| PTTEIQLTDYGALT | |
| PTSEIQLTDYGALT | |
| VLVQIKYEGT | |
| VLVQVKYEGT | |
| SRWSRKMLMTGTLAVFFLLI | |
| GTLAVFFLLIMGQLTWNDLI | |
| AVFFLLIMGQLTWNDLIRLC | |
| GTLAVFLLLIMGQLTWNDLI | |
| KMRPMFAVGLLFRR | |
| SLVASVELPNSLEELGDGLAMGIMI | |
| LTDFQSHQLWATLLSLTFIK | |
| ATLLSLTFIKTTFSLHYAWK | |
| TTFSLHYAWKTMAMVLSI | |
| VLLGSLGCKPLTMFLIAENK | |
| GSLGCKPLTMFLIAENKIWG | |
| VERAVLDDGI | |
| VAGILAQGKKMIR | |
| TSGTYVSAIAQAKASQE (NS3) | |
| KLPQHLTQRAQ | |
| FSPSELETPNLAE | |
| FLPSELETPNLAE | |
| 2 | GRMLNILNRRRRSAGMIIMLIPTVM |
| MMAAILAYTIGTTHFQRAL | |
| DTGKHGKEIK | |
| LPGADTQGSNWIQKET | |
| VIRVQYEGDGS | |
| EIMDLEKRHVL | |
| DSYIIIGVEPGQ | |
| QKAHEEGI | |
| IMTGDIKGIMQA | |
| MLSTESHNQTFL | |
| KQDVFCDSK | |
| GMALFLEEMLRTRVGTKHAILLVAVSFVTLITGNMSFRDLGRVMVMVGATMTDDI | |
| KELMMTTIGIVLLSQSTIPETILELTDALALGMMVLKMVRNMEKYQLAVTIMAILCVPNAVILQNAWKVSCT | |
| VVSVSPLLLTSSQQKTDWIPLALTIKGLNPTAIFLTTLSRTSKK | |
| DQAEISGSSPILSITISEDGSMSIKNEEEEQTLTILIRTGLLVI | |
| ITAAAWYLWEVKKQRA | |
| RAVQTKPGLFKTNAGT | |
| EKSIEDNPEIEDDIFR | |
| LTLNLITEMGRLPTFMTQKARD | |
| MTLGMCCIITASILLWYAQIQPH | |
| LGSIATQQPESNI | |
| SRLNALGKSEFQI | |
| RYKKATYEP | |
| NIGIESEIPNLDIIGKRIEKIKQEHETS | |
| KELGKKKTPRM | |
| FKSIQHLTITEEIAVQNWLARVGR | |
| 3 | SNMLSIINQRKKTSLCLMMILPAAL |
| ILALFLAHYIGTSLTQKVV | |
| ETQGVTAE | |
| ASGATTETPTWNRKEL | |
| LIKVEYKGEDA | |
| STEDGQGKAHN | |
| ESNIVIGIGDNA | |
| AGAWENGV | |
| VVVGDTLGVLEQ | |
| IVTAETQNSSFI | |
| VYTQLCDHR | |
| CLAILFEEVLRGKFGKKHMIAGVFFTFVLLLSGQITWRDMAHTLIMIGSNASDRM | |
| RENLLLGVGLAMATTLQLPEDIEQMANGVALGLMALKLITQFETYQLWTALVSLTCSNTIFTLTVAWRTATL | |
| GVSLLPVCQSSSMRKTDWLPMTVAAMGVPPLPLFIFSLKDTLKR | |
| EEAEQTGVSHNLMITVDDDGTMRIKDDETENILTVLLKTALLIV | |
| ATLLVWHTWQKQTQRS | |
| KNFQTTPGTFQTTTGE | |
| NAEPDGPTPELEEEMFK | |
| IALDLVTEIGRVPSHLAHRTRN | |
| TSIGLICVIASSGMLWMAEVPLQ | |
| MSKEPGVVSPTSY | |
| KKLNQLSRKEFDL | |
| THRRPTIEK | |
| HVNAEPETPNMDVIGERIKRIKEEHNST | |
| RTLGRNKRPRL | |
| LTKADLENPHLLEKKITQWLETKGV | |
| 4 | ALLAGFMAYMIGQTGIQRTVF |
| TKTTAKEVA | |
| SGKFSIDKEMA | |
| SNHGVTATITPRSPSVEVK | |
| RDVNKEKVVGRVISSTPLAE | |
| RVISSTPLAE NTNSVT | |
| VITLCAIILGGLTWMDLLRA | |
| WMDLLRALIMLGDTMSGRIG | |
| TALMVIGMAMTTTLSIPHDL | |
| AMTTTLSIPHDLMELIDGIS | |
| AMTTVLSIPHDLMELIDGIS | |
| IVTQFDNTQVGTLA | |
| IMAVLFVVTLIPLCRTSCLQ | |
| IPLCRTSCLQKQSHWVEI | |
| TNMITLLVKLALITVSGLYP | |
| ALITVSGLYP LAIPVTMTL | |
| ALITVSGLYPLAIPITMTL | |
| YEVDEDIFRKKR | |
| ILTEIASLPTYLSSRAKL | |
| IYVILTILTIIGLI | |
| KTKTDFGFYQVKTETTI | |
| EEIDKKDGDL | |
| EDIDKKDGDL | |
| LITEQMAPHHKILAKA | |
| ITQDDMQNPKGLKERVEKWL | |
| DDMQNPKGLKERVEKWLREC | |
| DDMQNPKGLKERVEKWLKEC | |
| YSAPSESEGVL |
Cultured ELISPOT assays
Cultured ELISPOT assays were performed on 20 of 24 healthy dengue immune adults. PBMC from each donor were incubated with the peptides of each DV serotype peptide pool consisting of all overlapping peptides. Cultured ELISPOT assays were performed as described previously [18]. Background (cells plus media) was subtracted and data expressed as number of spot-forming units (SFU) per 106 PBMC. Peptides of each DENV serotype were arranged into nine peptide pools, each pool consisting of five to eight peptides, with each peptide present in two different pools. Therefore, each peptide would drive a response in two different pools. In each instance, once a peptide was found to be antigenic by using the peptide matrix, it was retested with the identified peptide for confirmation of the response. Thus, each peptide response was confirmed at least twice. All peptides that induced an interferon (IFN)-γ response of more than mean ± 3 standard deviations (s.d.) of the irrelevant peptide were considered positive.
Ex-vivo ELISPOT assays
Ex-vivo ELISPOT assays were performed as described previously in 24 dengue-immune donors and five dengue seronegative donors. For ex-vivo ELISPOT assays, 0·1 × 106 PBMC were added to a final volume of 200 µl. Peptide was added at a final concentration of 10 µM. All peptides were tested in duplicate. Phytohaemagglutinin (PHA) was always included as a positive control and an irrelevant peptide [severe acute respiratory syndrome (SARS) peptide] was included as a negative control. Ex-vivo responses were assessed only for the immunogenic peptides identified by the cultured ELISPOT assays. Background (cells plus media) was subtracted and data expressed as number of SFU per 106 PBMC. All peptides that induced an IFN-γ response of more than mean ± 3 s.d. of the irrelevant peptide were considered positive.
Intracellular cytokine secreting assay
To determine IFN-γ production, ex-vivo PBMC or T cell lines were stimulated at 1 × 106–2 × 106/ml in RPMI-1640 plus 10% FCS with the relevant peptides (20 µl of µM peptide) for 16 h according to the manufacturer's instructions in the presence of Brefeldin A (BD GolgiStopTM). Cells were washed and stained with anti-CD3 [fluorescein isothiocyanate (FITC)], anti-CD4 [peridinin chlorophyll (PerCP)] (BD Biosciences) and anti-CD8 [phycoerythrin (PE)]. Cells were then permeabilized and fixed with Cytofix/Cytoperm (BD Biosciences, San Jose, CA, USA) and then stained for intracellular IFN-γ[allophycocyanin (APC)] according to the manufacturer's instructions and analysed using a fluorescence activated cell sorter (FACSCalibur) (Becton Dickinson) with CellQuest software (Becton Dickinson).
Serology and human leucocyte antigen (HLA) typing
Serum was analysed for indirect dengue immunoglobulin (Ig)G capture enzyme-linked immunosorbent assay (ELISA) (Panbio, Alere, Cheshire, UK). All PBMC and B cell lines were HLA-typed by polymerase chain reaction–sequence-specific primers (PCR–SSP) phototyping.
Maintenance of cell lines
Murine fibroblast cell lines transfected with HLA-DRB1*15 (kindly supplied by Professor Lars Fugger) were maintained in Dulbecco's modified Eagle medium (DMEM) (Gibco, Grand Island, NY, USA) supplemented with 10% FCS, 2 mM L-glutamine, 50 U/ml penicillin and 50 µg/ml streptomycin at 37°C with 5% CO2.
MHC class II HLA restriction
All MHC class II HLA restrictions were performed in triplicate. Cells from short-term cultures were incubated with 10 µl monoclonal antibodies at 0·2 mg/ml specific for HLA-DR (L243), HLA-DQ (SPV-L3) (kindly supplied by Prof. Lars Fugger) and HLA-DP (Leinco Technologies, St. Louis, MO, USA; H127) at 37°C for 1 h before addition of peptides.
Murine fibroblast cell lines were initially pulsed with 100 µl of 40 µM peptide for 1 h at 37°C, in 5% CO2. They were then washed three times in RPMI-1640 plus 10% FCS and used as antigen-presenting cells to washed T cells harvested from cell cultures.
Results
Identification of immunogenic, highly conserved, serotype-specific peptides in the four DENV serotypes
The peptides that were recognized by individuals who were naturally exposed to the DENV are shown in Table 2. The donors recognized four peptides of the 23 20-mer peptides in DENV-1, five peptides of the 35 20-mer peptides of DENV-2, five peptides of the 35 peptides of the DENV-3 and five peptides of the 28 20-mer peptides of DENV-4 (Table 2). All dengue immune donors responded to the peptides of at least two DENV serotypes. Two donors responded to peptides of all four DENV serotypes. The number of healthy donors responding to at least two peptides of the four DENV serotypes in the cultured ELISPOT assays is shown in Table 3. Eight of 20 (40%) of the individuals responded to at least two peptides of DENV-4 and responses to at least two peptides of other serotypes ranged from 30 to 50% (Table 3). The frequency of cultured ELISPOT responses to each of these peptides is shown in Fig. 1. These peptides had <15% homology between the four DENV serotypes except for 30% homology for four peptides (DENV-1 peptide with DENV-1 pep-11, DENV-2 pep-33, DENV-4 pep-12, DENV-2 pep-11, DENV-3 pep-11. DENV-2 peptide 17 with DENV-3 pep-21, DENV-3 pep-11 with DENV-4 pep-19).
Table 2.
The sequences, regions of the dengue virus and other details of the serotype-specific, non-cross-reactive and highly conserved peptides of the four dengue virus serotypes.
| Sequence | Virus serotype | Location | Peptide number | n * | Subset |
|---|---|---|---|---|---|
| LKTEVTNPAV | 1 | Env46–55 | Pep1 | 1 | |
| RDVNKEKVVGRVISSTPLAE | 4 | Env340–359 | Pep5 | 3 | CD4 |
| IMTGDIKGIMQA | 2 | NS188–99 | Pep18 | 6 | CD4 |
| SLVASVELPNSLEELGDGLAMGIMI | 1 | NS2A109–133 | Pep11 | 5 | CD4 |
| GSLGCKPLTMFLIAENKIWG | 1 | NS2A19–216 | Pep16 | 4 | |
| SSQQKTDWIPLALTIKGLNP | 2 | NS2A184–203 | Pep33 | 5 | CD4 |
| RENLLLGVGLAMATTLQLPE | 3 | NS2A99–118 | Pep28 | 9 | CD4 |
| AMTTTLSIPHDLMELIDGIS | 4 | NS2A108–127 | Pep10 | 6 | CD4/CD8 |
| IVTQFDNTQVGTLA | 4 | NS2A135–148 | Pep12 | 5 | |
| DQAEISGSSPILSITISEDG | 2 | NS2B63–82 | Pep1 | 7 | CD4 |
| TMRIKDDETENILTVLLKTA | 3 | NS2B83–102 | Pep3 | 4 | CD4 |
| TSGTYVSAIAQAKASQE | 1 | NS3157–173 | Pep20 | 6 | CD4/CD8 |
| LTLNLITEMGRLPTFMTQKA | 2 | NS4A2–21 | Pep11 | 4 | CD4 |
| IALDLVTEIGRVPSHLAHRT | 3 | NS4A2–21 | Pep11 | 5 | CD4 |
| ILTEIASLPTYLSSRAKL | 4 | NS4A6–23 | Pep19 | 9 | CD4 |
| IYVILTILTIIGLI | 4 | 2 K region between NS4A and NS4B8–21 | Pep20 | 6 | |
| SRLNALGKSEFQI | 2 | NS515–27 | Pep17 | 6 | CD4 |
| HVNAEPETPNMDVIGERIKR | 3 | NS5263–282 | Pep8 | 3 | CD4 |
| KKLNQLSRKEFDL | 3 | NS515–27 | Pep21 | 8 | CD4 |
n: Number of individuals who responded to each peptide.
Table 3.
Number of healthy individuals with past asymptomatic/mild infection who responded to at least two peptides of each dengue virus serotype by cultured enzyme-linked immunospot (ELISPOT) assays.
| DENV serotype | Number of individuals who responded to at least two peptides of each serotype, n (%) |
|---|---|
| DEN-1 | 6 (30%) |
| DEN-2 | 8 (40%) |
| DEN-3 | 10 (50%) |
| DEN-4 | 8 (40%) |
Fig. 1.
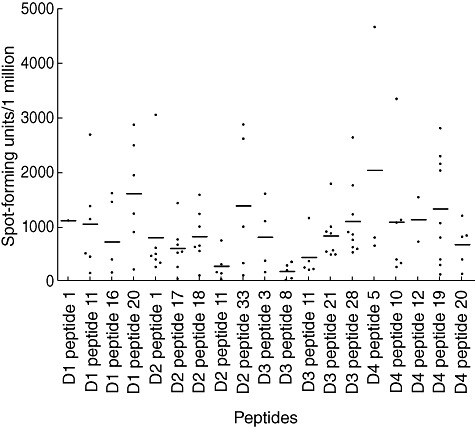
Cultured interferon (IFN)-γ enzyme-linked immunospot (ELISPOT) responses to the peptides from dengue virus serotype (DEN)-1, DEN-2, DEN-3 and DEN-4 in a cohort of healthy dengue immune donors following naturally acquired dengue infection.
Of the 19 conserved and non-cross-reactive regions identified from the four DENV serotypes, two peptides were from the envelope region, one peptide from the DENV-2 was from the NS1 region, six peptides were from the NS2A region, two peptides from the NS2B region, one peptide of DENV-1 was from the NS3 region, four peptides were from the NS4A region and three peptides were from the NS5 region (Table 2).
Serotype-specific and conserved peptides identified from the NS2A region of the DENV
Of the six peptides identified which were from the NS2A region, one peptide each was from DENV-2 and DENV-3, two peptides from DENV-4 and two of the peptides were from DENV-1. Three of six of these peptides were from the region represented by amino acids (aa) 99–133, and two of six peptides were from the region represented by aa 184–216. One peptide from DENV-4 was from the aa 135–148. Variants of all the peptides are shown in supplementary Table S1 and are based on NCBI Virus Variation website data. In the current study we have used the most common sequence, which accounted for >90% of the detected variation in the majority of cases.
The three peptides, from aa 99 to 133, were again found to be highly conserved. Of these three peptides, peptide 28 of DENV-3 (RENLLLGVGLAMATTLQLPE), which was the most frequently recognized peptide among all donors (nine of 20), had two changes in the amino acids in only two sequences. In these two variants, threonine in position 14 is replaced by alanine and arginine in position 17 was replaced by methionine. Peptide 10 of DENV-4 (AMTTTLSIPHDLMELIDGIS) had the amino acid leucine in position 6 replaced by isoleucine in some sequences. Although we also used this sequence in our peptide matrix, we did not detect any responses to the sequence with the altered amino acid. Peptide 11 of DENV-1 (SLVASVELPNSLEELGDGLAMGIMI) also had the last residue of the sequence isoleucine was replaced by methinone in some sequences.
Serotype-specific and conserved peptides identified from the NS4A region of the DV
Of the 20 conserved and non-cross-reactive peptides identified, four were from the NS4A region of the DENV. One of these peptides was from the 2 K region, which lies in between the NS4A and the NS4B region. The other three peptides were from regions 2–26 aa. Of these, peptide 19 (ILTEIASLPTYLSSRAKL) of DENV-4 was the most frequently recognized peptide of DENV-4.
HLA restriction of the peptides
Except for a few peptides in DENV-1 and -4 (peptide 10 in DENV-4 and peptide 20 in DENV-1), the majority of responses to these peptides were from the CD4+ subset of T cells. Therefore, we then proceeded to characterize the HLA restriction of the peptides recognized by the CD4+ subset of T cells. We initially used HLA-DR, -DQ and -DP blocking antibodies to determine which of these molecules were involved in presenting these peptides. We found that all three of these MHC class II molecules were involved in presenting these peptides. Interestingly, the most frequently recognized peptides (peptides 21 and 28 of DENV-3, peptide 19 of DENV-4, peptides 1 and 33 of DENV-2) were found to be restricted through HLA-DP. Of these peptides, peptide 18 of DENV-2 was found to include an epitope with restriction through HLA-DQ*06, as complete blocking of the responses to this peptide was achieved by HLA-DQ antibodies in two HLA-DQ*06 homozygous individuals.
Characterization of an epitope restricted through HLA-DRB1*1501
As responses to peptide 3 of the DENV-3 serotype were found to be blocked by HLA-DR antibodies (Fig. 2a), we proceeded to characterize further the HLA restriction of this peptide. PBMCs cultured with peptide 3 of the DENV was tested for IFN-γ production using peptide pulsed and unpulsed DRB1*1501 expressing transfected L cells for antigen presentation. Figure 2b shows that peptide 3 was indeed restricted through DRB1*1501. We then proceeded to determine the sensitivity of short-term T cell lines for peptide 3. We found that we could detect responses (mean 81·48, s.d. ± 12·83 SFU/1 million cells) to this peptide even at 0·001 µM/l concentrations of this peptide (Fig. 2c).
Fig. 2.
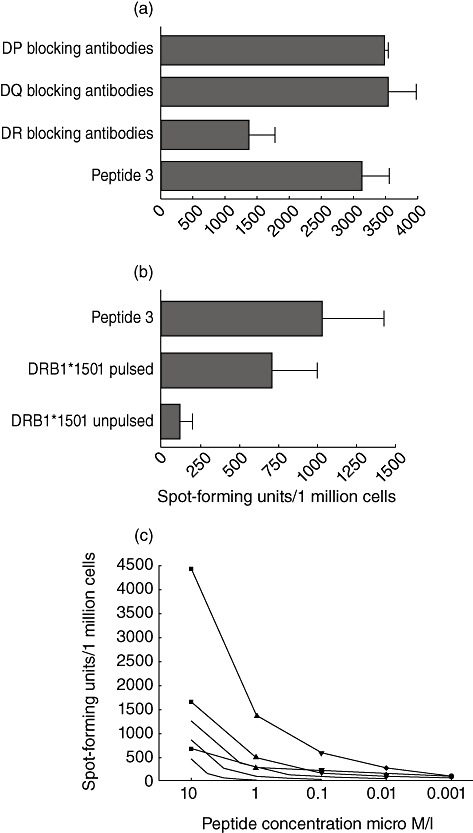
(a) Interferon (IFN)-γ enzyme-linked immunospot (ELISPOT) responses to dengue virus serotype (DEN)-3 peptide 3-specific line, in the presence or absence of anti-DR, anti-DP or anti-DQ antibodies. (b) IFN-γ ELISPOT responses using DEN-3-specific line, incubated with DEN-3 peptide 3 added directly to T cells and DRB1*1501-expressing L cells pulsed with the peptide and unpulsed L cells used as antigen-presenting cells to the T cell line. (c) IFN-γ ELISPOT responses using three DEN-3-specific lines of three different DRB1*1501 individuals in the presence of varying concentrations of DEN-3 peptide 3.
Frequency of memory T cell responses to these peptides in individuals with past symptomatic and asymptomatic dengue infection
Ex-vivo IFN-γ ELISPOT assays were used to assess the frequency of memory T cell responses to the peptides in healthy immune and five dengue seronegative donors. None of the dengue seronegative donors responded to any of the dengue peptides of the four DENVs. One donor with a past severe DI had a response of 1186·67 SFU/1 million PBMCs to peptide 21 of DENV-3, whereas this donor did not have responses of >100 SFU/1 million to any other peptides. A high frequency of responses (>500 SFU/1 million PBMCs) was also seen of peptide 3 of DENV-3, peptide 16 of DENV-1, peptide 20 of DENV-1 and peptide 19 of DENV-4 (Fig. 3). High responses to these peptides were seen in different donors. Although responses to DENV-1 peptide 1 and DENV-4 peptide 5, which represented the envelope region of the DENV, was detected in individuals, only two individuals responded to each of the peptides. In addition, no ex-vivo responses were detected to DENV-3 peptide 8, which represented the NS5 region. In the cultured ELISPOT assays, only four individuals responded to DENV-3 peptide 3, which was found to be restricted through DRB1*1501. However, eight individuals (all DRB1*1501) responded to this peptide in ex-vivo ELISPOT assays.
Fig. 3.
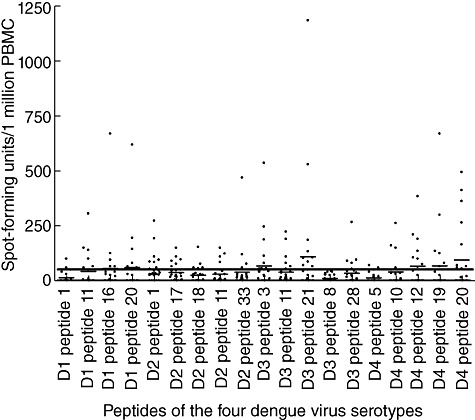
Ex-vivo interferon (IFN)-γ enzyme-linked immunospot (ELISPOT) responses to the identified serotype-specific, non-cross-reactive and highly conserved peptides of dengue virus serotype (DEN)-1, DEN-2, DEN-3 and DEN-4 serotypes, in a cohort of healthy immune donors following natural exposure. The horizontal bar shows the mean ± 3 standard deviations of the irrelevant peptide control.
Discussion
We have identified 19 serotype-specific and conserved peptides from the four DENV serotypes. The naturally exposed healthy immune donors in our study responded to peptides of at least two DENV serotypes, suggesting that they had been exposed to at least two DENV infections. This is not surprising, as we found that 50% of children aged 16, living in the suburban areas of the Colombo district in Sri Lanka, showed evidence of an apparent DI in 2003 [19]. Of the donors, only two had experienced a symptomatic secondary DI. Two of our donors responded to peptides of all four DENVs, suggesting that they had been exposed to all four of these DENVs without experiencing a severe DI.
Sri Lanka has been affected by epidemics of DHF for nearly two decades. In recent years, dengue has become the most common cause of mosquito-borne mortality [10]. Epidemiological data have suggested that DENV-2 and DENV-3 viruses were responsible for almost 95% of the infections during the last two decades up to 2009 [15]. Until 2009, DENV-1 and DENV-4 serotypes accounted for <10% of all symptomatic DIs. However, symptomatic infections due to DENV-4 remains at <5%. Despite DEN-4 not being detected in patients with symptomatic DIs, eight of 20 (40%) individuals recruited in our study responded to at least two peptides of the DENV-4, which was surprising. Therefore, it is possible that the majority of individuals exposed to DENV-4 develop mild/asymptomatic DI due to the low frequency of this serotype being detected among patients with acute DI. As dengue surveillance programmes, which are usually limited to patients with acute infection, may not detect ‘silent’ dengue transmission in the community. Although many individuals responded to DENV-4 peptides, only six of 20 responded to peptides of the DEN-1. This is perhaps not surprising, as until 2009 DEN-1 accounted for <10% of symptomatic DIs and most individuals were probably not exposed to this virus serotype until recently.
Many have investigated if certain DENV serotypes are associated with the development of severe DIs [20]. While all four DENV serotypes have been identified in patients with DHF/DSS, certain genotypes of DENV-2 and DENV-3 viruses are thought to be more virulent and able to cause more severe epidemics followed by DENV-1 [21]–[23]. DEN-4 has found to be associated with milder disease [24]. Although the DENV-4 serotype was not prevalent among patients with DHF/DSS in Sri Lanka, it is possible that it caused a majority of the silent DIs, as it resulted in milder clinical disease. As DENV isolation and serotyping by PCR or other methods have been carried out only in hospitalized patients in Sri Lanka [14],[15],[25], it is possible that milder clinical disease due to DENV-4 was not detected.
Although infection with the DV can result in DHF/DSS, the majority of infected individuals develop asymptomatic or mild clinical disease. Many genetic [3],[26] and virological factors [27] have been thought to predispose to severe disease along with the host immune response [27]. However, the correlates of a protective immune response have not been defined due to the inability to define DENV-serotype specific T cell responses. The lack of data regarding the constituents of a DENV-specific protective immune response has hampered the development of a safe and effective dengue vaccine. As we have identified serotype-specific and highly conserved peptides from all four DENV serotypes, these tools can be used to dissect DENV-specific immune responses in greater detail. As the peptides identified by us are serotype-specific and conserved, they can be used to determine past infecting DENV serotypes and would help us to understand the dynamics of the silent DIs in the community. This will be of value to address a number of questions, such as whether the sequence of infections with DENV serotypes and/or the timing of DIs determine severity. Such data would help us to define the correlates of a protective DV-specific immune response and help us to develop safe and effective vaccines.
In summary, we have shown that DENV-4 infection is likely to be more common than thought previously in Sri Lanka. We have identified T cell responses to 19 regions of the four DENV serotypes, which are serotype-specific and highly conserved from dengue immune donors who have had asymptomatic/mild DI. The use of conserved serotype-specific T cell epitopes to determine past infecting DENV serotypes will be of value to determine the silent and symptomatic transmission of the DENV in the community and to identify the correlates of a DENV-specific protective immune response.
Acknowledgments
Funding was provided by the Medical Research Council (UK). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Disclosure
An application has been made for protection of the intellectual property herein.
Supporting information
Table S1. Degree of conservation of theidentified peptides in the published dengue virus sequences. Degreeof conservation was assessed by the use of the virus variationresource on the dengue virus sequence database available at: http://www.ncbi.nlm.nih.gov/genomes/VirusVariation/Database/nph-select.cgi
References
- 1.Callaway E. Dengue fever climbs the social ladder. Nature. 2007;448:734–5. doi: 10.1038/448734a. [DOI] [PubMed] [Google Scholar]
- 2.Mathew A, Rothman AL. Understanding the contribution of cellular immunity to dengue disease pathogenesis. Immunol Rev. 2008;225:300–13. doi: 10.1111/j.1600-065X.2008.00678.x. [DOI] [PubMed] [Google Scholar]
- 3.Stephens HA, Klaythong R, Sirikong M, et al. HLA-A and -B allele associations with secondary dengue virus infections correlate with disease severity and the infecting viral serotype in ethnic Thais. Tissue Antigens. 2002;60:309–18. doi: 10.1034/j.1399-0039.2002.600405.x. [DOI] [PubMed] [Google Scholar]
- 4.Lan NT, Kikuchi M, Huong VT, et al. Protective and enhancing HLA alleles, HLA-DRB1*0901 and HLA-A*24, for severe forms of dengue virus infection, dengue hemorrhagic fever and dengue shock syndrome. PLoS Negl Trop Dis. 2008;2:e304. doi: 10.1371/journal.pntd.0000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weaver SC, Vasilakis N. Molecular evolution of dengue viruses: contributions of phylogenetics to understanding the history and epidemiology of the preeminent arboviral disease. Infect Genet Evol. 2009;9:523–40. doi: 10.1016/j.meegid.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Imrie A, Meeks J, Gurary A, et al. Differential functional avidity of dengue virus-specific T-cell clones for variant peptides representing heterologous and previously encountered serotypes. J Virol. 2007;81:10081–91. doi: 10.1128/JVI.00330-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Appanna R, Huat TL, See LL, Tan PL, Vadivelu J, Devi S. Cross-reactive T-cell responses to the nonstructural regions of dengue viruses among dengue fever and dengue hemorrhagic fever patients in Malaysia. Clin Vaccine Immunol. 2007;14:969–77. doi: 10.1128/CVI.00069-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong T, Moran E, Vinh Chau N, et al. High pro-inflammatory cytokine secretion and loss of high avidity cross-reactive cytotoxic T-cells during the course of secondary dengue virus infection. PLoS ONE. 2007;2:e1192. doi: 10.1371/journal.pone.0001192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mangada MM, Rothman AL. Altered cytokine responses of dengue-specific CD4+ T cells to heterologous serotypes. J Immunol. 2005;175:2676–83. doi: 10.4049/jimmunol.175.4.2676. [DOI] [PubMed] [Google Scholar]
- 10.Gagnon SJ, Mori M, Kurane I, et al. Cytokine gene expression and protein production in peripheral blood mononuclear cells of children with acute dengue virus infections. J Med Virol. 2002;67:41–6. doi: 10.1002/jmv.2190. [DOI] [PubMed] [Google Scholar]
- 11.Mongkolsapaya J, Duangchinda T, Dejnirattisai W, et al. T cell responses in dengue hemorrhagic fever: are cross-reactive T cells suboptimal? J Immunol. 2006;176:3821–9. doi: 10.4049/jimmunol.176.6.3821. [DOI] [PubMed] [Google Scholar]
- 12.Moran E, Simmons C, Vinh Chau N, et al. Preservation of a critical epitope core region is associated with the high degree of flaviviral cross-reactivity exhibited by a dengue-specific CD4+ T cell clone. Eur J Immunol. 2008;38:1050–7. doi: 10.1002/eji.200737699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simmons CP, Dong T, Chau NV, et al. Early T-cell responses to dengue virus epitopes in Vietnamese adults with secondary dengue virus infections. J Virol. 2005;79:5665–75. doi: 10.1128/JVI.79.9.5665-5675.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Messer WB, Vitarana UT, Sivananthan K, et al. Epidemiology of dengue in Sri Lanka before and after the emergence of epidemic dengue hemorrhagic fever. Am J Trop Med Hyg. 2002;66:765–73. doi: 10.4269/ajtmh.2002.66.765. [DOI] [PubMed] [Google Scholar]
- 15.Kanakaratne N, Wahala WM, Messer WB, et al. Severe dengue epidemics in Sri Lanka, 2003–2006. Emerg Infect Dis. 2009;15:192–9. doi: 10.3201/eid1502.080926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–10. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 17.Rice P, Longden I, Bleasby A. EMBOSS: the European Molecular Biology Open Software Suite. Trends Genet. 2000;16:276–7. doi: 10.1016/s0168-9525(00)02024-2. [DOI] [PubMed] [Google Scholar]
- 18.Malavige GN, Jones L, Black AP, Ogg GS. Varicella zoster virus glycoprotein E-specific CD4+ T cells show evidence of recent activation and effector differentiation, consistent with frequent exposure to replicative cycle antigens in healthy immune donors. Clin Exp Immunol. 2008;152:522–31. doi: 10.1111/j.1365-2249.2008.03633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malavige GN, Fernando S, Aaskov J, et al. Seroprevalence of anti-dengue virus antibodies in children in the Colombo district. Dengue Bull. 2006;30:68–71. [Google Scholar]
- 20.Rico-Hesse R. Microevolution and virulence of dengue viruses. Adv Virus Res. 2003;59:315–41. doi: 10.1016/s0065-3527(03)59009-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cologna R, Armstrong PM, Rico-Hesse R. Selection for virulent dengue viruses occurs in humans and mosquitoes. J Virol. 2005;79:853–9. doi: 10.1128/JVI.79.2.853-859.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rico-Hesse R, Harrison LM, Salas RA, et al. Origins of dengue type 2 viruses associated with increased pathogenicity in the Americas. Virology. 1997;230:244–51. doi: 10.1006/viro.1997.8504. [DOI] [PubMed] [Google Scholar]
- 23.Balmaseda A, Hammond SN, Perez L, et al. Serotype-specific differences in clinical manifestations of dengue. Am J Trop Med Hyg. 2006;74:449–56. [PubMed] [Google Scholar]
- 24.Nisalak A, Endy TP, Nimmannitya S, et al. Serotype-specific dengue virus circulation and dengue disease in Bangkok, Thailand from 1973 to 1999. Am J Trop Med Hyg. 2003;68:191–202. [PubMed] [Google Scholar]
- 25.Messer WB, Gubler DJ, Harris E, Sivananthan K, de Silva AM. Emergence and global spread of a dengue serotype 3, subtype III virus. Emerg Infect Dis. 2003;9:800–9. doi: 10.3201/eid0907.030038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Appanna R, Ponnampalavanar S, Lum Chai See L, Sekaran SD. Susceptible and protective HLA class 1 alleles against dengue fever and dengue hemorrhagic fever patients in a Malaysian population. PLoS ONE. 2010;5:e13029. doi: 10.1371/journal.pone.0013029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martina BE, Koraka P, Osterhaus AD. Dengue virus pathogenesis: an integrated view. Clin Microbiol Rev. 2009;22:564–81. doi: 10.1128/CMR.00035-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


