Abstract
Several assays to measure pre-existing allospecific T cell immunity in renal transplant candidates have been developed in the past years. In 46 patients, we used flow cytometry-based mixed lymphocyte culture to measure the precursor frequency and phenotype of alloreactive T cells before renal transplantation, using donor-specific or third-party cells for allostimulation. Allostimulation induced up-regulation of co-stimulatory molecules, chemokine receptors relevant for migration of T cells into the graft and effector proteins. Recipients prone for acute rejection had a higher precursor frequency of alloreactive CD8+ T cells and a lower percentage of interleukin (IL)-7Rα expressing alloreactive CD8+ T cells than non-rejectors. These data point to quantitative and qualitative differences between T cells of patients who will experience acute cellular rejection episodes from those who will not.
Keywords: alloreactivity, IL7Rα, kidney, rejection, T cell
Introduction
Despite an essential role for T cells in the pathogenesis of allograft rejection, in the selection of candidates for renal transplantation most attention has always been paid to the measurement of pre-existing allospecific B cell immunity. Although a relationship between precursor frequencies of alloreactive T cells and clinical outcome has been suggested in several studies [1],[2], only in the past years have reliable and sensitive methods for measurement of pre-existing allospecific T cell immunity been developed. Several groups have now shown that donor-specific interferon (IFN)-γ enzyme-linked immunospot (ELISPOT) enables prediction of the strength of the alloimmune response before transplantation [3]–[5]. In addition, the pretransplant differentiation status of alloreactive T cells has been shown to be predictive for transplant rejection [6]. However, these assays measure only part of the cellular immune reactivity against alloantigens, and one may question whether one parameter of cellular immunity will suffice to select patients at risk for mounting a high cellular T cell response to the allograft [7],[8].
Considering the cellular alloimmune response, several steps are involved. T cells recognize alloantigens through their antigen receptors [T cell receptors (TCR)] via the direct or indirect pathway [9]. Optimal activation of T cells by antigen depends on appropriate signalling through co-stimulatory receptors and the influence of inhibitory receptors [10]–[12]. The interaction of common-γ chain cytokines and their receptors are pivotal in the initiation and perpetuation of an immune response. These receptors are expressed differentially during the immune response, depending in part on the strength of activation signals [13],[14]. Alloactivated T cells are recruited into the graft by locally expressed chemokines [15]–[18]. Once in the graft, the CD4+ T cells function mainly by producing cytokines that activate and attract other immune cells. The CD8+ T cells can lyse tubular cells directly through their effector molecules, perforin and granzymes [19].
Also, the differentiation state of the alloreactive T cell pool may be important, where a preponderance of Th1 cells is predictive for allograft failure and regulatory T cells (Tregs) can inhibit potential damaging effector T cells [20],[21].
The ideal test to predict allospecific T cell immunity would include assessment of each of the above-mentioned properties of alloreactive T cells. Therefore, we used flow cytometry-based mixed lymphocyte culture (MLC), the so-called multi-parameter MLC–5-,6-carboxyfluorescein diacetate succinimidyl ester (CFSE)-assay, which can measure simultaneously the precursor frequency of both CD4+ and CD8+ alloreactive T cells, in combination with qualitative T cell properties [22]. We questioned whether this assay would detect differences between patients with various post-transplant outcomes. In this study we show that patients with a high precursor frequency of alloreactive T cells and low percentage of interleukin (IL)-7Rα expressing alloreactive CD8+ T cells before transplantation have an increased risk of acute rejection after transplantation.
Study subjects, materials and methods
Subjects, sample collection and isolation of lymphocytes
This study was approved by the Medical Ethics Committee of the Academic Medical Center, Amsterdam (METC 06/157) and informed consent was given by all participants. The study population consisted of 46 renal allograft recipients. Rejectors were selected based on the availability of both patient cells collected before transplantation and donor cells. The non-rejectors were matched for type of donor (i.e. post mortem and living related), age and sex (Table 1). Blood samples were obtained from healthy individuals and from renal transplant recipients on the day of transplantation before start of immunosuppressive treatment and before transplant surgery. Donor cells were derived from peripheral blood of living related donors and from spleen cells of post-mortem donors. As third-party cells, fully human leucocyte antigen (HLA)-A/B/DR mismatched spleen cells were used for post-mortem donor MLC and fully mismatched PBMC were used for living related donor MLC. PBMC were isolated from heparinized whole blood by Ficoll density centrifugation (Pharmacia Biotech AB, Uppsala, Sweden). All cells were frozen and stored in liquid nitrogen until the day of analysis.
Table 1.
Patient characteristics of the non-rejector group and the rejector group.
| Non-rejectors n = 22 | Rejectors n = 24 | |
|---|---|---|
| Age, years (s.d.) | 44 (13) | 40 (14) |
| Male/female, n | 14/8 | 14/10 |
| Women with previous pregnancies, n | 4 | 6 |
| Total no. of pregnancies, n | 10 | 17 |
| Cold ischaemia time, h (s.d.) | 11 (8) | 11 (10) |
| Panel reactivity, %, mean (s.d.) | 6 (14) | 6 (15) |
| Pretransplant infection, %: | ||
| CMV seropositive | 77 | 79 |
| EBV seropositive | 95 | 100 |
| Pretransplant bloodtransfusion, % | 100 | 100 |
| Type of donor | ||
| Post-mortem/living, n | 10/12 | 12/12 |
| Mismatches, n | ||
| HLA-A/B, median (range) | 2 (0–4) | 2 (0–4) |
| HLA-DR, median (range) | 1 (0–2) | 1 (0–2) |
| Total no. MM, median (range) | 3 (0–6) | 3 (0–6) |
| Primary renal disease, n | ||
| DM | 2 | 0 |
| Vascular | 5 | 3 |
| Glomerular | 6 | 6 |
| Interstitial | 4 | 6 |
| Hereditary | 3 | 5 |
| Unknown | 2 | 4 |
| Type of rejection, Banff, n | ||
| Type I | 10 | |
| Type II | 11 | |
| Type III | 1 | |
| Unknown | 2 |
CMV: cytomegalovirus; EBV: Epstein–Barr virus; HLA: human leucocyte antigen; MM: mismatches; DM: Diabetes Mellitus; s.d.: standard deviation.
Immunosuppressive treatment and rejection therapy
All patients received induction therapy with anti-CD25 monoclonal antibody (mAb) in combination with maintenance treatment, consisting of prednisolone, mycophenolate and cyclosporin. Twenty-two patients with an uncomplicated post-transplantation course and 24 patients who developed an episode of acute rejection during the first 3 months after transplantation were included. Diagnosis of acute rejection was based on clinical and laboratory criteria, and was followed by a core biopsy in all patients. Biopsies were scored blindly and independently by two pathologists, according to the Banff criteria [23] (Table 2). All rejection episodes, except for the one that was classified as type III, were treated with corticosteroids. The type III T cell-mediated rejection was treated successfully with anti-thymoglobulin (ATG) and plasmapheresis. Response to therapy was evaluated based on the change in plasma creatinine concentration. Return of the plasma creatinine concentration to maximally 125% of the prerejection value within 2 weeks after cessation of treatment was considered to represent a complete response [24]. All patients showed a complete response to anti-rejection treatment. Additional patient characteristics are presented in Table 1. None of the patients had active BK virus (BKV) or CMV infection in the time-period following transplantation until or during their acute rejection episode.
Table 2.
CD8+ donor-specific (dsp CD8 pf) and CD8+ third-party precursor frequency (third-party CD8 pf), cytomegalovirus (CMV) serostatus before transplantation, the number of days from transplantation to rejection and type of rejection of each individual transplant recipient.
| Non-rejectors | dsp CD8 pf | 3rd-party CD8 pf | CMV serology before transplantation | Days between Tx and acute rejection | Banff classification |
|---|---|---|---|---|---|
| 1 | 2·78 | 3·50 | + | ||
| 2 | 2·77 | 8·26 | + | ||
| 3 | 4·08 | 5·49 | + | ||
| 4 | 2·00 | 7·21 | − | ||
| 5 | 6·12 | 8·98 | + | ||
| 6 | 8·88 | 8·12 | + | ||
| 7 | 4·80 | 8·46 | − | ||
| 8 | 4·66 | 4·32 | + | ||
| 9 | 7·82 | 8·31 | + | ||
| 10 | 4·97 | 11·80 | - | ||
| 11 | 0·58 | 3·38 | + | ||
| 12 | 3·69 | 7·23 | − | ||
| 13 | 1·01 | 3·50 | + | ||
| 14 | 6·44 | 11·10 | + | ||
| 15 | 1·94 | 6·48 | + | ||
| 16 | 1·00 | 4·87 | + | ||
| 17 | 0·85 | 6·90 | + | ||
| 18 | 6·93 | 8·12 | + | ||
| 19 | 1·25 | 2·50 | − | ||
| 20 | 3·21 | 3·72 | + | ||
| 21 | 2·96 | 4·32 | + | ||
| 22 | 6·63 | 10·50 | + | ||
| Rejectors | |||||
| 1 | 7·78 | 7·95 | + | 45 | ACR IIa |
| 2 | 1·23 | 4·07 | + | 33 | ACR Ia |
| 3 | 7·80 | 10·90 | + | 8 | ACR IIa |
| 4 | 2·20 | 4·01 | + | 38 | ACR Ia |
| 5 | 8·54 | 5·04 | + | 9 | ACR Ib |
| 6 | 7·35 | 7·54 | + | 35 | ACR Ib |
| 7 | 6·97 | 19·20 | + | 28 | ACR IIa |
| 8 | 1·69 | 4·27 | − | 24 | ACR Ib |
| 9 | 5·32 | 15·70 | + | 10 | ACR Ia |
| 10 | 3·88 | 4·71 | − | 10 | ACR IIb |
| 11 | 7·29 | 6·29 | + | 7 | ACR IIa |
| 12 | 7·32 | 12·90 | − | 29 | No material |
| 13 | 7·17 | 5·00 | + | 27 | ACR IIb |
| 14 | 2·55 | 5·64 | − | 13 | ACR Ia |
| 15 | 2·59 | 3·61 | + | 9 | No material |
| 16 | 2·03 | 5·64 | − | 55 | ACR Ia |
| 17 | 3·82 | 4·80 | + | 41 | ACR IIa |
| 18 | 3·41 | 2·23 | + | 28 | ACR III |
| 19 | 1·00 | 3·97 | + | 31 | ACR IIa |
| 20 | 5·97 | 15·30 | + | 11 | ACR IIa |
| 21 | 7·31 | 13·90 | + | 6 | ACR IIa |
| 22 | 2·18 | 4·72 | − | 13 | ACR Ia |
| 23 | 17·00 | 11·40 | + | 23 | ACR Ib |
| 24 | 7·60 | 7·37 | + | 38 | ACR Ia |
CFSE labelling and cell culture
Responder peripheral blood mononuclear cells (PBMC) were labelled with CFSE (Molecular Probes Europe BV, Leiden, the Netherlands), as described previously [22], and cultured with irradiated donor cells or with irradiated third-party cells in a one-to-one ratio. The precursor frequency was calculated as follows: [Σn>=1(Pn/2n)]/[Σn>=0(Pn/2n)], where ‘n’ is the division number that cells have passed through and ‘Pn’ is the number of cells in division n [25] and equals the percentage of alloreactive cells at the start of the mixed lymphocyte reaction that participates in the alloresponse.
Fluorescence activated cell sorter (FACS) staining
Freshly thawed cells and cells obtained after 6 days' MLC were stained as follows: 500 000 PBMC were incubated with fluorescently labelled conjugated mAbs (at saturating concentrations) for 30 min at 4°C, protected from light. The necessary fluorochrome-conjugated antibodies were purchased from eBiosience, Inc. (San Diego, CA, USA), Becton Dickinson (BD) (San Jose, CA, USA) or Sanquin (Amsterdam, the Netherlands) Samples were measured using the FACS Canto flow cytometer from BD. Subsequent analysis was done using FlowJo version 8·8. The gating was performed using isotype controls.
ELISPOT assay
IFN-γ ELISPOT assay was performed as described previously in detail [26]. Briefly, 96-well plates (Millipore, Eschborn, Germany) were first coated with a primary IFN-γ antibody (BD Pharmingen, Heidelberg, Germany) and left at 4°C overnight. Next, 3 × 105 responder PBMC and 3 × 105 donor or third-party T cell-depleted cells were incubated in triplicate wells. Phytohaemagglutinin (PHA) was used as a positive control and as a negative control we used autologous MLC, recipient cells alone and stimulator cells alone. After 24 h of incubation at 37°C, 5% CO2, plates were washed with phosphate-buffered saline (PBS) and PBS-Tween-20. Biotinylated anti-IFN-γ antibody was added and incubated overnight at 4°C. Then, streptavidin–horseradish peroxidase conjugate (BD) was added for 2 h. After a final wash, plates were developed with 3-amino-9-ethylcarbazole. Results are presented as median values of ELISPOTs detected in triplicate wells containing responder PBMC plus donor stimulator cells after subtracting the response of wells with responder or donor cells only.
Cell sorting and restimulation
After 6 days' MLC, PBMC were stained with anti-IL-7Ra (CD127)-peridinin chlorophyll (PerCP)-cyanin 5·5 (Cy5·5), CD3-PE-Cy7 and CD8-PE-Alexa610 (all purchased from BD) and sorted in CFSE-negative, CD8+ IL-7Rα+ fraction and CFSE-negative CD8+ IL-7Rα- fraction using the Aria FACS (BD Biosciences). The purity of the sorted populations was assessed by FACS analysis and was >95%. Sorted cells were labelled subsequently with CFSE and restimulated with the radiated stimulator cells. After 4 days, cells were stained with CD3-PE-Cy7 (BD), CD4-APC-Alexa750 (eBioscience) and CD8-APC (BD).
Statistical analysis
Comparisons between two groups were performed using either the Mann–Whitney or Student's t-test. Spearman's test was used to correlate results obtained by flow cytometry and ELISPOT assay. If more than two groups were compared, we used the one-way analysis of variance (anova) and subsequent Dunnet's post-hoc test. P-values < 0·05 were considered statistically significant.
Results
T cells stimulated by allogeneic cells show changes in expression of cytokine and chemokine receptors as well as expression of activation and differentiation-associated markers
As we showed previously, the multi-parameter MLC–CFSE assay enables determination of a combination of quantitative and qualitative properties of alloreactive T cells in one assay [22]. Figure 1a shows examples of stainings from one representative patient without stimulation and after 6 days of allostimulation in the MLC–CFSE assay. The isotype control of the same experiment is shown in Fig. 1b. We analysed the expression of surface markers known to be functionally important in the alloresponse and compared expression on resting T cells to that on alloreactive cells against donor cells and third-party cells (Fig. 1c). We also analysed the expression of these receptors on non-responsive cells in MLC or after 6 days of autologous MLC. This showed no significant differences between unstimulated, uncultured cells and non-responsive cells after 6 days of culture, except for IL-2Rα, which increased after 6 days (data not shown). Alloreactive CD4+ and CD8+ T cells showed an activated phenotype with a decrease in percentage of CD45RA+ cells, but a marked increase in the percentage of cells expressing IL-2Rα and HLA-DR. Furthermore, alloreactive CD4+ and CD8+ T cells had a lower percentage of cells that express receptors of the common-γ chain cytokines other than IL-2Rα. The percentage of cells expressing the chemokine receptor CXCR3 was increased after stimulation, contrasting with cells expressing CCR1 and CCR5, where only small differences were observed. Changes in the percentage of cells expressing co-stimulatory proteins CD27, OX40 and inducible T cell co-stimulator (ICOS) were observed in both CD4+ and CD8+ T cells. CD28 expression did not changed in either subset. Expression of proteins associated with inhibitory functions, CTLA-4 and PD-1, was increased. Forkhead box protein 3 (FoxP3), a transcription factor present in regulatory cells but also associated with recently activated T cells [27], was increased after 6 days' MLC in both CD4+ and CD8+ T cells.
Fig. 1.
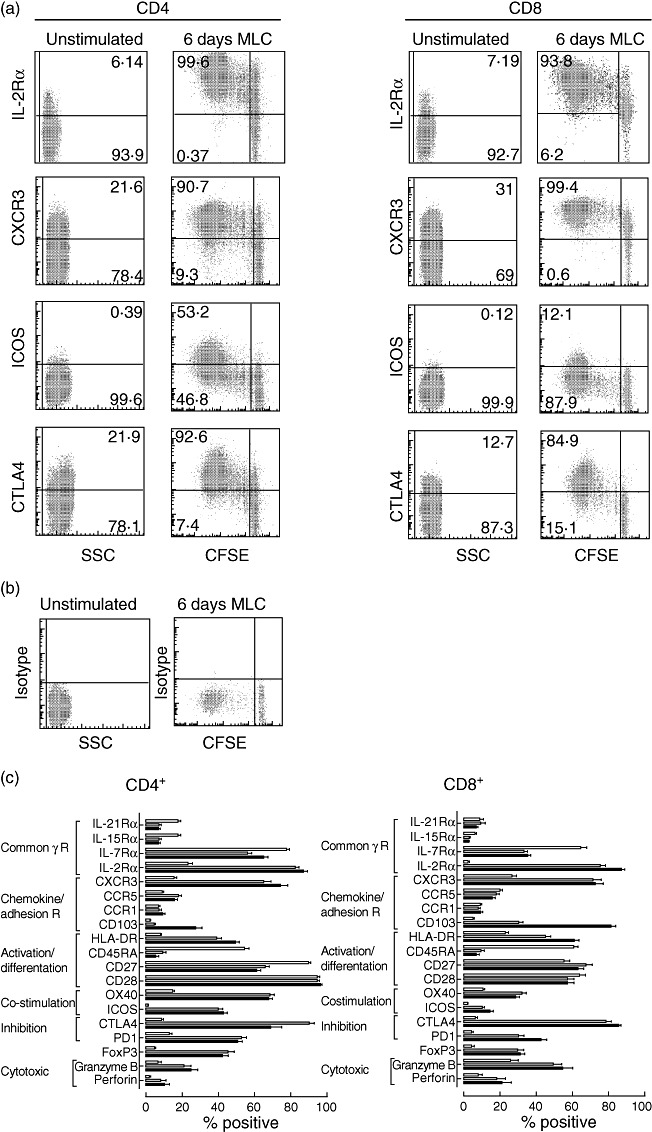
Phenotypic changes of CD4+ and CD8+ T cells after alloantigenic stimulation in vitro. (a) Examples from a representative transplant recipient for expression of common-γ chain receptor, chemokine receptor, co-stimulatory and inhibitory molecules (y-axis) on unstimulated and alloreactive T cells after 6 days mixed lymphocyte culture (MLC) for CD3+CD4+ (left) and CD3+CD8+ (right) T cell compartment. The x-axis shows side-scatter (SSC) for unstimulated cells and 5-,6-carboxyfluorescein diacetate succinimidyl ester (CFSE) for 6 days' MLC. (b) Plots show isotype controls of unstimulated cells and cells after 6 days' MLC. (c) The percentage of cells that express the designated protein in the CD4+ (left) and CD8+ (right) T cell compartment are shown. White bars represent the % positive cells at day 0 (unstimulated); grey bars represent the % positive within total CFSE negative (alloreactive) T cells after MLC using donor-specific cells as stimulators; black bars represent the % positive within total CFSE-negative (alloreactive) T cells after MLC using third-party cells as stimulators.
Donor-specific precursor frequency of CD8+ T cells but not of CD4+ T cells discriminates patients who will experience acute cellular rejection
To study whether we could discriminate before transplantation between patients who will experience acute cellular rejection episodes from those who will not, we studied retrospectively 24 patients who had suffered from acute cellular rejection episode(s) and compared them with 22 patients who had not. Figure 2a shows a representative plot of CFSE dilution of CD8+ T cells after 6 days' MLC of a non-rejector and a rejector. Based on these plots, precursor frequencies were calculated.
Fig. 2.
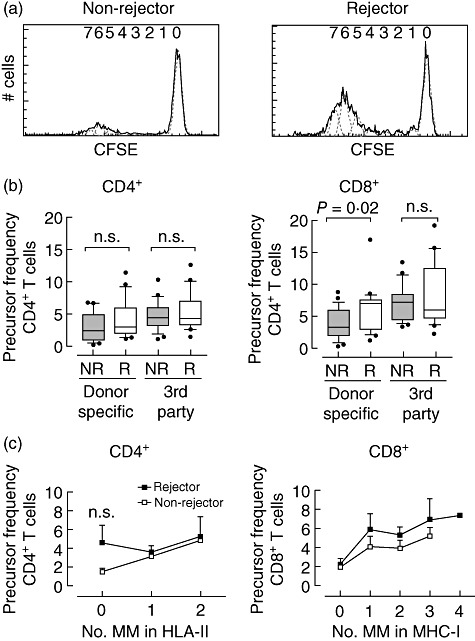
Donor-specific precursor frequency of CD8+ T cells but not of CD4+ T cells measured before transplantation discriminates between the group of patients who will experience acute cellular rejection episodes from the group without rejection. (a) Histograms show 5-,6-carboxyfluorescein diacetate succinimidyl ester (CFSE) dilution of alloreactive CD8+ T cells after 6 days' mixed lymphocyte culture (MLC) of a non-rejector and a rejector. Generations of divided cells were identified and the number of cells in each generation was used for calculation of the precursor frequency. (b) Precursor frequency of CD4+ and CD8+ T cells after stimulation with donor-specific (dsp) or third-party cells from non-rejectors (NR; n = 22) and rejectors (R; n = 24) are shown. The data are presented as box-plots; the whiskers depict 10–90 percentile. (c) The influence of the number of human leucocyte antigen (HLA) class II mismatches (MM) on dsp CD4 precursor frequency (pf) (left) and HLA class I, respectively, class II mismatches on dsp CD8 precursor frequency (pf) (right). The mean ± standard error of the mean of rejectors (black symbol) and non-rejectors (open symbol) are shown.
Figure 2b shows that although the precursor frequency (pf) of CD4+ T cells showed a trend to increase both after donor-specific (dsp) and third-party stimulation, the difference between rejector and non-rejector was not significant. However, the dsp CD8pf of the rejectors was significantly higher than that of the non-rejectors (P = 0·02), whereas no difference between rejector and non-rejector was observed after third-party stimulation. There was no relationship between the donor-specific CD8+ precursor frequency and the time interval between transplantation and acute rejection, nor with the severity of rejection.
CD4pf and CD8pf are dependent on the number of mismatches in HLA-DR and HLA-A/B, respectively. We found a trend towards a higher CD8pf in rejectors compared to non-rejectors with the same number of mismatches for HLA-A/B or HLA-DR (Fig. 2c).
Donor-specific precursor frequency of CD8+ but not of CD4+ T cells measured by MLC–CFSE correlates with the number of IFN-γ-positive spots using ELISPOT assay
Data from the literature show that the IFN-γ ELISPOT assay can predict cellular alloreactivity pre- and post-transplantation. We applied the IFN-γ ELISPOT assay to rejecting and non-rejecting patients from whom PBMC were still available and from whom the dsp CD8pf and CD4pf was already analysed using the MCL–CFSE assay. Indeed, the number of donor-specific IFN-γ-producing cells as detected by ELISPOT was significantly higher in the rejector than in the non-rejector groups (Fig. 3a). Moreover, we found that the number of IFN-γ spots did not correlate with the dsp CD4pf, but correlated significantly with the dsp CD8pf (Fig. 3b,c). We could not establish a relationship between number of IFN-γ spots and the number of mismatches, although this could be due to the small number of patients.
Fig. 3.
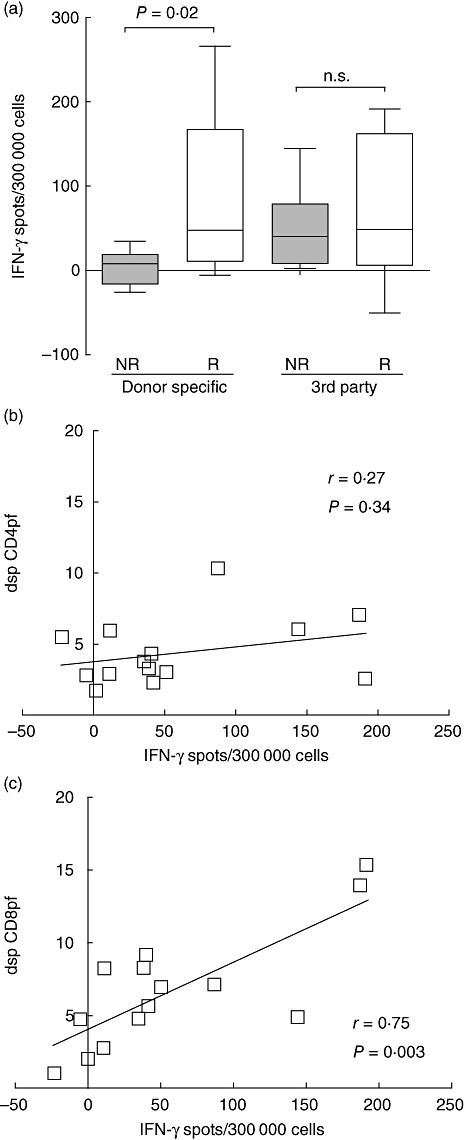
Donor-specific (dsp) CD8 precursor frequency correlates to donor-specific interferon (IFN)-γ enzyme-linked immunospot (ELISPOT) assay. (a) Box-plots show the number of IFN-γ spots measured by ELISPOT of non-rejectors (NR; n = 7) and rejectors (R; n = 7) after mixed lymphocyte culture (MLC) against donor-specific or third-party cells. The box-plot whiskers depict 10–90 percentile. (b) Correlation between number of IFN-γ spots and dsp CD4 precursor frequency (pf) (n = 14). (c) Correlation between number of IFN-γ spots and dsp CD8pf (n = 14). Correlations were calculated using Spearman's test.
No difference between rejectors and non-rejectors in the percentage of IL-2Rα- and IL-15Rα-expressing alloreactive T cells
The expression of common-γ cytokine receptors can be influenced by the differentiation status of T cells. We measured the expression of IL-2Rα on unstimulated and alloreactive CD4+ and CD8+ T cells. Before stimulation a low percentage of cells expressed the IL-2Rα chain; after allostimulation nearly all responsive cells expressed this receptor but there was no difference between rejectors and non-rejectors (data not shown). We also measured the expression of IL-15Rα on unstimulated and alloreactive T cells. The frequency of IL-15Rα expressing cells on unstimulated cells was low, and did not increase after donor-specific or third-party stimulation either in the CD4+ or in the CD8+ T cell subset (data not shown).
Rejectors have a lower percentage of alloreactive IL-7Rα-expressing CD8+ T cells after allostimulation
Before stimulation most CD4+ and CD8+ T cells expressed IL-7Rα, but after 6 days' MLC CD8+ T cells had a higher percentage of IL-7Rα- cells within the alloreactive pool than did CD4+ T cells (Fig. 4a). Importantly, rejectors had a higher percentage of alloreactive CD8+ T cells that lack IL-7Rα expression than the non-rejectors. This was the case for both donor-specific (P = 0·01) and third-party stimulation (P = 0·04) (Fig. 4b), suggesting that this is an intrinsic property of the recipient T cells. To test if the alloreactive IL-7Rα+ cells are functionally distinct from the IL-7Rα- cells, we sorted alloreactive, CFSE-negative, IL-7Rα+ and IL-7Rα- cells after 6 days' MLC using PBMC from healthy individuals. We labelled the sorted cells with CFSE again and evaluated the secondary proliferative response by MLC. We found that in contrast to IL-7Rα+ cells, sorted IL-7Rα- cells showed a low secondary proliferative response (Fig. 4c). Figure 4d shows a fair although not significant degree of relationship between the dsp CD8pf and the percentage of alloreactive IL-7Rα- CD8+ T cells.
Fig. 4.
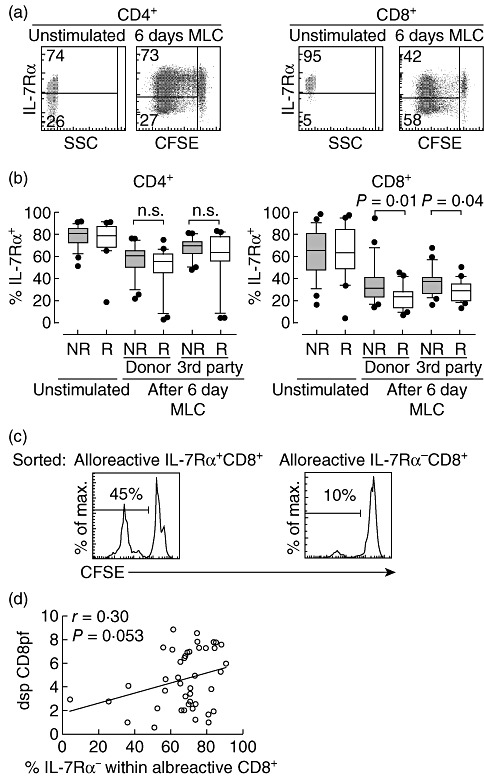
After allostimulation, the percentage of interleukin (IL)-7Rα+ CD8+ T cells is significantly lower in rejectors compared to non-rejectors. (a) Representative plots of IL-7Rα expression on unstimulated cells and alloreactive cells after 6 days' mixed lymphocyte culture (MLC) with donor-specific (dsp) stimulation for CD4+ (left) and CD8+ (right) T cells. (b) Box-plots show the % of IL-7Rα-positive cells in the CD4+ (left) and CD8+ (right) T cell compartment. Results from non-rejectors (NR) and rejectors (R) are shown for unstimulated cells and alloreactive cells after 6 days' MLC with donor-specific or third-party cells. The box-plot whiskers depict 10–90 percentile. (c) Histograms of a representative experiment show the proliferative response of sorted alloreactive IL-7Rα+ (left) and IL-7Rα- (right) CD8+ T cells after restimulation with the original stimulator cells. (d) Correlation between % IL-7Rα- cells within total alloreactive CD8+ T cells and dsp CD8 precursor frequency (pf) is shown (n = 43). Correlation was calculated using the Pearson's test.
Discussion
In this study we show that the multi-parameter MLC–CFSE-assay enables the simultaneous assessment of the proliferative capacity of T cells after allogeneic stimulation together with their phenotypic and functional characterization. In addition, the assay seems promising in detecting differences before transplantation between patients who are at risk for experiencing an acute cellular rejection episode from those who will not. Patients in the rejector group showed a significantly higher donor-specific precursor frequency of CD8+ T cells and a lower percentage of alloreactive IL-7Rα+ CD8+ T cells than patients in the non-rejector group.
First, we studied the differentiation of both CD4+ and CD8+ T cells after allostimulation in vitro. We found that the alloreactive T cells were activated and more differentiated. Due to the set-up of our experiment, we could not discern if alloreactive T cells were already activated and more differentiated before MLC or if they were recruited from the more undifferentiated cell population.
Next, we analysed whether the multi-parameter MLC–CFSE assay could discriminate before transplantation between patients who will experience acute cellular rejection episodes from those who will not. We hypothesized that measurement of several steps involved in the cellular alloimmune response, like allorecognition, co-stimulation, signalling by cytokines and chemokines, would reveal more discriminatory parameters than known until now. However, studying all these parameters, the two groups of patients could be discriminated based only on a significantly higher dsp CD8pf, a trend towards higher dsp CD4pf and a lower percentage of IL-7Rα+ cells within the alloreactive CD8+ T cells in patients of the rejector group. Apparently, measuring more parameters of the cellular immune response towards alloantigens offered minimal additional value.
Our finding of a higher dsp CD8pf in these patients confirms data in the literature obtained by limiting the dilution assay [2],[28]. Further analysis revealed that, with a similar number of HLA-mismatches, rejectors had a higher dsp CD8pf than non-rejectors. This may be due to a difference in mismatches that actually cause an immune response, the so-called permissive HLA-mismatches [29]. Another explanation may be a difference in infectious history or in the number of blood transfusions and pregnancies. This could result in a broader repertoire of pathogen-specific T cells that can cross-react with allogeneic HLA molecules, so-called heterologous immunity [22],[28]. However, comparing the two patient groups regarding alloimmune and infectious history, we found no difference (data not shown). Remarkably, we did not find a correlation between either severity of time to rejection and donor-specific CD8 precursor frequency, implying that other factors predominate in this respect. This could be due to differences in drug metabolism, concomitant with viral infections after transplantation that went unnoticed or the presence of Tregs that somehow delays the alloimmune response.
Several groups have shown the IFN-γ ELISPOT assay to be a sensitive assay in predicting cellular alloreactivity pre- and post-transplantation. We therefore compared the results of this assay with the results of the MLC–CFSE assay [4],[26]. Indeed, the number of IFN-γ-producing cells as detected by ELISPOT was increased significantly in rejectors compared to non-rejectors. In addition, we found a correlation between the number of IFN-γ-producing cells detected by ELISPOT and the dsp CD8 pf. This indicates that the CD8+ allospecific T cells are the most important IFN-γ-producing cells in the ELISPOT assay. However, in the relatively small populations studied, there was a great overlap between rejectors and non-rejectors both in the ELISPOT assay and the MLC–CFSE assay.
Because the difference in precursor frequency between rejectors and non-rejectors could not be explained by a difference in number of HLA-mismatches only, we measured the strength of alloreactive T cell activation by examining the difference in common-γ chain receptor expression after allostimulation. Importantly, we observed a significantly lower frequency of IL-7Rα expressing alloreactive CD8+ T cells after both donor-specific and third-party stimulation in rejectors compared to non-rejectors. A higher pretransplant number of alloreactive IL-7Ra- CD8+ cells could cause this increase in pf. Indeed, we found a fair correlation between dsp CD8pf and the percentage of alloreactive IL-7Rα- CD8+ T cells. An explanation for the difference in percentage of IL-7Rα+ CD8+ T cells between the two patient groups may be a genetic polymorphism that influences the down modulation of IL-7Rα surface expression induced after T cell receptor (TCR) signalling or IL-7 binding [26],[30],[31]. In line with this, there are known polymorphisms associated with rejection after bone marrow transplantation as well as polymorphisms associated with increased immune activation playing a role in multiple sclerosis [32]–[34].
The finding of a low proliferative recall response to alloantigens of sorted IL-7Rα- CD8+ T cells is consistent with data from murine and human anti-viral responses [31],[35]. These cells resemble the chronic antigen-addicted memory cells as described by Wherry et al. [36]. These cells resemble phenotypically distinct subsets of anti-viral CD8+ T cells defined by high perforin expression and low IL-7Rα expression, mediating proliferative or cytotoxic capacity [37]. Recently, in attempts to prolong allograft survival, the possibility of targeting alloreactive memory cells via their IL-7Rα was postulated [38]. Our current data indicate that this approach would attack only part of the alloreactive memory cells, leaving unaffected the IL-7Rα- cells which, on the contrary, seem the most harmful alloreactive memory/effector cells.
In conclusion, using the multi-parameter MLC–CFSE assay we have shown that allostimulated cells have a highly activated and differentiated phenotype with increased expression of chemokine receptors relevant for migration of T cells into the graft and high expression of effector molecules. In addition, our analysis of patients before transplantation who are at risk for experiencing an acute cellular rejection episode, versus those who are not, revealed a higher dsp CD8pf and lower percentage of alloreactive IL-7Rα+ CD8+ T cells. However, given the retrospective nature of our present study and the overlap in results of rejectors compared to non-rejectors, it is not possible to predict the outcome of the transplantation with respect to the occurrence of acute rejection on a per-patient basis. Our data point to quantitative and qualitative differences between T cells of a group of patients who will experience acute cellular rejection episodes and those who will not. The predictive value of these parameters needs to be established in a large prospective study.
Disclosure
All authors declare no conflicts of interest.
Acknowledgments
This study was supported financially by grants from the Dutch Kidney Foundation (grant C05·2141), the RISET consortium (Sixth Framework Programme of the European Commission) and Novartis Pharma BV.
References
- 1.Bestard O, Nickel P, Cruzado JM, et al. Circulating alloreactive T cells correlate with graft function in longstanding renal transplant recipients. J Am Soc Nephrol. 2008;19:1419–29. doi: 10.1681/ASN.2007050539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Besouw NM, van der Mast BJ, de KP, et al. Donor-specific T-cell reactivity identifies kidney transplant patients in whom immunosuppressive therapy can be safely reduced. Transplantation. 2000;70:136–43. [PubMed] [Google Scholar]
- 3.Augustine JJ, Poggio ED, Heeger PS, Hricik DE. Preferential benefit of antibody induction therapy in kidney recipients with high pretransplant frequencies of donor-reactive interferon-gamma enzyme-linked immunosorbent spots. Transplantation. 2008;86:529–34. doi: 10.1097/TP.0b013e31818046db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nickel P, Presber F, Bold G, et al. Enzyme-linked immunosorbent spot assay for donor-reactive interferon-gamma-producing cells identifies T-cell presensitization and correlates with graft function at 6 and 12 months in renal-transplant recipients. Transplantation. 2004;78:1640–6. doi: 10.1097/01.tp.0000144057.31799.6a. [DOI] [PubMed] [Google Scholar]
- 5.Poggio ED, Augustine JJ, Clemente M, et al. Pretransplant cellular alloimmunity as assessed by a panel of reactive T cells assay correlates with acute renal graft rejection. Transplantation. 2007;83:847–52. doi: 10.1097/01.tp.0000258730.75137.39. [DOI] [PubMed] [Google Scholar]
- 6.Brook MO, Wood KJ, Jones ND. The impact of memory T cells on rejection and the induction of tolerance. Transplantation. 2006;82:1–9. doi: 10.1097/01.tp.0000226082.17507.da. [DOI] [PubMed] [Google Scholar]
- 7.Dinavahi R, Heeger PS. T-cell immune monitoring in organ transplantation. Curr Opin Organ Transplant. 2008;13:419–24. doi: 10.1097/MOT.0b013e3283071463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sawitzki B, Pascher A, Babel N, Reinke P, Volk HD. Can we use biomarkers and functional assays to implement personalized therapies in transplantation? Transplantation. 2009;87:1595–601. doi: 10.1097/TP.0b013e3181a6b2cf. [DOI] [PubMed] [Google Scholar]
- 9.Poggio ED, Clemente M, Riley J, et al. Alloreactivity in renal transplant recipients with and without chronic allograft nephropathy. J Am Soc Nephrol. 2004;15:1952–60. doi: 10.1097/01.asn.0000129980.83334.79. [DOI] [PubMed] [Google Scholar]
- 10.Ito T, Ueno T, Clarkson MR, et al. Analysis of the role of negative T cell costimulatory pathways in CD4 and CD8 T cell-mediated alloimmune responses in vivo. J Immunol. 2005;174:6648–56. doi: 10.4049/jimmunol.174.11.6648. [DOI] [PubMed] [Google Scholar]
- 11.Judge TA, Wu Z, Zheng XG, Sharpe AH, Sayegh MH, Turka LA. The role of CD80, CD86, and CTLA4 in alloimmune responses and the induction of long-term allograft survival. J Immunol. 1999;162:1947–51. [PubMed] [Google Scholar]
- 12.Sayegh MH, Turka LA. The role of T-cell costimulatory activation pathways in transplant rejection. N Engl J Med. 1998;338:1813–21. doi: 10.1056/NEJM199806183382506. [DOI] [PubMed] [Google Scholar]
- 13.Alves NL, van Leeuwen EM, Derks IA, van Lier RA. Differential regulation of human IL-7 receptor alpha expression by IL-7 and TCR signaling. J Immunol. 2008;180:5201–10. doi: 10.4049/jimmunol.180.8.5201. [DOI] [PubMed] [Google Scholar]
- 14.Schluns KS, Lefrancois L. Cytokine control of memory T-cell development and survival. Nat Rev Immunol. 2003;3:269–79. doi: 10.1038/nri1052. [DOI] [PubMed] [Google Scholar]
- 15.Hancock WW, Wang L, Ye Q, Han R, Lee I. Chemokines and their receptors as markers of allograft rejection and targets for immunosuppression. Curr Opin Immunol. 2003;15:479–86. doi: 10.1016/s0952-7915(03)00103-1. [DOI] [PubMed] [Google Scholar]
- 16.Panzer U, Reinking RR, Steinmetz OM, et al. CXCR3 and CCR5 positive T-cell recruitment in acute human renal allograft rejection. Transplantation. 2004;78:1341–50. doi: 10.1097/01.tp.0000140483.59664.64. [DOI] [PubMed] [Google Scholar]
- 17.Schaub S, Nickerson P, Rush D, et al. Urinary CXCL9 and CXCL10 levels correlate with the extent of subclinical tubulitis. Am J Transplant. 2009;9:1347–53. doi: 10.1111/j.1600-6143.2009.02645.x. [DOI] [PubMed] [Google Scholar]
- 18.Tatapudi RR, Muthukumar T, Dadhania D, et al. Noninvasive detection of renal allograft inflammation by measurements of mRNA for IP-10 and CXCR3 in urine. Kidney Int. 2004;65:2390–7. doi: 10.1111/j.1523-1755.2004.00663.x. [DOI] [PubMed] [Google Scholar]
- 19.Wever PC, Boonstra JG, Laterveer JC, et al. Mechanisms of lymphocyte-mediated cytotoxicity in acute renal allograft rejection. Transplantation. 1998;66:259–64. doi: 10.1097/00007890-199807270-00021. [DOI] [PubMed] [Google Scholar]
- 20.Uss E, Rowshani AT, Hooibrink B, Lardy NM, van Lier RA, ten Berge IJ. CD103 is a marker for alloantigen-induced regulatory CD8+ T cells. J Immunol. 2006;177:2775–83. doi: 10.4049/jimmunol.177.5.2775. [DOI] [PubMed] [Google Scholar]
- 21.Wood KJ, Sakaguchi S. Regulatory T cells in transplantation tolerance. Nat Rev Immunol. 2003;3:199–210. doi: 10.1038/nri1027. [DOI] [PubMed] [Google Scholar]
- 22.Nikolaeva N, Uss E, van Leeuwen EM, van Lier RA, ten Berge IJ. Differentiation of human alloreactive CD4+ and CD8+ T cells in vitro. Transplantation. 2004;78:815–24. doi: 10.1097/01.tp.0000133308.60226.fa. [DOI] [PubMed] [Google Scholar]
- 23.Sis B, Mengel M, Haas M, et al. Banff '09 meeting report: antibody mediated graft deterioration and implementation of Banff working groups. Am J Transplant. 2010;10:464–71. doi: 10.1111/j.1600-6143.2009.02987.x. [DOI] [PubMed] [Google Scholar]
- 24.Gaber LW, Moore LW, Alloway RR, et al. Correlation between Banff classification, acute renal rejection scores and reversal of rejection. Kidney Int. 1996;49:481–7. doi: 10.1038/ki.1996.68. [DOI] [PubMed] [Google Scholar]
- 25.He X, Janeway CA, Jr, Levine M, et al. Dual receptor T cells extend the immune repertoire for foreign antigens. Nat Immunol. 2002;3:127–34. doi: 10.1038/ni751. [DOI] [PubMed] [Google Scholar]
- 26.Gebauer BS, Hricik DE, Atallah A, et al. Evolution of the enzyme-linked immunosorbent spot assay for post-transplant alloreactivity as a potentially useful immune monitoring tool. Am J Transplant. 2002;2:857–66. doi: 10.1034/j.1600-6143.2002.20908.x. [DOI] [PubMed] [Google Scholar]
- 27.Bacchetta R, Passerini L, Gambineri E, et al. Defective regulatory and effector T cell functions in patients with FOXP3 mutations. J Clin Invest. 2006;116:1713–22. doi: 10.1172/JCI25112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruiz P, Fuller J, Sanfilippo F. Donor-specific cellular immunity in rejecting and long-term-surviving class I-disparate rat renal allograft recipients. Transplantation. 1990;49:175–83. doi: 10.1097/00007890-199001000-00039. [DOI] [PubMed] [Google Scholar]
- 29.Dankers MK, Roelen DL, Korfage N, et al. Differential immunogenicity of paternal HLA class I antigens in pregnant women. Hum Immunol. 2003;64:600–6. doi: 10.1016/s0198-8859(03)00058-2. [DOI] [PubMed] [Google Scholar]
- 30.Alves NL, van Leeuwen EM, Remmerswaal EB, et al. A new subset of human naive CD8+ T cells defined by low expression of IL-7R alpha. J Immunol. 2007;179:221–8. doi: 10.4049/jimmunol.179.1.221. [DOI] [PubMed] [Google Scholar]
- 31.van Leeuwen EM, de Bree GJ, Remmerswaal EB, et al. IL-7 receptor alpha chain expression distinguishes functional subsets of virus-specific human CD8+ T cells. Blood. 2005;106:2091–8. doi: 10.1182/blood-2005-02-0449. [DOI] [PubMed] [Google Scholar]
- 32.Gregory SG, Schmidt S, Seth P, et al. Interleukin 7 receptor alpha chain (IL7R) shows allelic and functional association with multiple sclerosis. Nat Genet. 2007;39:1083–91. doi: 10.1038/ng2103. [DOI] [PubMed] [Google Scholar]
- 33.Hafler DA, Compston A, Sawcer S, et al. Risk alleles for multiple sclerosis identified by a genomewide study. N Engl J Med. 2007;357:851–62. doi: 10.1056/NEJMoa073493. [DOI] [PubMed] [Google Scholar]
- 34.Shamim Z, Ryder LP, Heilmann C, et al. Genetic polymorphisms in the genes encoding human interleukin-7 receptor-alpha: prognostic significance in allogeneic stem cell transplantation. Bone Marrow Transplant. 2006;37:485–91. doi: 10.1038/sj.bmt.1705277. [DOI] [PubMed] [Google Scholar]
- 35.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–8. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 36.Wherry EJ, Barber DL, Kaech SM, Blattman JN, Ahmed R. Antigen-independent memory CD8 T cells do not develop during chronic viral infection. Proc Natl Acad Sci USA. 2004;101:16004–9. doi: 10.1073/pnas.0407192101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cellerai C, Perreau M, Rozot V, Enders FB, Pantaleo G, Harari A. Proliferation capacity and cytotoxic activity are mediated by functionally and phenotypically distinct virus-specific CD8 T cells defined by interleukin-7R{alpha} (CD127) and perforin expression. J Virol. 2010;84:3868–78. doi: 10.1128/JVI.02565-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Racape M, Vanhove B, Soulillou JP, Brouard S. Interleukin 7 receptor alpha as a potential therapeutic target in transplantation. Arch Immunol Ther Exp (Warsz) 2009;57:253–61. doi: 10.1007/s00005-009-0036-7. [DOI] [PubMed] [Google Scholar]


