Abstract
The peripheral chemokine receptors chemokine receptor 3 (CXCR3) and CC chemokine receptor 5 (CCR5) have been reported to be associated with allograft rejection. The impact of the expression of immunosuppressive drugs on peripherally circulating CD4+ T cell subsets after renal transplantion is unknown. Expression of CXCR3 and CCR5 was investigated by flow cytometry in 20 renal allograft recipients participating in a prospective, randomized trial (NCT00514514). Initial immunosuppression consisted of basiliximab, cyclosporin A (CsA), mycophenolate sodium and corticosteroids. After 3 months, patients were treated either with CsA, mycophenolate sodium (MPA) plus corticosteroids (n = 6), CsA and everolimus plus corticosteroids (n = 8) or CsA-free (CsAfree) receiving everolimus, MPA and corticosteroids (n = 6). After initial reduction of CD4+forkhead box protein 3 (FoxP3)+ and CD4+CD25hiFoxP3+ regulatory T cells (Tregs) (P < 0·05; P < 0·01), 3-month post-transplant percentages of Tregs were reconstituted in CsAfree and CsAlo arms compared to CsAreg 12 months post transplant. Expression of CCR5 and CXCR3 on CD4+FoxP3+ and CD4+FoxP3- T cells 12 months post transplant was increased in CsAfreeversus CsAreg. Increase in CCR5+CXCR3+ co-expressing CD4+FoxP3- cells between 3 and 12 months correlated negatively with the glomerular filtration rate (GFR) slope/year [modification of diet in renal disease (MDRD); r = −0·59, P < 0·01]. CsA, but not everolimus, inhibits both Treg development and expression of CXCR3 and CCR5 on CD4+ T cell subsets. Increase in CCR5+CXCR3+ co-expressing CD4+FoxP3- T cells is associated with early loss in allograft function.
Keywords: chemokine receptors, chemotaxis, regulatory T cell, transplantation
Introduction
Co-ordinated T cell migration is required for the development of effective and regulated immunity. Trafficking of donor antigen-primed effector T cells from the lymphoid tissue to the allograft represents a key event during T cell-mediated rejection of allografts [1]. Following alloantigen priming, donor-specific T cells up-regulate tissue-selective integrins and peripheral chemokine receptors that enable them to enter the transplanted allograft [2]. Despite redundancy within the chemokine–chemokine receptor family, the peripheral CXC chemokine receptor 3 (CXCR3) and the CC chemokine receptor 5 (CCR5), as well as their respective chemokine ligands, are essential in directing activated T lymphocytes in acute rejection [1],[3]–[9]. In experimental murine studies, interruption of the CCR5- and/or CXCR3 axis by in-vivo neutralization or by using CCR5−/− or CXCR3−/− recipients has been associated with reduced cellular infiltration and prolongation of allograft survival [10],[11]. Consecutively, considerable effort has been directed to selective targeting of these two chemokine receptors and their ligands with the aim of interfering with leucocyte infiltration into the allograft in order to attenuate graft injury [12]–[16]. Similar to effector T cells, human peripheral circulating forkhead box protein 3 (FoxP3)+ memory-like regulatory T cells (Tregs) have been shown to modulate peripheral immune responses through selective migration by expressing a combination of adhesion molecules [17] and chemokine receptors [18]–[21]. Treg cell-mediated suppression of allograft rejection has been shown to play an important role in allotolerance [22]–[26]. Moreover, it was shown that effective immunoregulation in vivo was not achieved in the absence of defined patterns of Treg migration [24]. Hence, understanding the compartmentalization and especially the interplay in migration of both effector T cells (Teffs) and Tregs is an area of intense study, and is of importance for allograft function following solid organ transplantation [24],[27]–[29]. However, most studies have been performed using rodent models, and little is known about the profiles of trafficking receptors or the trafficking patterns of Tregs in humans after solid organ transplantation. Moreover, studies investigating the in-vivo effect of immunosuppressive drugs on peripheral chemokine receptor expression in renal transplant recipients are lacking so far. It would be desirable to select a combination of immunosuppressive drugs that favour not only Treg survival but also preserve their peripheral trafficking properties while inhibiting function and migration of alloreactive Teff cells.
The aim of this study was to investigate the expression of peripheral trafficking receptors on circulating CD4+ T cells in patients receiving cyclosporin A (CsA) and/or everolimus. To dissect the effects of mammalian target of rapamycin (mTOR)- and calcineurin inhibition on peripheral chemokine receptors, we analysed the longitudinal course of CXCR3 and CCR5 expression on CD4+ Treg and Teff cell subsets in 20 stable renal transplant recipients that were enrolled into a prospective and randomized trial.
Material and methods
Patients and blood samples
This study was designed to take advantage of a prospective, randomized, controlled trial in which renal transplant recipients received standardized dosages of CsA and/or everolimus (Herakles, NCT00514514; CRAD001ADE13). This trial was started in October 2007 and conducted in 84 patients of the University Hospital Essen Transplant Center. From 2009 to the end of the inclusion period in 2010, 20 transplant recipients were investigated for expression of CXCR3 and CCR5 on CD4+ T cell subsets.
None of these patients fulfilled the Herakles trial exclusion criteria: serum creatinine > 3·0 mg/dl, graft loss during the trial period, alterations in immunosuppressive regimen because of acute rejection events (Banff II), platelets < 75000/mm3, leucocytes < 2500/mm3 and haemoglobin < 6 g/dl, proteinuria > 1 g/day, clinically significant infection that required continuous treatment or occurrence of severe side effects caused by the immunosuppressive drugs.
None of these patients had biopsy-proven rejection events and they did not undergo an undefined change of immunosuppressive regimen. All patients received the following immunosuppression within the first 3 months after transplantation (Fig. 1): induction therapy with basiliximab (Simulect®; Novartis, Basel, Switzerland) 2 × 20 mg, CsA (trough level 150–220 ng/ml) and mycophenolate sodium (MPA; Myfortic®; Novartis) 2 × 720 mg/day plus corticosteroids. Three months after transplantation patients were randomized into one of the following three treatment arms receiving (1) CsA at a regular dosage (trough level 100–150 ng/ml; CsAreg) in combination with MPA (2 × 720 mg per day) plus corticosteroids; (2) CsA at a low dosage (trough level 50–75 ng/ml) combined with low-dose everolimus (trough level 4–6 ng/ml; CsAlow) plus corticosteroids without MPA; or (3) a completely CsA-free everolimus (trough level 6–10 ng/ml) plus MPA (2 × 720 mg per day) plus corticosteroids containing regimen (CsAfree). Six patients in the CsAreg arm, eight of the CsAlow and six in the CsAfree arm were investigated by flow cytometry (total n = 20). A group of 16 age- and sex-matched healthy adults served as healthy controls (HC).
Fig. 1.
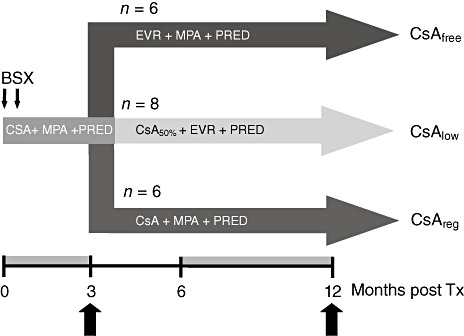
Study arms. The three study arms and their patient numbers are depicted. The orange-tinted parts of the time-line indicate time-periods in which samples were collected for fluorescence activated cell sorter (FACS) analysis and the block arrows the time-points at which expression data were used for statistical analysis. BSX, basiliximab; CsA, cyclosporin A; EVR, everolimus; MPA, mycophenolate sodium; PRED, prednisone; 144 × 123 mm (300 × 300 dots per inch).
Renal allograft function was assessed by serum creatinine, estimated as eGFR (estimated glomerular filtration rate) and calculated by modified diet in renal disease (MDRD). The longitudinal course of allograft function was evaluated by calculating the average rate of MDRD change (slope) per year of at least six comparable time-points at the time of randomization into the different treatment groups (3 months after transplantation) until 12 months after time of transplantation. The slope (s) of longitudinal courses of eGFR was calculated as s = MDRD (ml × min−1 × years−1) [30]. Demographic characteristics are shown in Table 1. None of the patients experienced biopsy-proven acute rejection. Blood samples were collected 1, 2, 3, 3, 9 and 12 months after transplantation. As some patients were followed-up in other centres, the analysis of immunomonitoring of all patients was focused between months 3 and 12 after transplantation. The study protocol was approved by the Ethics Committee of the University Duisburg-Essen. Informed consent was obtained before study entry from each patient. The results of this study had no impact on clinical decisions, as the study physicians had no insight into the flow cytometric analyses.
Table 1.
Information on demographic data of each treatment arm.
| Variables | CsAfree | CsAlow | CsAreg | P |
|---|---|---|---|---|
| Recipient age (years) | 46 ± 9·8* | 60 ± 11·5* | 50·2 ± 11·6 | <0·05 |
| Recipient gender (male/female) | 2/4 | 3/5 | 3/3 | n.s. |
| LRD (no/yes) | 5/1 | 7/1 | 5/1 | n.s. |
| CIT (min) | 875 ± 368 | 758 ± 372 | 605 ± 356 | n.s. |
| HLA mismatches A, B, DR (mean ± s.d.) | 2·3 ± 1·5 | 2·3 ± 1·9 | 2·5 ± 1·6 | n.s. |
| DGF (no/yes) | 6/0 | 8/0 | 6/0 | n.s. |
| CsA trough level (ng/ml) | ||||
| Month 3 | 125 ± 47 | 127 ± 43 | 135 ± 35·3 | n.s. |
| Month 12 | – | 68 ± 22 | 103 ± 7 | n.s. |
| MPA trough level (ng/ml) | ||||
| Month 3 | 3·1 ± 1·4 | 2·0 ± 0·3 | 2·6 ± 2·6 | n.s. |
| Month 12 | 2·1 ± 1·2 | – | 2·7 ± 1·6 | n.s. |
| EVR trough level (ng/ml) | ||||
| Month 3 | 4·3 ± 1·6 | 4·8 ± 0·9 | – | n.s. |
| Mean sCreatinin (mg/dl) | ||||
| Month 3 | 1·3 ± 0·3 | 1·5 ± 0·5 | 1·6 ± 0·8 | n.s. |
| Month 12 | 1·3 ± 0·4 | 1·6 ± 0·5 | 1·8 ± 0·9 | n.s. |
| Mean GFR (ml/min) | ||||
| Month 3 | 52·7 ± 12·2 | 42·0 ± 10·8 | 49·7 ± 22·1 | n.s. |
| Month 12 | 54·8 ± 14·4 | 42·9 ± 19·7 | 46·8 ± 23·5 | n.s. |
DGF, delayed graft function; CIT, cold ischaemia time; LRD, living related donor; s.d., standard deviation; GFR, glomerular filtration rate; MPA, mycophenolate sodium; CsA, cyclosporin A; HLA, human leucocyte antigen; n.s., not significant.
Reagents and monoclonal antibodies
Mouse anti-human CCR5-fluorescein isothiocyanate (FITC) (HEK/1/85a) was obtained from Biolegend (San Diego, CA, USA). Mouse anti-human CD4-peridinin chlorophlyll (PerCP) (SK3), anti-human CXCR3-phycoerythrin (PE) (1C6), anti-human CD45RO-FITC (UCHL1), anti-human CD25-FITC (M-A251) and the respective murine monoclonal subclass-specific isotype controls were purchased from BD Biosciences (San Jose, CA, USA). FoxP3 expression was performed by staining with anti-human FoxP3-allophycocyanin (APC) (259D/C7; BD Biosciences), a FoxP3 clone that has not been implicated with artificial staining of activated T cells [31].
Flow cytometry
Four-colour fluorescence activated cell sorter (FACS) analysis was performed on freshly isolated peripheral blood mononuclear cells (PBMCs) collected before (month 3) and after randomization (months 6–12). Recipient PBMCs were stained for up to three surface antigens with direct-conjugated fluorescent antibodies for 30 min at 4°C. After extensive washing, cells were fixed and permeabilized using the Fixation and Permeabilization Kit (BD Biosciences). Intracellular staining of FoxP3 was performed according to the manufacturer's recommendations. Phenotyping of T cell subsets was performed by gating first on the lymphocyte and subsequently on the CD4+ T cell population. Acquisition was performed on a FACSCalibur (BD Biosciences) using CellQuest software (BD Biosciences). Data were analysed using FlowJo Software, version 8·7.3 (TreeStar, Inc., Ashland, OR, USA).
Statistical analysis
Statistical analysis for flow cytometry assays was performed using GraphPad Prism version 4·0. Demographic data shown in Table 1 was analysed with standard one-way analysis of variance (anova). The unrelated groups of healthy individuals and allograft recipients shown in Fig. 2 were compared using the Mann–Whitney U-test. Related expression data, compared before and after randomization within one of the three treatment groups, were analysed using Wilcoxon's matched-pairs test (Figs 3–5). Correlation analysis of CD4+FoxP3+, CD4+CD25hiFoxP3+ or chemokine receptor expression with the slope of glomerular filtration rate as calculated by MDRD (eGFR) was performed with Pearson's test after proving normal distribution of the data. A P-value < 0·05 was considered statistically significant.
Fig. 2.
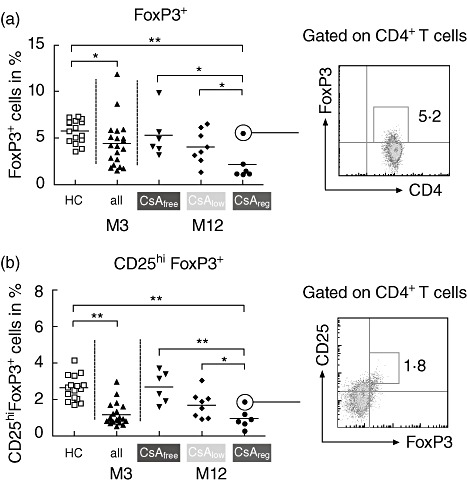
Longitudinal development of percentages of CD4+ regulatory T cells (Tregs) in the three treatment arms. The percentages of expression of forkhead box protein 3 (FoxP3) (a) and CD25hiFoxP3 (b) are shown after gating on the CD4+ T cell subset and summarized as scatter-plots with mean. Dot-blots on the right depict representatives of each Treg subset. The asterixes represent statistical significance (*P < 0·05; **P < 0·01); 123 × 88 mm (300 × 300 dots per inch).
Fig. 3.
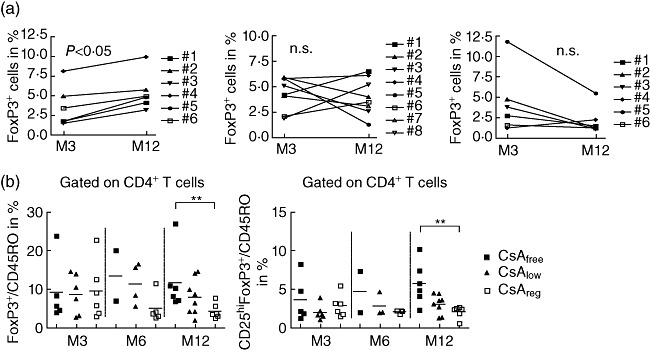
Cyclosporin inhibits CD4+ regulatory T cell (Treg) development. Scatter-plot graphs in (a) show expression in percentage of forkhead box protein 3 (FoxP3) after gating in CD4+ T cells at 3 (pre-randomization) and 12 months after transplantation for each patient in each study arm. The scatter-plot graph with mean in (b) depicts the ratio of either FoxP3/CD45RO (left panel) or CD25hiFoxP3/CD45RO (right panel) after gating on the CD4+ T cell subset in samples collected 3 (pre-randomization), 6 and 12 months after transplantation; 96 × 59 mm (300 × 300 dots per inch).
Results
Induction therapy with basiliximab in combination with a cyclosporin inhibitor-based immunosuppression reduces the amount of peripherally circulating regulatory CD4+ T cells
The percentages of both FoxP3 and CD25hiFoxP3-expressing CD4+ T cells was significantly lower in all patients (n = 20) after 3 months of standard calcineurin inhibition therapy regimen compared to healthy subjects (n = 16, Fig. 2).
Comparing the treatment groups 12 months post transplant with each other demonstrated significant differences in percentages of both CD4+FoxP3+- and CD4+CD25hiFoxP3+-expressing T cells for CsAfreeversus CsAreg and CsAlowversus CsAreg arms (Fig. 2a,b). In addition, after 3 months of standard CsA treatment and randomization into the trial arms, suppression of FoxP3+ and CD25hiFoxP3+ expression was abrogated in patients receiving a calcineurin inhibitor (CNI)-free immunosuppression 12 months post transplant (Fig. 3a, upper panel). Moreover, comparing the ratios of either FoxP3+/CD45RO+ or CD25hiFoxP3+/CD45RO+CD4+ T cells demonstrated higher percentages of CD4+ Tregs in patients receiving CsAfree immunosuppression (Fig. 3b). Therefore, CsA reduced the percentages of peripherally circulating regulatory CD4+ T cell subsets during the first year after transplantation.
CsA inhibits expression of the peripheral chemokine receptor CXCR3 on peripherally circulating CD4+ T cell subsets
The expression of CXCR3 on CD4+FoxP3+ T cells in all 20 patients receiving calcineurin inhibition 3 months after transplantation (41 ± 8%, n = 20) did not differ significantly compared to healthy subjects (44 ± 5%, n = 16; not shown). Similarly, no difference in expression of CXCR3 in CD4+FoxP3- T cells was observed between both groups (41 ± 8%, n = 20 versus 45 ± 8%, n = 16; not shown).
However, comparison of CXCR3 expression in CD4+ T cell subsets 3 months after transplantation (before randomization) and 12 months after transplantation revealed significant differences for each treatment arm (Fig. 4): randomization into the CNIfree arm led to an increase in CXCR3 expression in both CD4+FoxP3+ and CD4+FoxP3- T cells (P < 0·05, Fig. 4a). Similarly, patients receiving the CNIlow regimen displayed an increased expression of CXCR3 in both CD4+FoxP3+ Tregs and CD4+FoxP3- T cells (Fig. 4a). There was no difference in CXCR3 expression of patients treated with the standard CNI regimen 3 and 12 months after transplantation (Fig. 4a). These results demonstrate that CsA, applied in a regular clinical dosage, but not everolimus, inhibits expression of the peripheral chemokine receptor CXCR3 on both CD4+FoxP3+ and FoxP3- T cell subsets.
Fig. 4.
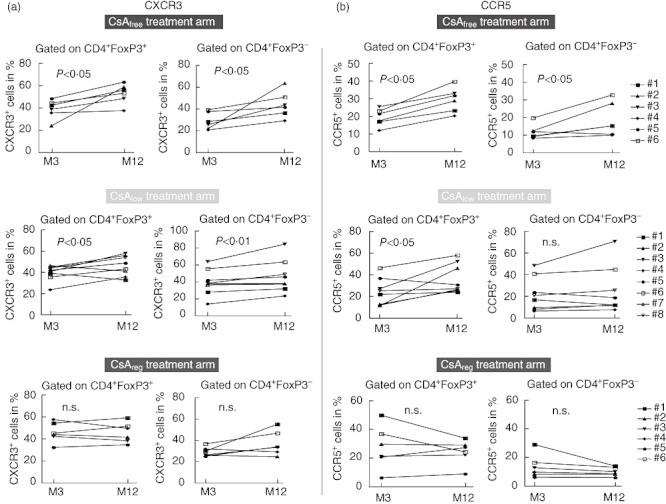
Cyclosporin inhibits expression of CXCR3 and CC chemokine receptor 5 (CCR5) in CD4+ T cell subsets. Scatter-plot graphs show expression of CXCR3 (a) and CCR5 (b) for the three treatment arms after gating in CD4+ forkhead box protein 3 (FoxP3)+ T cells (left) and CD4+FoxP3- T cells (right) at 3 (pre-randomization) and 12 months after transplantation; 157 × 126 mm (300 × 300 dots per inch).
CsA inhibits expression of the peripheral chemokine receptor CCR5 on peripherally circulating CD4+ T cell subsets
The expression of CCR5 was reduced significantly in CD4+FoxP3+ T cells of patients receiving calcineurin inhibition 3 months after transplantation (24 ± 11%, n = 20) compared to healthy subjects (31 ± 11%, n = 16, P < 0·01; not shown). For CD4+FoxP3- T cells no significant difference was demonstrated between both groups.
Comparison of CCR5 expression in CD4+ T cell subsets 3 months after transplantation (before randomization) and 12 months after transplantation revealed significant differences for each treatment arm (Fig. 4b): patients treated with the CNIfree regimen displayed an increase in CCR5 expression in both CD4+FoxP3+ and CD4+FoxP3- T cells (Fig. 4b). After randomization into the CNIlo arm, expression of CCR5 was increased in CD4+FoxP3+ Tregs but not in CD4+FoxP3- T cells (Fig. 4b). There was no difference in pre-randomization versus post-randomization samples of patients treated with the standard CNI regimen (CNIreg arm). Similar to CXCR3, these results demonstrate that CsA, but not everolimus, inhibits expression of CCR5 on both CD4+FoxP3+ and FoxP3- T cell subsets.
CsA inhibits co-expression of CCR5 and CXCR3 on CD4+FoxP3+ and CD4+FoxP3- T cells
Comparison of CCR5+CXCR3+ co-expression in CD4+ T cell subsets 3 (before randomization) and 12 months after transplantation revealed significant differences for each treatment arm: patients treated with the CNIfree regimen displayed a significant increase in CCR5+CXCR3+ co-expression in CD4+FoxP3+ (18 ± 8% versus 24 ± 7%, P < 0·05, not shown), but not in CD4+FoxP3- T cells (11 ± 4% versus 18 ± 10%, P = 0·06, not shown). After randomization into the CNIlo arm, co-expression of CCR5+CXCR3+ in CD4+FoxP3+ Tregs (16 ± 8% versus 24 ± 10%, P < 0·05), but not in CD4+FoxP3- T cells, was increased (22 ± 20% versus 23 ± 21%, not shown). There was no difference in pre-randomization versus post-randomization samples of patients treated with the standard CNI regimen (CNIreg arm), neither in CD4+FoxP3+ cells (24 ± 18% versus 19 ± 9%, not shown) nor in the CD4+FoxP3- subset (12 ± 7% versus 11 ± 5%, not shown). In contrast to the analysis of single chemokine receptors, these results demonstrate that therapy with everolimus seems to permit co-expression of CCR5 and CXCR3 preferably on CD4+FoxP3+ subsets, whereas no significant change of co-expression could be documented in patients receiving CsAreg treatment.
High percentages of peripheral circulating CD4+FoxP3- T cells that co-express CXCR3 and CCR5 are associated with loss in renal allograft function during the first year post transplantation
Correlation analysis revealed a negative association with the change of CXCR3 and CCR5 co-expression on CD4+FoxP3- T cells (ΔCXCR3+CCR5+) from 3 (pre-randomization) to 12 months after transplantation (r = −0·59, P < 0·01, n = 20, Fig. 5). Correlating the change in expression of CCR5 and/or CXCR3 on Treg cells (ΔCCR5+, ΔCXCR3+, ΔCCR5+CXCR3+) with renal allograft function showed no significant association. In addition, analysing the difference in percentages of the ratio of CD25hiFoxP3+/CD45RO+ CD4+ T cells (and also FoxP3+/CD45RO+) assessed after 3 and 12 months post transplantation (Δratio CD25hiFoxP3+/CD45RO+ CD4+ T cells) with the slopes of MDRD measurements showed no correlation.
Fig. 5.

Correlation of CD4+ T cell subsets with allograft function during the first year after transplantation. The scatter-plot graph depicts the difference in percentage of CC chemokine receptor 5 (CCR5) and chemokine receptor 3 (CXCR3) co-expression (ΔCCR5+CXCR3+) of CD4+ forkhead box protein 3 (FoxP3)- T cells (y-axis) over the slope of modified diet in renal disease (MDRD) (ml/min/year; x-axis) between 3 and 12 months post transplantation (n = 20); 117 × 84 mm (300 × 300 dots per inch).
High cyclosporin trough levels are associated with lower amounts of circulating CD4+FoxP3+ T cells that co-express CCR5+CXCR3+ during the first year post transplantation
To explore further whether cyclosporin is affecting expression of CXCR3 and/or CCR5 in a dose-related response, all patient samples that were collected during the first 3 months and samples of those patients who were randomized into the CsA standard arm were analysed. CsA trough levels were correlated with Treg markers and chemokine receptor surface expression. Expression of CD25 on CD4+ T cells demonstrated a negative correlation with CsA trough levels (r = −0·36, P < 0·05, n = 38; not shown). In addition, CsA trough levels were associated negatively with the expression of CCR5 (r = −0·36, P < 0·05, n = 38) and the co-expression of CCR5 and CXCR3 on CD4+FoxP3+ T cells (r = −0·48, P < 0·01, n = 38), but not on CD4+FoxP3- T cells (Fig. S1a,b). Expression of CXCR3 on CD4+FoxP3- correlated negatively with CsA trough levels (r = −0·34, P < 0·05, n = 38), in contrast to CXCR3 on CD4+FoxP3+ (r = −0·24, P = 0·13, n = 38; not shown). Therefore, CsA inhibits expression of CCR5 preferentially on CD4+FoxP3+ in a dose-related response. CD4+FoxP3- T cells that co-express CCR5 and CXCR3 were not affected by CsA.
Discussion
The results of this prospective study demonstrate that, in humans, cyclosporin but not everolimus inhibits not only the expansion of regulatory CD4+ T cells but also their expression of the peripheral trafficking receptors CXCR3 and CCR5 in the longitudinal course until 1 year post transplantation. Treatment with everolimus does not exert any inhibition on surface expression of both chemokine receptors and thus seems to permit an increase in expression of CXCR3 and CCR5 on both CD4+FoxP3+ and CD4+FoxP3- T cells in the same manner. The increase of CXCR3 surface expression over time after transplantation occurred also in both CD4+CD25hiFoxP3+ and CD4+CD25loFoxP3- subsets (not shown).
mTOR inhibitors have been shown to augment selective expansion of Tregs[32],[33] and promote tolerance induction in vivo[32],[34]–[36]. Increased FoxP3 mRNA levels have been observed in peripherally circulating PBMCs of renal transplant recipients after withdrawal from calcineurin inhibition [37]. The results presented here demonstrate that in patients receiving calcineurin-free immunosuppression Treg expansion was not achieved 12 months after transplantation compared to the healthy control group. Because pre-transplant samples for flow cytometric analysis were lacking, these results only allow the conclusion that treatment with everolimus led at least to a reconstitution of the Treg compartment 12 months after transplantation compared to patients treated with standard dosages of cyclosporin. Correlation analysis of CD25hiFoxP3+ Treg development in the peripheral blood between 3 and 12 months post transplantation failed to demonstrate a positive association with the slope of estimated GFR. That detection and quantification of Tregs circulating in human blood does not reflect suppressive function is a common finding; the majority of previous reports have associated the outcome of the allograft primarily with changes in the frequency of allograft residing Tregs[29],[38],[39]. Only few human studies demonstrated that increased FoxP3 expression in peripherally circulating T cells of renal transplant recipients was associated with long-term (8 years) stable renal function compared with patients exhibiting chronic rejection [40].
Experimental murine studies have shown that functionally active Tregs are present in secondary lymphoid organs as well as within the transplanted graft in order to suppress alloreactive immune responses [24]. The trafficking of Tregs into both compartments has been proposed to be important for alloimmune tolerance induction [24],[27], as well as for the prevention of chronic rejection [28]. In humans, Treg cells have been shown to accumulate in kidney allografts [29],[41]. Moreover, Bestard and co-workers demonstrated that the FoxP3+ Treg/CD3+ T cell ratio of intragraft T cell infiltrates correlated positively with graft function 2 years after transplantation [29]. This observation suggests that peripheral homing receptor expression on Tregs has the potential to define effective intragraft migration patterns and peripheral immunoregulation in vivo. Analysis of peripheral chemokine receptor expression on CD4+ T cell subsets circulating in the peripheral blood may thus provide a more distinct evaluation of those immune cells that are enabled to traffick into the allograft. In previous studies we demonstrated that the mTOR inhibitor rapamycin permits the expansion of CD4+CXCR3+ Tregsin vitro, and we found higher numbers of circulating FoxP3+CXCR3+ Tregs in transplant recipients treated with everolimus versus those treated with CsA as part of their maintenance immunosuppressive therapy [42]. In this prospective and controlled study with renal transplant patients receiving standardized immunosuppression, no association with expression of CXCR3 and/or CCR5 on peripherally circulating Tregs and allograft function could be shown. A reason for this may be similar drug effects on CCR5 and CXCR3 expression on both Treg and Teff cell subsets that may negate a beneficial effect of increased Treg recruitment into the allograft.
Interestingly, recent data by Lo et al. [43] provide evidence that intra-allograft transcript expression of the interferon (IFN)-γ-inducible ligands CXCL9-11 (CXCR3), as well as regulated upon activation normal T cell expressed and secreted (RANTES) and macrophage inflammatory proteins (MIP)-1α (CCR5) are elevated moderately in non-rejecting kidney transplants (up to 10-fold) compared to kidney biopsies of healthy living donors. Therefore, future studies should also include both the analysis of chemokine receptor expression and levels of their respective ligands (e.g. CXCL9-11, RANTES, MIP1α) to understand more clearly the in-vivo effects of immunosuppressive drugs on T cell trafficking.
That the difference of CCR5+CXCR3+ co-expression on CD4+FoxP3- T cells between 3 and 12 months after transplantation correlated negatively with the slope of MDRD development during the first year post transplantation is a new finding. It has been reported that CXCR3 and CCR5 expression is induced during the maturation from naive donor antigen-activated towards IFN-γ-producing/cytotoxic T cells [44]–[47]. Moreover, co-expression of both receptors is expressed preferentially on T helper type 1 (Th1) CD4+ Tmem cells and effector CD8+ Tmem cells [44]–[46],[48]. In addition, the ligands for CXCR3, monokine induced by IFN-γ (Mig or CXCL9), IFN-γ-inducible protein of 10 kDa (IP-10 or CXCL10) and IFN-γ-inducible T cell α-chemoattractant (I-Tac or CXCL11) and for CCR5, RANTES (CCL5) have been shown to be associated with both cardiac and renal allograft rejection [4]–[7],[11],[49],[50]. An increase of CXCR3+CCR5+ co-expressing CD4+ Teff cells over time may thus contain substantial numbers of donor-specific alloreactive Tmem cells and this may then lead, or at least predispose, to a loss of renal allograft function in the longitudinal course.
In this study, we took advantage of a prospective, controlled and randomized trial involving a homogeneous study cohort with three treatment arms (NCT00514514). The treatment protocol contains two caveats: first, the trial protocol determined that patients received induction therapy with basiliximab. Basiliximab is known to cause a sufficient saturation of the alpha subunit of the IL-2 receptor CD25 for 5–8 weeks [51]. Therefore, reduction of both expression of the T regulatory-associated molecules FoxP3 and CD25 as well as the chemokine receptors CXCR3 and CCR5 3 months after transplantation may represent an effect caused mainly by blocking IL-2 receptor signalling. However, the post-transplant course after 3 months demonstrates that CsA inhibits both Treg development as well as expression of the peripheral chemokine receptors CXCR3 and CCR5. Secondly, besides standardized trough levels of CsA and everolimus the CsAlow arm cannot be compared fully to the CsAreg group, as differences might also depend upon MPA. In addition, the trial protocol determined that patients had to be treated at baseline with CsA. However, tacrolimus-based therapy regimens currently represent the safest and most efficacious immunosuppression [52],[53]. Hence, it would also have been of interest to investigate Treg development and their expression of chemokine receptors in patients receiving tacrolimus.
In summary, these observations in human renal transplant recipients, derived from a prospective randomized calcineurin conversion trial, demonstrate that cyclosporin reduces not only the percentage of circulating Tregs but also their expression of the peripheral chemokine receptors CCR5 and CXCR3. More importantly, we show that cyclosporin is affecting both Treg and Teff cells. In contrast, everolimus enabled reconstitution of the Treg subset and permitted expression of CXCR3 and CCR5 on both Tregs and Teffs in the same manner. Finally, that increasing percentages of CCR5 and CXCR3 co-expressing CD4+FoxP3- T cells are associated with the loss of renal function after 12 months post transplantation is a new finding. Because the number of patients who were investigated is limited, a statement for an association with renal allograft function can be made only for this selected cohort of patients exhibiting a favourable post-transplant course, as biopsy-proven rejection events did not occur.
Further studies of sequential monitoring of CXCR3 and CCR5 co-expression on CD4+ Teff cells are needed to evaluate the relevance of this finding, and if confirmed in combination with renal allograft protocol biopsies their kinetics may help to predict imminent rejection or the development of chronic injury in renal allografts.
Acknowledgments
This work was supported by an IFORES Research fellowship from the University Duisburg-Essen Medical School to A. H.
Disclosures
O. W., A. K. and T. F. received honoraria and/or lecture fees from Astellas, Genzyme, Novartis and Roche. The authors declare that no conflict of interest exists. The results presented in this paper have not been published previously in whole or part, except in abstract format.
Supporting information
Fig. S1. Correlation of cyclosporin A (CsA)trough levels with CC chemokine receptor 5 (CCR5) expressingCD4+forkhead box protein 3 (FoxP3)+ T cellsubsets during the first year after transplantation. Thescatter-plot graph depicts the correlation analysis of CCR5expression (a) or chemokine receptor 3 (CXCR3) and CCR5co-expression (b) on CD4+FoxP3+ T cells withCsA trough levels (ng/ml; x-axis); 86 × 38 mm (300× 300 dots per inch).
References
- 1.Hancock WW. Chemokine receptor-dependent alloresponses. Immunol Rev. 2003;196:37–50. doi: 10.1046/j.1600-065x.2003.00084.x. [DOI] [PubMed] [Google Scholar]
- 2.Sallusto F, Baggiolini M. Chemokines and leukocyte traffic. Nat Immunol. 2008;9:949–52. doi: 10.1038/ni.f.214. [DOI] [PubMed] [Google Scholar]
- 3.Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565–8. doi: 10.1038/33340. [DOI] [PubMed] [Google Scholar]
- 4.Melter M, Exeni A, Reinders ME, et al. Expression of the chemokine receptor CXCR3 and its ligand IP-10 during human cardiac allograft rejection. Circulation. 2001;104:2558–64. doi: 10.1161/hc4601.098010. [DOI] [PubMed] [Google Scholar]
- 5.Fahmy NM, Yamani MH, Starling RC, et al. Chemokine and receptor-gene expression during early and late acute rejection episodes in human cardiac allografts. Transplantation. 2003;75:2044–7. doi: 10.1097/01.TP.0000069601.73079.94. [DOI] [PubMed] [Google Scholar]
- 6.Panzer U, Reinking RR, Steinmetz OM, et al. CXCR3 and CCR5 positive T-cell recruitment in acute human renal allograft rejection. Transplantation. 2004;78:1341–50. doi: 10.1097/01.tp.0000140483.59664.64. [DOI] [PubMed] [Google Scholar]
- 7.Tatapudi RR, Muthukumar T, Dadhania D, et al. Noninvasive detection of renal allograft inflammation by measurements of mRNA for IP-10 and CXCR3 in urine. Kidney Int. 2004;65:2390–7. doi: 10.1111/j.1523-1755.2004.00663.x. [DOI] [PubMed] [Google Scholar]
- 8.Schenk AD, Rosenblum JM, Fairchild RL. Chemokine-directed strategies to attenuate allograft rejection. Clin Lab Med. 2008;28:441–54. doi: 10.1016/j.cll.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lacotte S, Brun S, Muller S, Dumortier H. CXCR3, inflammation, and autoimmune diseases. Ann NY Acad Sci. 2009;1173:310–17. doi: 10.1111/j.1749-6632.2009.04813.x. [DOI] [PubMed] [Google Scholar]
- 10.Hancock WW, Gao W, Faia KL, Csizmadia V. Chemokines and their receptors in allograft rejection. Curr Opin Immunol. 2000;12:511–16. doi: 10.1016/s0952-7915(00)00130-8. [DOI] [PubMed] [Google Scholar]
- 11.Hancock WW, Lu B, Gao W, et al. Requirement of the chemokine receptor CXCR3 for acute allograft rejection. J Exp Med. 2000;192:1515–20. doi: 10.1084/jem.192.10.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson M, Li AR, Liu J, et al. Discovery and optimization of a series of quinazolinone-derived antagonists of CXCR3. Bioorg Med Chem Lett. 2007;17:3339–43. doi: 10.1016/j.bmcl.2007.03.106. [DOI] [PubMed] [Google Scholar]
- 13.Schnickel GT, Bastani S, Hsieh GR, et al. Combined CXCR3/CCR5 blockade attenuates acute and chronic rejection. J Immunol. 2008;180:4714–21. doi: 10.4049/jimmunol.180.7.4714. [DOI] [PubMed] [Google Scholar]
- 14.Bastani S, Sherman W, Schnickel GT, et al. Chemokine receptor blockade with a synthetic nonpeptide compound attenuates cardiac allograft vasculopathy. Transplantation. 2009;88:995–1001. doi: 10.1097/TP.0b013e3181b9ccd5. [DOI] [PubMed] [Google Scholar]
- 15.Liu J, Fu Z, Li AR, et al. Optimization of a series of quinazolinone-derived antagonists of CXCR3. Bioorg Med Chem Lett. 2009;19:5114–18. doi: 10.1016/j.bmcl.2009.07.032. [DOI] [PubMed] [Google Scholar]
- 16.Scholten D, Canals M, Maussang D, et al. Pharmacological modulation of chemokine receptor function. Br J Pharmacol. 2011 doi: 10.1111/j.1476-5381.2011.01551.x. doi: 10.1111/j.1476-5381.2011.01551.x [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huehn J, Siegmund K, Hamann A. Migration rules: functional properties of naive and effector/memory-like regulatory T cell subsets. Curr Top Microbiol Immunol. 2005;293:89–114. doi: 10.1007/3-540-27702-1_5. [DOI] [PubMed] [Google Scholar]
- 18.Huehn J, Hamann A. Homing to suppress: address codes for Treg migration. Trends Immunol. 2005;26:632–6. doi: 10.1016/j.it.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Sather BD, Treuting P, Perdue N, et al. Altering the distribution of Foxp3(+) regulatory T cells results in tissue-specific inflammatory disease. J Exp Med. 2007;204:1335–47. doi: 10.1084/jem.20070081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakaguchi S, Ono M, Setoguchi R, et al. Foxp3+CD25+CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 21.Piccirillo CA. Regulatory T cells in health and disease. Cytokine. 2008;43:395–401. doi: 10.1016/j.cyto.2008.07.469. [DOI] [PubMed] [Google Scholar]
- 22.Boehmer V. Mechanisms of suppression by suppressor T cells. Nat Immunol. 2005;6:338–44. doi: 10.1038/ni1180. [DOI] [PubMed] [Google Scholar]
- 23.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–87. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 24.Zhang N, Schroppel B, Lal G, et al. Regulatory T cells sequentially migrate from inflamed tissues to draining lymph nodes to suppress the alloimmune response. Immunity. 2009;30:458–69. doi: 10.1016/j.immuni.2008.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 26.Li XC, Turka LA. An update on regulatory T cells in transplant tolerance and rejection. Nat Rev Nephrol. 2010;6:577–83. doi: 10.1038/nrneph.2010.101. [DOI] [PubMed] [Google Scholar]
- 27.Long ET, Baker S, Oliveira V, Sawitzki B, Wood KJ. Alpha-1,2-mannosidase and hence N-glycosylation are required for regulatory T cell migration and allograft tolerance in mice. PLoS ONE. 2010;5:e8894. doi: 10.1371/journal.pone.0008894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nadig SN, Wieckiewicz J, Wu DC, et al. In vivo prevention of transplant arteriosclerosis by ex vivo-expanded human regulatory T cells. Nat Med. 2010;16:809–13. doi: 10.1038/nm.2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bestard O, Cruzado JM, Rama I, et al. Presence of FoxP3+ regulatory T cells predicts outcome of subclinical rejection of renal allografts. J Am Soc Nephrol. 2008;19:2020–6. doi: 10.1681/ASN.2007111174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gera M, Slezak JM, Rule AD, Larson TS, Stegall MD, Cosio FG. Assessment of changes in kidney allograft function using creatinine-based estimates of glomerular filtration rate. Am J Transplant. 2007;7:880–7. doi: 10.1111/j.1600-6143.2006.01690.x. [DOI] [PubMed] [Google Scholar]
- 31.Koenen HJ, Smeets RL, Vink PM, van Rijssen E, Boots AM, Joosten I. Human CD25highFoxp3pos regulatory T cells differentiate into IL-17-producing cells. Blood. 2008;112:2340–52. doi: 10.1182/blood-2008-01-133967. [DOI] [PubMed] [Google Scholar]
- 32.Zeiser R, Leveson-Gower DB, Zambricki EA, et al. Differential impact of mammalian target of rapamycin inhibition on CD4+CD25+Foxp3+ regulatory T cells compared with conventional CD4+ T cells. Blood. 2008;111:453–62. doi: 10.1182/blood-2007-06-094482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Delgoffe GM, Kole TP, Zheng Y, et al. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30:832–44. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bestard O, Cruzado JM, Grinyo JM. Inhibitors of the mammalian target of rapamycin and transplant tolerance. Transplantation. 2009;87(8 Suppl.):S27–9. doi: 10.1097/TP.0b013e3181a07b08. [DOI] [PubMed] [Google Scholar]
- 35.Gao W, Lu Y, Essawy BE, Oukka M, Kuchroo VK, Strom TB. Contrasting effects of cyclosporine and rapamycin in de novo generation of alloantigen-specific regulatory T cells. Am J Transplant. 2007;7:1722–32. doi: 10.1111/j.1600-6143.2007.01842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McMahon G, Weir MR, Li XC, Mandelbrot DA. The evolving role of mTOR inhibition in transplantation tolerance. J Am Soc Nephrol. 2011;22:408–15. doi: 10.1681/ASN.2010040351. [DOI] [PubMed] [Google Scholar]
- 37.van de Wetering J, Koumoutsakos P, Peeters A, et al. Discontinuation of calcineurin inhibitors treatment allows the development of FOXP3+ regulatory T-cells in patients after kidney transplantation. Clin Transplant. 2011;25:40–6. doi: 10.1111/j.1399-0012.2010.01311.x. [DOI] [PubMed] [Google Scholar]
- 38.Bestard O, Cuñetti L, Cruzado JM, Lucia M, et al. Intragraft regulatory T cells in protocol biopsies retain FoxP3 demethylation and are protective biomarkers for kidney graft outcome. Am J Transplant. 2011;11:2162–72. doi: 10.1111/j.1600-6143.2011.03633.x. [DOI] [PubMed] [Google Scholar]
- 39.DI Baan CC, Weimar W. Regulatory T cells in alloreactivity after clinical heart transplantation. Curr Opin Immunol. 2009;14:577–82. doi: 10.1097/MOT.0b013e32833037e8. [DOI] [PubMed] [Google Scholar]
- 40.Alvarez CM, Opelz G, Garcia LF, Susal C. Expression of regulatory T-cell-related molecule genes and clinical outcome in kidney transplant recipients. Transplantation. 2009;87:857–63. doi: 10.1097/TP.0b013e318199fa57. [DOI] [PubMed] [Google Scholar]
- 41.Muthukumar T, Dadhania D, Ding R, et al. Messenger RNA for FOXP3 in the urine of renal-allograft recipients. N Engl J Med. 2005;353:2342–51. doi: 10.1056/NEJMoa051907. [DOI] [PubMed] [Google Scholar]
- 42.Hoerning A, Koss K, Datta D, et al. Subsets of human CD4(+) T regulatory cells express the peripheral homing receptor CXCR3. Eur J Immunol. 2011;41:2291–302. doi: 10.1002/eji.201041095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lo DJ, Weaver TA, Kleiner DE, et al. Chemokines and their receptors in human renal allotransplantation. Transplantation. 2011;91:70–7. doi: 10.1097/TP.0b013e3181fe12fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Loetscher M, Loetscher P, Brass N, Meese E, Moser B. Lymphocyte-specific chemokine receptor CXCR3: regulation, chemokine binding and gene localization. Eur J Immunol. 1998;28:3696–705. doi: 10.1002/(SICI)1521-4141(199811)28:11<3696::AID-IMMU3696>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 45.Qin S, Rottman JB, Myers P, et al. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Invest. 1998;101:746–54. doi: 10.1172/JCI1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Loetscher P, Uguccioni M, Bordoli L, et al. CCR5 is characteristic of Th1 lymphocytes. Nature. 1998;391:344–5. doi: 10.1038/34814. [DOI] [PubMed] [Google Scholar]
- 47.Bonecchi R, Bianchi G, Bordignon PP, et al. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J Exp Med. 1998;187:129–34. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Groom JR, Luster AD. CXCR3 in T cell function. Exp Cell Res. 2011;317:620–31. doi: 10.1016/j.yexcr.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hancock WW, Gao W, Csizmadia V, Faia KL, Shemmeri N, Luster AD. Donor-derived IP-10 initiates development of acute allograft rejection. J Exp Med. 2001;193:975–80. doi: 10.1084/jem.193.8.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao DX, Hu Y, Miller GG, Luster AD, Mitchell RN, Libby P. Differential expression of the IFN-gamma-inducible CXCR3-binding chemokines, IFN-inducible protein 10, monokine induced by IFN, and IFN-inducible T cell alpha chemoattractant in human cardiac allografts: association with cardiac allograft vasculopathy and acute rejection. J Immunol. 2002;169:1556–60. doi: 10.4049/jimmunol.169.3.1556. [DOI] [PubMed] [Google Scholar]
- 51.Ramirez CB, Marino IR. The role of basiliximab induction therapy in organ transplantation. Expert Opin Biol Ther. 2007;7:137–48. doi: 10.1517/14712598.7.1.137. [DOI] [PubMed] [Google Scholar]
- 52.Ekberg H, Bernasconi C, Tedesco-Silva H, et al. Calcineurin inhibitor minimization in the Symphony study: observational results 3 years after transplantation. Am J Transplant. 2009;9:1876–85. doi: 10.1111/j.1600-6143.2009.02726.x. [DOI] [PubMed] [Google Scholar]
- 53.Ekberg H, Tedesco-Silva H, Demirbas A, et al. Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med. 2007;357:2562–75. doi: 10.1056/NEJMoa067411. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


