Abstract
It is a challenge to understand how barn owls (Tyto alba) reduce noise during flight to be able to hunt small mammals by audition. Several specializations of the wing and the wing feathers have been implicated in noise reduction. What has been overlooked so far are the fringes at the inner vanes of remiges. We demonstrated, by using precise imaging techniques combined with morphometric measurements and air-flow studies, that these fringes merge into neighboring feather vanes by gliding into the grooves at the lower wing surface that are formed by parallel-oriented barb shafts. The connection of adjacent feathers results in a smooth lower wing surface and thus reduces sharp and noisy edges. This finding sheds new light on the mechanisms underlying noise reduction of flying owls.
Keywords: barbs, barn owl, feather, flow control, fringes, silent flight
Introduction
A feather consists of a central shaft and two laterally attached vanes. Vanes are formed by barbs that are connected via hook and bow radiates. Tiny hooklets (hamuli) of the hook radiates (anterior barbules) cling into grooves of the bow radiates (posterior barbules) to form a flexible, lightweight and securely connected surface (pennaceous vane). Apart from the pennaceous vanes, most feathers have downy parts (plumulaceous vanes) at their bases (Fig. 1). Here, barbs are slender, flexible and fuzzy, creating vanes with a fluffy and thick texture (Stettenheim, 2000). The barbs remain separated and fluffy due to small barbicles at the pennula of the hook radiates that prevent alignment of the barb shafts. Functionally, these areas trap air and are used for thermal insulation and, in the case of wing feathers, for upholstery in necessary wing areas.
Fig. 1.

Representatives of the five different remiges investigated: (A) 10th primary; (B) 5th primary; (C) 1st primary; (D) 4th secondary; (E) 9th secondary. Note the basal downs (plumulaceous barbs) at the base of each feather. Here, barbs separate to create a fluffy structure, which is used among other things for thermal insulation. Scale bar: 5 cm.
Owls (Strigiformes) are renowned for their quiet flight. However, quantitative sound measurements of flying owls are rare. Neuhaus et al. (1973) were the first to measure and compare the noise intensity of flying tawny owls (Strix aluco) and mallard ducks (Anas platyrhynchos). They measured a main noise frequency band of 50–1500 Hz for the owl and 3–5 kHz for the duck. All frequencies above 1.5 kHz were significantly reduced in intensity for the tawny owl. In comparison, mallard ducks showed a high intensity throughout the frequency band. More recently, Sarradj et al. (2010) presented flyover noise measurements of a barn owl (Tyto alba), a common kestrel (Falco tinnunculus) and a Harris hawk (Parabuteo unicinctus). These authors showed that noise for frequency bands above 1.6 kHz was significantly lower for the owl, with a noise reduction of a few decibels. At high frequencies above 6.3 kHz the noise from the owl was so low that it could not be measured with the microphone array used (Sarradj et al. 2010). By damping the noise intensity above 1.6 kHz, the flight noise is reduced within the typical hearing spectrum of the owl’s prey (Neuhaus et al. 1973) and within the owl’s own best hearing range (Konishi, 1973). The understanding of the mechanisms underlying noise reduction in owl flight is a challenge. Several anatomical specializations of the plumage considered to play an important role in noise reduction during flight by either air-flow control or noise reduction have been reported for these birds. Graham (1934) was the first to mention serrations at the leading edge of the wing, a velvety dorsal surface of each feather, and fringes of the inner vanes of remiges. These plumage specifics are unique and have been qualitatively reported for almost all species of owls, except fish-eating owls that presumably do not rely on a silent flight (Sick, 1937).
In case of the fringes, the tips of barbs of the inner vane are separated due to a loss of hooklets in the most distal part similar to the plumulaceous barbs (Bachmann et al. 2007). Hook and bow radiates align with the barb shaft to support the formation of fringes. In barn owls (Tyto alba), fringes are found at the inner vanes of all remiges. Thus, fringes do not only occur at the trailing edge of each remex but also at the trailing edge of the wing (Graham, 1934; Bachmann et al. 2007). Bachmann et al. (2007) morphometrically compared feathers from barn owls with feathers from a non-silent flying bird, the pigeon (Columba livia). All remiges of the pigeon have sharp edges (inner and outer vane), and no fringes or fringe-like structures are found.
Functionally, Graham (1934) stated that fringes are designed to deal with the air flow alone. He claimed that small vortices, which occur at the trailing edge of the wing due to different velocities of the upper and lower streams (from which sound emanates), are delayed by the fringes. Hence, noise and fluttering of the trailing edge should be reduced during flight. The existence of fringes along the inner vanes was interpreted as a mechanism to reduce noise during fluttering, because wing feathers separate during the upstroke and the air flows through the wing (Rüppell, 1980). Consequently, each feather acts as a small airfoil and thus has its own trailing edge where noise needs to be reduced.
Kroeger et al. (1971) and Neuhaus et al. (1973) investigated the flow field and noise production of owl wings during flight. Although these authors mentioned fringes at the trailing edge of the feathers, no morphometric data or functional aspects were presented. Lilley (1998) and Lockard & Lilley (2004) restated that the function of fringes is to reduce turbulence at the trailing edge of the wing. They mentioned that the scattering phenomenon, which can be found when turbulent eddies pass over the posterior wing region that gradually transitions from the loaded wing to freestream conditions, can be interrupted by introducing a pressure-release mechanism such as fringes at the trailing edge. Thus, although some progress has been made recently, a quantitative morphometric characterization of the fringes is still missing, and it is not yet well understood how the fringes interact to reduce noise.
The aim of this study was to measure and characterize fringes of barn owl remiges quantitatively using digital photography and scanning electron microscopy. On the basis of these experimental data and on high-speed video analyses of fringes subjected to a flow field, we shall postulate formerly unrevealed functions of the fringes and advance beyond any phenomenological description provided so far. Fringes merge neighboring feather vanes by gliding into grooves at the lower wing surface to create a smooth airfoil without sharp and noisy edges. Further, fringes seem to stabilize the weak feather–feather connection and thus prevent friction between them to some extent.
Materials and methods
Feather material
Five different remiges (10th primary, 5th primary, 1st primary, 4th secondary, 9th secondary; Fig. 1) were investigated with respect to their fringes. Five individual feathers were obtained from each feather position. The five different feather positions chosen for this study cover representative remiges of the whole wing. In total, 25 remiges from seven barn owls (Tyto alba pratincola) bred at the Institute for Biology 2 (RWTH Aachen University, Germany) were investigated. Fixed wings of barn owls were available to obtain the orientation of the fringes within the extended wing. All wings and feathers were taken from specimens that had been used in other experiments under a permit of the local authorities [Landespräsidium für Natur, Umwelt und Verbraucherschutz Nordrhein Westfalen, Recklinghausen, Germany (LANUV)].
Two neighboring feathers (secondary 7 and 8) of a common buzzard (Buteo buteo), a harris hawk (Parabuteo unicinctus), a pigeon (Columba livia) and a mute swan (Cygnus olor) were generously provided by local breeders and feather collectors.
Morphometric analysis
Each feather was photographed with an 8-megapixel digital camera (Canon EOS 350D; Canon, Tokyo, Japan) equipped with a 50-mm Canon macro lens. Measurements took place at every 10% of the inner vane length. In total, 225 sampling areas were investigated. Length and density of the fringes were measured using the software Photoshop (Adobe Systems, San Jose, CA, USA). An overview of the morphometrical parameters is shown in Fig. 2.
Fig. 2.
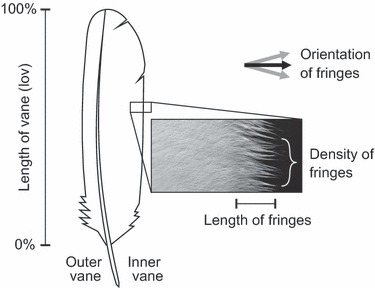
Morphometrical parameters measured for the characterization of fringes at barn owl feathers. The length of fringes (mm) and their density (fringes per mm) were measured every 10% of the inner vane.
Scanning electron microscopy
Selected barbs were examined with scanning electron microscopy. The barbs were cut at the rachis and placed with a glue pad on an aluminum specimen stub. Afterwards the specimens were gold coated with a sputter coater (Hummer; Technics, Alexandria, VA, USA; 10 mA, 1000 V, 7–9 min). Observations were made with a Cambridge Stereoscan 604 scanning electron microscope (Cambridge Instruments, Cambridge, UK).
High-speed video analysis
A set of secondary remiges from a barn owl wing were mounted onto an artificial leading edge (reinforced carbon fiber, NACA0012 profile, chord length = 25 mm; Fig. 3). This wing configuration was subjected to a flow field of varying velocities ranging between 0 and 5 m s−1 by blowing air over the surface (Fig. 3). The flight speed of a barn owl ranges between 2.5 and 7 m s−1 depending on the illumination of the environment (Mebs & Scherzinger, 2000). A high-speed digital video camera Phantom V12.1 (Vision Research, Wayne, NJ, USA) with a Nikon 85 mm lens (+ distances; Nikon, Tokyo, Japan) was used to capture a small area of overlapping vanes at the ventral side of the wing. Possible interactions of the fringes with the neighboring barbs during the experiment could be observed. The camera resolution was set to 1280 × 800 pixels at a recording speed of 400 frames s−1. The field of view was chosen to cover a medial section of the inner vane of the 7th secondary and the subjacent 8th secondary because this area was in the most central part of the artificial wing.
Fig. 3.
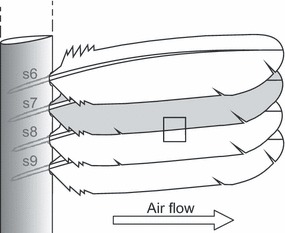
Schematic of the feather arrangement (sequential secondaries) for the high-speed video analysis. The feathers are positioned as in a spread wing of a barn owl. The artificial wing was subjected to a flow field of Vmax = 5 m s−1. The area of interest was chosen to cover a central part of overlapping vanes (square). The schematic is not to scale.
Comparison to other species of birds
To exemplify the exceptional position of owl fringes, trailing edges of five different species of birds, including a barn owl (Tyto alba), a common buzzard (Buteo buteo), a Harris hawk (Parabuteo unicinctus), a pigeon (Columba livia) and a mute swan (Cygnus olor), were compared. These species were chosen because buzzards and Harris hawks are diurnal birds of prey having comparable wing loadings to barn owls, pigeons are of comparable body mass, and mute swans are totally different in many aspects. The wing loadings of the species chosen are as follows: barn owl – 33 Nm−2; common buzzard – 48 Nm−2; Harris hawk – 39 Nm−2; pigeon – 97 Nm−2; mute swan – 196 Nm−2. Two neighboring secondaries (s7 and s8) were positioned as they are naturally found in a fully extended wing and subsequently photographed with a digital microscope (Keyance VHX-500FD; Keyance, Osaka, Japan). Although the measurements were performed under quiescent air, we ensured that the relevant trailing edge was as close to the neighboring vane as possible. Pictures were taken at the most central parts of the vane’s edge.
Statistics
Factorial analysis of variance (anova) was used to test for differences of length and density of fringes between the different feather positions (10th primary, 5th primary, 1st primary, 4th secondary, 9th secondary). If significant differences among data sets were found, post-hoc tests after Fisher LSD (least significant difference) were performed.
Results
Morphometry of inner vane fringes of barn owl feathers
The morphometric analysis of fringes revealed that they are formed by the most distal parts of the barbs. Hooklets of the hook radiates are absent (Fig. 4E), which cause the barb shafts to separate (Fig. 4C). Neighboring barbs are not able to interconnect any longer; hence, fringes are formed. Moreover, the radiates change their orientation slightly by bending towards the barb shaft and thus supporting the formation of fringes, which is exemplarily shown in Fig. 4E. All barn owl remiges are fringed at the edge of the inner vane.
Fig. 4.
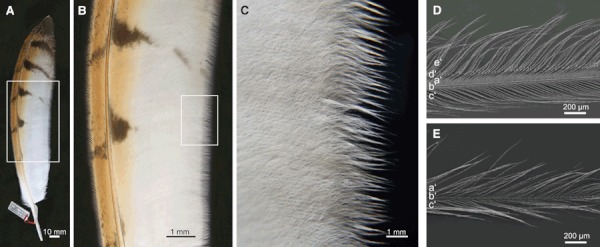
Example inner vane fringes of one 10th primary of the barn owl in different magnification: (A, B) dorsal; (C) ventral. Note also the serrated leading edge of the outer vane and the velvety dorsal surface of the feather. (D) SEM picture of an example barb of the inner vane (ventral). (a′) Hook radiates (anterior barbules); (b′) barb shaft; (c′) bow radiates (posterior barbules); (d′) hooklets (hamuli); (e′) pennula. (E) SEM picture of an example fringe (ventral). (a′) Hook radiates; (b′) barb shaft; (c′) bow radiates. Note the absence of hooklets.
In all feathers investigated, the fringe length decreases from the feather’s base towards the tip, except for the 10th primary. Differences between the residual feathers are quite small and not statistically significant. A typical fringe is 2–2.4 mm long in central feather regions. Shortest fringes (below 1 mm) are measured at the tip of the feather. In basal regions, the difference of fringe lengths between the feathers is more distinct due to the transition of the basal downs into the fringed structure. Here, longest fringes are measured to be 4.46 mm (Table 1). Between the 5th primaries, the 1st primaries, the 4th secondaries and the 9th secondaries, no statistical significant difference is found when comparing the length of the fringes (anova: df = 3, P = 0.91). By contrast, the 10th primaries have significantly longer fringes compared with the residual feathers (anova: df = 4, P = 0.0058; Table 1). Furthermore, the length of the fringes is differently distributed along the vane of the 10th primaries. While the longest fringes (4.16 mm) are found in the central and distal regions of the feather, the shortest fringes are located at the feather’s base (Table 1).
Table 1.
Length of fringes (mm) along the inner vane (0% = base) of five different remiges of the barn owl.
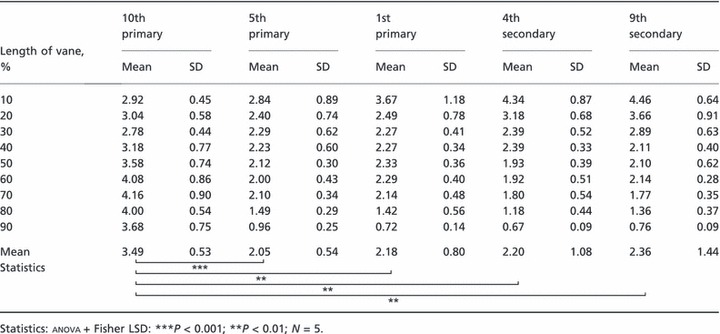
The density of fringes (fringes per mm) is more or less similar in all feathers investigated when comparing the relevant sampling areas. No statistical differences are found when comparing the density of fringes between the different feathers (anova: df = 4, P = 0.95). The number of fringes per mm decreases almost linearly from the feather’s base towards the tip. While five fringes per mm on average are counted in basal regions of each feather, approximately two fringes per mm are found in distal regions of each feather (Table 2).
Table 2.
Density of fringes (fringes per mm) along the inner vane (0% = base) of five different remiges of the barn owl.
| 10th primary | 5th primary | 1st primary | 4th secondary | 9th secondary | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Length of vane, % | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| 0–10 | 5.13 | 0.30 | 5.17 | 0.30 | 5.36 | 0.14 | 4.86 | 0.56 | 5.67 | 0.05 |
| 10–20 | 4.18 | 0.19 | 3.80 | 0.08 | 3.86 | 0.14 | 3.89 | 0.11 | 4.14 | 0.07 |
| 20–30 | 3.81 | 0.03 | 3.51 | 0.05 | 3.52 | 0.06 | 3.84 | 0.37 | 3.52 | 0.09 |
| 30–40 | 3.62 | 0.13 | 3.36 | 0.04 | 3.25 | 0.08 | 3.34 | 0.08 | 3.18 | 0.02 |
| 40–50 | 3.41 | 0.10 | 3.18 | 0.02 | 2.99 | 0.03 | 2.94 | 0.04 | 2.92 | 0.01 |
| 50–60 | 3.19 | 0.11 | 2.91 | 0.02 | 2.78 | 0.08 | 2.64 | 0.06 | 2.65 | 0.02 |
| 60–70 | 2.98 | 0.10 | 2.68 | 0.02 | 2.51 | 0.03 | 2.47 | 0.04 | 2.46 | 0.04 |
| 70–80 | 2.65 | 0.09 | 2.44 | 0.03 | 2.39 | 0.03 | 2.29 | 0.06 | 2.22 | 0.04 |
| 80–90 | 2.46 | 0.07 | 2.31 | 0.02 | 2.32 | 0.08 | 2.25 | 0.05 | 1.98 | 0.13 |
| 90–100 | 2.05 | 0.16 | 2.17 | 0.04 | 1.93 | 0.02 | 2.05 | 0.42 | 1.50 | 0.06 |
| Mean | 3.35 | 0.90 | 3.15 | 0.89 | 3.09 | 0.93 | 3.06 | 0.79 | 3.02 | 1.20 |
The mean values of the relevant positions are not statistically different between the feathers (anova, P = 0.95); N = 5.
Orientation of the fringes at the wing
A bird wing is highly dynamic and complex. The bird is able to change wing planform, camber and feather orientation by adjusting the joints of the forelimb and thus the position of the feathers relative to each other. Consequently, the edges of the inner vanes and, thus, the fringes are not fixed in place during flight. For this study, fixed and spread out barn owl wings are analyzed with respect to fringe orientation. However, note that the wings do not fit the exact geometry of a freely-flying bird. Therefore, we do not present exact orientation angles, but the orientation trend within the wing. As the feathers are dead material, only the positions of the feathers can be changed but not the orientation of the fringes within the feather. In a fully extended wing of an owl, feathers have an imbricate arrangement and large areas of the remiges overlap. Thereby, barbs of inner vanes of two neighboring feathers are more or less aligned due to the parallel orientation of the barb shafts within the vane. Because fringes are formed by barb endings, they are aligned with the barb shafts of the neighboring feathers as well.
In principle, two different orientations of the fringes are found within the wing. First, fringes at those edges that are oriented perpendicular to flight direction and thus to the air flow are arranged parallel to the flight direction. These regions are found at the trailing edge of the wing, the trailing edge of the 10th primary, and less pronounced the trailing edges of the 9th and 8th primaries. Second, fringes that are located within the wing area are arranged almost parallel to the neighboring barb shafts of adjacent feathers. This orientation is found in all remiges, except at their outermost tips (last 10% of vane). Figure 5 shows the orientation of fringes at an example wing in principle (ventral view).
Fig. 5.
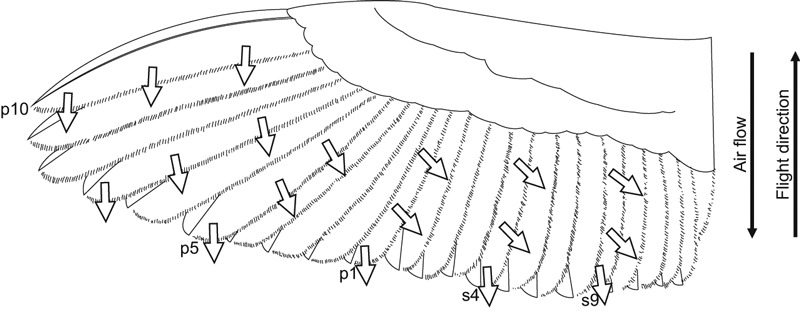
Scheme of a ventral barn owl wing. The arrows indicate the orientation of fringes in principle.
High-speed video analysis
High-speed video analysis was used to reveal whether the fringes interact with the subjacent barb shafts. For this a set of secondaries of a fixed wing were subjected to a flow field and a small area of overlapping vanes was filmed. Under quiescent air conditions, the fringes are approximately 500 μm apart from the subjacent feather (Fig. 6B). This distance vanishes immediately, and both feathers approach each other after the air flow hits the wing (Fig. 6C). Moreover, the fringes merge into the grooves formed by the parallel oriented barbs shafts of the adjacent feather vane. The fringes bend slightly to fit between the barb shafts. Because the density of the fringes and the barb shafts correlates and no significant differences between feather positions are found (Table 2), the number of fringes and grooves match perfectly. This interlocking of the feather vanes seems stable and immune to lateral movements to some degree. When the airfoil is bending due to the air flow, single feathers do not change their orientation relative to each other. The only movement of the feathers that can be observed is a sliding of the barbs in the groves of the neighboring vane, but the fringes hardly switch between grooves.
Fig. 6.
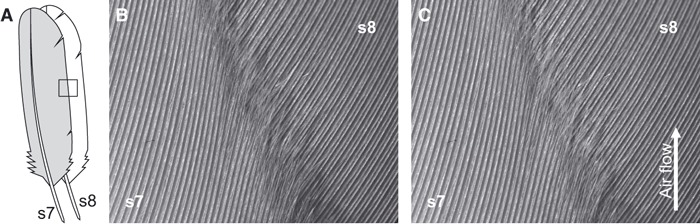
Selected images of a high-speed film of two adjacent secondaries (A: s7, s8) under static conditions (B) and subjected to a flow field (C) (ventral view). (B) Under static conditions, the fringes of s7 and the inner vane of s8 have a certain distance indicated by the shadow. The fringes are bent slightly but do not align with the adjacent barbs. (C) Both vanes approach when subjected to a flow field. Thereby, fringes merge tightly into the grooves formed by the adjacent barb shafts.
Comparison to other species of birds
Figure 7 shows the comparison of inner vanes of five different species of birds. While inner vanes of barn owl feathers are fringed and already close to the neighboring vane under quiescent air, vanes of all other feathers have sharp edges and show a certain distance to the neighboring feather indicated by a shadow (Fig. 7B–E). Although the distance between the feathers may decrease during flight, the edges will still remain sharp. During handling and preparation of the feathers we additionally observed that barn owl feathers were resistant to lateral movements of neighboring vanes. All feathers of the Harris hawk, common buzzard, pigeon and mute swan can easily be moved relative to each other.
Fig. 7.
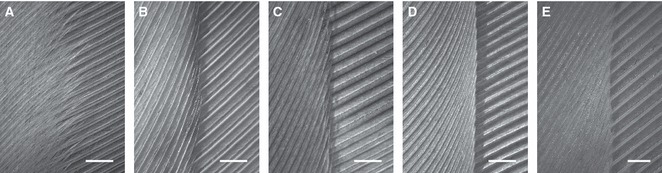
Comparison of inner vanes of neighboring secondaries (s7 and s8) from lower side. (A) Barn owl (Tyto alba); (B) common buzzard (Buteo buteo); (C) Harris hawk (Parabuteo unicinctus); (D) pigeon (Columba livia); (E) mute swan (Cygnus olor). Scale bars: 1 mm.
Discussion
Feathers are among the most complex outgrowth of the epidermis of vertebrates. In many species, including barn owls, the largest area of the wing is made up of remiges (Bachmann et al. 2007, 2011). Their geometry and arrangement are responsible for the wing planform and thus the aerodynamic performance. Furthermore, the edge and surface characteristics of the wing influence the flight performance (Rüppell, 1980). In owl species, many adaptations have been reported that affect the edges and the surface of the wing. The leading edge of the distal wing is serrated and the surface has a velvety texture. These structures presumably control the boundary layer of the wing passively to prevent flow separation in critical flight maneuvers (Graham, 1934; Neuhaus et al. 1973; Lilley, 1998; Klän et al. 2008; Ito, 2009). The function of fringes at the trailing edge of owl remiges is not yet well understood. All functional explanations are based on hypotheses or technical experiments neglecting the natural model. In the available literature, fringes are exclusively discussed to reduce fluttering of the feathers and thus turbulences behind the wing. The two different flow velocities of the upper and lower wing surface have to merge behind the wing. Lockard & Lilley (2004) stated that fringes serve as a pressure-release mechanism to interrupt scattering phenomena of turbulent eddies. The transmissivity of the wing surface might be increased by the porous structure that brings the two air flows in early contact (Müller & Patone, 1998). Furthermore, sharp trailing edges of wings, fluttering feathers (van Casteren et al. 2010; Clark et al. 2011) and flat plates (Moreau et al. 2011) produce a perceptible noise even at low Reynolds numbers (1 × 105). Fringes at the trailing edge of a wing effectively reduce such noise (Herr, 2006). As the owl relies on silent flight to be successful in hunting prey, it seems to be obvious that fringes have evolved to adapt the trailing edge of the wing. While fringes can be found on flight feathers of almost all species of owls, they vary in size depending on the species (Sick, 1937). Only some owls that hunt for fish such as Bubo peli or Bubo flavipes do not have fringes or serrations (Graham, 1934). Their feathers look similar to those of the other species of birds introduced in this paper. Until now it is still unclear why these birds do not develop the specifics and if they never did or the feature got lost secondarily in the course of evolution. Some hypotheses exist, for instance that these owls do not rely on a silent flight as the prey (fish) cannot hear the approaching owl or they simply suffer from getting wet, which would be intensified by the odd feather structures.
But, why are the central parts of the feathers fringed as well? With this paper, we provide one possible answer without any claim to completeness. During fluttering – for instance during striking – the remiges separate on the upstroke and air moves through the wing (Rüppell, 1980). Now, each remex serves as a single airfoil that has its own trailing edge where noise needs to be reduced. However, during normal cruise flight and during gliding flight, the remiges hardly separate in barn owls. The pressure gradient and the air transmissivity of the vanes prevent separation of the feathers (Müller & Patone, 1998). The feathers are rather pressed together by the air flow. Additionally, our experiments revealed that the fringes are able to merge into the neighboring feather vane. The orientation of the fringes and the barb shafts, and the density of both, support this merging effect. Furthermore, the fringes might be sucked into the grooves of the barb shafts, due to the lower pressure on the upper wing surface and the air transmissivity of the vanes (Müller & Patone, 1998). By this merging effect, sharp edges within the wing surface could be avoided; hence, noisy edges are absent. Additionally, the flexible feather vanes are presumably mechanically stabilized by the connection of two vanes. In this context it makes sense that fringes in central regions of the feather are longer than at the tip. The fringes need to fit into the grooves. If the fringes are too short, then they cannot merge into the grooved texture of the neighboring feather vane.
Interestingly, the length of fringes and the length distribution along the inner vane is different in the outermost flight feather of the wing, the 10th primary. Additionally, the trailing edge of the 10th primary is differently oriented in comparison to the other remiges. While the 10th primary is almost perpendicularly oriented to the air flow (in the fully extended wing), the remiges align more and more with the air-flow direction the further basal the feather is positioned on the wing. The 10th primary is unique in many aspects. As the outermost feather, it has to deal with strong aerodynamic forces that act on the vanes and on the rachis (Rüppell, 1980). Approximately 50% of the leading edge of the wing is formed by the outer vane of the 10th primary. Here, barb endings separate and bend upwards to form a serrated edge (Bachmann & Wagner, 2011). This structure most likely prevents flow separation in critical flight maneuvers (Neuhaus et al. 1973). The tip of the 10th primary forms the tip of the wing. Consequently, this feather is subjected to frequently changing conditions (Rüppell, 1980). As this feather is not embedded into a set of remiges on both sides, it seems quite reasonable that the fringes differ in length in comparison to the other remiges. Due to the special position within the wing, the 10th primary is prone to separate and noise is likely produced without fringes. Hence, fringes need to be long to efficiently reduce noise when separated or merge the inner vane to the 9th primary during normal cruise flight.
The orientation, length and density of barn owl fringes within the wing surface support the current view of fringes as a flow turbulence-reducing application; however, the data further suggest an additional and formerly unrevealed function. The merging of neighboring feather vanes during flight effectively creates a smooth wing surface without any sharp edges that may produce noise. Moreover, the connection of single feathers may stabilize the wing surface mechanically. Due to only few interconnections between single barbs, the feather vanes of barn owl feathers appear extremely flexible and pliant (Bachmann et al. 2007). The connection of several feathers within the wing may reinforce the stability of the wing without decreasing the flexibility of its single components, and may effectively reduce friction and fluttering between the feathers. Owls benefit from the smooth, lightweight and securely connected wing surface by a good flight performance and high maneuverability. This way, owls can sneak up on their prey without making a sound and if needed react rapidly on an escaping animal, which increases the hunting success and thus the fitness of the species.
Acknowledgments
The authors are in debt to Lars Opfer for his competent help with the high-speed camera system. We thank Martin Becker, Ulrich Goldbach and Olaf Sander for providing feather material. Financial support was provided by the German Research Foundation (WA606/15-2 and BA4191/1-1 within SPP1207).
References
- Bachmann T, Wagner H. The three-dimensional shape of serrations at barn owl wings: towards a typical natural serration as a role model for biomimetic applications. J Anat. 2011;219:192–202. doi: 10.1111/j.1469-7580.2011.01384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann T, Klän S, Baumgartner W, et al. Morphometric characterisation of wing feathers of the barn owl (Tyto alba pratincola) and the pigeon (Columba livia. Front Zool. 2007;4:23. doi: 10.1186/1742-9994-4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann T, Mühlenbruch G, Wagner H. The barn owl wing: an inspiration for silent flight in the aviation industry? Proc SPIE. 2011;7975:79750N. doi 10.1117/12.882703. [Google Scholar]
- van Casteren A, Codd JR, Gardiner JD, et al. Sonation in the male common snipe (Capella gallinago gallinago L.) is achieved by a flag-like fluttering of their tail feathers and consequent vortex shedding. J Exp Biol. 2010;213:1602–1608. doi: 10.1242/jeb.034207. [DOI] [PubMed] [Google Scholar]
- Clark CJ, Elias DO, Prum RO. Aeroelastic flutter produces hummingbird feather songs. Science. 2011;222:1430–1433. doi: 10.1126/science.1205222. [DOI] [PubMed] [Google Scholar]
- Graham R. The silent flight of owls. J Roy Aero Soc. 1934;38:837–843. [Google Scholar]
- Herr M. Experimental study on noise reduction through trailing edge brushes. 2006. pp. 365–372. Notes on numerical fluid mechanics and multidisciplinary design. 92/2006.
- Ito S. Aerodynamic influence of leading-edge serrations on an airfoil in a low Reynolds number. J Biomech Sci Eng. 2009;4/1:117–123. [Google Scholar]
- Klän S, Bachmann T, Klaas M, et al. Experimental analysis of the flow field over a novel owl based airfoil. Exp Fluids. 2008;46:975–989. [Google Scholar]
- Konishi M. How the owl tracks its prey. Am Sci. 1973;61:414–424. [Google Scholar]
- Kroeger R, Gruschka H, Helvey T. Low speed aerodynamics for ultraquiet flight. AFFDL. 1971;71–75:1–55. [Google Scholar]
- Lilley GM. A study of the silent flight of the owl. AIAA Paper. 1998;9:8–2340. [Google Scholar]
- Lockard DP, Lilley GM. The airframe noise reduction challenge. 2004. NASA/TM-2004-213013.
- Mebs T, Scherzinger W. Die Eulen Europas. Stuttgart: Frankh-Kosmos; 2000. [Google Scholar]
- Moreau DJ, Brooks LA, Doolan CJ. On the aeroacoustic tonal noise generation mechanism of a sharp-edged plate. JASA Expr Lett. 2011;129:EL154–EL160. doi: 10.1121/1.3565472. doi 10.1121/1.3565472. [DOI] [PubMed] [Google Scholar]
- Müller W, Patone G. Air transmissivity of feathers. J Exp Biol. 1998;201:2591–2599. doi: 10.1242/jeb.201.18.2591. [DOI] [PubMed] [Google Scholar]
- Neuhaus W, Bretting H, Schweizer B. Morphologische und funktionelle Untersuchungen über den lautlosen Flug der Eule (Strix aluco) im Vergleich zum Flug der Ente (Anas platyrhynchos. Biol Zentr. 1973;92:495–512. [Google Scholar]
- Rüppell G. Vogelflug. Hamburg: Rowohlt Taschenbuch; 1980. [Google Scholar]
- Sarradj E, Fritzsche C, Geyer T. Silent owl flight: bird flyover noise measurements; In Proceedings of 16th AIAA/CEAS Aeroacoustics Conference, Stockholm, 7–9 June 2010; 2010. pp. 1–17. [Google Scholar]
- Sick M. Morphologisch-funktionelle Untersuchungen über die Feinstruktur der Vogelfeder. J Ornith. 1937;85:206–372. [Google Scholar]
- Stettenheim RR. The integumentary morphology of modern birds – an overview. Am Zool. 2000;40:461–477. [Google Scholar]


