Abstract
A total histological grade does not necessarily distinguish between different manifestations of cartilage damage or degeneration. An accurate and reliable histological assessment method is required to separate normal and pathological tissue within a joint during treatment of degenerative joint conditions and to sub-classify the latter in meaningful ways. The Modified Mankin method may be adaptable for this purpose. We investigated how much detail may be lost by assigning one composite score/grade to represent different degenerative components of the osteoarthritic condition. We used four ovine injury models (sham surgery, anterior cruciate ligament/medial collateral ligament instability, simulated anatomic anterior cruciate ligament reconstruction and meniscal removal) to induce different degrees and potentially ‘types’ (mechanisms) of osteoarthritis. Articular cartilage was systematically harvested, prepared for histological examination and graded in a blinded fashion using a Modified Mankin grading method. Results showed that the possible permutations of cartilage damage were significant and far more varied than the current intended use that histological grading systems allow. Of 1352 cartilage specimens graded, 234 different manifestations of potential histological damage were observed across 23 potential individual grades of the Modified Mankin grading method. The results presented here show that current composite histological grading may contain additional information that could potentially discern different stages or mechanisms of cartilage damage and degeneration in a sheep model. This approach may be applicable to other grading systems.
Keywords: cartilage, grading, histology, Modified Mankin, osteoarthritis, ovine
Introduction
Cartilage degeneration may develop in response to structural and mechanical insults to ligaments and the joint capsule (Bobinac et al. 2003; Lahm et al. 2004; Yeow et al. 2009), and/or biochemical mechanisms within the joint (Heinegard et al. 2003). The degeneration is influenced by age (Meachim, 1982), weight (Madsen et al. 1997), environmental (Dykgraaf et al. 2008) and hereditary (Felson et al. 1998) factors. Consequently, osteoarthritis (OA) and its related disorders are considered a diverse group of degenerative conditions, a factor that is often overlooked when cartilage damage and degeneration is quantified histologically. An accurate and reliable assessment of cartilage degeneration is a prerequisite to the identification of stages and mechanisms of damage and the resulting treatment plan. Histological analysis provides detailed internal structural and physiological information of cartilage deterioration, serving as a gold standard for the validation of other potential assessment tools.
Histological grading methods rationalize the severity of OA by reducing disparate histological information to quantifiable, albeit subjective, discriminators. These grading methods broadly fall under two categories, namely, an assessment system based on sequential stages of increasing OA severity (Collins & Mcelligott, 1960; Pritzker et al. 2006), or based on the sum of independent indicators of OA severity (Mankin et al. 1971; Yoshimi et al. 1994; Little et al. 1997; Leroux et al. 2001). These grading methods allow for quantification of different forms of OA by assigning a total grade; however, this process does not reflect the contribution of different injury, repair, degenerative and OA processes of the conditions. For example, a total score does not indicate if the cartilage damage is primarily surface irregularities or fibrillation; if it is cellular; or related to proteoglycan loss. Therefore, cartilage sections awarded the same total histological grade may represent the development of two very different degenerative conditions.
In this paper we present an analysis of Mankin scoring systems in a sheep model by accounting for individual components of the total score, especially when different conditions produce the same total Modified Mankin score. It is envisioned that this extension could provide a more rigorous picture that could improve the accuracy and reliability of classifying the stages and mechanisms of OA. A Modified Mankin grading method was chosen for its comprehensive deconstruction of cartilage damage. We used an ovine injury model for this analysis due to some anatomical and biomechanical similarities to the human knee (Bellenger & Pickles, 1993; Armstrong et al. 1995; Burger et al. 2007), and the ability to induce post-traumatic damage by surgical removal of a meniscus or by transecting the anterior cruciate ligament. These injuries mimic those occurring in physically active people that are known to lead to post-traumatic cartilage damage and degeneration.
Methodology
The stifle joints of 45 skeletally mature female Suffolk-cross sheep were prepared for histological examination and grading. To characterize differences in degeneration, these 45 stifle joints had been subjected to either combined anterior cruciate/medial collateral ligament transection (n = 12), anatomic anterior cruciate ligament femoral core (n = 3), twist loose and twist tight anterior cruciate ligament femoral core (n = 4), lateral meniscectomy (n = 6), sham (n = 6) or non-operated age-matched controls (n = 14), as previously described (Tapper et al. 2008; Beveridge et al. 2011; Heard et al. 2011). All surgical procedures were reviewed and approved by the University of Calgary Animal Care Committee and complied with the guidelines of the Canadian Council on Animal Care.
Histological preparation
Cartilage biopsies were removed from the subchondral bone in 15 anatomically defined locations within the stifle joint, including the patella, femoral head and tibial plateau from the left and right knee joints to maximize the observable variation in cartilage architecture and degeneration. These locations included (Fig. 1): anterior patella; posterior patella; anterior femoral grove; central femoral groove; posterior femoral groove; anterior lateral femoral condyle; posterior lateral femoral condyle; anterior medial femoral condyle; posterior medial femoral condyle; anterior lateral tibial plateau; posterior lateral tibial plateau; central lateral tibial plateau; anterior medial tibial plateau; posterior medial tibial plateau; central medial tibial plateau. Cartilage sections were immediately embedded in optimal cutting temperature medium, and frozen at −70 °C for sectioning. Tissue blocks were cryosectioned at 7 μm thickness and mounted on slides, before drying in a 40 °C oven for up to 1 week.
Fig. 1.
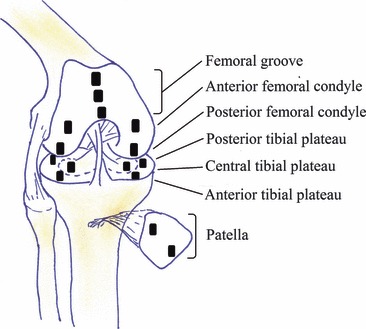
Standardized regions selected for histological assessment.
Prior to histological staining, sections were hydrated through graded ethanol (100%, 95%, 70%) and distilled water for 1 min each. Sections were then stained in trichrome stain, which includes three colour components for the purpose of staining the cells (black), proteoglycans (red), and remaining matrix absent of proteoglycans (blue) different colours. Slides were stained in Haematoxylin for 20 min, allowed to blue in running water for 15 min, counterstained in fast green for 5 min, and rinsed in 1% acetic acid for 1 min. Slides were quickly rinsed in distilled water, followed by proteoglycan staining in Safranin-O for 2 min. Finally, slides were dehydrated in 6 × 1 s dips of each graded ethanol (70%, 95%, 100%) before clearing in three changes of xylene for 5 min each, and then mounted in Permount.
Tissue grading
An established Modified Mankin grading system (Little et al. 1997; Oakley et al. 2004) provided histological grading of degeneration for Trichrome-stained cartilage sections. This method was chosen to enable the most thorough deconstruction of histological cartilage damage. The scores for each of the parameters used, namely: surface integrity; cellularity (change in cell number); cell cloning; and Safranin-O staining intensity (Safranin-O binds stoichiometrically to chondroitin 6-sulphate and keratan sulphate in cartilage tissue sections), are presented in Table 1. Scoring of all individual parameters incorporated the entire depth of cartilage: superficial zone; midzone; and deep zone.
Table 1.
Modified Mankin grading system.
| Category | Subcategory | Score |
|---|---|---|
| Surface integrity | Normal | 0 |
| Slight surface irregularities | 1 | |
| Moderate surface irregularities | 2 | |
| Severe surface irregularities | 3 | |
| Clefts to transitional zone | 4 | |
| Clefts to radial zone | 5 | |
| Clefts to calcified zone | 6 | |
| Fibrillation and/or loss to transitional zone | 7 | |
| Fibrillation and/or loss to radial zone | 8 | |
| Fibrillation and/or loss to calcified zone | 9 | |
| Fibrillation and/or loss to subchondral bone | 10 | |
| Cellularity | Normal | 0 |
| Increase or slight decrease | 1 | |
| Moderate decrease | 2 | |
| Severe decrease | 3 | |
| No cells | 4 | |
| Cell cloning | Normal | 0 |
| Several doublets | 1 | |
| Many doublets | 2 | |
| Doublets and triplets | 3 | |
| Multiple cell nests | 4 | |
| Safranin-O staining | Normal | 0 |
| Slight reduction | 1 | |
| Reduction in radial layer | 2 | |
| Reduction in interterritorial layer | 3 | |
| Only present in pericellular matrix | 4 | |
| No staining | 5 | |
| Total | 0–23 |
Statistical analysis
Cartilage samples were allocated into one of three compartments: patella and femoral groove; lateral condyle and tibial plateau; medial condyle and tibial plateau. Each compartment was sorted to return the sample location with the highest individual Modified Mankin scores for each sheep, in each treatment group. The data were then analysed using the Kruskal–Wallis test with Dunn’s Multiple Comparison test. Significance was accepted at P < 0.05.
Results and analysis
Histology
Histological grading was carried out on 1352 cartilage specimens taken from standardized regions across the patella, femoral groove, femoral condyles and tibial plateau. These were then graded with a Modified Mankin system on a scale from 0 (normal) to 23 (severely degenerated). Representative results for both the normal and degenerated samples are presented in Fig. 2. Normal cartilage with no degradation (Fig. 2A) accounted for 7.8% (n = 106) of the total. Cartilage with proteoglycan loss alone (Fig. 2B,C) accounted for 24.6% (n = 333), ranging from slight reduction in Safranin-O stain, through to complete loss of Safranin-O staining. In total, 84.0% (n = 1136) of all samples showed signs of Safranin-O stain loss, with Grade 3 Safranin-O stain (i.e. those with proteoglycan reduction in the interterritorial layers) occurring most frequently (31.4% of the subset, n = 357; Fig. 2B).
Fig. 2.
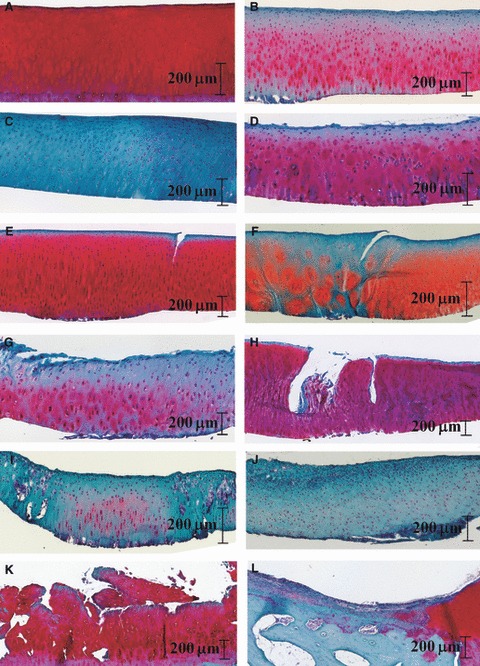
Sample of trichrome stained articular cartilage of varying levels of degeneration (A–L).
Slight to severe surface irregularities were observed in conjunction with Safranin-O stain loss and occasional cellular changes (39.7%, n = 537; Fig. 2D). Clefts were identified in cartilage samples containing only minor Safranin-O stain loss, with no other form of damage (6.6%, n = 89; Fig. 2E), or with additional damage types (6.0%, n = 81; Fig. 2F). Artefactual clefts resulting from tissue preparation were distinguishable from in vivo cartilage damage by a clean artefact cut of the scalpel blade compared with an irregular or torn appearance. Some specimens displayed fibrillation that appeared to vary in accordance with increasing severity of surface irregularities (Fig. 2G), or as a progression of cleft formation (Fig. 2H), both associated with varying degrees of additional types of damage (100% of the subset, n = 52). Cellular changes were observed with no other forms of damage (1.5%, n = 20), or with minor surface irregularities and loss of Safranin-O staining (22.8%, n = 308; Fig. 2I,J), through to major surface disruption (5.5%, n = 74; Fig. 2K), cleft formation (4.4%, n = 59; Fig. 2F) and fibrillation with loss of tissue (3.2%, n = 43; Fig. 2L).
Influence and relevance of individual parametric scores
Examination of individual parametric scores showed that all grading components of the matrix, including surface integrity, cell configuration and proteoglycan loss (Safranin-O staining intensity), may depreciate without concomitant effect from the other components (Fig. 3). Individual parametric degradation without concomitant effect from other parameters accounted for 30.9% (n = 418) of all samples. Of these, surface integrity accounted for 16.0% (n = 67) of single parametric degradation, with 10.5% (n = 44) due to slight surface irregularities (score = 1), and the remainder due to moderate and severe surface irregularities, and cracks, possibly caused by impact blows. Similarly, changes to cellularity (cell number) and cell cloning were minor (4.3%, n = 18), with no damage to other matrix scoring parameters. Loss of Safranin-O staining was most common, attributable to 79.7% (n = 333) of single parametric degradation without contribution from other damage indicators. Of these, the most commonly observed type were samples with reduction of Safranin-O stain in the interterritorial layer of the matrix (score = 3), with 28.0% (n = 117) of single parametric degradation specimens showing this form of degeneration.
Fig. 3.
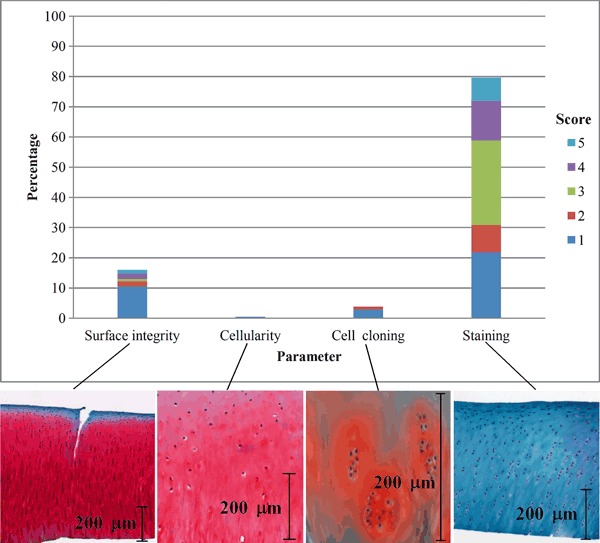
Occurrence of single parametric degeneration and their scores.
Critical appraisal of the combined parametric scores
The individual parametric scores of the Modified Mankin grading system were combined (concatenated) to form a sequence of numbers that allowed relative contributions by individual and combined components, and comparison between the total scores for each sample. Of the 1980 possible permutations of the combined four major signs of OA, 234 different combinations were observed.
The large and varied distributions of the degenerative component/parameters contributing to the total Modified Mankin grade are illustrated in Fig. 4. This is especially pronounced at the lower values of the total Modified Mankin grading scores where the grading is usually dominated by surface integrity and Safranin-O staining scores only; both of which exhibited inconsistencies and significant variability.
Fig. 4.
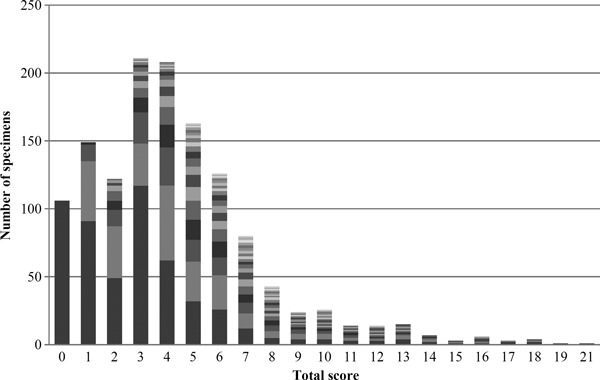
Variability of individual parametric scores within total Modified Mankin score (different shades represent different concatenations/combinations of individual scores making up a total score).
Histological examples of cartilage samples of ‘similar severity’ as represented by the total grading score are presented in Fig. 5. These display a large variation in their patterns of degeneration or manifestation of damage. For example, both the samples in Fig. 5E,F received a total Modified Mankin grade of 11; however, the cartilage shown in Fig. 5E suffered extensive fibrillation, earning a surface integrity score of 7, while that in Fig. 5F exhibited minor surface irregularities, with a higher cell cloning and Safranin-O staining score.
Fig. 5.
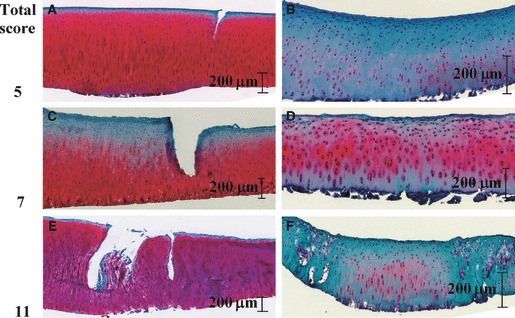
Different manifestations of cartilage damage with the same total score: samples with a total score of 5 (A and B); 7 (C and D); and 11 (E and F).
By observing the individual parametric scores, it was possible to determine that the highest total score recorded for cellular/proteoglycan-related damage was 14 (where surface integrity scored between 0 and 3 was indicative of surface irregularities only). This type of damage is similar to that depicted in Fig. 5B,D and F. The highest total score recorded for surface integrity-related damage, similar to Fig. 5A,C, was 8 (where cellular components scored 0 and proteoglycan scored between 0 and 3). Combined cellular, proteoglycan and surface integrity, for example Fig. 5E, scored between 2 and 23. These observations are depicted in Fig. 6.
Fig. 6.
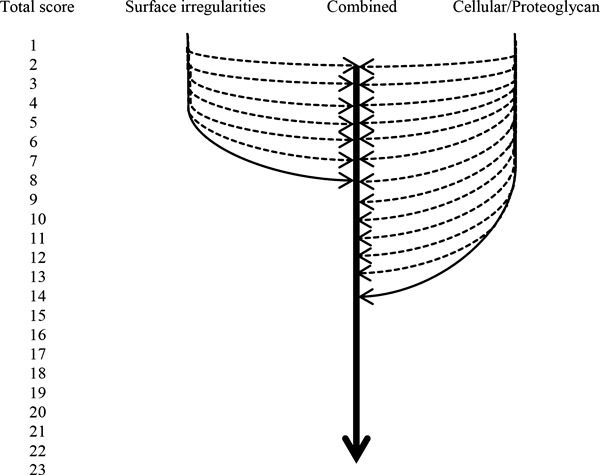
Different manifestations of cartilage degeneration and their maximum total scores.
Statistical analysis of animal model degeneration
Not surprisingly, sheep that underwent lateral meniscectomy showed significantly higher total Modified Mankin grades in the lateral compartment than other sheep (Fig. 7). When this score was broken down into the individual parametric scores, meniscectomized sheep showed much higher scoring in the lateral compartment than any other treatment group for surface integrity, cellularity and cloning, but lower scoring for proteoglycan loss. Furthermore, when the highest scores from each location in each sheep were examined, the scores for structural integrity, cellularity and cell cloning showed an increasing trend in the severity of damage with more destructive treatment. Proteoglycans, however, while showing a slight decrease in staining loss with increasing treatment severity, exhibited near the full range of scoring from 1 to 4 for four of the five treatment groups.
Fig. 7.
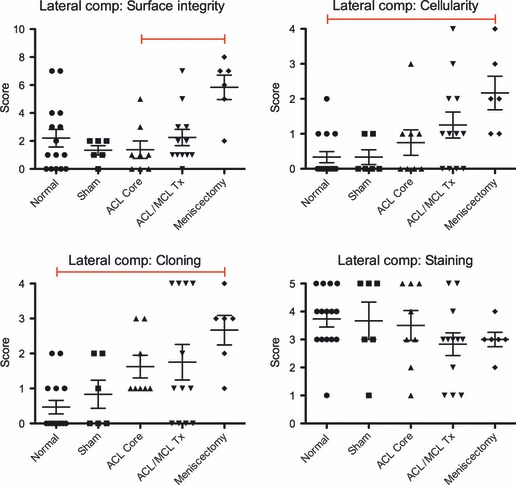
Summary of treatment group comparisons of lateral compartment grading protocols. Sample size for treatment groups: Normal (n = 15); Sham (n = 6); anterior cruciate ligament (ACL) Core (n = 8); ACL/medial collateral ligament (MCL) Tx (n = 12); and Meniscectomy (n = 6). Each score is represented by a point on the graph along with treatment group means ± SEM, red bar indicates significant difference (Kruskal–Willis test with Dunn’s multiple comparison test: significance accepted at P ≤ 0.05).
Discussion
Results of this study show that the Modified Mankin histological grading method appears to be capable of identifying different manifestations of cartilage damage and degeneration through observation of the individual parametric scores, that is, structural integrity, cellularity, cell cloning and loss in proteoglycan staining. Analysis of the individual parametric scores in the four sheep joint injury models used created a spectrum of joint damage and degeneration. The results show that a total score can indicate a level of destruction that has occurred; however, it does not necessarily reflect the contribution of different components of cartilage damage and degeneration and, more importantly, the asymmetric nature of the development of the OA condition, unless the individual parametric scores are consulted. For example, a total score of 11 represents a moderately high level of cartilage degeneration; however, consultation of the individual parametric scores indicates that this total score may represent: a specimen with deep clefts (deep damage), severe surface irregularities (surface damage) and some proteoglycan loss (matrix degeneration); or it could represent a specimen with extensive cellular changes and proteoglycan loss (damage and degeneration involving both cellular and matrix changes). This variability in the manifestation of cartilage damage was evident at every level of degeneration/total score.
The occurrence of extensive cellular/proteoglycan changes with surface irregularities only (no cracks or fibrillation) were observed to score up to a total of 14 out of 23, which, when considered as a total score alone, may be termed severe OA. However, this form of degeneration may be undetectable using current macroscopic assessment tools. Visually normal cartilage is not necessarily healthy (Heinegard et al. 2003), as is supported here. If this extended histological grading was used for validation of emerging non-destructive, real-time imaging instruments, it may be possible for surgeons to identify different treatment groups aimed at cellular therapies, assuming damage in human cartilage manifests in many different forms as it does in the ovine model. It is therefore envisioned that by extending current histological grading methods to account for individual components, a more rigorous picture of cartilage health within the joint can be obtained.
Analysis of the individual parametric scores further enables potential insight into the different manifestations of the degenerative condition resulting from different surgical treatments. The meniscectomy group displayed more severe damage to the surface integrity, cellularity and cell cloning parameters; however, exhibited lower proteoglycan loss. This supports the findings that proteoglycan levels fluctuate greatly in health and repair (Burton-Wurster et al. 1993), and likely increase in concentration in damaged cartilage. This could lead to a temporary decrease in the total score as proteoglycan levels increase until damage is too extensive and proteoglycans are irreversibly lost. This could result in a difference of 5 points in the total score, which may or may not be significant in later stage cartilage degeneration.
Further research is clearly required to validate the potential functional or clinical importance of these sub-categories, and also to assess the reproducibility of the individual parametric histological grading method, as it is based on Mankin grading, which has been shown to be less reliable than succeeding methods (Custers et al. 2007). The Modified Mankin method applied here was chosen for its extensive breakdown of the individual stages of cartilage damage and degeneration to attempt to represent all stages of the condition. However, because in this study, grading was performed by only one experienced histologist, further testing is required to assess the reproducibility and reliability of this approach across observers. Furthermore, biopsies were removed from fixed locations in the treatment groups, so lesions outside of these locations were overlooked and the significance of that is not yet known. Further testing is recommended to determine if human cartilage produces similar variability in patterns of degeneration and therefore if they would benefit from the use of individual histological parametric analysis.
The aim of this paper is to highlight the potential benefits of utilizing all of the information obtained at the time of histological grading. This may enable a more accurate and detailed validation of current and new assessment tools, and potentially improve the assessment and treatment of different manifestations of cartilage degradation in the whole joint with new potential therapeutic options for different sub-types (or stages) of OA (Fig. 8).
Fig. 8.
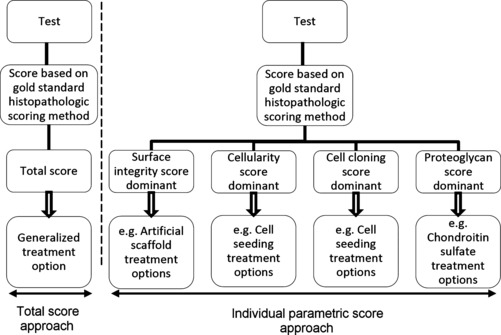
Potential treatment benefits of extending current histopathology ranking to include individual parameters containing information on the nature of degeneration.
Acknowledgments
The Queensland Government Smart Futures Scholarship awarded to Ms Moody is gratefully acknowledged. The authors also thank the Canadian Institutes of Health Research for the operating grants (MOP84436 and MOP9858) and the funding provided by the McCaig Institute for Bone & Joint Health for support of these investigations. The assistance of Ms Leona Barclay with the histology work is gratefully acknowledged. Nigel G. Shrive holds the Killam Memorial Chair, and Cy Frank is McCaig Professor in Arthritis Research.
Disclaimer
The authors of this work wish to disclose that we do not have any conflicts of interest.
References
- Armstrong SJ, Read RA, Price R. Topographical variation within the articular cartilage and subchondral bone of the normal and ovine knee joint: a histological approach. Osteoarthritis Cartilage. 1995;5:25–33. doi: 10.1016/s1063-4584(05)80035-4. [DOI] [PubMed] [Google Scholar]
- Bellenger C, Pickles D. Load bearing in the ovine medial tibial condyle: effect of minescectomy. Vet Comp Orthop Traumatol. 1993;6:100–104. [Google Scholar]
- Beveridge JE, Shrive NG, Frank CB. Minescectomy causes significant in vivo kinematic changes and mechanically induced focal chondral lesions in a sheep model. J Orthop Res. 2011;29:1397–1405. doi: 10.1002/jor.21395. [DOI] [PubMed] [Google Scholar]
- Bobinac D, Spanjol J, Zoricic S, et al. Changes in articular cartilage and subchondral bone histomorphometry in osteoarthritic knee joints in humans. Bone. 2003;32:284–290. doi: 10.1016/s8756-3282(02)00982-1. [DOI] [PubMed] [Google Scholar]
- Burger C, Mueller M, Wlodarczyk P, et al. The sheep as a knee osteoarthritis model: early cartilage changes after meniscus injury and repair. Lab Anim. 2007;41:420–431. doi: 10.1258/002367707782314265. [DOI] [PubMed] [Google Scholar]
- Burton-Wurster N, Todhunter JF, Lust G. Animal models of osteoarthritis. In: Woessner J, Howell DS, editors. Joint Cartilage Degradation: Basic and Clinical Aspects. New York: Marcel Dekker; 1993. pp. 347–384. [Google Scholar]
- Collins DH, Mcelligott TF. Sulphate uptake by chondrocytes in relation to histological changes in osteoarthritic articular cartilage. Ann Rheum Dis. 1960;19:318–330. doi: 10.1136/ard.19.4.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Custers RJH, Creemers LB, Verbout AJ, et al. Reliability, reproducibility and variability of the traditional histologic/histochemical grading system vs the new OARSI osteoarthritis cartilage histopathology assessment system. Osteoarthritis Cartilage. 2007;15:1241–1248. doi: 10.1016/j.joca.2007.04.017. [DOI] [PubMed] [Google Scholar]
- Dykgraaf S, Firth EC, Rogers CW, et al. Effects of exercise on chondrocyte viability and subchondral bone sclerosis in the distal third metacarpal and metatarsal bones of young horses. Vet J. 2008;178:53–61. doi: 10.1016/j.tvjl.2007.08.016. [DOI] [PubMed] [Google Scholar]
- Felson DT, Couropmitree NN, Chaisson CE, et al. Evidence for a mendelian gene in a segregation analysis of generalized radiographic osteoarthritis: the framingham study. Arthritis Rheum. 1998;41:1064–1071. doi: 10.1002/1529-0131(199806)41:6<1064::AID-ART13>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Heard BJ, Achari Y, Chung M, et al. Early joint tissue changes are highly correlated with a set of inflammatory and degradative synovial biomarkers after anatomic ACL autograft and its sham surgery in an ovine model. J Orthop Res. 2011;29:1185–1192. doi: 10.1002/jor.21404. [DOI] [PubMed] [Google Scholar]
- Heinegard D, Bayliss M, Lorenzo P. Biochemistry and metabolism of normal and osteoarthritis cartilage. In: Brandt KD, Doherty M, Lohmander LS, editors. Osteoarthritis. 2nd edn. New York: Oxford University Press; 2003. pp. 73–82. [Google Scholar]
- Lahm A, Uhl M, Erggelet C, et al. Articular cartilage degeneration after acute subchondral bone damage. Acta Orthop Scand. 2004;75:762–767. doi: 10.1080/00016470410004166. [DOI] [PubMed] [Google Scholar]
- Leroux MA, Cheung HS, Bau JL, et al. Altered mechanics and histomorphometry of canine tibial cartilage following joint immobilization. Osteoarthritis Cartilage. 2001;9:633–640. doi: 10.1053/joca.2001.0432. [DOI] [PubMed] [Google Scholar]
- Little C, Smith S, Ghosh P, et al. Histomorphological and immunohistochemical evaluation of joint changes in a model of osteoarthritis induced by lateral meniscectomy in sheep. J Rheumatol. 1997;24:2199–2209. [PubMed] [Google Scholar]
- Madsen OR, Brot C, Peterson MM, et al. Body composition and muscle strength in women scheduled for a knee or hip replacement: a comparative study of two groups of osteoarthritic women. Clin Rheumatol. 1997;16:39–44. doi: 10.1007/BF02238761. [DOI] [PubMed] [Google Scholar]
- Mankin HJ, Dorfman H, Lippiello L, et al. Biochemical and metabolic abnormalities in articular cartilage from osteoarthritis human hip. II. Correlations of morphology with biochemical and metabolic data. J Bone Joint Surg Am. 1971;53:523–537. [PubMed] [Google Scholar]
- Meachim G. Age-related degeneration of patella articular cartilage. J Anat. 1982;134:365–371. [PMC free article] [PubMed] [Google Scholar]
- Oakley SP, Lassere MN, Portek L, et al. Biomechanical, histological and macroscopic assessment of articular cartilage in a sheep model of osteoarthritis. Osteoarthritis Cartilage. 2004;12:667–679. doi: 10.1016/j.joca.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Pritzker KPH, Gay S, Jimenez SA, et al. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis Cartilage. 2006;14:13–29. doi: 10.1016/j.joca.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Tapper J, Fukushima S, Azuma H, et al. Dynamic in vivo three-dimensional (3D) kinematics of the anterior cruciate ligament/medial collateral ligament transected ovine stifle joint. J Orthop Res. 2008;26:660–672. doi: 10.1002/jor.20557. [DOI] [PubMed] [Google Scholar]
- Yeow CH, Lau SW, Lee PVS, et al. Damage and degenerative changes in menisci-covered and exposed tibial osteochondral regions after simulated landing impact compression – a porcine study. J Orthop Res. 2009;27:1100–1108. doi: 10.1002/jor.20861. [DOI] [PubMed] [Google Scholar]
- Yoshimi T, Kikuchi T, Obara T, et al. Effects of high-molecular-weight sodium hyaluronate on experimental osteoarthrosis induced by the resection of rabbit anterior cruciate ligament. Clin Orthop Relat Res. 1994;298:296–304. [PubMed] [Google Scholar]


