Abstract
Objective:
Both brain and body temperature rise after stroke but the cause of each is uncertain. We investigated the relationship between circulating markers of inflammation with brain and body temperature after stroke.
Methods:
We recruited patients with acute ischemic stroke and measured brain temperature at hospital admission and 5 days after stroke with multivoxel magnetic resonance spectroscopic imaging in normal brain and the acute ischemic lesion (defined by diffusion-weighted imaging [DWI]). We measured body temperature with digital aural thermometers 4-hourly and drew blood daily to measure interleukin-6, C-reactive protein, and fibrinogen, for 5 days after stroke.
Results:
In 44 stroke patients, the mean temperature in DWI-ischemic brain soon after admission was 38.4°C (95% confidence interval [CI] 38.2–38.6), in DWI-normal brain was 37.7°C (95% CI 37.6–37.7), and mean body temperature was 36.6°C (95% CI 36.3–37.0). Higher mean levels of interleukin-6, C-reactive protein, and fibrinogen were associated with higher temperature in DWI-normal brain at admission and 5 days, and higher overall mean body temperature, but only with higher temperature in DWI-ischemic brain on admission.
Conclusions:
Systemic inflammation after stroke is associated with elevated temperature in normal brain and the body but not with later ischemic brain temperature. Elevated brain temperature is a potential mechanism for the poorer outcome observed in stroke patients with higher levels of circulating inflammatory markers.
After ischemic stroke, the temperature in areas of brain affected by ischemia is higher than in unaffected brain and the rest of the body.1 Investigators have proposed clinical trials of therapeutic hypothermia, in patients with ischemic stroke,2 based on the observations that hypothermia more than halves the size of cerebral infarcts in animal models,3 and in stroke patients higher body temperature is associated with poorer outcome.4
The processes that determine brain temperature after human ischemic stroke are uncertain. Animal studies suggest that in health, brain temperature is a balance between heat-generating metabolic processes and cooling by cerebral blood flow,5 though in disease other mechanisms may be important. We have shown previously that there may be dissociation between metabolic activity and heat generation in ischemic brain.
A systemic response to the rise in systemic inflammatory cytokines after stroke could also increase brain temperature. Interleukin-6 (IL-6) triggers the release of other proinflammatory cytokines, and its presence is important for the generation of fever.6 Higher levels of IL-6 and acute phase proteins are associated with poorer functional outcome after stroke,7,8 and one potential mechanism for their association with poor outcome is a rise in brain temperature.
We aimed to determine the association between levels of IL-6, or downstream acute phase proteins such as C-reactive protein or fibrinogen, and changes in brain or body temperatures over the first 5 days after stroke.
METHODS
Ethical review.
We conducted the study according to the principles expressed in the Declaration of Helsinki. Scotland A Research Ethics Committee (REC Ref. No: 06/MRE00/119) approved the study. All patients or their proxies provided written informed consent for the collection of data and subsequent analysis.
Patient recruitment.
We prospectively recruited patients with ischemic stroke who presented to the emergency department of our hospital and who could undergo magnetic resonance spectroscopy (MRS) within 24 hours of symptom onset. At the patient's admission to hospital, a stroke physician measured neurologic impairment with the NIH Stroke Scale (NIHSS),9 blood pressure, and body temperature with a First Temp Genius aural digital thermometer. During the hospital admission, the patient's nurse used a First Temp Genius thermometer to measure aural temperature once every 4 hours, and at the time of the magnetic resonance (MR) brain scans. Tympanic thermometry agrees well with other methods of measuring body temperature in acute stroke patients.10 A stroke physician examined each patient during their admission for potential alternative sources of raised body temperature: sepsis, deep venous thrombosis (DVT), or surgery.
Brain imaging.
Each patient had 2 MR brain scans: 1) as soon as practicable after admission to hospital, and 2) around 5 days after stroke onset. Both scans measured brain temperature of normal and abnormal (i.e., ischemic) brain regions. We used a GE Signa Echospeed LX 1.5T (General Electric, Milwaukee, WI) MR scanner with self-shielding gradients (33 mT/m maximum) and birdcage quadrature head coil. In each patient on both occasions we performed axial T2*-weighted gradient echo imaging; fluid-attenuated inversion recovery (FLAIR) imaging; axial DWI or diffusion tensor imagingwith field of view (FOV) 240 × 240 mm, 15 axial slices of thickness 5 mm, slice gap 1 mm, acquisition matrix 128 × 128, echo time 97.4 msec, repetition time 10 s, and diffusion sensitizing gradients with scalar b values of 1,000 s/mm2 applied in 6 noncollinear directions; and multivoxel point resolved spectroscopy (PRESS)–localized proton MRS imaging (1H MRSI, FOV 320 × 320 mm, slice thickness 10 mm, acquisition matrix 24 × 24, echo time 145 msec, and repetition time 1,000 msec, 512 complex data points acquired with a sampling interval of 1 msec).
Brain temperature measurement.
MRSI technique.
We used an MR technique that has been described in detail in previous publications.1,11
Brain temperature estimation.
We calculated cerebral temperature for each voxel from the relative chemical shifts of water and NAA. Temperature-dependent changes in hydrogen bonding cause the water chemical shift to vary linearly with temperature at 0.01 ppm per °C,12,13 while the chemical shift of NAA is independent of temperature. Both chemical shifts are essentially independent of pH.14
Tissue classification.
We classified brain voxels based on the DWI appearance using an operational classification as described previously,1 into abnormal (definitely and possibly abnormal, i.e., “ischemic,” on DWI) and normal (ipsilateral and contralateral). We calculated the mean temperature values for each tissue type, blind to body temperature readings and blood marker levels.
Measurement of blood markers.
We aimed to draw blood daily for 5 days following stroke as close to the same time each day as possible. We drew blood as soon as possible after initial clinical assessment, quickly transferred it on water ice for centrifugation at 3,000 rpm for 10 minutes, and froze plasma at −80°C until analysis. We measured CRP and fibrinogen by immunonephelometry (Prospec, Dade Behring Milton Keynes, UK) and IL-6 by ELISA (R & D Systems, Oxford, UK), blind to stroke outcome. Intra-assay and interassay coefficients of variation were for CRP 4.7% and 8.3%, for fibrinogen 2.6% and 5.3%, and for IL-6 7.5% and 8.9%.
Statistical analysis.
We used Stata 11 (2009 StataCorp) for statistical analysis. We analyzed only those cases or scans where complete data were available for each analysis, and did not impute missing data. We compared normally distributed data with Student t tests and skewed data with the Wilcoxon rank sum test. We assessed correlation between variables with Spearman rank correlation coefficients. We used linear regression to measure the association between mean blood marker or mean body temperature, and marker levels at admission and gradients with brain temperature. We report the gradient, β, and its 95% confidence interval (CI), which we have interpreted as the change in brain temperature in °C per unit increase in blood marker or per °C increase in body temperature. Our primary analysis of brain temperature compared DWI-abnormal (“ischemic”) with DWI-normal brain tissue. We considered a 2-sided p < 0.05 to be statistically significant.
RESULTS
Patient characteristics.
We recruited 44 patients with acute ischemic stroke between December 2007 and March 2009. The clinical characteristics of the patients are summarized in table 1.
Table 1.
Characteristics of patients enrolled into the study
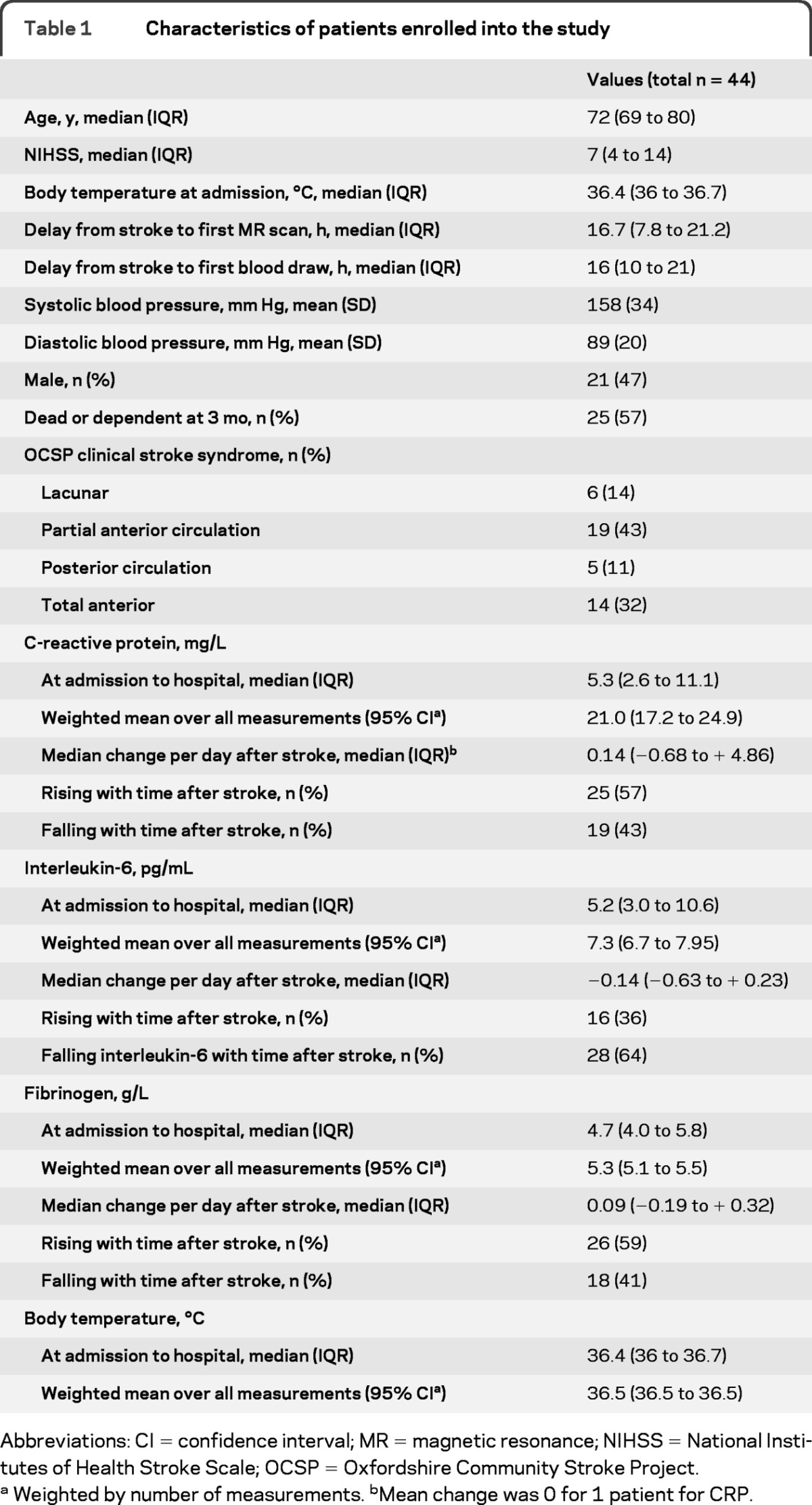
Abbreviations: CI = confidence interval; MR = magnetic resonance; NIHSS = National Institutes of Health Stroke Scale; OCSP = Oxfordshire Community Stroke Project.
Weighted by number of measurements. bMean change was 0 for 1 patient for CRP.
Inflammatory markers.
Inflammatory markers are described in table 1. Patients had a median of 4 blood samples (IQR 3 to 5) drawn over a median of 120 hours after stroke onset (interquartile range [IQR] 103 to 138 hours): 12/44 (27%) patients had blood drawn on 5 occasions. Patients with fewer than 5 blood samples had similar neurologic impairment to those with 5 blood samples (NIHSS 10 vs 12 p = 0.23), but were older (age 80 vs 73 years p = 0.03).
At each individual time point after stroke in each patient, all markers were correlated (IL-6 vs fibrinogen r = 0.38; IL-6 vs CRP r = 0.46, fibrinogen vs CRP r = 0.68; p < 0.0001 for each relationship), though mean marker levels were more strongly intercorrelated than at any individual timepoint (IL-6 vs fibrinogen r = 0.68; IL-6 vs CRP r = 0.79; fibrinogen vs CRP r = 0.72; p < 0.0001 for each relationship).
Body temperature.
Body temperature is described in table 1. Median admission aural temperature was 36.4°C (IQR 36 to 36.7°C) and at the time of the first scan was 36.6°C (IQR 36.3 to 37.0°C). During the period of observation, 4 patients had a fever (>38°C) and 16 patients had a body temperature ≥37.5°C. A potential source of temperature (sepsis, surgical procedure, nasogastric tube, or urinary catheter) was found in 9/16 (56%) of those with body temperature >37.5°C. Body temperature was observed for a median of 4.5 days (IQR 4.1 to 4.8 days), and the median number of readings per patient was 13 (IQR 11 to 17 observations). Higher mean body temperature was associated with higher mean levels of IL-6 (p = 0.003), CRP (p = 0.02), and fibrinogen (p = 0.01). Body temperature at admission was not significantly correlated with the admission levels of circulating inflammatory markers.
Brain temperature.
Brain temperature is described in table 2. At the time of the first scan (n = 44, median 16.7 hours after stroke onset, IQR 7.8 to 21.2 hours), brain temperature was higher in DWI-abnormal (“ischemic”) than in DWI-normal brain. By the time of the second scan (n = 40, median 4.8 days after stroke, IQR 4.3 to 6.4), mean brain temperature had risen in DWI-normal brain and remained elevated in DWI-abnormal brain, though temperature in DWI-abnormal brain was similar (though slightly higher) than DWI-normal brain.
Table 2.
Brain temperatures (°C)
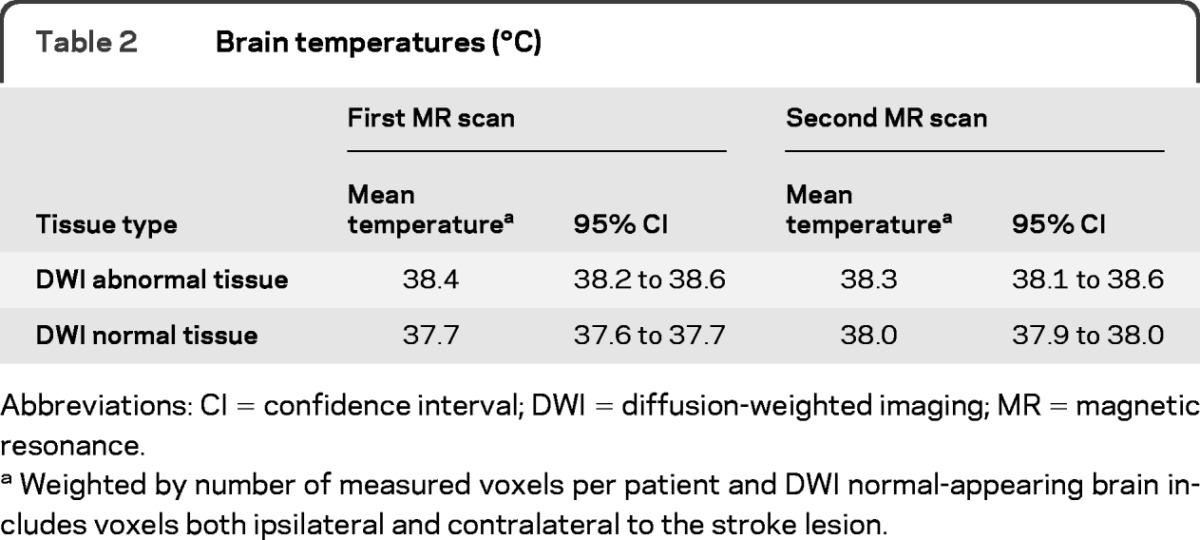
Abbreviations: CI = confidence interval; DWI = diffusion-weighted imaging; MR = magnetic resonance.
Weighted by number of measured voxels per patient and DWI normal-appearing brain includes voxels both ipsilateral and contralateral to the stroke lesion.
Relationship between brain and body temperature and inflammatory markers.
Relationship between brain and body temperature and inflammatory markers is described in table 3. Temperature in DWI-abnormal brain was not associated with IL-6 level at either first or second scan, but was associated with higher levels of CRP (overall mean p < 0.01, admission level p = 0.014) and fibrinogen (overall mean p < 0.01) but only at the time of the first scan. Higher temperature in DWI-abnormal brain was not associated with higher body temperature (admission, mean or contemporaneous) at the time of the first scan, but was associated with higher contemporaneous body temperature at the time of the second scan (figure 1).
Table 3.
Associations between brain temperature and body temperature with markers of inflammationa
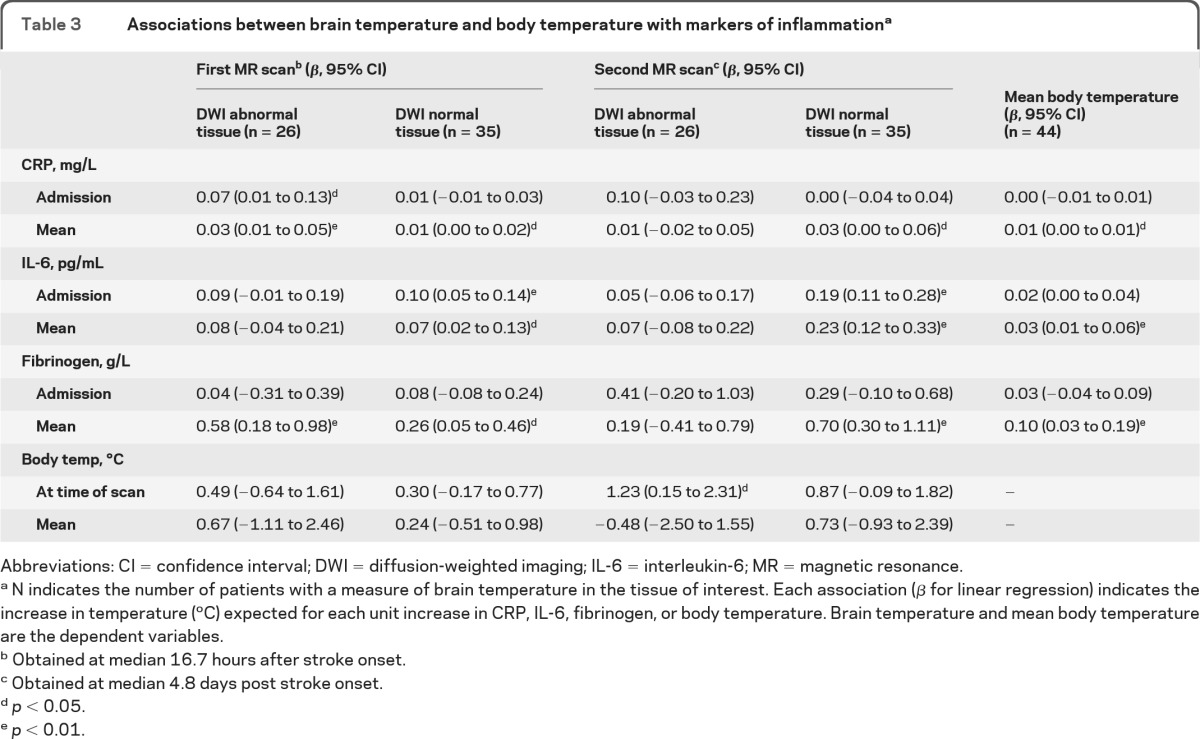
Abbreviations: CI = confidence interval; DWI = diffusion-weighted imaging; IL-6 = interleukin-6; MR = magnetic resonance.
N indicates the number of patients with a measure of brain temperature in the tissue of interest. Each association (β for linear regression) indicates the increase in temperature (°C) expected for each unit increase in CRP, IL-6, fibrinogen, or body temperature. Brain temperature and mean body temperature are the dependent variables.
Obtained at median 16.7 hours after stroke onset.
Obtained at median 4.8 days post stroke onset.
p < 0.05.
p < 0.01.
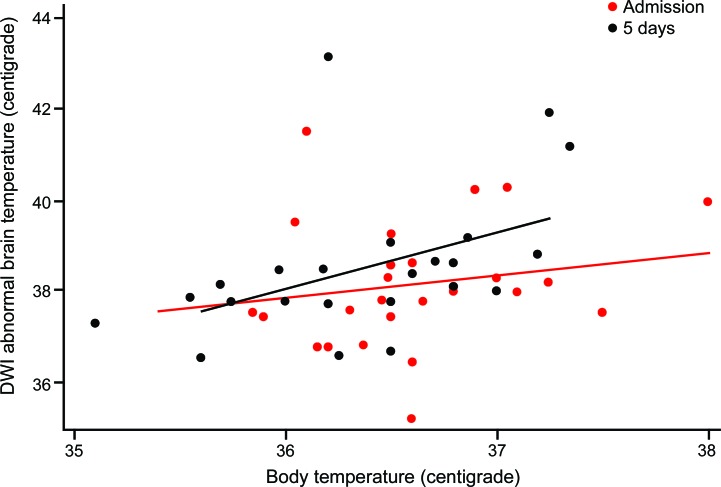
Figure 1. Temperature in diffusion-weighted imaging (DWI) abnormal (“ischemic”) brain is more strongly associated with body temperature later after stroke.
A 1°C higher body temperature is associated with a 0.5°C (95% confidence interval [CI] −0.6 to 1.6) higher temperature in DWI-abnormal brain at 16 hours (red line and dots), and a 1.2°C (95% CI 0.2 to 2.3) higher temperature in DWI-abnormal brain at 5 days (black line and dots).
Higher temperature in DWI-normal brain at the time of both the first and the second scans was associated with higher mean and admission levels of IL-6, and mean levels of CRP and fibrinogen (figure 2). Neither mean body temperature nor body temperature at the time of scanning was associated with temperature in DWI-normal brain.
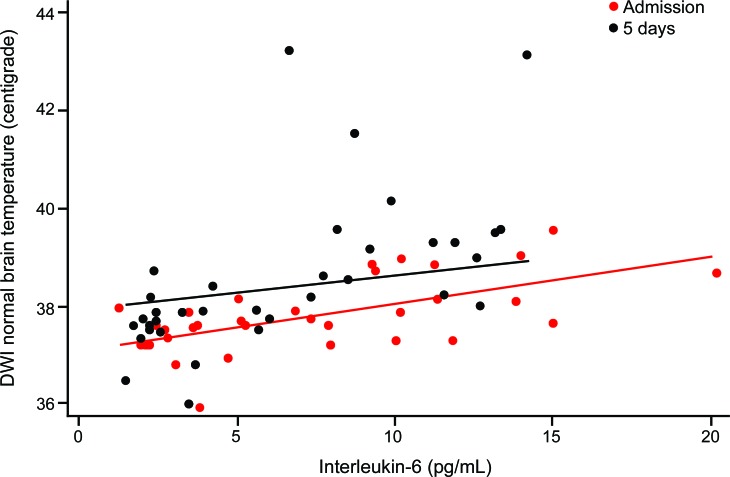
Figure 2. Temperature of diffusion-weighted imaging (DWI) normal brain is associated with higher blood levels of interleukin-6.
A 1 pg/mL higher overall mean level of interleukin-6 was associated with a 0.1°C (0 to 0.1) higher temperature in DWI-normal brain at admission and a 0.5°C (0.1 to 0.3) higher temperature in DWI-normal brain at 5 days poststroke.
DISCUSSION
In this study of acute stroke patients, we found that higher levels of circulating markers of the acute inflammatory response were associated with higher temperatures in normal brain. Later after stroke, higher body temperature was associated with higher temperature in DWI-abnormal brain. We also confirmed our previous finding that tissue temperature of DWI-abnormal (ischemic) brain was higher than in areas of normal brain ipsi- or contralateral to the stroke.1
We used a validated noninvasive imaging technique to measure brain temperature in patients with ischemic stroke. Previous studies of brain diseases15,16 have measured local brain temperature invasively in patients on intensive care units, who have other reasons—usually neurosurgical—for invasive monitoring devices. Such monitoring is impractical in a less intensive ward-based setting and only measures temperature in tissue close to the probe whereas MRS measures temperature across a slice of brain allowing direct comparison between DWI-abnormal and normal-appearing brain. While the recruitment of patients for our study is not immune from a number of selection biases, these patients are more likely to be more representative of most populations of stroke patients than invasively monitored patients, and therefore of the potential target population for hypothermic treatments.
Our study has several limitations. First, we recruited relatively few patients, a reflection of the difficulties inherent in performing complex MRI in acute stroke patients. This has limited the strength of our conclusions, and the power of our analyses. While we have not found a significant difference between the association of blood markers of inflammation and brain temperature in different regions of the brain, it is possible that one exists. Only a larger number of patients would strengthen this conclusion. Second, because we took blood and measured brain temperature serially in a group of patients where clinical priorities led to early discharge from hospital, or some patients were too ill, we had missing data for a number of patients. It is likely therefore that those patients in whom data were missing differed systematically from those patients in whom we had complete data even though we were not able to demonstrate a statistical difference. This may have biased our results and either overestimated or underestimated the strength of any associations. Third, as an observational study, we were not able to draw strong conclusions about the causative role of inflammatory markers on body or brain temperature, or body on brain temperature or vice versa, nor can we comment on potential effects of therapeutic hypothermia. Fourth, we measured only 3 inflammatory markers at daily intervals; measurement more frequently, or of different markers, might provide more information.
The data from this study support the following conclusions: first, that the rise in brain temperature early after stroke in ischemic brain is not associated strongly and consistently with body temperature (though we cannot exclude such an association). Second, later after stroke, body temperature is associated with brain temperature, most strongly in abnormal brain tissue. Third, brain temperature is associated with markers of inflammation. However, although our results do not show whether this effect is significantly stronger in DWI-normal or DWI-abnormal brain, significant relationships were more frequent with DWI-normal than abnormal brain. Fourth, mean body temperature is associated with mean markers of inflammation.
The relationship between body and temperature in DWI-normal and DWI-abnormal brain is therefore complex, and could imply that early temperature elevation in DWI-abnormal brain is due to different mechanisms than temperature elevation in DWI-normal brain and the rest of the body. Early and rapid cooling of body temperature may not result in an equally rapid brain cooling, particularly of the ischemic tissue.
Studies of therapeutic hypothermia in acute stroke are of great interest, and we look forward to the successful completion of randomized controlled trials; they are the only way to reliably explore the causal relationship between body temperature and poor outcome after stroke, and embedded studies of biomarkers could explore the influence of body temperature on circulating markers of inflammation.
This study should inform the design of those trials: although body temperature may be measured as a surrogate endpoint of the efficacy of body cooling, a more relevant biomarker is brain cooling, as body temperature and brain temperature have a complex relationship that is not always easy to predict. Early temperature elevation in ischemic tissue may result from a different mechanism from that which elevates body temperature, as body temperature may not peak until several days after stroke17 and temperature in DWI-normal brain was higher at the second scan. The mechanisms by which brain or body cooling lead to improved outcomes in animal models after stroke—or potentially in patients—are uncertain, and there may be other important intermediate biomarkers of treatment efficacy. The association between higher levels of inflammation and higher brain temperature hints at a causal relationship between the two and is an important question for further research.
ACKNOWLEDGMENT
The authors thank the radiographers of the Brain Research Imaging Centre Edinburgh (BRIC), the Wellcome Trust Clinical Research Facility in Edinburgh, the stroke fellows who helped to recruit patients, and in particular the patients themselves.
GLOSSARY
- CI
confidence interval
- DVT
deep venous thrombosis
- DWI
diffusion-weighted imaging
- FLAIR
fluid-attenuated inversion recovery
- FOV
field of view
- 1H MRSI
proton magnetic resonance spectroscopy imaging
- IL-6
interleukin-6
- IQR
interquartile range
- MR
magnetic resonance
- MRS
magnetic resonance spectroscopy
- NIHSS
NIH Stroke Scale
- PRESS
point resolved spectroscopy
AUTHOR CONTRIBUTIONS
Drafting/revising manuscript: Dr. Whiteley, R. Thomas, Dr. Lowe, Dr. Karaszewski, Dr. Armitage, Dr. Marshall, Dr. Dennis, Dr. Wardlaw. Study design or concept: Dr. Wardlaw, Dr. Dennis, Dr. Whiteley, Dr. Armitage, Dr. Marshall. Analysis or interpretation of data: Dr. Whiteley, R. Thomas, Dr. Lowe, Dr. Rumley, Dr. Armitage, Dr. Marshall, Dr. Dennis, Dr. Wardlaw. Laboratory assays: Dr. Lowe, Dr. Rumley. Data acquisition: Dr. Whiteley, R. Thomas, Dr. Karaszewski, Dr. Armitage, Dr. Marshall, Dr. Lymer, Dr. Wardlaw. Study supervision: Dr. Wardlaw. Obtained funding: Dr. Wardlaw.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1. Karaszewski B, Wardlaw JM, Marshall I, et al. Measurement of brain temperature with magnetic resonance spectroscopy in acute ischemic stroke. Ann Neurol 2006; 60: 438– 446 [DOI] [PubMed] [Google Scholar]
- 2. Yenari MA, Hemmen TM. Therapeutic hypothermia for brain ischemia: where have we come and where do we go? Stroke 2010; 41: S72– S74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van der Worp HB, Sena ES, Donnan GA, Howells DW, Macleod MR. Hypothermia in animal models of acute ischaemic stroke: a systematic review and meta-analysis. Brain 2007; 130: 3063– 3074 [DOI] [PubMed] [Google Scholar]
- 4. Greer DM, Funk SE, Reaven NL, Ouzounelli M, Uman GC. Impact of fever on outcome in patients with stroke and neurologic injury: a comprehensive meta-analysis. Stroke 2008; 39: 3029– 3035 [DOI] [PubMed] [Google Scholar]
- 5. Kiyatkin EA. Brain temperature fluctuations during physiological and pathological conditions. Eur J Appl Physiol 2007; 101: 3– 17 [DOI] [PubMed] [Google Scholar]
- 6. Epstein FH, Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med 1999; 340: 448– 454 [DOI] [PubMed] [Google Scholar]
- 7. Whiteley W, Chong WL, Sengupta A, Sandercock P. Blood markers for the prognosis of ischemic stroke: a systematic review. Stroke 2009; 40: e380– e389 [DOI] [PubMed] [Google Scholar]
- 8. Whiteley W, Jackson C, Lewis S, et al. Inflammatory markers and poor outcome after stroke: a prospective cohort study and systematic review of interleukin-6. PLoS Med 2009; 6: e1000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brott T, Adams HP, Jr, Olinger CP, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke 1989; 20: 864– 870 [DOI] [PubMed] [Google Scholar]
- 10. Christensen H, Boysen G. Acceptable agreement between tympanic and rectal temperature in acute stroke patients. Int J Clin Pract 2002; 56: 82– 84 [PubMed] [Google Scholar]
- 11. Marshall I, Karaszewski B, Wardlaw JM, et al. Measurement of regional brain temperature using proton spectroscopic imaging: validation and application to acute ischemic stroke. Magn Reson Imaging 2006; 24: 699– 706 [DOI] [PubMed] [Google Scholar]
- 12. Cady EB, D'Souza PC, Penrice J, Lorek A. The estimation of local brain temperature by in vivo 1H magnetic resonance spectroscopy. Magn Reson Med 1995; 33: 862– 867 [DOI] [PubMed] [Google Scholar]
- 13. Germain D, Chevallier P, Laurent A, Saint-Jalmes H. MR monitoring of tumour thermal therapy. MAGMA 2001; 13: 47– 59 [DOI] [PubMed] [Google Scholar]
- 14. Martin M, Labouesse J, Canioni P, Merle M. N-acetyl-L-aspartate and acetate 1H NMR signal overlapping under mild acidic pH conditions. Magn Reson Med 1993; 29: 692– 694 [DOI] [PubMed] [Google Scholar]
- 15. Otawara Y, Ogasawara K, Kubo Y, Tomitsuka N, Ogawa A, Suzuki M. Brain and systemic temperature in patients with severe subarachnoid hemorrhage. Surg Neurol 2003; 60: 159– 164 [DOI] [PubMed] [Google Scholar]
- 16. Verlooy J, Heytens L, Veeckmans G, Selosse P. Intracerebral temperature monitoring in severely head injured patients. Acta Neurochir 1995; 134: 76– 78 [DOI] [PubMed] [Google Scholar]
- 17. Saini M, Saqqur M, Kamruzzaman A, Lees KR, Shuaib A. on behalf of the VISTA Investigators. Effect of hyperthermia on prognosis after acute ischemic stroke. Stroke 2009; 40: 3051– 3059 [DOI] [PubMed] [Google Scholar]