Abstract
Objective:
We determined whether site of care explains a previously identified racial disparity in carotid artery imaging.
Methods:
In this retrospective cohort study, data were obtained from a chart review of veterans hospitalized with ischemic stroke at 127 Veterans Administration hospitals in 2007. Extensive exclusion criteria were applied to obtain a sample who should have received carotid artery imaging. Minority-serving hospitals were defined as the top 10% of hospitals ranked by the proportion of stroke patients who were black. Population level multivariate logistic regression models with adjustment for correlation of patients in hospitals were used to calculate predictive probabilities of carotid artery imaging by race and minority-service hospital status. Bootstrapping was used to obtain 95% confidence intervals (CIs).
Results:
The sample consisted of 1,534 white patients and 628 black patients. Nearly 40% of all black patients were admitted to 1 of 13 minority-serving hospitals. No racial disparity in receipt of carotid artery imaging was detected within nonminority serving hospitals. However, the predicted probability of receiving carotid artery imaging for white patients at nonminority-serving hospitals (89.7%, 95% CI [87.3%, 92.1%]) was significantly higher than both white patients (78.0% [68.3%, 87.8%] and black patients (70.5% [59.3%, 81.6%]) at minority-serving hospitals.
Conclusions:
Underuse of carotid artery imaging occurred most often among patients hospitalized at minority-serving hospitals. Further work is required to explore why site of care is a mechanism for racial disparities in this clinically important diagnostic test.
Racial disparities in health care are widely reported.1 Commonly cited mechanisms for disparities include unequal access to care, association of race with vulnerable socioeconomic characteristics, and the quality of interaction and communication between clinicians and patients. A mechanism that is receiving further attention is the site of care.2–4 Black patients receive care from a concentrated set of hospitals.5,6 If those hospitals deliver a lower quality of care, then population-level disparities would be detected, even if no disparity exists within a hospital.
Carotid artery stenosis is an important, modifiable risk factor for stroke.7,8 Carotid artery stenosis is detected by carotid artery imaging, and therefore these diagnostic tests are routinely performed in the workup of patients presenting with ischemic stroke.
In a recent analysis, we unexpectedly found that black veterans hospitalized with ischemic stroke were less likely than white patients to receive carotid artery imaging.9 Several explanations are cited why carotid interventions occur less commonly among black patients. However, these reasons do not address why there would be disparities of diagnostic tests to detect carotid stenosis.10,11
Carotid artery imaging would seem to be driven by provider behavior or system characteristics instead of patient behavior. In a prior Veterans Health Administration (VA) study, minority-serving hospitals were less likely (44% vs 74%, p < 0.001) to have MRI availability during a study period of 1996–2002.6 Therefore, we analyzed whether site of care could be a mechanism for the observed racial disparities in receipt of carotid artery imaging in the VA.
METHODS
Sample.
In 2009, the VA Offices of Quality and Performance (OQP), Patient Care Services (PCS), and the Stroke QUERI collaborated to conduct the Office of Quality and Performance Stroke Special Study.12 The design of this study is a retrospective cohort of veterans admitted to all 127 Veterans Affairs Medical Centers (VAMCs) in fiscal year (FY) 2007 (October 1, 2006, to September 30, 2007) with a primary discharge diagnosis of ischemic stroke. A sample of 5,000 patients was identified using all patients at low-volume facilities (≤55 patients with ischemic stroke in FY2007) and an 80% sample of patients at high-volume facilities (>55 patients with ischemic stroke in FY2007).
Trained nurses at the West Virginia Medical Institute (WVMI) conducted chart reviews for this project. This organization has been conducting large-scale chart reviews of the VA for the past decade. For over 10 years, all VA Medical Centers have used an enterprise-wide electronic health care record system, thus allowing the WVMI to remotely retrieve and review care delivered in the VA from a central location. The nurses confirmed a hospitalization of ischemic stroke in 3,987 patients, then proceeded with a chart review of 307 data elements among the confirmed patients. Inter-rater reliability was greater than 70% for over 90% of data elements.
Exclusion criteria.
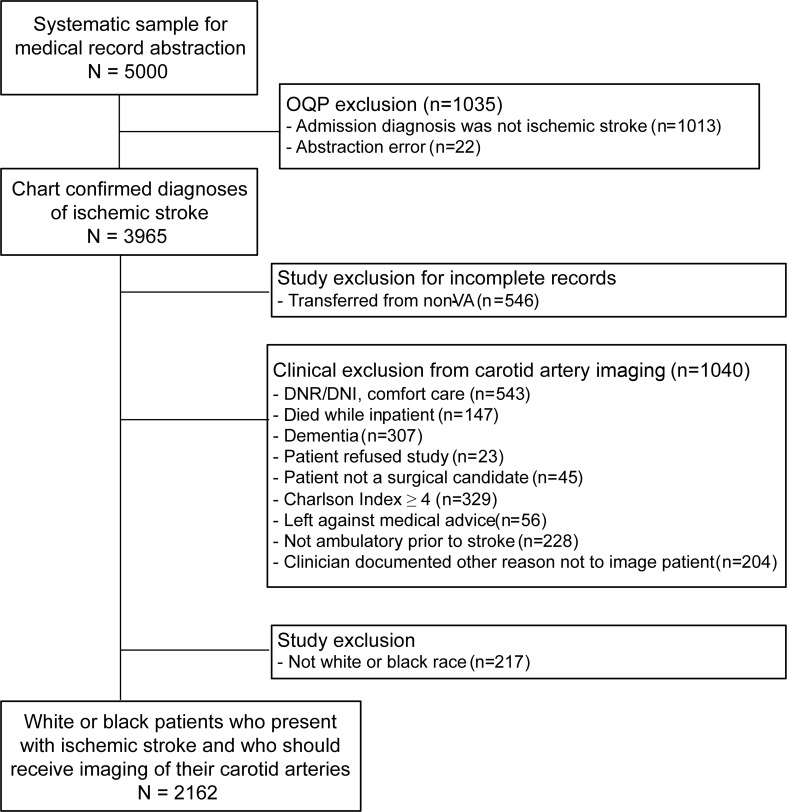
Patients were excluded if they were transferred from a non-VA hospital because carotid artery imaging performed at a non-VA hospital may not be documented in the VA medical records (figure 1). Patients were also excluded if clinicians documented a reason why carotid artery imaging was not performed or if patients refused the test. Patients were excluded if they died in the hospital, if there was documentation that they wanted less aggressive care, or if there was an indication of poor health status that may make a person ineligible for carotid interventions (Charlson comorbidity greater than 4, dementia, not ambulatory prior to admission, deemed not to be a surgical candidate). Although these extensive criteria may have excluded some patients who should have received carotid imaging, the goal was to identify a final sample for whom there is little doubt that carotid artery imaging should have been performed.
Figure 1. Inclusion and exclusion criteria of study to examine receipt of carotid imaging among persons presenting with acute stroke.
DNR/DNI = do not resuscitate/do not intubate; OQP = Offices of Quality and Performance; VA = Veterans Health Administration.
Race of the patient was obtained from various sources in the medical record, chiefly from the Functional Status and Outcomes Database13 and the Centers for Medicare and Medicaid Services (CMS) Vital Status File. We excluded veterans if they were not of white or black race, because the numbers of veterans in other race categories were too few to compare across racial groups. After applying exclusion criteria, there were 1,534 white and 628 black patients in the sample, for a total of 2,162 patients.
Dependent variable.
Our dependent variable was receipt of carotid artery imaging from 12 months prior to the date of admission to 2 months after the date of admission. Even though many clinicians would repeat carotid imaging after a stroke, the length of the preadmission time period was chosen because the degree of stenosis of the carotid artery is not expected to change significantly over 12 months. The postadmission time period was chosen because carotid procedures may have a greater impact on risk reduction if done within the first 8 weeks poststroke.14 Carotid imaging tests could consist of any of the following: carotid artery ultrasound, magnetic resonance angiography (MRA) of the neck, CT angiography (CTA) of the neck, and catheter angiography.15
Independent variables.
The 2 independent variables of interest were race of the patient and minority-serving status of the hospital. For the latter variable, the proportion of stroke patients in the study sample who were black in each hospital was calculated. While all VA hospitals treat minority patients, for purposes of this study, minority-serving hospitals were defined as the top 10th percentile of hospitals by proportion of stroke patients who were black.3
Patient-level characteristics included age and sex. Clinical history included prior history of stroke, TIA, carotid procedures, and atrial fibrillation. The NIH Stroke Scale score, a measure of clinical stroke severity, was derived based upon review of the documented neurologic examination upon admission.16 The Charlson score, a measure of comorbidity, was calculated for each patient.17
Hospital-level characteristics included the annual number of stroke patients, urban/rural status, and complexity level (a VA composite measure of patient volume, comorbidity of the patient population, teaching hospital status, and intensive care unit capability).18
Analysis.
We conducted bivariate comparisons of receipt of carotid artery imaging and other characteristics between white and black patients using t tests and Wilcoxon tests for continuous measures and χ2 and Fisher exact tests for categorical measures. We then conducted bivariate comparisons of receipt of carotid artery imaging, patient characteristics, and hospital characteristics between patients seen at non-minority-serving and minority-serving hospitals using these same statistical tests. We fit a series of logistic regression models which first included indicators for patient race, minority-serving status of the hospital, and an interaction term of those 2 variables. We then added patient-level characteristics that were significantly associated with carotid imaging as well as hospital characteristics.3 Models were fit using generalized estimating equations with an exchangeable covariance structure to incorporate the correlation of imaging for patients from the same hospital.19 Predictive margins were used to calculate predictive probabilities and risk differences.20 The 95% confidence intervals (CIs) for probabilities and differences were obtained by fitting the models to 5,000 bootstrap samples, which we selected by randomly selecting hospitals with replacement. Analyses were conducted using SAS for Windows version 9.1 (SAS Institute, Cary, NC) and Stata/SE 10 (StataCorp LP, College Station, TX).
As a sensitivity analysis of the designation of minority-serving hospitals, we redefined it as the top 25th percentile instead of the top 10th percentile of all hospitals.3 A second sensitivity analysis was run on a broader sample that dropped the exclusion criteria of do not resuscitate/do not intubate status, dementia, high Charlson score, and ambulation status. A third sensitivity analysis redefined the dependent variable as receipt of carotid artery imaging in the 1-week time period after presentation for acute ischemic stroke because early recurrence of stroke can occur.21
Standard protocol approvals, registrations, and patient consents.
All data were stored at the Richard L. Roudebush VA Medical Center, Indianapolis, IN. The Institutional Review Board at that facility approved the research protocol, including a waiver of consent.
RESULTS
The final sample consisted of 1,534 white patients and 628 black patients (table 1). The unadjusted racial disparity in carotid artery imaging was 7.2% (88.6% of white patients vs 81.4% of black patients, p < 0.001). Use of ultrasound was more common among white patients (60.4% vs 41.9%, p < 0.001), while use of MRA was less common (32.9% vs 39.6%, p = 0.002). Black patients were more likely to be younger than age 55, have had a prior stroke, and to have had a more severe stroke (table 1). Black patients had similar Charlson scores but a longer length of stay.
Table 1.
Characteristics of white and black veterans presenting with ischemic stroke (n = 2,162)a

Values are % or median (interquartile range).
p < 0.001.
p < 0.05.
The concentration of black patients by site of care is demonstrated in table 2. Among the 114 non-minority-serving hospitals, the median proportion of black patients in each was 9.6%, whereas the 13 minority-serving hospitals had a median proportion of black patients in each hospital of 57.1% (p < 0.001). About 40% of black patients in the entire sample were admitted to 1 of the 13 minority-serving hospitals. Patients admitted to non-minority-serving hospitals were more likely to receive carotid artery imaging than patients admitted to minority-serving hospitals (88.6% vs 77.7%, p < 0.001), and this disparity appeared to be driven by a differential use of carotid ultrasound (60.4% vs 39.6%, p < 0.001).
Table 2.
Characteristics of patients admitted to non-minority-serving and minority-serving hospitals (n = 2,162 patients in 127 hospitals)a

Values are % or median (interquartile range).
p < 0.001.
p < 0.05.
Complexity score is a composite measure of patient volume, comorbidity of the patient population, teaching hospital status, and intensive care unit capability.
In logistic models which accounted for clustering, minority-serving status of a hospital (p = 0.04) and patient race (p = 0.07) had similar levels of association with carotid artery imaging (table e-1 on the Neurology® Web site at www.neurology.org). The interaction term between patient race and minority-serving hospital was not significant (data not shown). In generalized estimating equation regression models that adjusted for age, clinical characteristics, and hospital characteristics, the predicted probabilities of receiving carotid artery imaging were similar between white patients (89.7% [95% CI 87.3%, 92.1%]) and black patients (87.2% [95% CI 83.1%, 91.4%], p = 0.18) at non-minority-serving hospitals (table e-4). However, the predicted probabilities among white patients (78.0% [68.3%, 87.8%]) and black patients (70.5% [59.3%, 81.6%]) at minority-serving hospitals were both significantly lower (p < 0.001) than white patients at non-minority-serving hospitals (figure 2). In post hoc analyses using black patients at minority-serving hospitals as the reference group, the 3 other groups had significantly higher use of carotid artery imaging (p < 0.01, table e-2).
Figure 2. Predicted probabilities of receipt of carotid artery imaging by patient race and minority-serving status of the hospital.
Similar results were found in sensitivity analyses defining minority-serving hospitals as the top 25% of hospitals by proportion of black patients and in analyses using the larger sample (n = 2,792 patients) constructed using fewer exclusion criteria than originally applied to the sample (table e-3). When receipt of carotid imaging was limited to the week after presentation, we found a similar disparity by race (table 1) and site of care (table 2). Of the patients who did receive carotid artery imaging, 94% did so in the first week after presentation. In multivariate models, receipt of carotid imaging among white patients at non-minority-serving hospitals was again significantly higher than white patients and black patients at minority-serving hospitals.
DISCUSSION
In our study, we showed that black patients were less likely than white patients to receive carotid artery imaging. We also showed that black patients receive their care at different facilities from white patients. At these predominantly minority-serving facilities, both racial groups were less likely to receive carotid artery imaging compared to white patients at non-minority-serving facilities. The overall use of carotid artery imaging in this 2007 study has improved from prior VA studies completed a decade earlier,10,22 but this improvement in overall carotid imaging is now accompanied by a racial disparity that was not detected in prior VA studies. Non-VA studies also have not consistently detected racial disparities in carotid artery imaging,23–25 though studies have also reported that men consistently receive carotid artery imaging about 5%–10% higher than women.26–29 The use of carotid imaging among black patients and within minority-serving hospitals in our study actually appears to be as high or higher than in other studies, but the use of carotid imaging among white patients and within non-minority-serving hospitals were still significantly higher.
Although disparities in other stroke processes and outcomes of care have been well-documented,30,31 commonly cited explanations for disparities do not readily apply to carotid artery imaging. First, access to care is unlikely to be the cause because all patients in the sample are hospitalized. In addition, all patients in this sample have health insurance coverage for services rendered by the VA system. Second, these disparities are unlikely to be due to differential perceived risk of carotid artery imaging, as may be the case for carotid endarterectomy.11 The most common methods of imaging the carotid arteries, carotid ultrasound and MRA, are essentially risk-free and do not require informed consent. Third, these disparities are unlikely due to issues with clinician-patient interaction, such as cultural competency or shared decision-making. A study of over 300,000 patients receiving care showed considerable racial disparities for processes that require substantial interaction (such as smoking cessation) but much smaller disparities for processes that require little interaction between patient and clinician (such as prescription of β-blockers upon discharge).2 Carotid artery imaging also involves very little interaction and is typically ordered by the clinician with little to no patient input. Finally, in the VA, the impact of financial incentives and barriers are greatly mitigated, which is a commonly cited reason why disparities are reduced (though not completely eliminated).32 Therefore, other mechanisms likely underlie disparities in carotid artery imaging, although it is not readily apparent why site of care has such a strong association with disparities.
We expected that racial disparities could be driven by differential access to more advanced technology, such as MRA and CTA, but our findings were driven by differential use of carotid ultrasound, an older, more inexpensive, and more universally available technology. We do not know the reasons for this, but we note that a study of Medicare recipients also noted that black patients were significantly less likely to receive carotid ultrasound than white patients but significantly more likely to receive MRA than white patients.23
Several limitations should be mentioned. Our definition of minority-serving hospital was based on the proportion of patients who were black, regardless of the number.3 A prior VA study defined minority-serving hospital according to the top decile of number of black patients instead of proportion.6 However, 9 of the 13 hospitals defined as minority-serving in our study would have also been defined as minority-serving hospital according to the alternative definition. Second, we did not have information on the specialty of the providers managing the veterans in our study. An older study determined that involvement of neurologists was associated with carotid artery imaging among persons with TIA.33 Third, we could not determine which patients had strokes in the posterior circulation, which could not arise from embolism from the carotid artery. Two prior studies of carotid imaging did include data about the location of stroke. One study found no association between anterior vs posterior circulation and receipt of carotid artery imaging.29 The other study found that anterior circulation location was a predictor of receipt of carotid artery imaging, though it was no longer so when the sample was restricted to patients who were eligible for surgery.28 In our study, a substantial proportion of patients received MRA or CTA, and these tests assess both the carotid and vertebral arteries. Finally, VA users are predominantly male and have low socioeconomic status, high levels of comorbidity, and high levels of disability,34 so results may not be generalizable to the non-VA population.
A strength of the study is the number of hospitals included in the sample. Most prior studies were conducted at too few sites to assess whether site of care is a mechanism for disparities.
Site of care should be explored as an explanation of disparities by race or ethnicity if the comparison groups are obtaining medical care from different facilities. In our study, black veterans are concentrated within a few VA hospitals. Both white patients and black patients at minority-serving hospitals were a vulnerable group with regard to the receipt of carotid artery imaging. The omission of carotid artery imaging in a patient with a new ischemic stroke represents poor quality of care because eligibility for more aggressive treatment options is not ascertained. Further investigations will focus on specific sites of care instead of the national VA system to understand the barriers to performing this clinically important diagnostic test in a hospital system that has the resources to provide access to care for its patients.
Supplementary Material
GLOSSARY
- CI
confidence interval
- CMS
Centers for Medicare and Medicaid Services
- CTA
CT angiography
- FY
fiscal year
- MRA
magnetic resonance angiography
- OQP
Offices of Quality and Performance
- PCS
Patient Care Services
- VA
Veterans Health Administration
- VAMC
Veterans Affairs Medical Center
- WVMI
West Virginia Medical Institute
Footnotes
Editorial, page 117
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Drs. Cheng, Keyhani, and Bravata participated in drafting and revising the manuscript for content, the study concept, analysis, and interpretation of data. Drs. Williams and Ordin participated in revising the manuscript for content and interpretation of data. Dr. Hebert and Ms. Ofner participated in revising the manuscript for content, analysis, and interpretation of data.
STUDY FUNDING
Supported by Veterans Administration RRP 09-184 (PI: Dr. Bravata) and RRP 09-185 (PI: Dr. Keyhani) and by the VHA HSR&D Center of Excellence on Implementing Evidence-Based Practice (CIEBP), Richard L. Roudebush VA Medical Center, Indianapolis, and the VHA HSR&D Stroke Quality Enhancement Research Initiative (QUERI) Program, Indianapolis, IN. Dr. Cheng is supported by a Career Development Award from NIH/NINDS (K23NS058571), by Project EXPORT (NCMHD grants P20MD000182), and by the UCLA Outcomes Research Center, funded through the American Heart Association Pharmaceutical Roundtable and David and Stevie Spina. Dr. Keyhani is supported by a Career Development Award from the Veterans Administration. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1. US Department of Health & Human Services HHS Action Plan to Reduce Racial and Ethnic Health Disparities. Available at: http://minorityhealth.hhs.gov/npa/templates/content.aspx?lvl=1&lvlid=33&ID=285 Accessed June 24, 2011
- 2. Hasnain-Wynia R, Baker DW, Nerenz D, et al. Disparities in health care are driven by where minority patients seek care: examination of the hospital quality alliance measures. Arch Intern Med 2007;167:1233–1239 [DOI] [PubMed] [Google Scholar]
- 3. Joynt KE, Orav EJ, Jha AK. Thirty-day readmission rates for Medicare beneficiaries by race and site of care. JAMA 2011;305:675–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li Y, Yin J, Cai X, Temkin-Greener J, Mukamel DB. Association of race and sites of care with pressure ulcers in high-risk nursing home residents. JAMA 2011;306:179–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jha AK, Orav EJ, Li Z, Epstein AM. Concentration and quality of hospitals that care for elderly black patients. Arch Intern Med 2007;167:1177–1182 [DOI] [PubMed] [Google Scholar]
- 6. Jha AK, Stone R, Lave J, Chen H, Klusaritz H, Volpp K. The concentration of hospital care for black veterans in veterans affairs hospitals: implications for clinical outcomes. J Healthc Qual 2010;32:52–61 [DOI] [PubMed] [Google Scholar]
- 7. Adams H, Jr, Bendixen B, Kappelle L, et al. Classification of subtype of acute ischemic stroke: definitions for use in a multicenter clinical trial: TOAST: Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993;24:35–41 [DOI] [PubMed] [Google Scholar]
- 8. Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011;42:227–276 [DOI] [PubMed] [Google Scholar]
- 9. Cheng EM, Keyhani S, Ofner S, Williams L. Use of carotid imaging among patients admitted with ischemic stroke. In: 63rd American Academy of Neurology annual meeting; Honolulu, Hawaii; 2011: A308 Abstract [Google Scholar]
- 10. Oddone EZ, Horner RD, Sloane R, et al. Race, presenting signs and symptoms, use of carotid artery imaging, and appropriateness of carotid endarterectomy. Stroke 1999;30:1350–1356 [DOI] [PubMed] [Google Scholar]
- 11. Horner RD, Oddone EZ, Matchar DB. Theories explaining racial differences in the utilization of diagnostic and therapeutic procedures for cerebrovascular disease. Milbank Q 1995;73:443–462 [PubMed] [Google Scholar]
- 12. Bravata D, Ordin D, Vogel B, Williams L. The Quality of VA Inpatient Ischemic Stroke Care, FY2007: Final National and Medical Center Results of the VHA Office of Quality and Performance (OQP) Special Study, 2009. VHA Office of Quality and Performance; 2009. [Google Scholar]
- 13. Stansbury JP, Reid KJ, Reker DM, Duncan PW, Marshall CR, Rittman M. Why ethnic designation matters for stroke rehabilitation: comparing VA administrative data and clinical records. J Rehabil Res Dev 2004;41:269–278 [DOI] [PubMed] [Google Scholar]
- 14. Rothwell PM, Eliasziw M, Gutnikov SA, Warlow CP, Barnett HJM. Endarterectomy for symptomatic carotid stenosis in relation to clinical subgroups and timing of surgery. Lancet 2004;363:915–924 [DOI] [PubMed] [Google Scholar]
- 15. Brott TG, Halperin JL, Abbara S, et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS Guideline on the Management of Patients With Extracranial Carotid and Vertebral Artery Disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American Stroke Association, American Association of Neuroscience Nurses, American Association of Neurological Surgeons, American College of Radiology, American Society of Neuroradiol, Congress of Neurological Surgeons, Society of Atherosclerosis Imaging and Prevention, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of NeuroInterventional Surgery, Society for Vascular Medicine, and Society for Vascular Surgery, developed in collaboration with the American Academy of Neurology and Society of Cardiovascular Computed Tomography. J Am Coll Cardiol 2011;57:e16–e94 [DOI] [PubMed] [Google Scholar]
- 16. Williams LS, Yilmaz EY, Lopez-Yunez AM. Retrospective assessment of initial stroke severity with the NIH Stroke Scale. Stroke 2000;31:858–862 [DOI] [PubMed] [Google Scholar]
- 17. de Groot V, Beckerman H, Lankhorst GJ, Bouter LM. How to measure comorbidity: a critical review of available methods. J Clin Epidemiol 2003;56:221–229 [DOI] [PubMed] [Google Scholar]
- 18. Szabo C. Veterans Health Administration NLB Human Resources Committee 2005 Facility Complexity Model. Veterans Health Administration; 2005 [Google Scholar]
- 19. Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika 1986;73:13–22 [Google Scholar]
- 20. Kleinman LC, Norton EC. What's the risk? A simple approach for estimating adjusted risk measures from nonlinear models including logistic regression. Health Serv Res 2009;44:288–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Marnane M, Ni Chroinin D, Callaly E, et al. Stroke recurrence within the time window recommended for carotid endarterectomy. Neurology 2011;77:738–743 [DOI] [PubMed] [Google Scholar]
- 22. Goldstein LB, Matchar DB, Hoff-Lindquist J, Samsa GP, Horner RD. Veterans Administration Acute Stroke (VASt) Study: lack of race/ethnic-based differences in utilization of stroke-related procedures or services. Stroke 2003;34:999–1004 [DOI] [PubMed] [Google Scholar]
- 23. Martin KD, Naert L, Goldstein LB, Kasl S, Molinaro AM, Lichtman JH. Comparing the use of diagnostic imaging and receipt of carotid endarterectomy in elderly black and white stroke patients. J Stroke Cerebrovasc Dis Epub 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tuhrim S, Cooperman A, Rojas M, et al. The association of race and sex with the underuse of stroke prevention measures. J Stroke Cerebrovasc Dis 2008;17:226–234 [DOI] [PubMed] [Google Scholar]
- 25. Jacobs BS, Birbeck G, Mullard AJ, et al. Quality of hospital care in African American and white patients with ischemic stroke and TIA. Neurology 2006;66:809–814 [DOI] [PubMed] [Google Scholar]
- 26. Kapral MK, Degani N, Hall R, et al. Gender differences in stroke care and outcomes in Ontario. Women's Health Issues 2011;21:171–176 [DOI] [PubMed] [Google Scholar]
- 27. Turaj W, Słowik A, Wnuk M, Szczudlik A. Gender-related differences in diagnostic evaluation and outcome of ischemic stroke in Poland. Stroke 2009;40:980–982 [DOI] [PubMed] [Google Scholar]
- 28. Kapral MK, Ben-Yakov M, Fang J, et al. Gender differences in carotid imaging and revascularization following stroke. Neurology 2009;73:1969–1974 [DOI] [PubMed] [Google Scholar]
- 29. Smith MA, Lisabeth LD, Brown DL, Morgenstern LB. Gender comparisons of diagnostic evaluation for ischemic stroke patients. Neurology 2005;65:855–858 [DOI] [PubMed] [Google Scholar]
- 30. Stansbury JP, Jia H, Williams LS, Vogel WB, Duncan PW. Ethnic disparities in stroke: Epidemiology, acute care, and postacute outcomes. Stroke 2005;36:374–386 [DOI] [PubMed] [Google Scholar]
- 31. Cruz-Flores S, Rabinstein A, Biller J, et al. Racial-ethnic disparities in stroke care: the American experience: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011;42:2091–2116 [DOI] [PubMed] [Google Scholar]
- 32. Saha S, Freeman M, Toure J, Tippens KM, Weeks C, Ibrahim S. Racial and ethnic disparities in the VA health care system: a systematic review. J Gen Intern Med 2008;23:654–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mitchell JB, Ballard DJ, Matchar DB, Whisnant JP, Samsa GP. Racial variation in treatment for transient ischemic attacks: Impact of participation by neurologists. Health Serv Res 2000;34:1413–1428 [PMC free article] [PubMed] [Google Scholar]
- 34. Wilson NJ, Kizer KW. The VA health care system: an unrecognized national safety net. Health Aff 1997;16:200–204 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.