Abstract
Objective:
To utilize high-throughput sequencing to determine the etiology of juvenile-onset neurodegeneration in a 19-year-old woman with progressive motor and cognitive decline.
Methods:
Exome sequencing identified an initial list of 133,555 variants in the proband's family, which were filtered using segregation analysis, presence in dbSNP, and an empirically derived gene exclusion list. The filtered list comprised 52 genes: 21 homozygous variants and 31 compound heterozygous variants. These variants were subsequently scrutinized with predicted pathogenicity programs and for association with appropriate clinical syndromes.
Results:
Exome sequencing data identified 2 GLB1 variants (c.602G>A, p.R201H; c.785G>T, p.G262V). β-Galactosidase enzyme analysis prior to our evaluation was reported as normal; however, subsequent testing was consistent with juvenile-onset GM1-gangliosidosis. Urine oligosaccharide analysis was positive for multiple oligosaccharides with terminal galactose residues.
Conclusions:
We describe a patient with juvenile-onset neurodegeneration that had eluded diagnosis for over a decade. GM1-gangliosidosis had previously been excluded from consideration, but was subsequently identified as the correct diagnosis using exome sequencing. Exome sequencing can evaluate genes not previously associated with neurodegeneration, as well as most known neurodegeneration-associated genes. Our results demonstrate the utility of “agnostic” exome sequencing to evaluate patients with undiagnosed disorders, without prejudice from prior testing results.
Inherited juvenile-onset neurodegeneration can present with a range of phenotypes due to a variety of genetic etiologies.1 Further complicating the diagnosis of these disorders is the phenotypic similarity shared among diverse genetic causes. Conversely, many individuals with the same genetic disorder have variable phenotypic expression. Additionally, juvenile neurodegenerative disorders are extremely rare and experience with their clinical presentation is often limited. The diagnostic tools used to diagnose neurodegenerative disorders include history and physical examination, radiologic studies, and biochemical screening tests. These data often provide the physician with sufficient information to categorize the nature of the patient's disorder so that diagnostic biochemical analysis or sequencing of candidate genes can be performed. Unfortunately, assembly of such information can be extremely challenging, resulting in slow, expensive, and unproductive evaluations and delay in therapeutic intervention.
Exome sequencing is a powerful tool for evaluating the coding sequences of an individual patient in a comparatively affordable manner.2 This technique employs high-throughput sequencing to identify protein-coding variants in an individual's genome (when compared with reference databases). Filtering algorithms are then used to narrow the number of possible disease-causing variants to manageable levels. In addition, exome sequencing of an entire family allows for intrafamilial comparison of variants in order to eliminate those that do not fit suspected mendelian inheritance. The remaining variants can then be further filtered for novelty, estimates of potential pathogenicity, or known associations with disease.2–4 Since exome sequencing profiles most known exons, such testing includes genes that have not been previously associated with neurodegeneration, as well as neurodegeneration-associated genes that may or may not have been eliminated from the differential diagnosis.
We report a patient with juvenile-onset GM1-gangliosidosis (GLB1, MIM# 230500), an autosomal-recessive disorder due to decreased β-galactosidase activity and leading to gray matter degeneration. Although her presentation was consistent with GM1-gangliosidosis (as well as other central gray matter degenerative disorders), prior urine oligosaccharide and β-galactosidase testing at outside facilities were reportedly normal. Evaluation with exome sequencing revealed compound heterozygous mutations for GLB1, and repeat measurement of β-galactosidase enzyme activity was consistent with juvenile-onset GM1-gangliosidosis.5
METHODS
Patient.
The proband (II-2) was born to nonconsanguineous healthy parents and has a healthy brother (II-1) and half-sister (II-3) (figure 1). The proband was evaluated by the NIH Undiagnosed Diseases Program at 19 years of age, after a 16-year history of neurologic decline. Development was normal until age 3, when she developed lower extremity spasticity. This was followed by progressive motor and cognitive decline. At age 5, she developed a stuttering dysarthria, which evolved into global aphasia. Her spasticity progressed to involve all extremities and she developed generalized dystonia. Wechsler Intelligence Scale for Children–IV testing at 10 years estimated her full-scale IQ at 42 (normal > 70). She developed partial epilepsy at 18 years of age.
Figure 1. Pedigree and GLB1 mutations.
(A) Familial pedigree. The proband is indicated by an arrow. Affected individuals are indicated in black. (B) Segregation of mutations with sequencing chromatograms (arrows represent mutations). (C) Conservation of altered amino acids across species.
Multiple lysosomal enzymes, including β-galactosidase, were previously reported as normal by an outside testing facility (specific activity values were not available; however, other normal enzymes included α-galactosidase, β-mannosidase, β-hexosaminidase, and arylsulfatase A). Other negative evaluations included filipin staining and gene sequencing for CDKL5, CLN3, DYT1, DRPLA, FOXG1, FRDA, HTT, MECP2, PANK2, PARKIN, and SCA3.
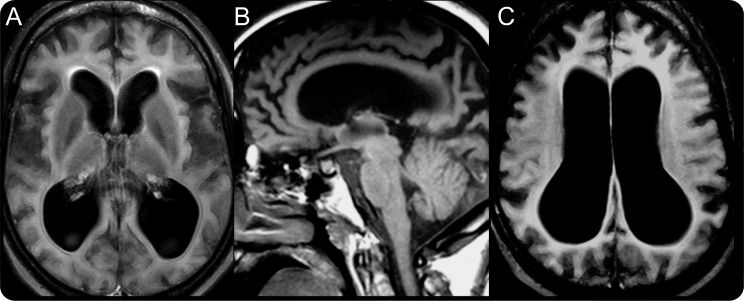
Previous brain MRIs revealed progressive cerebral cortical atrophy and thin corpus callosum. A recent study also included mild atrophy of the basal ganglia and thalamus (figure 2). At 10 years, an EEG was normal; however, 5 years later it showed diffuse slowing suggestive of bihemispheric dysfunction. Recent EEGs have been uninformative due to patient noncompliance.
Figure 2. Brain MRI of subject.
(A) Ventriculomegaly, cortical atrophy, and mild atrophy of the basal ganglia (axial fluid-attenuated inversion recovery). (B) Thin corpus callosum, ventriculomegaly, and cortical atrophy (sagittal T1). (C) Diffuse cortical atrophy and ventriculomegaly (axial fluid-attenuated inversion recovery) (3 Tesla).
On examination, the patient was awake and alert. She had expressive and receptive aphasia and did not follow commands. Ophthalmologic examination was normal. She had normal muscle bulk and strength. She was spastic in all extremities and had a mild intention tremor. Reflexes were increased throughout with extensor plantar responses. Sensation was intact. She had a spastic gait and required a walker for ambulation.
Standard protocol approvals, registrations, and patient consents.
Studies were approved by the NHGRI Institutional Review Board. Family members gave written informed consent for protocol 76-HG-0238.
Exome sequencing.
Exome sequencing, assembly, genotyping, and annotation were carried out on 5 of the family members by NISC.4,5 Capture utilized the Sureselect Human All-Exon System (Agilent Technologies, Santa Clara, CA). Captured regions totaled approximately 38 Mb. Flow cell preparation and 76-bp paired end read sequencing were performed per protocol of GAIIx sequencer (Illumina, San Diego CA). The percentage of the Consensus Coding Sequence exome with most probable genotype quality scores of >10 exceeded 85%.4 DNA variant list manipulation was performed using VarSifter (Jamie Teer, unpublished data). Variants detected on exome sequencing were scrutinized using criteria such as predicted pathogenicity (by CDPred) or association with an appropriate clinical syndrome.
Urine oligosaccharide and β-galactosidase analysis.
Urine oligosaccharide analysis was performed at 3 separate CLIA-certified laboratories with thin layer chromatography or MALDI-TOF/TOF technology. Serum β-galactosidase activity was evaluated at 2 separate CLIA-certified laboratories.
RESULTS
Exome sequencing of the 5 family members generated an initial list of 133,555 variants. This list was filtered using segregation analysis (autosomal recessive model), dbSNP (annotated heterozygosity >1%), and an empirically derived gene exclusion list (e.g., pseudogenes). Initial analysis focused on indel, nonsense, missense, and canonical splice site variants. The filtered list consisted of 21 homozygous and 31 compound heterozygous variants. One of the genes on this list was GLB1, which contained 2 variants (c.602G>A, p.R201H; c.785G>T, p.G262V). The p.R201H mutation had been reported as pathogenic for juvenile-onset GM1-gangliosidosis,3 whereas the p.G262V mutation had not been previously reported. Both mutations resulted in amino acid substitutions in regions conserved across numerous species (figure 1).
Four years prior to our evaluation, β-galactosidase testing at an outside facility was reported to be normal; however, subsequent repeat evaluation was abnormal with values consistent with juvenile-onset GM1-gangliosidosis (1.4 [45.7–140.1]; reference enzymes were nonpathologic: α-galactosidase 40.3 [20.3–60.9], β-mannosidase 128.0 [14.5–110.7], β-hexosaminidase 300.9 [37.4–242.7], arylsulfatase A 49.6 [23.7–79.7]; all values in nmol/mg protein/h). In addition, previous urine oligosaccharide screening with thin layer chromatography was also reported to be normal on 2 separate occasions; however, subsequent analysis with MALDI-TOF/TOF mass spectrometry analysis showed multiple oligosaccharides with terminal galactose residues consistent with GM1-gangliosidosis. A skeletal survey noted minimal irregularity of the cervical, thoracic, and lumbar vertebral endplates; however, there was no evidence of dysplasia in the long bones, hips, feet, wrists, or hands.
DISCUSSION
GM1-gangliosidosis is a lysosomal storage disease associated with a deficiency of the hydrolytic enzyme β-galactosidase.6,7 The neurodegeneration associated with this disorder is the result of inappropriate accumulation of GM1-ganglioside in the CNS causing neurotoxicity.8,9 Clinically, GM1-gangliosidoses can be classified into 3 subtypes based on the age at onset and rate of progression. The infantile-onset subtype involves rapid and severe neurodegeneration leading to death by 1–2 years of age; the juvenile-onset variant has a later onset and slower progression of motor and cognitive decline; the adult-onset disease often involves only late-onset extrapyramidal dysfunction.6 In general, disease severity is correlated with residual enzyme activity.9 Like other lysosomal diseases, the juvenile and adult-onset variants of GM1-gangliosidosis typically have variable presentations. The diagnosis is based on clinical evaluation and laboratory testing of β-galactosidase activity; however, the average time from onset to symptoms to diagnosis is often greater than 5 years.10
We describe a patient with juvenile-onset GM1-gangliosidosis diagnosed using exome sequencing. Her clinical examination was consistent with this disorder, but prior β-galactosidase activity had been normal. It is difficult to determine why the first evaluation was normal, since it is unlikely that her endogenous activity changed. A more likely explanation is an error in sample handling or evaluation. Fortunately, the capacity of exome sequencing to agnostically screen a large number of candidate genes, regardless of previous results, allowed for the detection of the GLB1 mutations and diagnosis of this patient.
The false-negative result led to years of diagnostic uncertainty and continued diagnostic testing. This case illustrates the utility of exome sequencing to resolve complex disorders that have eluded diagnosis and further illustrates that the cost of exome sequencing as a secondary screening tool may often be less than the cost of continued genetic workup involving multiple individual gene studies. At our facility, with costs continuing to decrease, the estimated price for exome sequencing is ∼$2,000 per sample or ∼$10,000 for the family quintet. Additional diagnostic testing after the false-negative result (including sequencing for the genes noted above) was estimated at ∼$14,000. As more therapeutic options for neurodegenerative disorders become available, the use of high-throughput sequencing will be an important means to rapidly diagnose patients and facilitate timely therapy. Using the entire family for exome analysis greatly aids in filtering large numbers of genetic variants consistent with the principles of mendelian inheritance. One of the confounding factors of high-throughput sequencing for clinical diagnostics is manipulating large amounts of data. With the advent of more robust bioinformatic tools, high-throughput sequencing has the potential both to revolutionize the diagnostic evaluation of neurogenetic disorders and to expand our knowledge of disease pathogenesis.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Shannon McNeil, Ronald Austin, Jose Salas, and Cheryl Hipple for administrative assistance; Eva Baker, Joy Bryant, Barrington Burnett, Kenneth Fischbeck, Roxanne Fischer, Miao He, Edwin Kolodny, C.J. Malanga, Greg Pastores, Chevalia Robinson, Bryan Traynor, and Lynne Wolfe for clinical and technical assistance and critical analysis; and the family of the patient for their cooperation with the work.
Footnotes
AUTHOR CONTRIBUTIONS
Study concept and design: Pierson, Adams, Markello, Simeonov, Sincan, Boerkoel, Gahl, and Tifft. Acquisition of data: Pierson, Adams, Markello, Golas, Yang, Simeonov, Sincan, Fuentes-Fajardo, NISC, Mullikin, Boerkoel, Gahl, and Tifft. Analysis and interpretation of data: Pierson, Adams, Markello, Golas, Simeonov, Sincan, NISC, Hansen, Cherukuri, Cruz, Teer, Mullikin, Boerkoel, Gahl, and Tifft. Drafting of the manuscript: Pierson, Adams, Simeonov, Boerkoel, Gahl, and Tifft. Critical revision of the manuscript for important intellectual content: Pierson, Adams, Markello, Simeonov, Sincan, Boerkoel, Gahl, and Tifft. Statistical analysis: Pierson, Adams, Markello, and Boerkoel. Obtained funding: Gahl and Tifft. Administrative, technical, and material support: Pierson, Adams, Markello, Simeonov, Fuentes-Fajardo, and Boerkoel. Study supervision: Pierson, Adams, Boerkoel, Gahl, and Tifft.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1. Zoghbi HY, Warren ST. Neurogenetics: advancing the “next-generation” of brain research. Neuron 2010; 68: 165– 173 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ng SB, Buckingham KJ, Lee C, et al. Exome sequencing identifies the cause of a mendelian disorder. Nat Genet 2010; 42: 30– 35 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Teer JK, Bonnycastle LL, Chines PS, et al. Systematic comparison of three genomic enrichment methods for massively parallel DNA sequencing. Genome Res 2010; 20: 1420– 1431 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Johnston JJ, Teer JK, Cherikuri PF, et al. Massively parallel sequencing of exons on the X chromosome identifies RBM10 as the gene that causes a syndromic form of cleft palate. Am J Hum Genet 2010; 86: 743– 748 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kaye EM, Shalish C, Livermore J, Taylor HA, Stevenson RE, Breakefield XO. β-Galactosidase gene mutations in patients with slowly progressive GM1 gangliosidosis. J Child Neurol 1997; 12: 242– 247 . [DOI] [PubMed] [Google Scholar]
- 6. Yoshida K, Oshima A, Shimmoto M, et al. Human β-galactosidase gene mutations in GM1-gangliosidosis: a common mutation among Japanese adult/chronic cases. Am J Hum Genet 1991; 49: 435– 442 . [PMC free article] [PubMed] [Google Scholar]
- 7. Nishimoto J, Nanba E, Inui K, Okada S, Suzuki K. GM1-gangliosidosis (genetic β-galactosidase deficiency): identification of four mutations in different clinical phenotypes among Japanese patients. Am J Hum Genet 1991; 49: 566– 574 . [PMC free article] [PubMed] [Google Scholar]
- 8. Brunetti-Pierri N, Scaglia F. GM1 gangliosidosis: review of clinical, molecular, and therapeutic aspects. Mol Genet Metab 2008; 94: 391– 396 . [DOI] [PubMed] [Google Scholar]
- 9. Caciotti A, Garman SC, Rivera-Colón Y, et al. GM1 gangliosidosis and Morquio B disease: An update on genetic alterations and clinical findings. Biochim Biophys Acta 2011; 1812: 782– 790 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Okada S, O'Brien JS. Generalized gangliosidosis: β-galactosidase deficiency. Science 1968; 160: 1002– 1004 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.