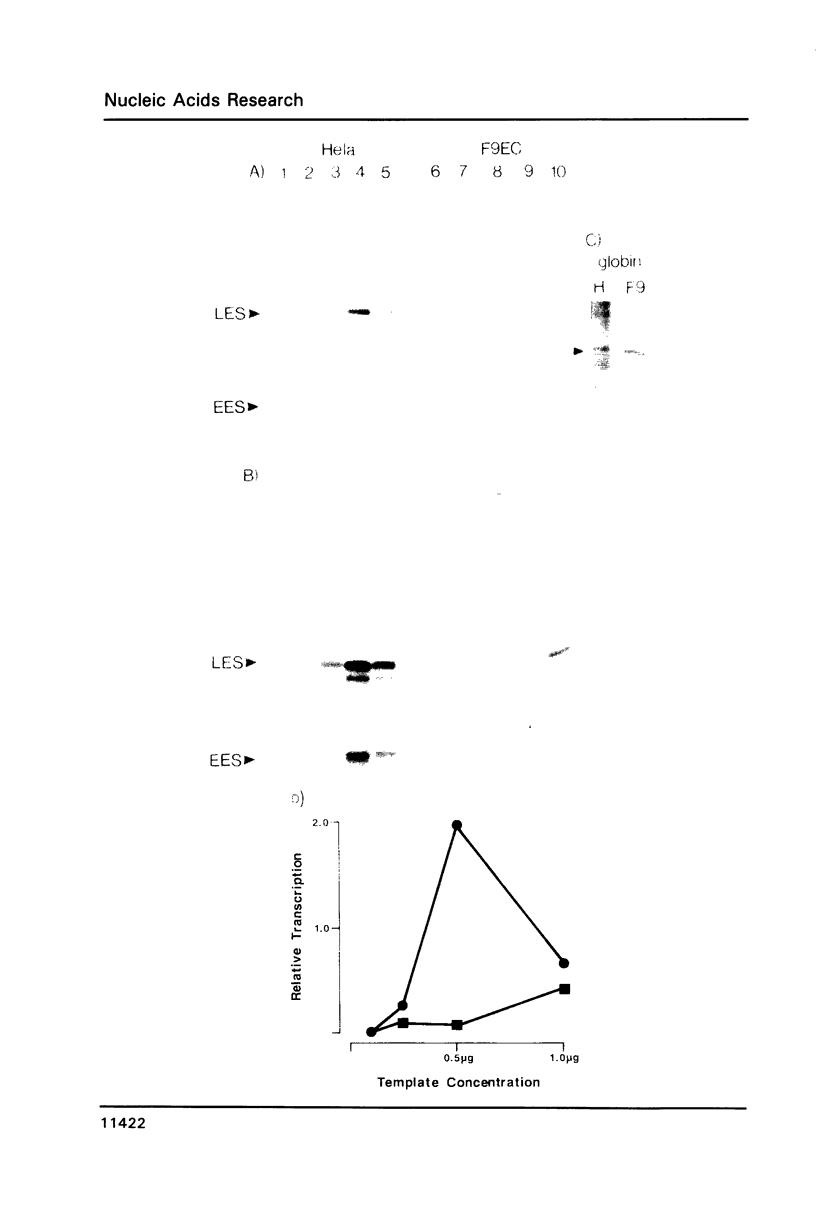
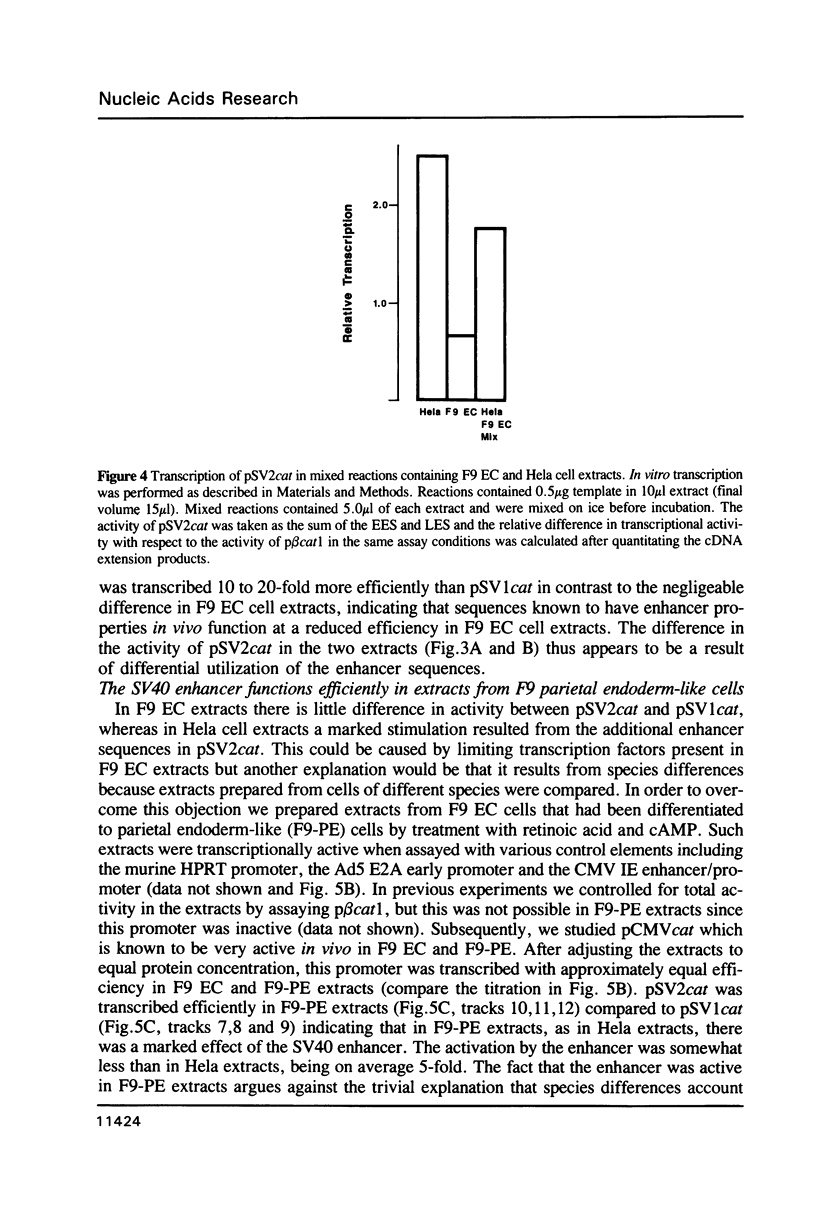
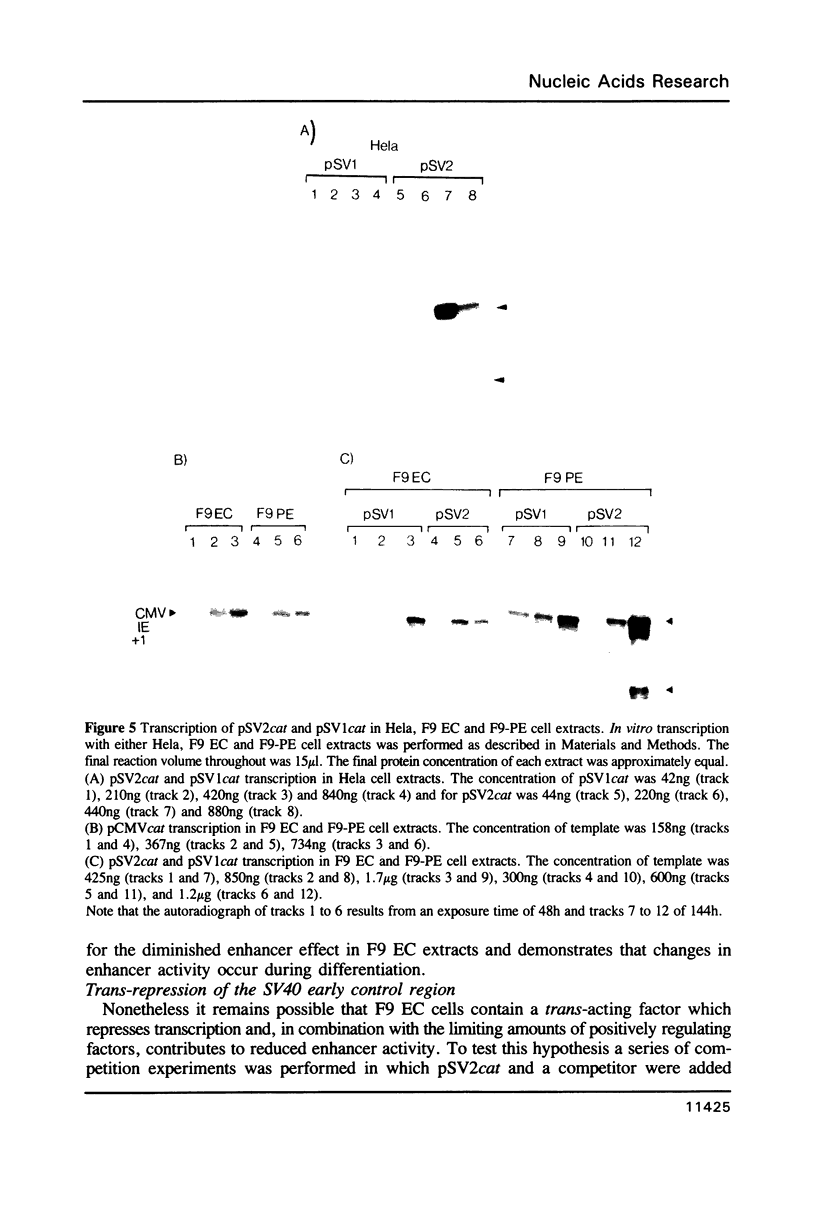
Abstract
We have derived from F9 murine embryonal carcinoma stem cells, and from differentiated derivatives induced with retinoic acid and cAMP, whole cell extracts which efficiently and accurately transcribe a variety of supercoiled DNA templates in vitro. These extracts and control elements from viral genomes have been used to study changes in transcriptional activity which accompany differentiation. The SV40 enhancer is inefficiently utilized in stem cells but is activated upon differentiation to parietal endoderm and the in vitro systems mimick this regulation. Mixing experiments demonstrate that the differentiated cell phenotype is dominant, suggesting that stem cells contain limiting amounts of factors required for enhancer activity. Our results may explain the developmental regulation of cellular genes with enhancer motifs in their control sequences.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bensaude O., Babinet C., Morange M., Jacob F. Heat shock proteins, first major products of zygotic gene activity in mouse embryo. Nature. 1983 Sep 22;305(5932):331–333. doi: 10.1038/305331a0. [DOI] [PubMed] [Google Scholar]
- Davidson I., Fromental C., Augereau P., Wildeman A., Zenke M., Chambon P. Cell-type specific protein binding to the enhancer of simian virus 40 in nuclear extracts. Nature. 1986 Oct 9;323(6088):544–548. doi: 10.1038/323544a0. [DOI] [PubMed] [Google Scholar]
- Dynan W. S., Tjian R. The promoter-specific transcription factor Sp1 binds to upstream sequences in the SV40 early promoter. Cell. 1983 Nov;35(1):79–87. doi: 10.1016/0092-8674(83)90210-6. [DOI] [PubMed] [Google Scholar]
- Dziadek M., Adamson E. Localization and synthesis of alphafoetoprotein in post-implantation mouse embryos. J Embryol Exp Morphol. 1978 Feb;43:289–313. [PubMed] [Google Scholar]
- Everett R. D., Dunlop M. Trans activation of plasmid-borne promoters by adenovirus and several herpes group viruses. Nucleic Acids Res. 1984 Aug 10;12(15):5969–5978. doi: 10.1093/nar/12.15.5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett R. D. The products of herpes simplex virus type 1 (HSV-1) immediate early genes 1, 2 and 3 can activate HSV-1 gene expression in trans. J Gen Virol. 1986 Nov;67(Pt 11):2507–2513. doi: 10.1099/0022-1317-67-11-2507. [DOI] [PubMed] [Google Scholar]
- Ghosh P. K., Lebowitz P. Simian virus 40 early mRNA's contain multiple 5' termini upstream and downstream from a Hogness-Goldberg sequence; a shift in 5' termini during the lytic cycle is mediated by large T antigen. J Virol. 1981 Oct;40(1):224–240. doi: 10.1128/jvi.40.1.224-240.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Merlino G. T., Willingham M. C., Pastan I., Howard B. H. The Rous sarcoma virus long terminal repeat is a strong promoter when introduced into a variety of eukaryotic cells by DNA-mediated transfection. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6777–6781. doi: 10.1073/pnas.79.22.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Rigby P. W., Lane D. P. Negative regulation of viral enhancers in undifferentiated embryonic stem cells. Cell. 1985 Sep;42(2):519–526. doi: 10.1016/0092-8674(85)90109-6. [DOI] [PubMed] [Google Scholar]
- Herbomel P., Bourachot B., Yaniv M. Two distinct enhancers with different cell specificities coexist in the regulatory region of polyoma. Cell. 1984 Dec;39(3 Pt 2):653–662. doi: 10.1016/0092-8674(84)90472-0. [DOI] [PubMed] [Google Scholar]
- Imperiale M. J., Kao H. T., Feldman L. T., Nevins J. R., Strickland S. Common control of the heat shock gene and early adenovirus genes: evidence for a cellular E1A-like activity. Mol Cell Biol. 1984 May;4(5):867–874. doi: 10.1128/mcb.4.5.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N. C., Rigby P. W., Ziff E. B. Trans-acting protein factors and the regulation of eukaryotic transcription: lessons from studies on DNA tumor viruses. Genes Dev. 1988 Mar;2(3):267–281. doi: 10.1101/gad.2.3.267. [DOI] [PubMed] [Google Scholar]
- Kimura A., Israël A., Le Bail O., Kourilsky P. Detailed analysis of the mouse H-2Kb promoter: enhancer-like sequences and their role in the regulation of class I gene expression. Cell. 1986 Jan 31;44(2):261–272. doi: 10.1016/0092-8674(86)90760-9. [DOI] [PubMed] [Google Scholar]
- Kryszke M. H., Piette J., Yaniv M. Induction of a factor that binds to the polyoma virus A enhancer on differentiation of embryonal carcinoma cells. Nature. 1987 Jul 16;328(6127):254–256. doi: 10.1038/328254a0. [DOI] [PubMed] [Google Scholar]
- La Thangue N. B., Rigby P. W. An adenovirus E1A-like transcription factor is regulated during the differentiation of murine embryonal carcinoma stem cells. Cell. 1987 May 22;49(4):507–513. doi: 10.1016/0092-8674(87)90453-3. [DOI] [PubMed] [Google Scholar]
- Lee W., Haslinger A., Karin M., Tjian R. Activation of transcription by two factors that bind promoter and enhancer sequences of the human metallothionein gene and SV40. Nature. 1987 Jan 22;325(6102):368–372. doi: 10.1038/325368a0. [DOI] [PubMed] [Google Scholar]
- Lee W., Mitchell P., Tjian R. Purified transcription factor AP-1 interacts with TPA-inducible enhancer elements. Cell. 1987 Jun 19;49(6):741–752. doi: 10.1016/0092-8674(87)90612-x. [DOI] [PubMed] [Google Scholar]
- Linnenbach A., Huebner K., Croce C. M. DNA-transformed murine teratocarcinoma cells: regulation of expression of simian virus 40 tumor antigen in stem versus differentiated cells. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4875–4879. doi: 10.1073/pnas.77.8.4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linney E., Davis B., Overhauser J., Chao E., Fan H. Non-function of a Moloney murine leukaemia virus regulatory sequence in F9 embryonal carcinoma cells. 1984 Mar 29-Apr 4Nature. 308(5958):470–472. doi: 10.1038/308470a0. [DOI] [PubMed] [Google Scholar]
- Luciw P. A., Bishop J. M., Varmus H. E., Capecchi M. R. Location and function of retroviral and SV40 sequences that enhance biochemical transformation after microinjection of DNA. Cell. 1983 Jul;33(3):705–716. doi: 10.1016/0092-8674(83)90013-2. [DOI] [PubMed] [Google Scholar]
- Manley J. L., Fire A., Cano A., Sharp P. A., Gefter M. L. DNA-dependent transcription of adenovirus genes in a soluble whole-cell extract. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3855–3859. doi: 10.1073/pnas.77.7.3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight S. L., Kingsbury R. Transcriptional control signals of a eukaryotic protein-coding gene. Science. 1982 Jul 23;217(4557):316–324. doi: 10.1126/science.6283634. [DOI] [PubMed] [Google Scholar]
- Mitchell P. J., Wang C., Tjian R. Positive and negative regulation of transcription in vitro: enhancer-binding protein AP-2 is inhibited by SV40 T antigen. Cell. 1987 Sep 11;50(6):847–861. doi: 10.1016/0092-8674(87)90512-5. [DOI] [PubMed] [Google Scholar]
- Nevins J. R. Mechanism of activation of early viral transcription by the adenovirus E1A gene product. Cell. 1981 Oct;26(2 Pt 2):213–220. doi: 10.1016/0092-8674(81)90304-4. [DOI] [PubMed] [Google Scholar]
- Nomiyama H., Fromental C., Xiao J. H., Chambon P. Cell-specific activity of the constituent elements of the simian virus 40 enhancer. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7881–7885. doi: 10.1073/pnas.84.22.7881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petterson M., Schaffner W. A purine-rich DNA sequence motif present in SV40 and lymphotropic papovavirus binds a lymphoid-specific factor and contributes to enhancer activity in lymphoid cells. Genes Dev. 1987 Nov;1(9):962–972. doi: 10.1101/gad.1.9.962. [DOI] [PubMed] [Google Scholar]
- Rosales R., Vigneron M., Macchi M., Davidson I., Xiao J. H., Chambon P. In vitro binding of cell-specific and ubiquitous nuclear proteins to the octamer motif of the SV40 enhancer and related motifs present in other promoters and enhancers. EMBO J. 1987 Oct;6(10):3015–3025. doi: 10.1002/j.1460-2075.1987.tb02607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal A., Wright S., Cedar H., Flavell R., Grosveld F. Regulated expression of an introduced MHC H-2K bm1 gene in murine embryonal carcinoma cells. Nature. 1984 Aug 2;310(5976):415–418. doi: 10.1038/310415a0. [DOI] [PubMed] [Google Scholar]
- Sassone-Corsi P., Dougherty J. P., Wasylyk B., Chambon P. Stimulation of in vitro transcription from heterologous promoters by the simian virus 40 enhancer. Proc Natl Acad Sci U S A. 1984 Jan;81(2):308–312. doi: 10.1073/pnas.81.2.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassone-Corsi P., Fromental C., Chambon P. A trans-acting factor represses the activity of the polyoma virus enhancer in undifferentiated embryonal carcinoma cells. Oncogene Res. 1987 Jul;1(2):113–119. [PubMed] [Google Scholar]
- Segal S., Khoury G. Differentiation as a requirement for simian virus 40 gene expression in F-9 embryonal carcinoma cells. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5611–5615. doi: 10.1073/pnas.76.11.5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirayoshi Y., Miyazaki J., Burke P. A., Hamada K., Appella E., Ozato K. Binding of multiple nuclear factors to the 5' upstream regulatory element of the murine major histocompatibility class I gene. Mol Cell Biol. 1987 Dec;7(12):4542–4548. doi: 10.1128/mcb.7.12.4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleigh M. J. Differential regulation of viral and cellular genes in F9 mouse embryonal carcinoma cells. Nucleic Acids Res. 1987 Nov 25;15(22):9379–9395. doi: 10.1093/nar/15.22.9379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleigh M. J., Lockett T. J., Kelly J., Lewy D. Competition studies with repressors and activators of viral enhancer function in F9 mouse embryonal carcinoma cells. Nucleic Acids Res. 1987 May 26;15(10):4307–4324. doi: 10.1093/nar/15.10.4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleigh M. J., Lockett T. J. SV40 enhancer activation during retinoic acid-induced differentiation of F9 embryonal carcinoma cells. EMBO J. 1985 Dec 30;4(13B):3831–3837. doi: 10.1002/j.1460-2075.1985.tb04155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley J. R., Swan H., Pater M. M., Pater A., Halpern M. E. Positive control of the herpes simplex virus thymidine kinase gene requires upstream DNA sequences. J Virol. 1983 Aug;47(2):301–310. doi: 10.1128/jvi.47.2.301-310.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speck N. A., Baltimore D. Six distinct nuclear factors interact with the 75-base-pair repeat of the Moloney murine leukemia virus enhancer. Mol Cell Biol. 1987 Mar;7(3):1101–1110. doi: 10.1128/mcb.7.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm R., Baumruker T., Franza B. R., Jr, Herr W. A 100-kD HeLa cell octamer binding protein (OBP100) interacts differently with two separate octamer-related sequences within the SV40 enhancer. Genes Dev. 1987 Dec;1(10):1147–1160. doi: 10.1101/gad.1.10.1147. [DOI] [PubMed] [Google Scholar]
- Swartzendruber D. E., Lehman J. M. Neoplastic differentiation: interaction of simian virus 40 and polyoma virus with murine teratocarcinoma cells in vitro. J Cell Physiol. 1975 Apr;85(2 Pt 1):179–187. doi: 10.1002/jcp.1040850204. [DOI] [PubMed] [Google Scholar]
- Teich N. M., Weiss R. A., Martin G. R., Lowy D. R. Virus infection of murine teratocarcinoma stem cell lines. Cell. 1977 Dec;12(4):973–982. doi: 10.1016/0092-8674(77)90162-3. [DOI] [PubMed] [Google Scholar]
- Vogt T. F., Compton R. S., Scott R. W., Tilghman S. M. Differential requirements for cellular enhancers in stem and differentiated cells. Nucleic Acids Res. 1988 Jan 25;16(2):487–500. doi: 10.1093/nar/16.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildeman A. G., Sassone-Corsi P., Grundström T., Zenke M., Chambon P. Stimulation of in vitro transcription from the SV40 early promoter by the enhancer involves a specific trans-acting factor. EMBO J. 1984 Dec 20;3(13):3129–3133. doi: 10.1002/j.1460-2075.1984.tb02269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildeman A. G., Zenke M., Schatz C., Wintzerith M., Grundström T., Matthes H., Takahashi K., Chambon P. Specific protein binding to the simian virus 40 enhancer in vitro. Mol Cell Biol. 1986 Jun;6(6):2098–2105. doi: 10.1128/mcb.6.6.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J. H., Davidson I., Ferrandon D., Rosales R., Vigneron M., Macchi M., Ruffenach F., Chambon P. One cell-specific and three ubiquitous nuclear proteins bind in vitro to overlapping motifs in the domain B1 of the SV40 enhancer. EMBO J. 1987 Oct;6(10):3005–3013. doi: 10.1002/j.1460-2075.1987.tb02606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenke M., Grundström T., Matthes H., Wintzerith M., Schatz C., Wildeman A., Chambon P. Multiple sequence motifs are involved in SV40 enhancer function. EMBO J. 1986 Feb;5(2):387–397. doi: 10.1002/j.1460-2075.1986.tb04224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]