Abstract
Introduction:
Research has identified at least two positive reinforcement-related effects of nicotine: (a) primary reinforcement and (b) enhancement of reinforcement from concurrently available stimuli. Prior examples of the reinforcement-enhancing effects with rats showed that repeated, intermittent nicotine exposure increased responding for non-nicotine reinforcers, and this effect remained robust over several weeks. However, the effects of continuous nicotine exposure on responding for a non-nicotine reinforcer are unknown, as are the effects of abruptly withdrawing continuous nicotine on behavior maintained by the same reinforcer.
Methods:
Lever pressing for a visual reinforcer under a fixed ratio schedule was assessed while rats were maintained on a chronic, continuous infusion of nicotine (3.16 mg/kg/day; osmotic minipump). The effects of precipitated withdrawal on responding, following 16 days of continuous nicotine exposure, were assessed by pre-session subcutaneous injections of mecamylamine (1.0 mg/kg).
Results:
Continuous nicotine initially increased active responding for the visual reinforcer; however, continued exposure resulted in an attenuation of this effect. Precipitated withdrawal from nicotine resulted in a significant decline in active responding.
Conclusions:
The initial increase in responding for the visual reinforcer with chronic nicotine exposure is consistent with prior research showing that intermittent exposure to nicotine acts as a reinforcement enhancer. However, the attenuation of this enhancement following prolonged nicotine exposure is in contrast with the persistent effects previously reported. Finally, the decrease in visual reinforcers below control levels (nicotine-naive animals) following nicotine withdrawal highlights a potential for affective withdrawal, which may serve as a motive for continued nicotine use.
Introduction
Nicotine, the primary psychoactive ingredient in tobacco, is a widely abused substance (Rose & Corrigall, 1997; Stolerman & Jarvis, 1995). However, the high dependence potential of nicotine has been difficult to explain on the basis of its relatively weak primary reinforcing effects. Whereas animals will self-administer modest amounts of nicotine when presented alone, they robustly self-administer nicotine when it is paired with other reinforcers (Caggiula, Donny, Chaudhri, et al., 2002; Caggiula, Donny, White, et al., 2002). Furthermore, nicotine dramatically increases responding for other reinforcers even when nicotine delivery is not contingent on the animal’s behavior (Donny et al., 2003) or when nicotine is concurrently available through another operant (Palmatier et al., 2006). These outcomes have led to the dual reinforcement model, which recognizes two actions of nicotine: (a) it is a primary reinforcer and (b) it enhances the reinforcing effects of concurrently available stimuli (Caggiula et al., 2009). These effects are consistent with data from studies measuring intracranial self-stimulation that show nicotine increases sensitivity of the neural pathways mediating reward (Bauco & Wise, 1994; Huston-Lyons & Kornetsky, 1992; Kenny & Markou, 2006). Although early tests of the reinforcement-enhancing effect with humans have been equivocal (Barr, Pizzagalli, Culhane, Goff, & Evins, 2008; Perkins, Grottenthaler, & Wilson, 2009), the dual reinforcement model has the potential to explain the apparent paradox between high rates of nicotine dependence despite its mild primary reinforcing properties.
In addition to the effects outlined in the dual reinforcement model, continued nicotine use by humans may be motivated by a desire to alleviate or prevent withdrawal symptoms (Kenny & Markou, 2001; Koob & Le Moal, 1997; Watkins, Stinus, Koob, & Markou, 2000). Withdrawal from prolonged nicotine exposure in rodent models is associated with changes in somatic (e.g., writhing, ptosis) and “affective” symptoms (e.g., increased brain stimulation reward thresholds). These clusters of symptoms have been dissociated (Epping-Jordan, Watkins, Koob, & Markou, 1998), and the latter affective reward decrements are hypothesized to play a greater role in the motivation to relapse (Koob, Markou, Weiss, & Schulteis, 1993; Markou, Kosten, & Koob, 1998).
This experiment included two aims: (a) to characterize the reinforcement-enhancing effects of continuous nicotine and (b) to assess potential decrements in reinforced behavior during precipitated withdrawal. Meeting these aims was accomplished by allowing rats to respond for an unconditioned visual stimulus when continuously exposed to nicotine via osmotic minipump and during mecamylamine-precipitated withdrawal from nicotine. Experiments using constant infusion of nicotine for at least 7 days have consistently shown evidence of withdrawal following mecamylamine injection (O’Dell et al., 2006; Watkins, Koob, & Markou, 2000; Watkins, Stinus, Koob, & Markou, 2000; Wilmouth & Spear, 2006).
Methods
Subjects
Male Sprague-Dawley rats (Harlan Farms®) weighing between 200 and 225 g, upon arrival, were individually housed in wire bottom cages in a temperature-controlled environment. Rats were exposed to a 12-hr reversed light/dark cycle with the dark cycle beginning at 7:00 a.m. Rats had ad libitum access to Purina Rat Chow® and water until the start of the study. During the study, rats were restricted to 20 g of food per day (Donny, Caggiula, Knopf, & Brown, 1995), but still had free access to water in the home cage. Experiments 1 and 2 included 27 and 68 rats, respectively.
Apparatus
Experimental sessions occurred in 14 operant conditioning chambers (BRS/LVE Model RTC-020, MD), which were enclosed in sound-attenuated cubicles. One wall of the chamber included two horizontally aligned retractable levers that were positioned 3 cm above the floor. A pellet dispenser was centrally positioned between the levers, 2 cm above the floor. Three 125-V horizontally aligned stimulus lights were located 5 cm above each lever, but only the central light (white) was operable. A 28-V houselight was centrally located 1 cm from the ceiling on the opposite wall. Extraneous sounds were masked by a fan located within the cubicle. Experimental events were controlled and recorded by Med-PC® software (Med Associates, St Albans, VT).
Procedures
Drugs
Solutions were delivered via subcutaneous (s.c.) injections or 28-day osmotic minipumps (Model-2004 Alzet®, Cupertino, CA). Nicotine bitartrate (3.16 mg/kg/day; dose expressed as free base) for the chronic phase was administered via osmotic minipump; to maximize stability in solution, pH was not adjusted (Matta et al., 2007). This dose of nicotine has been reported to produce stable nicotine plasma levels of 44 ng/ml for up to 28 days (Kenny, Gasparini, & Markou, 2003), which are equivalent to those obtained in humans who smoke approximately 30 cigarettes/day (Benowitz, 1988). For acute injections, nicotine was dissolved into 0.9% saline solution, and the pH was adjusted to 7.0 ± 0.2 with NaOH. An acute injection of nicotine (0.4 mg/kg) or saline occurred 5 min before or 1 hr after the session in the subjects’ home cage. Mecamylamine was dissolved into 0.9% saline solution with pH adjusted to 7.0 ± 0.2 with NaOH. Injections of mecamylamine (1.0 mg/kg s.c.) were delivered 1 hr before the session. All s.c. injections were located caudal to the minipump, near the hip.
Surgery
Minipumps containing either nicotine or saline were implanted under isoflurane anesthesia. Minipumps were filled 24 hr prior to surgery and incubated in a saline bath. An incision was made in the skin between the scapulae, and the minipump was inserted into an s.c. pocket with the flow moderator pointed away from the incision. An analgesic agent (0.5% bupivacaine) was applied topically before the incision and when the site was closed with sutures. Isoflurane was terminated after the incision site was closed; the next test session began 2–3 hr later.
Behavioral Procedure
Sessions occurred once daily at roughly the same time during the dark cycle and lasted for 1 hr. Response shaping procedures, which reinforced responding equally on both the left and the right levers with 45 mg food pellets, are described in detail elsewhere (Palmatier et al., 2006). After shaping behavior on both levers, one was randomly assigned to be active and the other inactive. Both levers were available during sessions, but the inactive lever was not associated with any programmed consequence. The chamber was illuminated by a white houselight during experimental sessions. Active lever responses were reinforced by an unconditioned visual reinforcer (visual stimulus [VS]), which consisted of a 1-s stimulus light onset followed by termination of the houselight lasting 1 min (e.g., Caggiula, Donny, Chaudhri, et al., 2002; Caggiula, Donny, White, et al., 2002). Responding was maintained under a fixed ratio (FR)1 for the first five sessions and then an FR2 for the remainder of the experiment. Active lever responses made during the VS were recorded but had no programmed consequence.
Experiment 1
Baseline responding for the VS was measured over 10 weekday sessions. s.c. saline injections occurred prior to sessions 8–10 as a means to habituate the animals to the injection procedure, and all rats were given pre-session injections of nicotine on sessions 11–15 (Monday–Friday: acute injection phase). Minipumps containing either nicotine (n = 14) or saline (n = 13) were implanted approximately 3 hr before session 16, which followed 2 days (weekend) without nicotine injections (chronic nicotine phase). Pre-session mecamylamine or saline injections occurred on sessions 27–30 (mecamylamine antagonism phase). Injection order was counterbalanced within groups, so that half of the group received two consecutive sessions of mecamylamine exposure and then two consecutive sessions of saline exposure, while the order was reversed for the other half.
Experiment 2
Experiment 2 was identical in design to Experiment 1 with one exception: the acute injection phase (sessions 11–15) consisted of pre-session nicotine (N = 22; nicotine administered 5 min prior to session), post-session nicotine (N = 22; nicotine administered 1 hr after session), or pre-session saline (N = 24; saline administered 5 min prior to session).
Data Analysis
Primary data analyses and discussion were based on active lever responding. Outcomes were analyzed using analysis of variance (ANOVA) with one or more of the following factors: session, chronic nicotine (nicotine vs. saline), mecamylamine antagonism (mecamylamine vs. saline), acute injection (pre-session nicotine vs. post-session nicotine vs. pre-session saline), and phase (baseline, acute injection, chronic nicotine, and/or mecamylamine antagonism). Additionally, supplementary ANOVAs included a lever (active vs. inactive) factor. Planned comparisons were used to further describe some effects within treatment phases. Results using active lever data were similar to those using VS presentations, and, for the sake of brevity, the latter were not reported. All estimates of within-subject effects of session were specified using linear contrasts. All alpha levels were set to p < .05, and data are presented as mean ± SEM.
Results
Experiment 1: Active Response Data
Intermittent pre-session nicotine (sessions 11–15: acute injection phase; all animals received nicotine) significantly increased the mean number of active responses above baseline levels, F(1, 26) = 100.9, p < .001 (Figure 1).
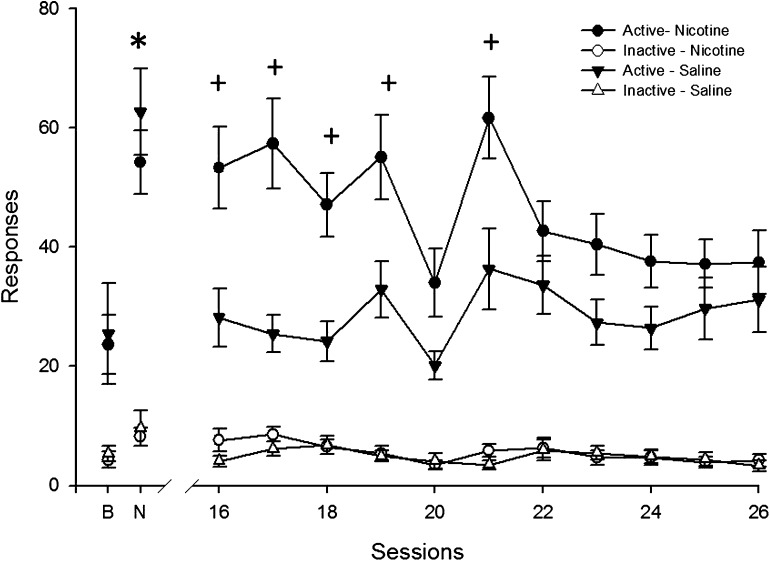
Figure 1.
Mean active and inactive responding for the visual stimulus during all sessions of chronic nicotine or saline exposure are presented for Experiment 1. Mean responding from the final day of baseline and acute nicotine injections are presented above “B” and “N,” respectively. Circles correspond to data from the group chronically exposed to nicotine (N = 14) and triangles represent the group chronically exposed to saline (N = 13). Filled symbols pertain to active responses and open symbols inactive response data. Error bars represent SEM. An asterisk indicates a significant within-subject effect of acute nicotine on active responding (p < .001). Crosses indicate a statistically significant effect of nicotine-infused pumps for individual sessions (p < .001). Active responding was significantly greater than inactive responding in all phases (ps < .001), and there were no significant interactions or main effects when data analysis was restricted to inactive responding.
Continuous nicotine (chronic nicotine phase) significantly increased the number of active responses above continuous saline, F(1, 25) = 3.2, p < .01 (Figure 1). This effect decreased over time, chronic nicotine by session interaction: F(1, 25) = 10.2, p < .01, such that differences that were statistically significant during the first session (session 16: p < .001) were no longer statistically significant by the final session before mecamylamine antagonism (session 26: nonsignificant; Figure 1).
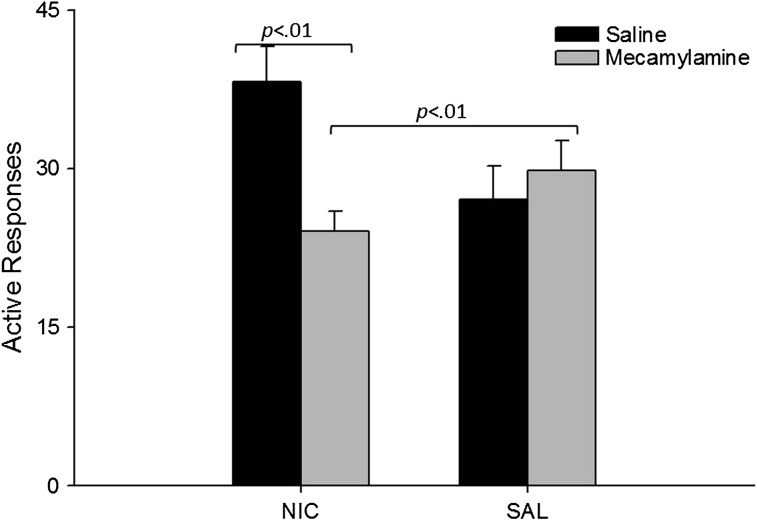
Mecamylamine antagonism resulted in a significant decrease in active responding that was dependent on pump solution, F(1, 25) = 6.5, p < .01 (Figure 2). In animals exposed to nicotine-filled minipumps, mecamylamine significantly reduced responding relative to saline injections, F(1, 13) = 14.5, p < .01 (Figure 2). There were no significant differences between animals exposed to nicotine pumps and mecamylamine injections relative to the drug-naive group.
Figure 2.
Mean active responding for the visual stimulus during sessions preceded by mecamylamine or saline (counterbalanced) for Experiment 1 are presented as a function of minipump solution. Black bars correspond to data from animals exposed to pre-session saline, and the gray bars correspond to pre-session mecamylamine. Error bars represent the SEM. Although not presented, active responses were greater than inactive responses (p < .001), and there were no significant interactions or main effects when data analysis was restricted to inactive responding.
Experiment 1: Analyses Including Inactive Responding
All above analyses were performed with the addition of a lever factor, and these outcomes conformed to those reported previously. Additionally, an omnibus ANOVA showed that active responses (31.4 ± 2.4) were significantly greater than inactive responses (12.8 ± 2.4), F(1, 54) = 29.7, p < .001. Finally, there were no statistically significant outcomes when the analyses were restricted to inactive responding.
Experiment 2: Active Response Data
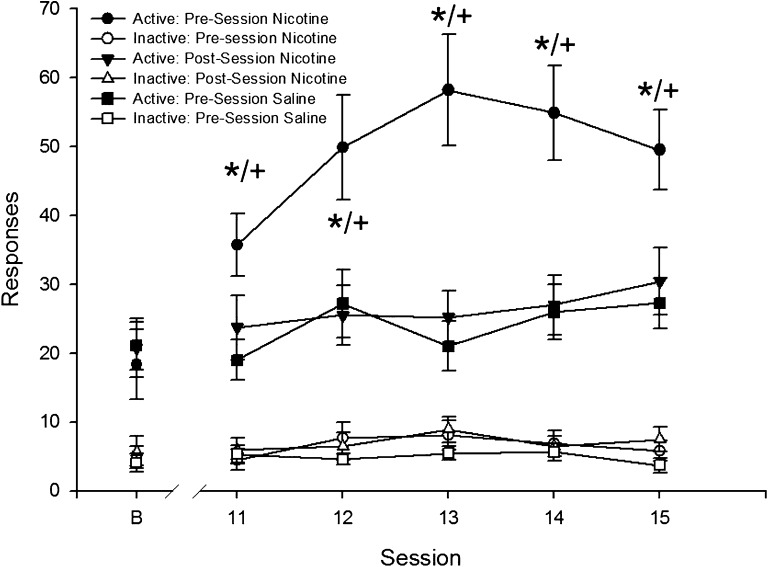
Pre-session treatment with nicotine led to greater responding than post-session nicotine or pre-session saline, F(2, 64) = 11.8, p < .001 (Figure 3). Pairwise tests revealed greater active responding in the pre-session nicotine group compared with both saline pre-session (p < .001) and nicotine post-session (p < .001).
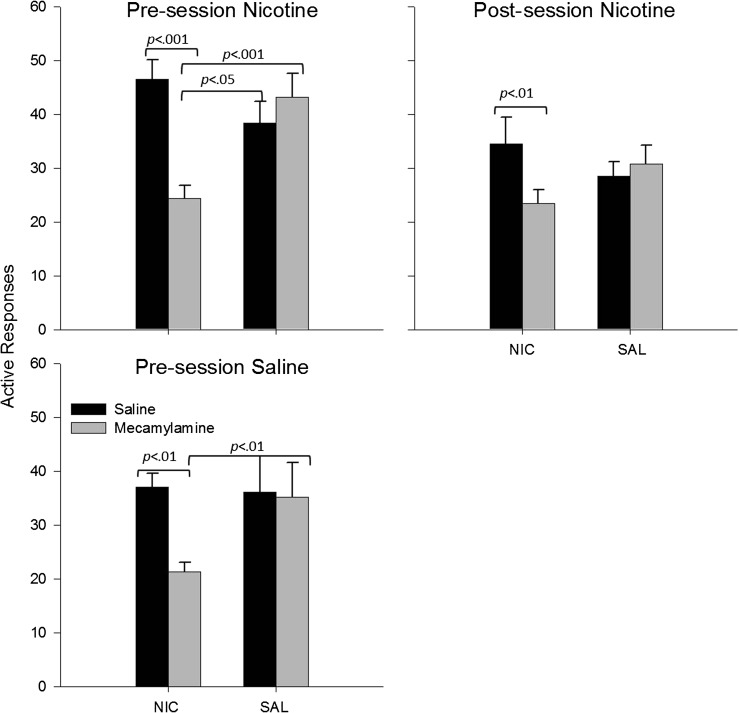
Figure 3.
Mean active and inactive responding for the visual stimulus during the acute injection phase of Experiment 2 are presented as a function of session. Mean responding from the final day of baseline is presented above “B.” Circles represent the group injected with nicotine prior to the session; triangles, data from the group injected with nicotine after the session; and squares, the group injected with saline before the session. Filled symbols represent active response data and open symbols inactive responses. Error bars represent the SEM. Asterisks represent a significant pairwise differences between pre-session and post-session nicotine (p < .001), and crosses represent significant pairwise differences between pre-session nicotine and saline (p < .001). Active responding was significantly greater than inactive responding in all phases (ps < .001), and there were no significant interactions or main effects when data analysis was restricted to inactive responding.
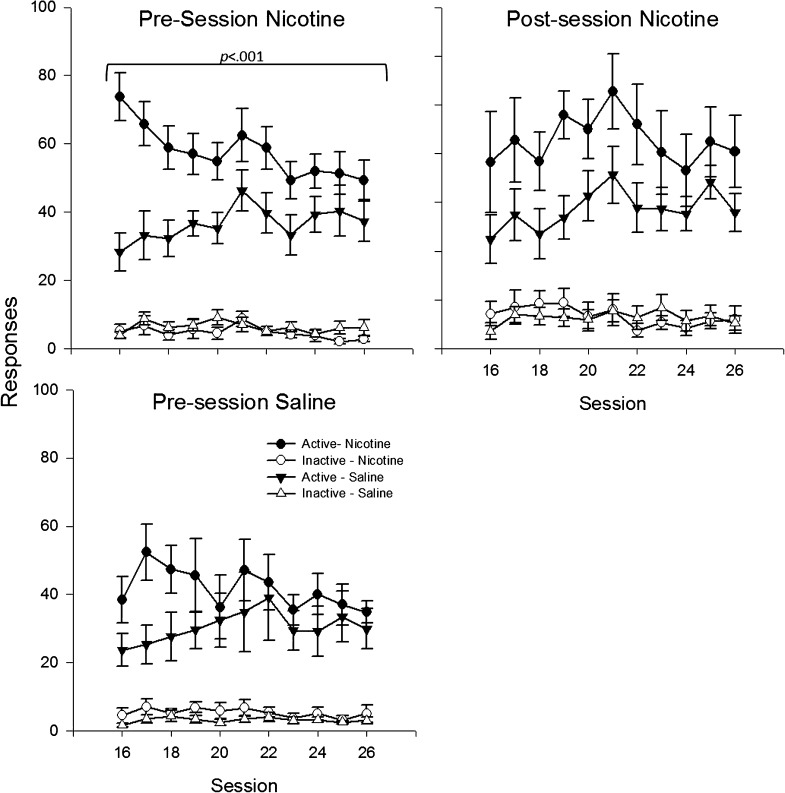
Continuous nicotine, via the minipump, increased responding relative to continuous saline across groups with different acute drug injection histories, F(1, 61) = 8.4, p < .01 (Figure 4); the overall ANOVA failed to reveal a significant main effect of acute injection or an interaction between acute injection and chronic nicotine. Comparisons restricted to the three different acute injection groups revealed a significant main effect of chronic nicotine in the nicotine pre-session animals, F(1, 22) = 6.9, p < .01, a trend toward significance in the nicotine post-session animals, F(1, 20) = 3.4, p < .07, but failed to reveal either a trend or significant differences for rats exposed to pre-session saline. In addition to the main effect of chronic nicotine, there was a chronic nicotine by linear session interaction, F(1, 61) = 12.0, p < .001, which indicated an overall decrease in the enhancement effect over time; however, the three-way interaction (acute injection by chronic nicotine by session) was not significant. ANOVAs restricted to the different acute injection groups revealed a chronic nicotine by session interaction only for the pre-session nicotine group, F(1, 22) = 11.0, p < .01. Finally, chronic nicotine effects were not significant in any acute injection groups by session 26 (Figure 4).
Figure 4.
Mean active and inactive responses for the visual stimulus during the chronic phase of Experiment 2 are presented as a function of session. Data are presented separately for each acute injection condition and are labeled accordingly. Circles represent data from groups that received nicotine-filled minipumps, and triangles indicate groups that received saline-filled minipumps. Filled symbols indicate active response data and open symbols inactive response data.
A comparison within the animals exposed to nicotine pumps revealed that mecamylamine significantly reduced active responses relative to saline injections, F(1, 61) = 11.1, p < .001. As in Experiment 1, mecamylamine antagonism significantly reduced active responding in animals with nicotine-filled pumps relative to animals with saline-filled pumps, F(1, 61) = 37.8, p < .001. Furthermore, a comparison between animals with nicotine-filled pumps that were exposed to mecamylamine and animals naive to both solutions also showed a significant difference, F(1, 62) = 9.5, p < .01 (Figure 5).
Figure 5.
Mean active responses for the visual stimulus during the mecamylamine antagonism phase of Experiment 2 are presented as a function of minipump solution. Data are presented separately for each acute injection condition and are labeled accordingly. Black bars represent data collected following pre-session saline injections, and gray bars correspond to data collected following mecamylamine injections. Mecamylamine and saline were presented in counterbalanced order. Error bars represent SEM. Although not presented, active responses were significantly greater than inactive responses (p < .001), and there were no significant interactions or main effects when data analysis was restricted to inactive responding.
Planned comparisons restricted to the three different acute injection groups were generally consistent with these overall findings. Comparisons within nicotine pump groups showed that mecamylamine significantly reduced active responding in the pre-session nicotine, F(1, 44) = 20.8, p < .001, post-session nicotine, F(1, 40) = 10.8, p < .01, and pre-session saline, F(1, 40) = 9.5, p < .01. Mecamylamine antagonism also significantly reduced active responding for animals with nicotine-filled pumps relative to saline pumps for pre-session nicotine, F(1, 44) = 19.2, p < .001, and pre-session saline groups, F(1, 40) = 13.2, p < .01, but not the post-session nicotine group. Finally, relative to drug-naive animals, responding by animals exposed to continuous nicotine and mecamylamine were significantly lower in pre-session nicotine group, F(1, 22) = 5.0, p < .05, and tended to be lower in pre-session saline, F(1, 20) = 3.8, p = .06, but were not significantly different in post-session nicotine group (Figure 5).
Experiment 2: Analyses Including Inactive Responding
Overall, active responses (33.6 ± 1.4) were significantly greater than inactive responses (4.8 ± 143), F(1, 132) = 194.1, p < .001 (Figure 3). Again, as with Experiment 1, all above analyses were performed with the addition of a lever factor and these too conformed to the reported outcomes. Likewise, there were no statistically significant outcomes when the analyses were restricted to inactive responding.
Discussion
The effectiveness of nicotine in enhancing responding for other reinforcers may be a major factor contributing to its abuse potential. These studies were designed to further assess the effects of nicotine on other reinforcers when nicotine was continuously administered via osmotic minipumps and when withdrawal from nicotine was precipitated with mecamylamine. The significant differences found between active and inactive responding provided evidence that responding on the active lever was maintained by reinforcing actions of the visual stimulus, which is similar to previous studies (Caggiula, Donny, Chaudhri, et al., 2002; Donny et al., 2003; Palmatier et al., 2006). Both experiments revealed enhanced responding for the visual stimulus as a consequence of continuously infused nicotine that dissipated with prolonged exposure. Moreover, withdrawal from continuous nicotine, precipitated by mecamylamine, reduced active responding below control (non-enhanced) levels. This decrement in reinforcer efficacy is consistent with affective withdrawal, which may be a potential motivation for relapse.
The observed decrement in reinforcer efficacy following mecamylamine-precipitated withdrawal is consistent with previous experiments that showed decrements in conditioning to novelty reward (Besheer & Bevins, 2003) and brain reward pathways (Epping-Jordan et al., 1998; Kenny & Markou, 2005; Skjei & Markou, 2003) after nicotine withdrawal. These previous findings have been interpreted as affective symptoms of nicotine withdrawal and potential motivators for relapse, namely, through negative reinforcement theory. The application of negative reinforcement theory to drug dependence posits that abstinence following chronic drug exposure is an aversive event, which in turn motivates further drug use (Kenny & Markou 2001; Koob & Le Moal, 1997; Koob et al., 1993; Watkins, Stinus, Koob, & Markou, 2000). The decrement in reinforcer efficacy for non-nicotine stimuli is a putative aversive event and affective symptom of withdrawal that may motivate subsequent nicotine use.
The current investigation focused on affective symptoms, a reduction in responding for another reinforcer, related to precipitated withdrawal from nicotine. Evidence suggests that precipitated withdrawal, using a variety of compounds, may have differential effects on affective symptoms when compared with spontaneous withdrawal (Markou & Paterson, 2009); therefore, a complete characterization of nicotine withdrawal on motivation to obtain non-nicotine stimuli will require further testing with other compounds (e.g., antagonists) and spontaneous withdrawal. Additionally, data (Donny, Caggiula, Weaver, Levin, & Sved, 2011; Harris, Pentel, Burroughs, Staley, & Lesage, 2011) suggest that withdrawal of nicotine may lead to different outcomes at earlier, as opposed to later, points in the timeline of withdrawal. The current investigation, however, was limited to 2 days of precipitated withdrawal; therefore, a complete characterization calls for a more temporally extended assessment.
In both experiments, continuous nicotine initially resulted in reinforcement enhancement, which was similar in magnitude to that observed in our previous studies with intermittent nicotine. However, the enhancement effect dissipated over days, and by 2 weeks (i.e., 10 sessions and 4 weekend days), the nicotine-exposed group did not significantly differ from saline. This attenuation of the reinforcement-enhancing effects of nicotine has not previously been observed in experiments involving intermittent nicotine exposure (Caggiula, Donny, Chaudhri, et al., 2002; Donny et al., 2003; Palmatier et al., 2006). The attenuation observed in the current experiments may be indicative of tolerance to the reinforcement-enhancing effects of nicotine or extinction of an association between nicotine and the VS established during the injection phase. The latter explanation is similar to one proposed by Rose, Behm, Westman, and Kukovich (2006), suggesting that transdermal nicotine may facilitate extinctions of the conditioned reinforcing properties of smoking stimuli. This interpretation is bolstered by the observation that chronic nicotine had the most robust effect in animals that previously received pre-session acute nicotine injections. Future studies should attempt to disentangle possible extinction processes from an attenuation of the enhancement effect. Likewise, the interpretation of the attenuation based on tolerance to the enhancement will require a thorough dose–response assessment.
Experiment 1 included rats that all had a history of intermittent nicotine exposure prior to minipump implantation to demonstrate the development of the reinforcement enhancement effect, which until the current experiment had only been observed with intermittent dosing regimens (e.g., s.c. and intravenous routes of administration). Experiment 2 was designed to address the effects of nicotine history while simultaneously increasing statistical power regarding effects related to chronic nicotine treatment and mecamylamine antagonism. Although results across pre-treatment conditions were qualitatively similar, the effects of continuous nicotine and mecamylamine were more robust in animals with a history of pre-session nicotine when compared with those with post-session nicotine or saline history. Future studies should further examine the role of nicotine history as a potential moderator of the effects of continuous, chronic nicotine exposure and subsequent withdrawal from nicotine.
It may be important to note that rats in the current experiment were continuously exposed to nicotine as a means to induce withdrawal, but this state of continuous exposure may have limited generality to human tobacco use. That is, unlike the current experimental arrangement, patterns of human tobacco use are not typically continuous but are instead intermittent throughout the waking hours. Thus, further research with methods that may more closely model extended, intermittent human exposure to tobacco (e.g., O’Dell et al., 2007) may also be necessary to generalize the current time-related dissipation of the reinforcement enhancement to human behavior. Additionally, the current dose of nicotine delivered via osmotic minipump (3.16 mg/kg/day) reportedly results in plasma levels of 44 ng/ml (Kenny et al., 2003), but other reports have variable estimates of plasma levels from similar doses and route of administration (Ghosheh, Dwoskin, Miller, & Crooks, 2001; LeSage et al., 2002; Lockman et al., 2005; Nguyen, Rasmussen, & Perry, 2004). Given this discrepancy in the literature, the current model system may not be in complete correspondence with plasma levels observed in human research although it is problematic to equate plasma levels across species such as humans and rats.
In summary, the current investigation included two experiments that both showed continuous nicotine initially enhanced nondrug reinforcement, but this effect dissipated following prolonged exposure. Furthermore, mecamylamine-precipitated withdrawal from chronic nicotine induced a decrement in reinforcement, which establishes the loss in reinforcer efficacy as an affective withdrawal symptom and a potential motivation for relapse.
Funding
This work was supported by the National Institutes of Health (DA10464).
Declaration of Interests
None declared.
References
- Barr RS, Pizzagalli DA, Culhane MA, Goff DC, Evins AE. A single dose of nicotine enhances reward responsiveness in nonsmokers: Implications for development of dependence. Biological Psychiatry. 2008;63:1061–1065. doi: 10.1016/j.biopsych.2007.09.015. doi:S0006-3223(07)00890-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauco P, Wise RA. Potentiation of lateral hypothalamic and midline mesencephalic brain stimulation reinforcement by nicotine: Examination of repeated treatment. Journal of Pharmacology and Experimental Therapeutics. 1994;271:294–301. [PubMed] [Google Scholar]
- Benowitz NL. Drug therapy. Pharmacologic aspects of cigarette smoking and nicotine addition. New England Journal of Medicine. 1988;319:1318–1330. doi: 10.1056/NEJM198811173192005. doi:10.1056/NEJM198811173192005. [DOI] [PubMed] [Google Scholar]
- Besheer J, Bevins RA. Impact of nicotine withdrawal on novelty reward and related behaviors. Behavioral Neuroscience. 2003;117:327–340. doi: 10.1037/0735-7044.117.2.327. doi:10.1037/0735-7044.117.2.327. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, Chaudhri N, Perkins KA, Evans-Martin FF, Sved AF. Importance of nonpharmacological factors in nicotine self-administration. Physiology & Behavior. 2002;77:683–687. doi: 10.1016/s0031-9384(02)00918-6. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, Palmatier MI, Liu X, Chaudhri N, Sved AF. The role of nicotine in smoking: A dual-reinforcement model. Nebraska Symposium on Motivation. 2009;55:91–109. doi: 10.1007/978-0-387-78748-0_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, et al. Environmental stimuli promote the acquisition of nicotine self-administration in rats. Psychopharmacology (Berl) 2002;163:230–237. doi: 10.1007/s00213-002-1156-5. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Knopf S, Brown C. Nicotine self-administration in rats. Psychopharmacology (Berl) 1995;122:390–394. doi: 10.1007/BF02246272. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Weaver MT, Levin ME, Sved AF. The reinforcement-enhancing effects of nicotine: Implications for the relationship between smoking, eating and weight. Physiology & Behavior. 2011;104:143–148. doi: 10.1016/j.physbeh.2011.04.043. doi:10.1016/j.physbeh.2011.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donny EC, Chaudhri N, Caggiula AR, Evans-Martin FF, Booth S, Gharib MA, et al. Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: Implications for nicotine self-administration and reinforcement. Psychopharmacology (Berl) 2003;169:68–76. doi: 10.1007/s00213-003-1473-3. doi:10.1007/s00213-003-1473-3. [DOI] [PubMed] [Google Scholar]
- Epping-Jordan MP, Watkins SS, Koob GF, Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393:76–79. doi: 10.1038/30001. doi:10.1038/30001. [DOI] [PubMed] [Google Scholar]
- Ghosheh OA, Dwoskin LP, Miller DK, Crooks PA. Accumulation of nicotine and its metabolites in rat brain after intermittent or continuous peripheral administration of [2′-(14)C]nicotine. Drug Metabolism and Disposition. 2001;29:645–651. [PubMed] [Google Scholar]
- Harris AC, Pentel PR, Burroughs D, Staley MD, Lesage MG. A lack of association between severity of nicotine withdrawal and individual differences in compensatory nicotine self-administration in rats. Psychopharmacology (Berl) 2011;217:153–166. doi: 10.1007/s00213-011-2273-9. doi:10.1007/s00213-011-2273-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huston-Lyons D, Kornetsky C. Effects of nicotine on the threshold for rewarding brain stimulation in rats. Pharmacology, Biochemistry and Behavior. 1992;41:755–759. doi: 10.1016/0091-3057(92)90223-3. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Gasparini F, Markou A. Group II metabotropic and alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA)/kainate glutamate receptors regulate the deficit in brain reward function associated with nicotine withdrawal in rats. Journal of Pharmacology and Experimental Therapeutics. 2003;306:1068–1076. doi: 10.1124/jpet.103.052027. doi:10.1124/jpet.103.052027. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Markou A. Neurobiology of the nicotine withdrawal syndrome. Pharmacology, Biochemistry and Behavior. 2001;70:531–549. doi: 10.1016/s0091-3057(01)00651-7. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Markou A. Conditioned nicotine withdrawal profoundly decreases the activity of brain reward systems. Journal of Neuroscience. 2005;25:6208–6212. doi: 10.1523/JNEUROSCI.4785-04.2005. doi:25/26/6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ, Markou A. Nicotine self-administration acutely activates brain reward systems and induces a long-lasting increase in reward sensitivity. Neuropsychopharmacology. 2006;31:1203–1211. doi: 10.1038/sj.npp.1300905. doi:1300905. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: Hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Koob GF, Markou A, Weiss F, Schulteis G. Opponent process and drug dependence: Neurobiological mechanisms. Seminars in the Neurosciences. 1993;5:351–358. [Google Scholar]
- LeSage MG, Keyler DE, Shoeman D, Raphael D, Collins G, Pentel PR. Continuous nicotine infusion reduces nicotine self-administration in rats with 23-h/day access to nicotine. Pharmacology, Biochemistry and Behavior. 2002;72:279–289. doi: 10.1016/s0091-3057(01)00775-4. doi:S0091305701007754. [DOI] [PubMed] [Google Scholar]
- Lockman PR, McAfee G, Geldenhuys WJ, Van der Schyf CJ, Abbruscato TJ, Allen DD. Brain uptake kinetics of nicotine and cotinine after chronic nicotine exposure. Journal of Pharmacology and Experimental Therapeutics. 2005;314:636–642. doi: 10.1124/jpet.105.085381. doi:jpet.105.085381. [DOI] [PubMed] [Google Scholar]
- Markou A, Kosten TR, Koob GF. Neurobiological similarities in depression and drug dependence: A self-medication hypothesis. Neuropsychopharmacology. 1998;18:135–174. doi: 10.1016/S0893-133X(97)00113-9. doi:S0893-133X(97)00113-9. [DOI] [PubMed] [Google Scholar]
- Markou A, Paterson NE. Multiple motivational forces contribute to nicotine dependence. Nebraska Symposium on Motivation. 2009;55:65–89. doi: 10.1007/978-0-387-78748-0_5. [DOI] [PubMed] [Google Scholar]
- Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, et al. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology (Berl) 2007;190:269–319. doi: 10.1007/s00213-006-0441-0. doi:10.1007/s00213-006-0441-0. [DOI] [PubMed] [Google Scholar]
- Nguyen HN, Rasmussen BA, Perry DC. Binding and functional activity of nicotinic cholinergic receptors in selected rat brain regions are increased following long-term but not short-term nicotine treatment. Journal of Neurochemistry. 2004;90:40–49. doi: 10.1111/j.1471-4159.2004.02482.x. doi:10.1111/j.1471-4159.2004.02482.x. [DOI] [PubMed] [Google Scholar]
- O’Dell LE, Bruijnzeel AW, Smith RT, Parsons LH, Merves ML, Goldberger BA, et al. Diminished nicotine withdrawal in adolescent rats: Implications for vulnerability to addiction. Psychopharmacology (Berl) 2006;186:612–619. doi: 10.1007/s00213-006-0383-6. doi:10.1007/s00213-006-0383-6. [DOI] [PubMed] [Google Scholar]
- O’Dell LE, Chen SA, Smith RT, Specio SE, Balster RL, Paterson NE, et al. Extended access to nicotine self-administration leads to dependence: Circadian measures, withdrawal measures, and extinction behavior in rats. Journal of Pharmacology and Experimental Therapeutics. 2007;320:180–193. doi: 10.1124/jpet.106.105270. doi:jpet.106.105270. [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Evans-Martin FF, Hoffman A, Caggiula AR, Chaudhri N, Donny EC, et al. Dissociating the primary reinforcing and reinforcement-enhancing effects of nicotine using a rat self-administration paradigm with concurrently available drug and environmental reinforcers. Psychopharmacology (Berl) 2006;184:391–400. doi: 10.1007/s00213-005-0183-4. doi:10.1007/s00213-005-0183-4. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Grottenthaler A, Wilson AS. Lack of reinforcement enhancing effects of nicotine in non-dependent smokers. Psychopharmacology (Berl) 2009;205:635–645. doi: 10.1007/s00213-009-1574-8. doi:10.1007/s00213-009-1574-8. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC, Kukovich P. Precessation treatment with nicotine skin patch facilitates smoking cessation. Nicotine & Tobacco Research. 2006;8:89–101. doi: 10.1080/14622200500431866. [DOI] [PubMed] [Google Scholar]
- Rose JE, Corrigall WA. Nicotine self-administration in animals and humans: Similarities and differences. Psychopharmacology (Berl) 1997;130:28–40. doi: 10.1007/s002130050209. [DOI] [PubMed] [Google Scholar]
- Skjei KL, Markou A. Effects of repeated withdrawal episodes, nicotine dose, and duration of nicotine exposure on the severity and duration of nicotine withdrawal in rats. Psychopharmacology (Berl) 2003;168:280–292. doi: 10.1007/s00213-003-1414-1. doi:10.1007/s00213-003-1414-1. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Jarvis MJ. The scientific case that nicotine is addictive. Psychopharmacology (Berl) 1995;117:2–10. doi: 10.1007/BF02245088. ; discussion 14–20. [DOI] [PubMed] [Google Scholar]
- Watkins SS, Koob GF, Markou A. Neural mechanisms underlying nicotine addiction: Acute positive reinforcement and withdrawal. Nicotine & Tobacco Research. 2000;2:19–37. doi: 10.1080/14622200050011277. [DOI] [PubMed] [Google Scholar]
- Watkins SS, Stinus L, Koob GF, Markou A. Reward and somatic changes during precipitated nicotine withdrawal in rats: Centrally and peripherally mediated effects. Journal of Pharmacology and Experimental Therapeutics. 2000;292:1053–1064. [PubMed] [Google Scholar]
- Wilmouth CE, Spear LP. Withdrawal from chronic nicotine in adolescent and adult rats. Pharmacology, Biochemistry and Behavior. 2006;85:648–657. doi: 10.1016/j.pbb.2006.10.021. doi:S0091-3057(06)00356-X. [DOI] [PMC free article] [PubMed] [Google Scholar]