Abstract
Many epidemiological studies have demonstrated that ambient particulate matter poses consistent risks for respiratory and cardiovascular disorders. The translocation of inhaled particles is one hypothesis that could explain such systemic effects. The objectives of this study were to conduct a systematic review of previous reports on particle translocation from the respiratory system and to discuss factors important for translocation. A PubMed search was conducted in August 2011 for the period from 1967 with four main keyword domains (particle, translocation, detection site, and exposure route). The systematic review identified 61 original articles written in English that met the specified criteria (i.e., information on experiment and particle detection). Categorical regression analysis was performed with the site of particle detection as the objective variable, and particle size, particle material, animal species, and exposure route as the explanatory variables. All explanatory variables showed statistically significant effects. The effects for particle size and particle material were large, while the effects for animal species and exposure route were relatively small. There was a broad relationship between particle size and detection site: ≤50 nm for brain and remote organs; ≤1 μm for blood; and ≤10 μm for lung tissues. However, these results should be considered within the context of several limitations, such as deficiency of information.
Keywords: Particle, Translocation, Respiratory system, Systematic review, Categorical regression
Introduction
A number of epidemiological studies have shown that airborne particulate matter (PM) is associated not only with respiratory diseases but also with cardiovascular diseases [1–3]. The existence of very small particles with diameters of <100 nm (i.e., ultrafine particles; UFPs) could explain, at least in part, the effects of airborne PM on the cardiovascular system [4, 5]. Predictive mathematical models for the deposition of inhaled particles indicate that particles of <100 nm are commonly deposited in the alveoli [6, 7]. The high surface-to-volume ratio of UFPs allows them to adsorb large amounts of substances, including various organic and inorganic compounds that induce oxidative stress and/or inflammation [8]. Inhalation of UFPs therefore triggers inflammatory responses in the lung and increases the release of inflammatory mediators into the blood. This, in turn, can lead to various changes in the cardiovascular system, such as an increase in blood coagulability and the progression of atherosclerotic lesions [1, 4].
In addition to such indirect effects via inflammatory responses, UFPs may directly affect the cardiovascular system by translocation from the respiratory system to the systemic circulation [9]. UFPs deposited in the lung may pass through the epithelial barrier because of their very small size; some particles may move into lung capillaries and then into the systemic circulation. Furthermore, inhaled UFPs could migrate to the brain from the nasal cavity via the olfactory nerve [9].
To evaluate the possibility of particle translocation from the airway or alveolar lumen, various studies have been conducted in which artificial particles were inhaled or administered into the airway. Afterwards, various organs or tissues, including the lymph nodes, blood, liver, heart, spleen, kidney, and brain, were examined for the presence of particles. Although several review articles concerning particle translocation have been published [9–12], no systematic reviews have been performed. In addition, important factors that affect inhaled particle translocation could be identified by a multivariate analysis of integrated data on particle translocation. Therefore, the purposes of this study were to summarize the studies published to date on particle translocation from the respiratory system, and to discuss factors important for the translocation of solid particles. In the literature search for the present study we did not limit particle size because it was felt that information on the translocation of large particles (i.e., >100 nm) would be useful for the subsequent statistical analysis. To the best of the authors’ knowledge, this is the first systematic review of particle translocation including statistical examination.
Materials and methods
Data source and keywords for literature search
A systematic search of the PubMed database (http://www.ncbi.nlm.nih.gov/sites/entrez) was performed for articles published in peer-reviewed journals from February 1967 through August 2011. The search was conducted using four main keyword categories, with terms related to: (1) particle (i.e., particle, particulate, nanoparticle, or *sphere); (2) translocation (i.e., pass, passage, translocat*, move*, migrat*, transport*, or incorporat*); (3) detection site (i.e., systemic, circulat*, blood, brain, liver, lymph*, nerv*, epithel*, or interstiti*); and (4) methods by which particles were exposed or the site into which particles were administered (i.e., inhal*, intratracheal*, respir*, airway, trache*, bronc*, alveol*, lung, nose, nostril, or nasal).
Selection of studies
Five main criteria were used for study selection.
Type of report: Original reports written in English.
Design of experiment: Animal or human studies in which particles were either inhaled or administered into the airway. Neither in vitro studies nor studies using isolated respiratory organs were included.
Information on experiment: Material and size of the particle, animal species, route of exposure, and the major results were specified. Major results should be shown with figures, tables, or photographs. Information on the particle concentration and duration in inhalation experiments or the amount of administered particles in administration experiments was not required, but was included for reference.
Detection of solid particles: Studies focusing on the translocation of solid particles. In reality, the constituents of the particles may dissolve or labels may be released from the particles. Such soluble fractions pass the epithelial barrier more easily than solid particles. An attempt was therefore made to exclude the cases where soluble fractions were detected. More specifically, papers were excluded in which such possibilities were pointed out by the authors themselves or by other researchers.
Validity of detection: Reports in which translocated particles were detected were evaluated for the validity of detection. Data that met at least one of the following four additional criteria were regarded as having successful detection at the translocated sites: [A] particles were observed histologically in the target tissue; [B] statistically significant amounts were detected in the target tissue; [C] a dose-response relationship was observed for more than two different doses or concentrations; or [D] more than 0.1% of the administered dose or lung deposition was observed in the target tissue.
Statistical analysis
Categorical regression (CATREG) analysis was performed using the studies that met the criteria described in the previous section. The objective variable was the site where particles were detected, and the explanatory variables were particle diameter, particle material, animal species, and exposure route. Doses or concentrations were not adopted as the explanatory variables for the following reasons: (1) there were several reports in which such information was not available; (2) varying descriptions were used in the inhalation studies (e.g., initial lung burden or initial body burden instead of concentration and time); and (3) subgroup analyses would have been required because of the difficulty in standardizing the exposure conditions in different styles of experiments, but there were not enough data to conduct such analyses.
The sites where particles were detected were classified into five nominal categories: (1) no translocation (remained in the lumen of the airways or alveoli); (2) lung tissues (in the epithelial cells of the airways or alveoli, submucosal tissue, interstitium, lung-associated lymph nodes, or endothelial cells of capillaries); (3) blood (in pulmonary capillary lumens, systemic blood circulation, liver, or spleen); (4) remote organs (organs other than lung, liver, spleen, or brain); and (5) brain (olfactory bulb, cerebrum, limbic system, brainstem, or cerebellum). The liver and the spleen were categorized as “blood” because these organs inherently function as eliminators of particles from the blood. Studies in which particles could not be detected in certain tissues were not included in the statistical analysis and were considered “negative results.” However, in studies in which particles were not detected in the lung tissue, the results were adopted as “no translocation.” Studies in which particles were detected in alveolar macrophages were also classified as “no translocation” because alveolar macrophages phagocytize foreign materials in the alveoli, and most of the cells migrate to terminal bronchioles, where they are cleared by the mucociliary escalator.
Particle diameter was classified into five ordinal categories: (1) ≤50 nm (nanoparticles); (2) >50 and ≤100 nm (UFPs without nanoparticles); (3) >100 and ≤1 μm (accumulation mode particles); (4) >1 and ≤10 μm (inhalable particles); and (5) >10 μm (non-inhalable particles). Particle material was classified into six nominal categories: (1) heavy metals and salts [gold (Au), silver (Ag), platinum (Pt), copper (Cu), iridium (Ir), tantalum (Ta), and barium sulfate (BaSO4)]; (2) metal oxides [manganese dioxide (MnO2), trimanganese tetraoxide (Mn3O4), cadmium oxide (CdO), titanium dioxide (TiO2), iron(II,III) oxide (Fe3O4), and cerium(IV) oxide (CeO2)]; (3) inorganic carbons (carbon black, C60 fullerene, and carbon particles generated with a Palas® (Palas, Karlsruhe, Germany) aerosol generator); (4) silicates (fly ash, silica, and aluminosilicate); (5) plastics [polystyrene and its conjugates, Teflon® (DuPont, Wilmington, DE, USA), polyacrylamide, polylactic acid, and methoxypoly (ethylene glycol) (mPEG)]; and (6) proteins (albumin and ferritin). BaSO4 had only one datum; it was combined with heavy metals because a preliminary CATREG analysis indicated that quantification of BaSO4 was similar to that of heavy metals. Animal species was classified into four nominal categories: (1) rat; (2) mouse; (3) guinea pig and hamster; and (4) dog. There were no human data available for CATREG analysis because all the data showed negative results. There was one datum each for guinea pig and hamster; these data were combined because a preliminary CATREG analysis indicated that quantifications of these species were similar. Exposure route was classified into three nominal categories: inhalation, intratracheal administration, and intranasal administration.
Statistical analysis was performed with IBM SPSS Statistics v. 19 (IBM, Armonk, NY, USA) and IBM SPSS Categories v. 19 (IBM).
Results and discussion
Selection of studies
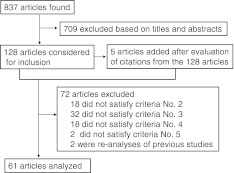
The literature search was conducted in August 2011. Eight hundred thirty-seven reports met all four main subject heading domains. Figure 1 illustrates the selection process. First, based on their titles and abstracts, 709 articles were excluded because they were not obviously related to the purpose of this study (e.g., review articles, in vitro studies, and articles on drug delivery). Of the remaining 128 articles (plus 5 articles that were added after evaluation of citations within the 128 articles), 61 articles met the criteria for study selection. Table 1 shows summaries of these articles.
Fig. 1.
Flow diagram of the study selection process. Criteria for the exclusion of 64 articles are described under the heading “Selection of studies” in the “Materials and methods” section
Table 1.
Summaries of the selected studies on particle translocation
| Material | Diameter (nm) | Animal | Route | Dose or concentration | Results | Reference |
|---|---|---|---|---|---|---|
| Fe3O4 | 1,300 | Rat | Inhalation | 0.01, 0.02, 0.05, 0.1 mg/m3, 4 weeks | Detected in lung-associated lymph node [B, C] | [13] |
| Human serum albumin | 7 | Rat | Intratracheal | 10 pmol/g wt | Detected in kidney, liver [A], and lymph node [A, D] | [14] |
| mPEG 20 kDa | 9 | Rat | Intratracheal | 10 pmol/g wt | Detected in lymph node [D] | [14] |
| Polystyrene–polyacrylate | 34 | Rat | Intratracheal | 10 pmol/g wt | Detected in lymph node [D] | [14] |
| Polystyrene–polyacrylate | 48 | Rat | Intratracheal | 10 pmol/g wt | <0.02% in lymph node | [14] |
| Polystyrene–polyacrylate | 68 | Rat | Intratracheal | 10 pmol/g wt | <0.02% in lymph node | [14] |
| Polystyrene–polyacrylate | 120 | Rat | Intratracheal | 10 pmol/g wt | <0.02% in lymph node | [14] |
| Polystyrene–polyacrylate | 270 | Rat | Intratracheal | 10 pmol/g wt | <0.02% in lymph node | [14] |
| TiO2 | 3 | Mouse | Intratracheal | 0.1 mg × 4 | Detected in brain [B] | [15] |
| CeO2 | 7 | Rat | Intratracheal | 0.2 mg | Detected in blood [B]. <0.01% in heart, kidney, brain, testis | [16] |
| Cu | 24 | Mouse | Intranasal | 1 mg, 40 mg | Detected in blood, liver, kidney, olfactory bulb [B]. Not significant in spleen, heart, brain | [17] |
| Cu | 17,000 | Mouse | Intranasal | 1 mg | Not significant in blood, liver, kidney, olfactory bulb, spleen, heart, brain | [17] |
| Polyacrylamide | 31 | Mouse | Intratracheal | 0.1 mg | Detected in blood, liver, heart, thyroid [D] | [18] |
| Polyacrylamide | 1,800 | Mouse | Intratracheal | 0.1 mg | Detected in blood, liver, spleen, kidney, heart, thyroid [D] | [18] |
| Ir | 20 | Rat | Inhalation | Detected in blood, liver, spleen, kidney, heart, brain [D] | [19] | |
| Ir | 80 | Rat | Inhalation | Not detected in blood, liver, spleen, kidney, heart, brain | [19] | |
| TiO2 | 700–800 | Rat | Inhalation | 2, 10, 50 mg/m3, 6 h/day, 5 days | Detected in mediastinal lymph node [D]. Not detected in liver, kidney, spleen, olfactory bulb, brain | [20] |
| Polystyrene | 100 | Mouse | Intranasal | 6.8 × 108 particles | Detected in apical cells of olfactory epithelium [A] | [21] |
| C60 | 0.68 | Mouse | Intratracheal | 0.625, 1 mg | Detected in lung capillary, lymph nodes, type I epithelial cells [A] | [22] |
| Au | 2 | Mouse | Intratracheal | 3 mg | Detected in liver [D] | [23] |
| Au | 40 | Mouse | Intratracheal | 15 mg | Not detected in liver | [23] |
| Au | 100 | Mouse | Intratracheal | 15 mg | Not detected in liver | [23] |
| TiO2 | 1,100 | Rat | Inhalation | 250 mg/m3, 6 h/day, 5 days | Detected in mediastinal lymph node [D] | [24] |
| Silica | 1,200 | Rat | Inhalation | 100 mg/m3, 6 h | Detected in mediastinal lymph node [D] | [24] |
| TiO2 | 80 | Mouse | Intranasal | 0.5 mg | Detected in olfactory bulb, hippocampus, cerebral cortex, cerebellum [B] | [25] |
| TiO2 | 150 | Mouse | Intranasal | 0.5 mg | Detected in olfactory bulb, hippocampus, cerebral cortex, cerebellum [B] | [25] |
| TiO2 | 21 | Rat | Intratracheal | 0.52 mg | Detected in lymph node [B] | [26] |
| TiO2 | 1,000 | Rat | Intratracheal | 10.7 mg | Not significant in lymph node | [26] |
| Carbon black | 120 | Rat | Inhalation | 16 mg/m3, 4 weeks | Detected in endothelial cells of blood capillary [A]. Not detected in liver, spleen, aortic endothelial cells | [27] |
| Carbon (Technegas) | 100 | Human | Inhalation | Not detected in liver | [28] | |
| MnO2 | 30 | Rat | Inhalation | 0.5 mg/m3 | Detected in olfactory bulb, striatum, frontal cortex, cerebellum [B] | [29] |
| Polystyrene | 56 | Rat | Intratracheal | 0.6 mg | Detected in liver, blood [D] | [30] |
| Polystyrene | 202 | Rat | Intratracheal | 0.6 mg | Detected in liver, blood [D] | [30] |
| Carbon (Technegas) | 108 | Human | Inhalation | No obvious uptake by liver or spleen | [31] | |
| Carbon black | 14 | Mouse | Intratracheal | 1 mg | Detected in interstitium, capillary lumen [A] | [32] |
| Au | 16 | Rat | Inhalation | 0.088 mg/m3, 6 h | Detected in blood [B], alveolar epithelial cells [A] | [33] |
| Carbon (Technegas) | 35 | Human | Inhalation | 4.6 × 105 particles/cm3, 6 min | No significant translocation to systemic circulation | [34] |
| TiO2 | 22 | Rat | Inhalation | 0.11 mg/m3 | Detected in capillary lumen [D], interstitium [A, D], alveolar epithelial cells [D] | [35] |
| Carbon black | 14 | Mouse | Intratracheal | (25, 125, 625 μg) × 4 | Detected in mediastinal lymph node [A, B, C] | [36] |
| Carbon black | 95 | Mouse | Intratracheal | (25, 125, 625 μg) × 4 | Detected in mediastinal lymph node [A, B, C] | [36] |
| Carbon | 36 | Rat | Inhalation | 0.16 mg/m3 | Detected in olfactory bulb, cerebrum, cerebellum [B] | [37] |
| Ir | 15–20 | Rat | Inhalation | 0.2 mg/m3 | Detected in liver, spleen, kidney, brain [D] | [38] |
| CdO | 40 | Rat | Inhalation | 0.07, 0.55 mg/m3, 6 h | Detected in liver, kidney [B] | [39] |
| Polylactic acid | 1,500 | Rat | Intranasal | Detected in submucosal layer [A] | [40] | |
| Polystyrene | 240 | Rat | Intratracheal | 9.5 mg | Not observed in alveolar epithelial cells, endothelial cells, capillary lumen | [41] |
| Carbon (Technegas) | 33 | Human | Inhalation | No accumulation was observed in vicinity of liver | [42] | |
| MnO2 | 1,300 | Rat | Inhalation | 3 mg/m3, 15 days | Detected in cerebral cortex [B]. Not significant in liver, olfactory bulb, cerebellum, brainstem, diencephalon, basal ganglia | [43] |
| MnO2 | 18,000 | Rat | Inhalation | 3 mg/m3, 15 days | Not significant in liver, olfactory bulb, cerebral cortex, cerebellum, brainstem, diencephalon, basal ganglia | [43] |
| Ir | 15 | Rat | Inhalation | 2.5 mg/m3, 1 h | Detected in liver [D] | [44] |
| Ir | 80 | Rat | Inhalation | 2.5 mg/m3, 1 h | <0.1% in liver | [44] |
| Carbon | 20-29 | Rat | Inhalation | 0.08, 0.18 mg/m3, 6 h | Detected in liver [B] | [45] |
| Ag | 15 | Rat | Inhalation | 0.113 mg/m3, 6 h | Detected in liver, kidney [D] | [46] |
| Ag | 15 | Rat | Intratracheal | 0.05 mg | Detected in liver [D] | [46] |
| Polystyrene | 20 | Rat | Intranasal | 0.2 mg | Detected in blood [D]. <0.1% in liver, kidney, spleen, brain | [47] |
| Polystyrene | 100 | Rat | Intranasal | 0.2 mg | Detected in blood, liver [D]. <0.1% in kidney, spleen, brain | [47] |
| Polystyrene | 500 | Rat | Intranasal | 0.2 mg | Detected in blood, liver [D]. <0.1% in kidney, spleen, brain | [47] |
| Polystyrene | 1,000 | Rat | Intranasal | 0.2 mg | Detected in blood, liver [D]. <0.1% in kidney, spleen, brain | [47] |
| Polystyrene | 1,100 | Mouse | Intranasal | 1 mg | Detected in nasal-associated lymphoid tissues, posterior lymph nodes, mediastinal lymph nodes [A, D]. <0.1% of lung burden in spleen | [48] |
| Colloidal albumin | <80 | Hamster | Intratracheal | 0.1 mg | Detected in blood, liver [D]. <0.1% in spleen, kidney, brain | [49] |
| Teflon | 18 | Rat | Inhalation | 5 × 105 particles/cm3 | Detected in interstitium [A] | [50] |
| Carbon | 25 | Mouse | Inhalation | Detected in alveolar epithelial cells [A] | [50] | |
| Pt | 13 | Rat | Inhalation | 5.6 × 106 particles/cm3 | Detected in liver [D] | [50] |
| TiO2 | 2,100 | Rat | Inhalation | 25, 50 mg/m3 | Detected in lymph nodes [C] | [51] |
| BaSO4 | 4,300 | Rat | Inhalation | 37.5, 75 mg/m3 | Detected in lymph nodes [C] | [51] |
| Polystyrene | 2,130 | Rat | Intratracheal | 2.4 × 108 particles | Detected in lymph nodes [D] | [52] |
| Mn phosphate | 4,900 | Rat | Intratracheal | 40, 80, 160 μg Mn/kg | No significant increase in striatum | [53] |
| Mn phosphate | 1,670–15,500 | Rat | Intratracheal | 40, 80, 160 μg Mn/kg | No significant increase in striatum | [53] |
| Mn3O4 | 730 | Rat | Intratracheal | 40, 80, 160 μg Mn/kg | No significant increase in striatum | [53] |
| Polystyrene | 40,000 | Guinea pig | Intratracheal | Did not penetrate basement membrane | [54] | |
| Polystyrene | 830 | Rat | Intranasal | 8 × 109 particles | Detected in blood [A] | [55] |
| TiO2 | 20 | Rat | Inhalation | 23 mg/m3, 12 weeks | Detected in lymph nodes, interstitium [D] | [56] |
| TiO2 | 250 | Rat | Inhalation | 23 mg/m3, 12 weeks | Detected in lymph nodes, interstitium [D] | [56] |
| Silica | 300 | Mouse | Intratracheal | 1 mg | Detected in interstitium [A, B] | [57] |
| TiO2 | 21 | Rat | Inhalation | 23.5 mg/m3, 12 weeks | Detected in hilar lymph nodes [D], alveolar epithelial cells [A] | [58] |
| TiO2 | 250 | Rat | Inhalation | 23 mg/m3, 12 weeks | Detected in hilar lymph nodes [D], alveolar epithelial cells [A] | [58] |
| TiO2 | 20 | Rat | Intratracheal | 0.065, 0.1, 0.2, 0.5, 1 mg | Detected in epithelial cells/interstitium [C, D] | [59] |
| TiO2 | 250 | Rat | Intratracheal | 0.5, 1 mg | Detected in epithelial cells/interstitium [D] | [59] |
| TiO2 | 12 | Rat | Intratracheal | 0.5 mg | Detected in epithelial cells/interstitium [D] | [59] |
| TiO2 | 220 | Rat | Intratracheal | 0.5 mg | Detected in epithelial cells/interstitium [D] | [59] |
| Carbon black | 20 | Rat | Intratracheal | 0.5 mg | Detected in epithelial cells/interstitium [D] | [59] |
| Polystyrene | 1,700 | Rat | Intratracheal | 107, 109 particles | Detected in interstitium [A, C, D] | [60] |
| Polystyrene | 1,700 | Dog | Intratracheal | 107, 109 particles | Detected in interstitium [A, D] | [60] |
| Carbon black | 240 | Rat | Inhalation | 7 mg/m3, 1, 3, 6 weeks | Detected in hilar lymph nodes [C, D] | [61] |
| Fly ash | 2,700 | Rat | Inhalation | 1.7 mg/m3, 1 year | Particles less than 2,300 nm transported to bronchopulmonary lymph nodes [A] | [62] |
| Polystyrene | 1,900 | Rat | Intratracheal | 4 × 108 particles | Detected in tracheobronchial lymph nodes [A] | [63] |
| Polystyrene | 1300 | Dog | Intratracheal | 5 × 1010 particles | Detected in lymph nodes [A, D] | [64] |
| TiO2 | 400 | Rat | Inhalation | 10, 50, 250 mg/m3, 2 years | Detected in liver [A, C], spleen [A, C], lymph node [C], type I epithelial cells [A] | [65] |
| Polystyrene | 3,000 | Dog | Intratracheal | 11 mg | Detected in tracheobronchial lymph nodes [D] | [66] |
| Polystyrene | 7,000 | Dog | Intratracheal | 0.7 mg | Detected in tracheobronchial lymph nodes [D] | [66] |
| Polystyrene | 13,000 | Dog | Intratracheal | 0.4 mg | Not detected in tracheobronchial lymph nodes | [66] |
| Aluminosilicate | 700 | Dog | Inhalation | IBB 11 μCi | Detected in lung-associated lymph nodes [D] | [67] |
| Aluminosilicate | 1,500 | Dog | Inhalation | IBB 21 μCi | Detected in lung-associated lymph nodes [D] | [67] |
| Aluminosilicate | 2,800 | Dog | Inhalation | IBB 29 μCi | Detected in lung-associated lymph nodes [D] | [67] |
| Aluminosilicate | 700 | Rat | Inhalation | IBB 0.2 μCi | <0.1% in lung-associated lymph nodes | [67] |
| Aluminosilicate | 1,500 | Rat | Inhalation | IBB 2.4 μCi | <0.1% in lung-associated lymph nodes | [67] |
| Aluminosilicate | 2,800 | Rat | Inhalation | IBB 0.43 μCi | <0.1% in lung-associated lymph nodes | [67] |
| Aluminosilicate | 700 | Mouse | Inhalation | IBB 0.075 μCi | <0.1% in lung-associated lymph nodes | [67] |
| Aluminosilicate | 1,500 | Mouse | Inhalation | IBB 0.56 μCi | <0.1% in lung-associated lymph nodes | [67] |
| Aluminosilicate | 2,800 | Mouse | Inhalation | IBB 2.5 μCi | <0.1% in lung-associated lymph nodes | [67] |
| Aluminosilicate | 1,700 | Dog | Intratracheal | ILB 0.5–64 μCi | Detected in thoracic lymph nodes [D] | [68] |
| Silica | 1,400 | Rat | Inhalation | 109 mg/m3 | Detected in alveolar epithelial cells, interstitium [A] | [69] |
| Carbon | 30 | Mouse | Intratracheal | 4 mg | Detected in type I alveolar epithelial cells and interstitial cells [A] | [70] |
| Polystyrene | 100 | Mouse | Intratracheal | 4 mg | Detected in interstitial cells and type I alveolar epithelial cells [A] | [70] |
| Polystyrene | 1,000 | Mouse | Intratracheal | 4 mg | Detected in type I alveolar epithelial cells [A] | [70] |
| Colloidal ferritin | 8 | Rat | Intratracheal | Detected in vesicles in lymphatic endothelium [A] | [71] | |
| Carbon | 35 | Rat | Intratracheal | Detected in a vesicle within epithelial lining of alveolar wall, vesicles within lymphatic endothelium [A] | [71] | |
| Au | 30 | Rat | Intratracheal | 0.65 mg | Detected in intracapillary platelets [A] | [72] |
| 182Ta | 1,000 | Dog | Inhalation | ILB 30–7230 mg | Detected in lymph nodes [A, D] | [73] |
| 182Ta | 5,000 | Dog | Inhalation | ILB 2700–14000 mg | Detected in lymph nodes [A, D] | [73] |
| 182Ta | 10,000 | Dog | Inhalation | ILB 5240–5950 mg | Detected in lymph nodes [D] | [73] |
[A]–[D] indicate the criteria for validity of particle detection (see “5. Validity of detection” under the heading “Selection of studies“ in the “Materials and methods” section)
wt weight, IBB initial body burden, ILB initial lung burden, mPEG methoxypoly (ethylene glycol)
After 2000, many reports examined the possibilities of particle translocation to the systemic circulation, remote organs, or brain. Most reports before 2000 evaluated translocation to the lung interstitium or the lung-associated lymph nodes. As for particle materials, 29 kinds of materials were used in the articles analyzed in this report: 14 metals and their oxides and salts; 7 inorganic carbons and silicates; and 8 organic compounds. Polystyrene and its conjugates were the most frequently used (26 reports), at sizes that were usually >100 nm in diameter. Titanium dioxide (18 reports) and inorganic carbons (16 reports) were also frequently used. In general, inorganic materials other than silica were frequent among particles whose diameters were ≤100 nm, and plastics and silica were popular among particles of >100 nm. The smallest particle was C60 fullerene (0.68 nm), and the largest was polystyrene (40 μm). As for the animal species, rat was the most frequently used (68 reports, approximately 60%), followed by mouse (24 reports), dog (12 reports), and human (4 reports). All of the human reports were on inhalation of Technegas, and reported that no translocation to the systemic circulation or remote organs could be detected. As for the sites where particles were detected, there were 9 reports for the brain, 7 reports for the kidney, 3 reports for the heart, 2 reports for the thyroid, 20 reports for the liver, 4 reports for the spleen, 14 reports for blood, and 4 reports for lung capillary lumens.
Statistical analysis of translocation
Evaluation of factors that affect translocation
One characteristic of this study was that the factors associated with particle translocation from the airway were statistically evaluated using information contained in preceding systematic reviews. For this present study, CATREG analysis was conducted with 113 sets of available data found in 61 previous studies. The objective variable was the particle detection site, and the explanatory variables were particle diameter, particle material, animal species, and exposure route. The F value of the model was 6.933 (p < 0.001). The coefficient of determination (R2) was 0.477 (unadjusted value; adjusted R2 was 0.408), indicating that <50% of the variance was explainable with these four variables. This model does not seem to be sufficient to predict particle translocation, but is reasonably acceptable to evaluate the relative importance of the explanatory variables for particle translocation. Table 2 summarizes the results of the analysis. The standardized partial regression coefficient (β) values of all the explanatory variables were statistically significant. Both particle size and particle material showed large effects. On the other hand, the effects of exposure route and animal species were relatively small. Furthermore, Pratt’s measure of relative importance, which is useful for comparing the contribution of each explanatory variable [74], also showed large contributions of particle size and particle material, followed by exposure route. The effect of animal species on particle translocation was very small.
Table 2.
Results of categorical regression analysis
| Explanatory variable | Scale (number of categories) | Coefficient | p | Pratt’s relative importance | |
|---|---|---|---|---|---|
| β | SE | ||||
| Size | Ordinal (5) | 0.427 | 0.131 | 0.000 | 0.43 |
| Material | Nominal (6) | 0.441 | 0.102 | 0.000 | 0.49 |
| Animal species | Nominal (4) | 0.204 | 0.103 | 0.011 | −0.05 |
| Route of exposure | Nominal (3) | 0.258 | 0.093 | 0.001 | 0.14 |
β standardized partial regression coefficient
Effects of particle size
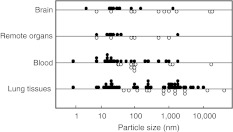
As shown in Table 2, particle size was a strong factor for translocation. In addition, because particle size is a numerical variable, it can easily be expressed on the abscissa. Figure 2 shows the relationship between particle size and detection site. Negative data for which particles were not detected are also included (shown by open circles in Fig. 2). Particles that were translocated to various sites were observed to have the following sizes: ≤50 nm for remote organs, ≤1 μm for blood, and ≤10 μm for lung tissues. In order to be detected in the blood, particles that have passed through the epithelial barrier must migrate into the capillaries. In addition, in order to be detected in remote organs, particles must be incorporated from the systemic blood circulation. Therefore, the conditions under which particles can be detected should be strictest for the remote organs, followed by the blood, and, finally, the lung tissues. In this context, it seems reasonable that detected particle size would be smallest for remote organs and largest for the lung tissues.
Fig. 2.
Relationship between particle size and site of particle detection. Closed circles show the data of successful particle detection. Open circles show the data of negative results
To further confirm the relationship between particle size and translocation, the odds ratios (ORs) for various cutoff points of size were calculated (Table 3). The OR was largest at the following cutoff points: 50 nm for brain and remote organs; 1 μm for blood; and 10 μm for lung tissues. The observations recorded in Fig. 2 were therefore also supported statistically.
Table 3.
Odds ratios for various cutoff points of particle diameter
| Cutoff point (nm) | Brain | Remote organs | Blood | Lung tissues |
|---|---|---|---|---|
| 50 | 8.0 | 9.3 | 6.3 | 5.4 |
| 100 | 5.3 | 4.0 | 1.8 | 3.1 |
| 1,000 | 3.4 | 0.4 | 9.0 | 0.9 |
| 10,000 | 5.6 | 1.5 | 7.4 | 28.7 |
Translocation to the brain
The largest OR for translocation to the brain was observed at a 50-nm cutoff size (Table 3). However, MnO2 particles as large as 1.3 μm have also been detected in the cerebral cortex [43]. Translocation to the brain via the systemic circulation would be very difficult, as the blood–brain barrier (BBB) restricts intrusion into the brain. There are three possibilities as to why such large particles were detected in the brain. The first is that translocation to the brain occurs via a pathway other than the systemic circulation. The second is that Mn particles easily pass through the BBB. The third is that a small amount of soluble Mn was detected with a sensitive detection method.
As for the first possibility, Oberdörster et al. [9] advocated a mechanism in which particles deposited in the olfactory mucosa of the nasal cavity are subsequently taken into axons of the olfactory nerve and migrate into the olfactory bulb of the brain ventricle. In the present review, successful detection of particles in the brain was found mostly in data from inhalation and intranasal administration studies (8 sets of data), but in only one study of intratracheal administration [15]. The success rate of detection was 0.8 for inhalation and intranasal administration (8 successful detections out of 10 inhalations or administrations), but was less than 0.2 for intratracheal administration (1 successful detection in 6). This finding seems to support the hypothesis of Oberdörster et al., at least in part, that both inhalation and intranasal administration give particles a chance to contact olfactory nerves in the nasal cavity, while intratracheal administration, which skips the nasal cavity, does not. Although the statistical analysis suggested that the effect of the exposure route was relatively small for translocation to the brain, the exposure route might, nevertheless, be important.
As for the second and third possibilities, Yokel and Crossgrove [75] demonstrated that soluble Mn was transported into the brain via some transporting system(s) other than a divalent metal transporter (i.e., DMT-1). However, it is questionable as to whether MnO2 particles that exceed 1 μm in diameter pass through the BBB with the help of some transporting system(s) similar to that for soluble Mn. On the other hand, various neurobehavioral effects have been reported among workers in the dry battery industry who had inhaled MnO2 aerosol [76, 77]; however, olfactory disorders were not specified among the workers. These findings suggest that the translocation of a substantial amount of Mn into the brain occurs via a route other than through the olfactory system. At present, the reason why 1.3-μm MnO2 particles were detected in the brain remains unclear.
Study limitations
Deficiency of information
The statistical analysis conducted in the present study was based on currently available data; therefore, it is possible that the results of statistical evaluation will differ in the future when more information on particle translocation is accumulated. Particle materials and animal species varied, and some categories had only a few cases. Such types of information bias may have increased the uncertainty of the statistical analysis. Moreover, differences in crystal structures and fabrications of the particle surfaces were not considered because such information was limited.
Publication bias
This study was conducted based only on the information in published reports. There were 97 sets of data that reported successful detection of translocated particles. On the other hand, there were 42 sets of data indicating that particles were not detected. The degree of publication bias could not be evaluated objectively (e.g., by funnel plot) because of the characteristics of the collected data. Therefore, the possibility cannot be denied that publication bias was incorporated into the present review.
Restriction by purpose of study
There were several reports in which only neighboring tissues were examined because of the purposes of the studies, even though particles that could possibly translocate to distant tissues were used. It seemed, however, that a statistical analysis with plenty of data would make it possible to discern some broad trend, although some of the data in the reports mentioned above were included.
Particle characteristics and detection
Varying definitions of particle size and concentration were used (e.g., average and median for size, mass and particle number for concentration). In addition, size distribution can also vary among products. However, such differences were not considered. Particle detection was evaluated qualitatively; differences in the efficiency of translocation were not considered. Also, as for the method of particle detection, instead of morphological observation, the elements or labels of particles were measured in many studies. Regarding the measurement of elements or labels, the possibility cannot be ruled out that a translocated soluble fraction was detected. Reports with such a possibility were excluded by setting criteria on particle detection; however, it is possible that some papers that should have been excluded were included in this analysis.
No consideration of interaction among the explanatory variables
A lack of consideration of interaction among the explanatory variables may be one of the reasons why R2 was less than 0.5.
No consideration of the effects caused by particles
Based on the purpose of this study, any effects caused by the translocated particles were not considered.
Conclusions
This present study gives the results of a systematic review and statistical analysis of particle translocation from the respiratory system. A categorical regression analysis based on currently available data showed that all of the effects of particle size, particle material, animal species, and exposure route were statistically significant. The effects were large for particle size and particle material, and small for exposure route and animal species. These results suggest that, in an experiment to evaluate the translocation of solid particles, the characteristics of the particles (i.e., size and material) should be considered carefully. On the other hand, the selection of the types of experimental animals might be less important. When translocation to the brain is to be evaluated, inhalation and intranasal administration would be recommended. However, the results of this study should be considered within the context of several limitations. Similar analyses should be conducted when more information becomes available, especially related to the surface characteristics of particles.
Acknowledgments
The author thanks Dr. Hideo Tanaka of the Aichi Cancer Center Research Institute for helpful advice on statistical analyses.
Conflicts of interest
The author belongs to the Atmospheric Environment Laboratory, Toyota Central R&D Labs., Inc. This work has been conducted within the scope of the research plan of the company.
References
- 1.Brook RD, Franklin B, Cascio W, Hong Y, Howard G, Lipsett M, et al. A statement for healthcare professionals from expert panel on population and prevention science of the American Heart Association. Circulation. 2004;109:2655–2671. doi: 10.1161/01.CIR.0000128587.30041.C8. [DOI] [PubMed] [Google Scholar]
- 2.Peters A. Particulate matter and heart disease: evidence from epidemiological studies. Toxicol Appl Pharmacol. 2005;207:S477–S482. doi: 10.1016/j.taap.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 3.Pope CA, III, Dockery DW. Health effects of fine particulate air pollution: lines that connect. J Air Waste Manag Assoc. 2006;56:709–742. doi: 10.1080/10473289.2006.10464485. [DOI] [PubMed] [Google Scholar]
- 4.Delfino RJ, Sioutas C, Malik S. Potential role of UFPs in associations between airborne particle mass and cardiovascular health. Environ Health Perspect. 2005;113:947–955. doi: 10.1289/ehp.7938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seaton A, MacNee W, Donaldson K, Godden D. Particulate air pollution and acute health effects. Lancet. 1995;345:176–178. doi: 10.1016/S0140-6736(95)90173-6. [DOI] [PubMed] [Google Scholar]
- 6.International Commission on Radiological Protection. Human respiratory tract model for radiological protection. (ICRP publication 66; Ann. ICRP 24). Oxford: Pergamon; 1994. [PubMed]
- 7.de Winter-Sorkina R, Cassee FR. From concentration to dose: factors influencing airborne particulate matter deposition in humans and rats. Report no. 650010031/2002. Retrieved September 29, 2009, from National Institute of Public Health and the Environment (RIVM) Website: http://www.rivm.nl/bibliotheek/rapporten/650010031.html.
- 8.Gwinn MR, Vallyathan V. Nanoparticles: health effects—pro and cons. Environ Health Perspect. 2006;114:1818–1825. doi: 10.1289/ehp.8871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oberdörster G, Oberdörster E, Oberdörster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect. 2005;113:823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elder A, Oberdörster G. Translocation and effects of ultrafine particles outside of the lung. Clin Occup Environ Med. 2006;5:785–796. doi: 10.1016/j.coem.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 11.Kreyling WG, Semmler-Behnke M, Möller W. Ultrafine particle-lung interactions: does size matter? J Aerosol Med. 2006;19:74–83. doi: 10.1089/jam.2006.19.74. [DOI] [PubMed] [Google Scholar]
- 12.Peters A, Veronesi B, Calderón-Garciudeñas L, Gehr P, Chen LC, Geiser M, et al. Translocation and potential neurological effects of fine and ultrafine particles a critical update. Particle Fiber Toxicol. 2006;3:13. doi: 10.1186/1743-8977-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pauluhn J. Subchronic inhalation toxicity of iron oxide (magnetite, Fe3O4) in rats: pulmonary toxicity is determined by the particle kinetics typical of poorly soluble particles. J Appl Toxicol. 2011. doi: 10.1002/jat.1668 (Article first published online: 1 APR 2011). [DOI] [PubMed]
- 14.Choi HS, Ashitate Y, Lee JH, Kim SH, Matsui A, Insin N, et al. Rapid translocation of nanoparticles from the lung airspaces to the body. Nat Biotechnol. 2010;28:1300–1303. doi: 10.1038/nbt.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Y, Li J, Yin J, Li W, Kang C, Huang Q, et al. Systematic influence induced by 3 nm titanium dioxide following intratracheal instillation of mice. J Nanosci Nanotechnol. 2010;10:8544–8549. doi: 10.1166/jnn.2010.2690. [DOI] [PubMed] [Google Scholar]
- 16.He X, Zhang H, Ma Y, Bai W, Zhang Z, Lu K, et al. Lung deposition and extrapulmonary translocation of nano-ceria after intratracheal instillation. Nanotechnology. 2010;21. doi:10.1088/0957-4484/21/28/285103. [DOI] [PubMed]
- 17.Liu Y, Gao Y, Zhang L, Wang T, Wang J, Jiao F, et al. Potential health impact on mice after nasal instillation of nano-sized copper particles and their translocation in mice. J Nanosci Nanotechnol. 2009;9:6335–6343. doi: 10.1166/jnn.2009.1320. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, Ibricevic A, Cohen JA, Cohen JL, Gunsten SP, Fréchet JM, et al. Impact of hydrogel nanoparticle size and functionalization on in vivo behavior for lung imaging and therapeutics. Mol Pharm. 2009;6:1891–1902. doi: 10.1021/mp900215p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kreyling WG, Semmler-Behnke M, Seitz J, Scymczak W, Wenk A, Mayer P, et al. Size dependence of the translocation of inhaled iridium and carbon nanoparticle aggregates from the lung of rats to the blood and secondary target organs. Inhal Toxicol. 2009;21(suppl.1):55–60. [DOI] [PubMed]
- 20.Ma-Hock L, Burkhardt S, Strauss V, Gamer AO, Wiench K, Ravenzwaay B, et al. Development of a short-term inhalation test in the rat using nano-titanium dioxide as a model substance. Inhal Toxicol. 2009;21:102–118. doi: 10.1080/08958370802361057. [DOI] [PubMed] [Google Scholar]
- 21.Mistry A, Glud SZ, Kjems J, Randel J, Howard KA, Stolnik S, et al. Effect of physicochemical properties on intranasal nanoparticle transit into murine olfactory epithelium. J Drug Target. 2009;17:543–552. doi: 10.1080/10611860903055470. [DOI] [PubMed] [Google Scholar]
- 22.Naota M, Shimada A, Morita T, Inoue K, Takano H. Translocation pathway of the intratracheally instilled C60 fullerene from the lung into the blood circulation in the mouse: possible association of diffusion and caveolae-mediated pinocytosis. Toxicol Pathol. 2009;37:456–462. doi: 10.1177/0192623309335059. [DOI] [PubMed] [Google Scholar]
- 23.Sadauskas E, Jacobsen NR, Danscher G, Stoltenberg M, Vogel U, Larsen A, et al. Biodistribution of gold nanoparticles in mouse lung following intratracheal instillation. Chem Cent J. 2009;3:16. doi: 10.1186/1752-153X-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ravenzwaay B, Landsiedel R, Fabian E, Burkhardt S, Strauss V, Ma-Hock L. Comparing fate and effects of three particles of different surface properties: nano-TiO2, pigmentary TiO2 and quartz. Toxicol Lett. 2009;186:152–159. doi: 10.1016/j.toxlet.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 25.Wang J, Chen C, Liu Y, Jiao F, Li W, Lao F, et al. Potential neurological lesion after nasal instillation of TiO2 nanoparticles in the anatase and rutile crystal phases. Toxicol Lett. 2008;183:72–80. doi: 10.1016/j.toxlet.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 26.Sager TM, Kommineni C, Castranova V. Pulmonary response to intratracheal instillation of ultrafine versus fine titanium dioxide: role of particle surface area. Particle Fiber Toxicol. 2008;5:17. doi: 10.1186/1743-8977-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niwa Y, Hiura Y, Sawamura H, Iwai N. Inhalation exposure to carbon black induces inflammatory response in rats. Circ J. 2008;72:144–149. doi: 10.1253/circj.72.144. [DOI] [PubMed] [Google Scholar]
- 28.Möller W, Felten K, Sommerer K, Scheuch G, Meyer G, Meyer P, et al. Deposition, retention, and translocation of ultrafine particles from the central airways and lung periphery. Am J Respir Crit Care Med. 2008;177:426–432. doi: 10.1164/rccm.200602-301OC. [DOI] [PubMed] [Google Scholar]
- 29.Elder A, Gelein R, Silva V, Feikert T, Opanashuk L, Carter J, et al. Translocation of inhaled ultrafine manganese oxide particles to the central nervous system. Environ Health Perspect. 2006;114:1172–1178. doi: 10.1289/ehp.9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen J, Tan M, Nemmar A, Song W, Dong M, Zhang G, et al. Quantification of extrapulmonary translocation of intratracheal-instilled particles in vivo in rats: effect of lipopolysaccharide. Toxicology. 2006;222:195–201. doi: 10.1016/j.tox.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 31.Mills NL, Amin N, Robinson SD, Anand A, Davies J, Patel D, et al. Do inhaled carbon nanoparticles translocate directly into the circulation in humans? Am J Respir Crit Care Med. 2006;173:426–431. doi: 10.1164/rccm.200506-865OC. [DOI] [PubMed] [Google Scholar]
- 32.Shimada A, Kawamura N, Okajima N, Kaewamatawong T, Inoue H, Morita T. Translocation pathway of the intratracheally instilled ultrafine particles from the lung into the blood circulation in the mouse. Toxicol Pathol. 2006;34:949–957. doi: 10.1080/01926230601080502. [DOI] [PubMed] [Google Scholar]
- 33.Takenaka S, Karg E, Kreyling WG, Lentner B, Möller W, Behnke-Semmler M, et al. Distribution pattern of inhaled ultrafine gold particles in the rat lung. Inhal Toxicol. 2006;18:733–740. doi: 10.1080/08958370600748281. [DOI] [PubMed] [Google Scholar]
- 34.Wiebert P, Sanchez-Crespo A, Falk R, Philipson K, Lundin A, Larsson S, et al. No significant translocation of inhaled 35-nm carbon particles to the circulation in humans. Inhal Toxicol. 2006;18:741–747. doi: 10.1080/08958370600748455. [DOI] [PubMed] [Google Scholar]
- 35.Geiser M, Rothen-Rutishauser B, Kapp N, Schürch S, Kreyling W, Schulz H, et al. Ultrafine particles cross cellular membranes by nonphagocytic mechanisms in lungs and in cultured cells. Environ Health Perspect. 2005;113:1555–1560. doi: 10.1289/ehp.8006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shwe TT, Yamamoto S, Kakeyama M, Kobayashi T, Fujimaki H. Effect of intratracheal instillation of ultrafine carbon black on proinflammatory cytokine and chemokine release and mRNA expression in lung and lymph nodes of mice. Toxicol Appl Pharmacol. 2005;209:51–61. doi: 10.1016/j.taap.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 37.Oberdörster G, Sharp Z, Atudorei V, Elder A, Gelein R, Kreyling W, et al. Translocation of inhaled ultrafine particles to the brain. Inhal Toxicol. 2004;16:437–445. doi: 10.1080/08958370490439597. [DOI] [PubMed] [Google Scholar]
- 38.Semmler M, Seitz J, Erbe F, Mayer P, Heyder J, Oberdörster G, et al. Long-term clearance kinetics of inhaled ultrafine insoluble iridium particles from the rat lung, including transient translocation into secondary organs. Inhal Toxicol. 2004;16:453–459. doi: 10.1080/08958370490439650. [DOI] [PubMed] [Google Scholar]
- 39.Takenaka S, Karg E, Kreyling WG, Lentner B, Schulz H, Ziesenis A, et al. Fate and toxic effects of inhaled ultrafine cadmium oxide particles in the rat lung. Inhal Toxicol. 2004;16(suppl. 1):83–92. doi: 10.1080/08958370490443141. [DOI] [PubMed] [Google Scholar]
- 40.Vila A, Sánchez A, Évora C, Soriano I, Vila Jato JL, Alonso MJ. PEG-PLA nanoparticles as carriers for nasal vaccine delivery. J Aerosol Med. 2004;17:174–185. doi: 10.1089/0894268041457183. [DOI] [PubMed] [Google Scholar]
- 41.Kato T, Yashiro T, Murata Y, Herbert DC, Oshikawa K, Bando M, et al. Evidence that exogenous substances can be phagocytized by alveolar epithelial cells and transported into blood capillaries. Cell Tissue Res. 2003;311:47–51. doi: 10.1007/s00441-002-0647-3. [DOI] [PubMed] [Google Scholar]
- 42.Brown JS, Zeman KL, Bennett WD. Ultrafine particle deposition and clearance in the healthy and obstructed lung. Am J Respir Crit Care Med. 2002;166:1240–1247. doi: 10.1164/rccm.200205-399OC. [DOI] [PubMed] [Google Scholar]
- 43.Fechter LD, Johnson DL, Lynch RA. The relationship of particle size to olfactory nerve uptake of a non-soluble form of manganese into brain. Neurotoxicology. 2002;23:177–183. doi: 10.1016/S0161-813X(02)00013-X. [DOI] [PubMed] [Google Scholar]
- 44.Kreyling WG, Semmler M, Erbe F, Mayer P, Takenaka S, Schulz H, et al. Translocation of ultrafine insoluble iridium particles from lung epithelium to extrapulmonary organs is size dependent but very low. J Toxicol Environ Health A. 2002;65:1513–1530. doi: 10.1080/00984100290071649. [DOI] [PubMed] [Google Scholar]
- 45.Oberdörster G, Sharp Z, Atudorei V, Elder A, Gelein R, Lunts A, et al. Extrapulmonary translocation of ultrafine carbon particles following whole-body inhalation exposure of rats. J Toxicol Environ Health A. 2002;65:1531–1543. doi: 10.1080/00984100290071658. [DOI] [PubMed] [Google Scholar]
- 46.Takenaka S, Karg E, Roth C, Schulz H, Ziesenis A, Heinzmann U, et al. Pulmonary and systemic distribution of inhaled ultrafine silver particles in rats. Environ Health Perspect. 2001;109(suppl. 4):547–551. doi: 10.1289/ehp.01109s4547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brooking J, Davis SS, Illum L. Transport of nanoparticles across the rat nasal mucosa. J Drug Target. 2001;9:267–279. doi: 10.3109/10611860108997935. [DOI] [PubMed] [Google Scholar]
- 48.Eyles JE, Bramwell VW, Williamson ED, Alpar HO. Microsphere translocation and immunopotentiation in systemic tissues following intranasal administration. Vaccine. 2001;19:4732–4742. doi: 10.1016/S0264-410X(01)00220-1. [DOI] [PubMed] [Google Scholar]
- 49.Nemmar A, Vanbilloen H, Hoylaerts MF, Hoet PHM, Berbruggen A, Nemery B. Passage of intratracheally instilled ultrafine particles form the lung into the systemic circulation in hamster. Am J Respir Crit Care Med. 2001;164:1665–1668. doi: 10.1164/ajrccm.164.9.2101036. [DOI] [PubMed] [Google Scholar]
- 50.Oberdörster G, Finkelstein JN, Johnston C, Gelein R, Cox C, Baggs R, et al. Acute pulmonary effects of ultrafine particles in rats and mice. Res Rep Health Eff Inst. 2000;96:5–74. [PubMed] [Google Scholar]
- 51.Tran CL, Buchanan D, Cullen RT, Searl A, Jones AD, Donaldson K. Inhalation of poorly soluble particles. II. Influence of particle surface area on inflammation and clearance. Inhal Toxicol. 2000;12:1113–1126. doi: 10.1080/08958370050166796. [DOI] [PubMed] [Google Scholar]
- 52.Trošić I, Mataušić-Pišl M, Horš N. Pathways and quantification of insoluble particles in the lung compartments of the rat. Int J Hyg Environ Health. 2000;203:39–43. doi: 10.1078/S1438-4639(04)70006-1. [DOI] [PubMed] [Google Scholar]
- 53.Vitarella D, Moss O, Dorman DC. Pulmonary clearance of manganese phosphate, manganese sulfate, and manganese tetraoxide by CD rats following intratracheal instillation. Inhal Toxicol. 2000;12:941–957. doi: 10.1080/08958370050138003. [DOI] [PubMed] [Google Scholar]
- 54.Hunter DD, Undem BJ. Identification and substance P content of vagal afferent neurons innervating the epithelium of the guinea pig trachea. Am J Respir Crit Care Med. 1999;159:1943–1948. doi: 10.1164/ajrccm.159.6.9808078. [DOI] [PubMed] [Google Scholar]
- 55.Alpar HO, Almeida AJ, Brown MRW. Microsphere absorption by the nasal mucosa of the rat. J Drug Target. 1994;2:147–149. doi: 10.3109/10611869409015903. [DOI] [PubMed] [Google Scholar]
- 56.Oberdörster G, Ferin J, Lehnert BE. Correlation between particle size, in vivo particle persistence, and lung injury. Environ Health Perspect. 1994;102(suppl. 5):173–179. doi: 10.1289/ehp.102-1567252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Adamson IYR. Radiation enhances silica translocation to the pulmonary interstitium and increases fibrosis in mice. Environ Health Perspect. 1992;97:233–238. doi: 10.1289/ehp.9297233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ferrin J, Oberdörster G, Penney DP. Pulmonary retention of ultrafine and fine particles in rats. Am J Respir Crit Care Med. 1992;6:535–542. doi: 10.1165/ajrcmb/6.5.535. [DOI] [PubMed] [Google Scholar]
- 59.Oberdörster G, Ferin J, Gelein R, Soderholm SC, Finkelstein J. Role of the alveolar macrophage in lung injury: studies with ultrafine particles. Environ Health Perspect. 1982;97:193–199. doi: 10.1289/ehp.97-1519541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mueller HL, Robinson B, Muggenburg BA, Gillett NA, Guilmette RA. Particle distribution in lung and lymph node tissues of rats and dogs and the migration of particle-containing alveolar cells in vitro. J Toxicol Environ Health. 1990;30:141–165. doi: 10.1080/15287399009531419. [DOI] [PubMed] [Google Scholar]
- 61.Strom KA, Johnson JT, Chan TL. Retention and clearance of inhaled submicron carbon black particles. J Toxicol Environ Health. 1989;26:183–202. doi: 10.1080/15287398909531244. [DOI] [PubMed] [Google Scholar]
- 62.Tanaka I. Particle size distributions in lungs and bronchopulmonary lymph nodes due to long-term exposure to coal fly ash aerosol in rats. J UOEH. 1987;9:361–367. doi: 10.7888/juoeh.9.361. [DOI] [PubMed] [Google Scholar]
- 63.Lehnert BE, Valdez YE, Stewart CC. Translocation of particles to the tracheobronchial lymph nodes after lung deposition: kinetics and particle–cell relationships. Exp Lung Res. 1986;10:245–266. doi: 10.3109/01902148609061496. [DOI] [PubMed] [Google Scholar]
- 64.Harmsen AG, Muggenburg BA, Snipes MB, Bice DE. The role of macrophages in particle translocation from lungs to lymph nodes. Science. 1985;230:1277–1280. doi: 10.1126/science.4071052. [DOI] [PubMed] [Google Scholar]
- 65.Lee KP, Trochimowicz HJ, Reinhardt CF. Transmigration of titanium dioxide (TiO2) particles in rats after inhalation exposure. Exp Mol Pathol. 1985;42:331–343. doi: 10.1016/0014-4800(85)90083-8. [DOI] [PubMed] [Google Scholar]
- 66.Snipes MB, Chaves GT, Muggenburg BA. Disposition of 3-, 7-, and 13-μm microspheres instilled into lungs of dogs. Environ Res. 1984;33:333–342. doi: 10.1016/0013-9351(84)90031-8. [DOI] [PubMed] [Google Scholar]
- 67.Snipes MB, Boecker BB, McClellan RO. Retention of monodisperse or polydisperse aluminosilicate particles inhaled by dogs, rats, and mice. Toxicol Appl Pharmacol. 1983;69:345–362. doi: 10.1016/0041-008X(83)90258-2. [DOI] [PubMed] [Google Scholar]
- 68.Snipes MB, Muggenburg BA, Bice DE. Translocation of particles from lung lobes or the peritoneal cavity to regional lymph nodes in beagle dogs. J Toxicol Environ Health. 1983;11:703–712. doi: 10.1080/15287398309530378. [DOI] [PubMed] [Google Scholar]
- 69.Brody AR, Roe MW, Evans JN, Davis GS. Deposition and translocation of inhaled silica in rats. Quantification of particle distribution, macrophage participation, and function. Lab Invest. 1982;6:533–542. [PubMed] [Google Scholar]
- 70.Adamson IYR, Bowden DH. Dose response of the pulmonary macrophagic system to various particulates and its relationship to transepithelial passage of free particles. Exp Lung Res. 1981;2:165–175. doi: 10.3109/01902148109052312. [DOI] [PubMed] [Google Scholar]
- 71.Leak LV. Lymphatic removal of fluids and particles in the mammalian lung. Environ Health Perspect. 1980;35:55–76. doi: 10.1289/ehp.803555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Berry JP, Arnoux B, Stanislas G, Galle P, Chretien J. A microanalytic study of particles transport across the alveoli: role of blood platelets. Biomedicine. 1977;27:354–357. [PubMed] [Google Scholar]
- 73.Morrow PE, Kilpper RW, Beiter HE, Gibb FR. Pulmonary retention and translocation of insufflated tantalum. Radiology. 1976;121:415–421. doi: 10.1148/121.2.415. [DOI] [PubMed] [Google Scholar]
- 74.Pratt JW. Dividing the indivisible: using simple symmetry to partition variance explained. In: Pukkila T, Puntanen S, editors. Proceedings of the second international Tampere conference in statistics. Department of Mathematical Sciences, University of Tampere, Tampere; 1987. p. 245–260.
- 75.Yokel RA, Crossgrove JS. Manganese toxicokinetics at the blood–brain barrier. Res Rep Health Eff Inst. 2004;119:7–58. [PubMed] [Google Scholar]
- 76.Dietz MC, Ihrig A, Wrazidlo W, Bader M, Jansen O, Triebig G. Results of magnetic resonance imaging in long-term manganese dioxide-exposed workers. Environ Res. 2001;85:37–40. doi: 10.1006/enrs.2000.4068. [DOI] [PubMed] [Google Scholar]
- 77.Emara AM, El-Ghawabi SH, Madkour OI, El-Samra GH. Chronic manganese poisoning in the dry battery industry. Br J Ind Med. 1971;28:78–82. doi: 10.1136/oem.28.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]