Abstract
Objective
We aimed to evaluate the hypothesis that the presence of an interaction between smoking and being overweight increases the risks of lifestyle-related diseases (hypertension, diabetes mellitus, dyslipidemia, and cardiovascular disease) in outpatients with mood disorders.
Methods
In this cross-sectional survey, using data from 213 outpatients with mood disorders (95 men, 118 women), we calculated the adjusted odds ratios (ORs) with 95% confidence intervals (CIs) for each of hypertension, diabetes, dyslipedemia, and cardiovascular disease, using a binary logistic regression model; we then calculated the adjusted OR values for smokers and non-smokers with body mass indexes (BMIs) of <25 or ≥25 kg/m2. Next, we examined the data for the presence of an interaction between smoking and being overweight, using three measures of additive interaction: relative excess risk due to the interaction (RERI), attributable proportion due to the interaction (AP), and the synergy index (S).
Results
Smokers with BMI <25 kg/m2 had a significantly lower risk of hypertension (OR 0.27, 95% CI 0.09–0.81) than non-smokers with BMI <25 kg/m2 (reference group). Compared with the reference group, overweight non-smokers had a significantly higher risk (2.82, 1.34–6.19) of hypertension, and overweight smokers had a higher risk (4.43, 1.28–15.26) of hypertension and very high risks of diabetes (8.24, 2.47–27.42) and cardiovascular disease (13.12, 1.95–88.41). The highest RERI was derived from the relation with cardiovascular disease. The highest AP and S were derived from the relation with type 2 diabetes. There was no interaction of smoking and being overweight with dyslipidemia.
Conclusion
The presence of an interaction between smoking and being overweight exacerbates the risks of hypertension, diabetes, and cardiovascular disease in outpatients with mood disorders.
Keywords: Mood disorders, Lifestyle-related diseases, Interaction, Smoking, Body mass index
Introduction
People with mood disorders, including those with unipolar depression and bipolar disorder, have poor physical health and a higher mortality rate compared with the general population [1–5]. Furthermore, patients with mood disorders are more likely to develop lifestyle-related diseases [6–13].
In Japanese men, major depression was found to be associated with raised serum cholesterol levels [11, 12]. It was also reported that metabolic syndrome was significantly related to depression in Japanese men [13]. Furthermore, Shiotani et al. [14] reported that depression was a significant risk factor for cardiac events in elderly patients.
It is reasonable to consider that lifestyle-related diseases are caused by unhealthy lifestyle habits. Therefore, patients with mood disorders might have unhealthier habits than the general population. In studies investigating lifestyle among psychiatric inpatients and outpatients [15, 16], alcohol consumption and unhealthy dietary habits were suggested to be risk factors for depression [15], and a diagnosis of bipolar disorder was found to be independently related to smoking status [15, 17, 18] and being overweight or obese [10, 17, 18]. In the present study we considered the effect of both these risk factors–smoking and being overweight. de Mutsert et al. [19] reported that the presence of an interaction between risk factors could be examined. Akbartabartoori et al. [20] reported that the combination of smoking and being overweight or obese increased cardiovascular disease risk factors, particularly levels of high-density lipoprotein-cholesterol and C-reactive protein, in the general population. We have previously examined the combined risk of smoking and being overweight among patients with schizophrenia [21]. Thus, here we focused our attention on the interaction between being overweight and smoking among outpatients with mood disorders, where the combination of smoking and being overweight might exacerbate the risks of lifestyle-related diseases. The aim of this study was to evaluate the hypothesis that the presence of an interaction between smoking and being overweight increases the risks of hypertension, diabetes mellitus, dyslipidemia, and cardiovascular disease in outpatients with mood disorders.
Patients, materials, and methods
Survey of patients in a psychiatric hospital
This study was conducted as part of a cross-sectional survey of psychiatric inpatients and outpatients in a psychiatric hospital in Tochigi prefecture, Japan, between 2007 and 2008. The survey was carried out by a clinical doctor working in the hospital and included information about gender, age, diagnosed psychiatric diseases, complicated physical diseases, inpatient or outpatient status, smoking status, alcohol consumption, habitual exercise, and intake of healthy foods. All participants who could understand the meaning of this survey and agreed to participate were asked for information about smoking, alcohol use, habitual exercise, and intake of healthy foods, using the following questions: “Are you a current smoker?”; “Do you regularly drink alcohol?”; “Do you take part in regular exercise?”; and “Do you have a healthy balanced diet?”. All questions could be answered with “Yes” or “No”. Data on the presence or absence of physical diseases, such as hypertension, dyslipidemia, type 2 diabetes, and cardiovascular disease, were acquired from medical records by a clinical doctor who used Japanese clinical criteria. Hypertension was defined as systolic blood pressure of ≥140 mmHg or diastolic pressure of ≥90 mmHg [22], or as the use of antihypertensive medication. Diabetes was defined as a plasma glucose level of ≥126 mg dL−1 during fasting or ≥200 mg dL−1 during a 75-g oral glucose tolerance test [23], or as the use of antidiabetic medication. Dyslipidemia was defined as the use of cholesterol-lowing medication or at least one of the following criteria: total cholesterol ≥140 mg dL−1; high-density lipoprotein cholesterol <40 mg dL−1; or triglycerides ≥150 mg dL−1 [24]. Patients were defined as having cardiovascular disease if they had already received treatment, including medication, for this condition.
Patients’ weights and heights were measured, and the body mass index (BMI) was calculated as weight/height2 (kg/m2). A total of 919 psychiatric patients (448 men, 471 women) participated in the cross-sectional survey. Patients were diagnosed using the World Health Organization International Classification of Disease 10th revision (ICD-10) [25] criteria, and data were collected from structured clinical interviews by specialists in psychiatric medicine. Schizophrenia included schizotypal and delusional disorders (ICD-10 F20–29). Mood disorders included bipolar affective disorders and depressive disorders (ICD-10 F30–39). Neuroticism included neurotic, stress-related, and somatoform disorders (ICD-10 F40–48).
Outpatient survey
Only outpatients were selected for this psychiatric patient survey, because the lifestyle of outpatients is more dependent on self-management than that of inpatients. Patient characteristics were compared between patients with schizophrenia (125 men and 114 women), those with mood disorders (95 men and 118 women), those with neuroticism (70 men and 90 women), and those with other disorders (12 men and 15 women) using data from medical records obtained by clinicians.
Study subjects and statistical analyses
The characteristics of patients with mental disorders were compared using Pearson’s χ2 test and one-way analysis of variance (ANOVA) with the Bonferroni post hoc test. For the present study, data from 213 outpatients with mood disorders who had no values missing from the cross-sectional survey were used for further analysis.
In the patients with mood disorders, we calculated multivariate adjusted odds ratios (ORs) for hypertension, type 2 diabetes, dyslipidemia, and cardiovascular disease, and used binary logistic regression analyses controlled for possible confounders, including age (continuous), sex (male or female), food intake (balanced or unbalanced), regular exercise (yes or no), current smoking (yes or no), and daily alcohol drinking (yes or no). Furthermore, patients were classified as non-smokers with BMI <25 kg/m2 (− −; reference), non-smokers with BMI ≥25 kg/m2 (− +; group 1), smokers with BMI <25 kg/m2 (+ −; group 2), or smokers with BMI ≥25 kg/m2 (+ +; group 3). Adjusted ORs for groups 1, 2, and 3 for hypertension, type 2 diabetes, dyslipidemia, and cardiovascular diseases were calculated by binary logistic regression analyses including age (continuous), sex (male or female), food intake (balanced or unbalanced), regular exercise (yes or no), and daily alcohol drinking (yes or no) as covariates.
After carrying out the logistic regression analyses, we examined for the presence of an interaction between smoking and being overweight in association with the prevalence of each lifestyle-related disease, using the following three measures of additive interaction derived from multiplicative models according to the method of de Mutsert et al. [19].
- The relative excess risk due to the interaction (RERI) calculated as the difference between the expected risk and the observed risk (OR):
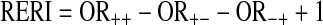
The attributable proportion due to the interaction (AP); that is, due to the interaction in subjects with both exposures: AP = RERI/OR++
- The synergy index (S), which can be interpreted as the excess risk from both exposures when there is interaction relative to the risk from exposure without interaction:
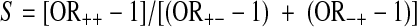
Because, in the absence of an interaction effect, RERI and AP equal 0 and S equals 1, some consider every departure from 0 (RERI and AP) or 1 (S) as evidence for the presence of an interaction, as reported by de Mutsert et al. [19].
All data were analyzed using the statistical software package PASW Statistics 18.0 (IBM Japan, Tokyo, Japan). A p value of <0.05 was considered significant for the χ2 test, ANOVA, and binary logistic regression analyses.
Approval
Approval for this study was obtained from the Ethics Committee at Dokkyo Medical University and written informed consent was obtained from all patients.
Results
Table 1 shows the characteristics of the subjects with mood disorders compared with the characteristics of those with other mental disorders (schizophrenia, neuroticism, and other mood disorders [others]). The mean age in the subjects with mood disorders was higher than that in the subjects with schizophrenia. In the subjects with mood disorders, the mean BMI and smoking rate, and the proportions of subjects with BMI of ≥25 kg/m2, those with an unbalanced diet, and those with lack of exercise were all lower than those in the subjects with schizophrenia. However, the prevalence of hypertension was highest in subjects with mood disorders compared with the prevalences in all subjects with mental disorders.
Table 1.
Characteristics and complications of lifestyle-related diseases among outpatients with mood disorders compared with those with other mental disorders
| Mental disorders | Mood disorders (n = 213) | Schizophrenia (n = 239) | Neuroticism (n = 160) | Others (n = 27) |
|---|---|---|---|---|
| Mean age, years (SD)** | 59.73 (14.39) | 51.85 (12.94) | 58.42 (17.27) | 57.52 (18.15) |
| Mean BMI (SD)* | 23.84 (3.86) | 25.15 (4.91) | 23.51 (3.92) | 23.76 (4.41) |
| Men | 95 (44.6%) | 125 (52.3%) | 70 (43.8%) | 12 (44.4%) |
| Alcohol drinking | 57 (26.8%) | 44 (18.4%) | 34 (21.3%) | 7 (25.9%) |
| BMI ≥25 mg/kg2* | 73 (34.3%) | 114 (47.7%) | 55 (34.4%) | 11 (40.7%) |
| Smoking* | 65 (30.5%) | 96 (40.2%) | 43 (26.9%) | 5 (18.5%) |
| Unbalanced diet | 116 (54.5%) | 157 (65.7%) | 79 (49.4%) | 22 (81.5%) |
| Lack of exercise* | 138 (64.8%) | 176 (73.6%) | 87 (54.4%) | 16 (59.3%) |
| Hypertension** | 87 (40.9%) | 52 (21.8%) | 54 (33.8%) | 4 (14.8%) |
| Type 2 diabetes | 38 (17.8%) | 40 (16.7%) | 16 (10.0%) | 1 (3.7%) |
| Dyslipidemia** | 66 (31.0%) | 60 (25.1%) | 29 (18.1%) | 2 (7.4%) |
| Cardiovascular disease | 12 (5.6%) | 8 (3.3%) | 12 (7.5%) | 0 (0.0%) |
All values except for mean age and mean body mass index (BMI) are given as numbers (%), unless otherwise stated
The p values were determined by analysis of variance (ANOVA) or Pearson’s χ2 test; * p < 0.05, ** p < 0.001
Associations between hypertension, type 2 diabetes, dyslipidemia, and cardiovascular disease and alcohol consumption, smoking status, diet, and excess weight were examined in outpatients with mood disorders. Multivariate adjusted ORs for hypertension, type 2 diabetes, dyslipidemia, and cardiovascular disease in these patients are shown in Table 2. Alcohol consumption [OR 2.56, 95% confidence interval (CI) 1.10–5.95] and BMI ≥25 kg/m2 (OR 3.55, 95% CI 1.82–6.90) significantly increased the risk for hypertension. A significantly higher risk for type 2 diabetes was seen in current smokers (OR 2.38, 95% CI 1.00–5.66) and a significantly higher risk for cardiovascular disease was seen in patients with BMI ≥25 kg/m2 (OR 4.52, 95% CI 1.22–16.75). Table 3 shows the adjusted ORs for all subgroups of subjects with mood disorders for hypertension, type 2 diabetes, dyslipidemia, and cardiovascular disease. Smokers with BMI <25 kg/m2 had a significantly lower risk (OR 0.27, 95% CI 0.09–0.81) for hypertension compared with non-smokers with BMI <25 kg/m2 (reference group). However, overweight non-smokers (OR 2.82, 95% CI 1.34–6.19) had a higher risk of hypertension compared with the reference group. Moreover, overweight smokers had a higher risk (OR 4.43, 95% CI 1.28–15.26) of hypertension and very high risks of diabetes (OR 8.24, 95% CI 2.47–27.42) and cardiovascular disease (OR 13.12, 95% CI 1.95–88.41) compared with the reference group.
Table 2.
Adjusted odds ratios for hypertension, type 2 diabetes, dyslipidemia, and cardiovascular disease among outpatients with mood disorders (n = 213)
| Hypertension OR (95% CI) |
Type 2 diabetes OR (95% CI) |
Dyslipidemia OR (95% CI) |
Cardiovascular disease OR (95% CI) |
|
|---|---|---|---|---|
| Alcohol drinking | ||||
| No | 1.00 | 1.00 | 1.00 | 1.00 |
| Yes | 2.56 (1.10–5.95)* | 1.34 (0.53–3.40) | 1.64 (0.74–3.61) | 0.56 (0.01–3.28) |
| BMI | ||||
| <25 kg/m2 | 1.00 | 1.00 | 1.00 | 1.00 |
| ≥25 kg/m2 | 3.55 (1.82–6.90)** | 1.86 (0.87–3.97) | 0.99 (0.52–1.89) | 4.52 (1.22–16.75)* |
| Smoking | ||||
| Non-smoker | 1.00 | 1.00 | 1.00 | 1.00 |
| Current smoker | 0.68 (0.31–1.49) | 2.38 (1.00–5.66)* | 0.71 (0.34–1.50) | 2.58 (0.58–11.53) |
| Diet | ||||
| Balanced | 1.00 | 1.00 | 1.00 | 1.00 |
| Unbalanced | 0.76 (0.40–1.47) | 1.00 (0.47–2.14) | 1.47 (0.78–2.78) | 0.50 (0.14–1.79) |
| Exercise | ||||
| Yes | 1.00 | 1.00 | 1.00 | 1.00 |
| No | 0.90 (0.46–1.78) | 1.49 (0.66–3.39) | 1.80 (0.91–3.57) | 0.92 (0.25–3.46) |
95% CI 95% confidence interval, OR odds ratio adjusted for age, sex, alcohol drinking, BMI, smoking, diet, and exercise using binary logistic regression
* p < 0.05, ** p < 0.01
Table 3.
Adjusted odds ratios for hypertension, type 2 diabetes, dyslipidemia, and cardiovascular disease among outpatients in relation to the combination of smoking and being overweight (n = 213)
| Hypertension OR (95% CI) |
Type 2 diabetes OR (95% CI) |
Dyslipidemia OR (95% CI) |
Cardiovascular disease OR (95% CI) |
|
|---|---|---|---|---|
| Non-smoker | ||||
| BMI <25 kg/m2 (n = 91) | 1.00 | 1.00 | 1.00 | 1.00 |
| BMI ≥25 kg/m2 (n = 57) | 2.82 (1.34–6.19)** | 1.12 (0.43–2.88) | 1.08 (0.51–2.27) | 2.89 (0.61–13.74) |
| Smoker | ||||
| BMI <25 kg/m2 (n = 49) | 0.27 (0.09–0.81)* | 0.84 (0.26–2.70) | 0.67 (0.27–1.65) | 1.02 (0.09–11.35) |
| BMI ≥25 kg/m2 (n = 16) | 4.43 (1.28–15.26)* | 8.24 (2.47–27.42)** | 1.12 (0.36–3.50) | 13.12 (1.95–88.41)** |
95% CI 95% confidence interval, OR odds ratio adjusted for age, sex, alcohol drinking, diet, and exercise using binary logistic regression
* p < 0.05, ** p < 0.01
Table 4 shows measures of additive interaction between smoking and being overweight derived from the multiplicative models RERI, AP, and S for each group of patients with hypertension, type 2 diabetes, dyslipidemia, and cardiovascular disease. The highest RERI was derived from the relation with cardiovascular disease. The highest AP and S were derived from the relation with type 2 diabetes. There was no interaction of smoking and being overweight with dyslipidemia.
Table 4.
Measures of additive interaction between smoking and being overweight derived from multiplicative models
| Hypertension | Type 2 diabetes | Dyslipidemia | Cardiovascular disease | |
|---|---|---|---|---|
| RERI | 2.34 | 7.28 | 0.37 | 10.21 |
| AP | 0.53 | 0.88 | 0.33 | 0.78 |
| S | 3.15 | −181 | −0.48 | 6.87 |
RERI Relative excess risk due to interaction, AP attributable proportion due to interaction, S synergy index
Discussion
The results of the present cross-sectional study show that the interaction between smoking and being overweight was significantly associated with an increased prevalence of hypertension, type 2 diabetes, and cardiovascular disease in outpatients with mood disorders.
From our study, the overall prevalences of hypertension, type 2 diabetes, dyslipidemia, and cardiovascular disease were 40.8, 17.8, 31.0, and 5.6%, respectively. From a survey of middle-aged Japanese individuals done by the Ministry of Health, Labour and Welfare of Japan, the proportions of people who were diagnosed with hypertension, diabetes, dyslipidemia, and cardiovascular disease were 17.0, 7.0, 8.5, and 2.6%, respectively [26]. We found that the values in our study participants were approximately two-fold higher than those in the general Japanese population. This difference might be due to an unhealthier lifestyle, including obesity, sedentary habits, smoking, and poor adherence to medical regimens, as reported by Katon [27]. Furthermore, type A behavior, which has been found to be associated with coronary heart disease, is similar to the immodithymic character that was reported to be a pre-pathological characteristic of mood disorders [28]. Metabolic syndrome, which is associated with lifestyle-related diseases, was found to be more common in patients with mood disorders [10, 13]. However, in the present study, waist circumference was not measured, and therefore we could not diagnose metabolic syndrome or explore any possible association of the syndrome with mood disorders.
We found that the smoking rate in patients with mood disorders was 30.5% (46.3% in men, 17.8% in women), which was a little higher than that in the general Japanese population, at 23.8% (39.4% in men, 11.0% in women) according to the 2007 National Health and Nutrition Survey [29]. According to Kilian et al. [15], the smoking rate was 57.9% in patients with bipolar disorder and 44.8% in those with depression; again, these rates were higher than those in the general population. Vanable et al. [17] also reported high rates of smoking in patients with bipolar disorder (66%) or depression (60%). According to a Japanese psychiatry hospital-based survey, smoking rates were 87.5% in males and 100% in females with bipolar mood disorders, and 69.6% and 5.4% in men and women, respectively, with depression [30]. The smoking rates in our study were considerably lower than those in previous studies. The psychiatric hospital in which this study was conducted has recently started a smoking cessation program for outpatients. Recently, in Japan, an anti-smoking declaration promoted by the Japanese Circulation Society [31] has probably had some effect, even among psychiatric patients. According to a survey of patients and staff in Japanese psychiatric hospitals, 75.7% of smokers wanted to quit, and 49.0% were in favor of smoking being prohibited in hospitals [30].
We found that non-overweight smokers had a significantly lower risk for hypertension (OR 0.27, 95% CI 0.09–0.81) compared with the reference group (non-overweight non-smokers). However, this result might be the reverse cause and effect that is commonly seen in a cross-sectional study. The cross-sectional design will often increasingly tend to give misleading result. Because some of patients with hypertension might quit smoking after diagnosis. Akbartabartoori et al. [20] have reported that smoking combined with overweight or obesity markedly elevates the cardiovascular risk in the general population. However, according to Katano et al. [32], current smoking combined with obesity was not a contributing factor to metabolic syndrome components in general. Nevertheless, the originality of the present study is that the reference group was composed of non-smokers who were not overweight, meaning that the interaction of the combined risk of smoking and being overweight was clearly shown. Furthermore, there has been little study of the presence of smoking and being overweight in association with the prevalence of lifestyle-related diseases. This study used three different measures to examine the interaction between smoking and being overweight, using the method of de Mustert et al. [19]. The results clearly showed the presence of a very high interaction between smoking and being overweight in association with the prevalence of type 2 diabetes and cardiovascular disease. It was previously reported that the severity of depression and anxiety symptoms strongly indicated the prevalence of coronary heart disease [33].
Smoking and obesity have been independently associated with specific food cravings and mood states. Pepino et al. [34] reported that current smokers craved high-fat foods more frequently and felt more depressed and angry than never-smokers; this was also the case for obese women, compared with non-obese women. In addition, they found that symptoms of depression were intensified with increasing body weight. Therefore, there might be an association between smoking and being overweight and mood disorders.
Unexpectedly, a study in Germany reported that inpatients with depression were more physically active than the general population [14], although another German study reported that physical fitness and heart rate recovery were reduced in patients with major depressive disorders [35]. In addition, Ng et al. [36] demonstrated that physical activity was efficacious for reducing depression and anxiety. In research on the effectiveness of cessation programs for the general population of smokers, Faulkner et al. [37] provide preliminary support for the effectiveness of a physical activity program for helping smokers to quit, and Cosci et al. [38] have proposed adding psychological or pharmacological support to complement smoking cessation programs. Thus, the introduction of a physical activity program alongside health promotional education might be important for the prevention of lifestyle-related diseases in patients with mood disorders, in the recovery stage when the depressive symptoms are decreasing.
The present study has several limitations. First, as the design of this study was cross-sectional and examined one point in time, no cause-effect relationship for the factors identified can be established. Second, as there were no healthy controls, it is unclear whether the risks for hypertension, dyslipidemia, and type 2 diabetes were higher than those in the general population. Third, the questionnaire consisted of some items with only yes/no answers, so we were not able to determine the subjects’ lifestyles in detail. Fourth, the study participants were outpatients from only one psychiatric hospital in Japan. Further investigation is needed to be able to provide generalized results. Thus, a further large longitudinal study will be needed to explore the combined risk of smoking and being overweight for lifestyle-related diseases in patients with mood disorders.
The results of this study suggest that overweight patients with mood disorders who smoke may carry an increased risk of hypertension, diabetes, or cardiovascular disease. Therefore, health promotion education for patients with depressive disorders is important for the prevention of these lifestyle-related diseases. The introduction of combined smoking cessation and weight loss programs is also likely to be beneficial for these patients.
Acknowledgments
We are grateful to Dr. Naotake Muroi, Director of Muroi Hospital, for assistance with our study. We would like to thank all participants and medical staff for their cooperation in this study.
References
- 1.Harris EC, Barraclough B. Excess mortality of mental disorder. Br J Psychiatry. 1998;173:11–53. doi: 10.1192/bjp.173.1.11. [DOI] [PubMed] [Google Scholar]
- 2.Angst F, Strassen HH, Clayton PJ, Angst J. Mortality of patients with mood disorders: follow-up over 34–38 years. J Affect Disord. 2002;68:167–181. doi: 10.1016/S0165-0327(01)00377-9. [DOI] [PubMed] [Google Scholar]
- 3.Newcomer JW. Medical risk in patients with bipolar disorder and schizophrenia. J Clin Psychiatry. 2006;67:25–30. doi: 10.4088/JCP.1106e16. [DOI] [PubMed] [Google Scholar]
- 4.Hert M, Dekker JM, Wood D, Kahl KG, Holt RIG, Möller HJ. Cardiovascular disease and diabetes in people with severe mental illness: position statement from the European Psychiatric Association (EPA), supported by the European Association for the Study of Diabetes (EASD) and the European Society of Cardiology (ESC) Eur Psychiatry. 2009;24:412–424. doi: 10.1016/j.eurpsy.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Osby U, Brandt L, Correia N, Ekbom A, Sparén P. Excess mortality in bipolar and unipolar disorder in Sweden. Arch Gen Psychiatry. 2001;58:844–850. doi: 10.1001/archpsyc.58.9.844. [DOI] [PubMed] [Google Scholar]
- 6.Barth J, Schumacher M, Herrmann-Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: a meta-analysis. Psychosom Med. 2004;66:802–813. doi: 10.1097/01.psy.0000146332.53619.b2. [DOI] [PubMed] [Google Scholar]
- 7.Melle JP, Jonge P, Spijkerman TA, Tijssen JG, Ormel J, Veldhuisen DJ, et al. Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: meta-analysis. Psychosom Med. 2004;66:814–822. doi: 10.1097/01.psy.0000146294.82810.9c. [DOI] [PubMed] [Google Scholar]
- 8.Goldstein BI, Fagioline A, Houck P, Kupfer DJ. Cardiovascular disease and hypertension among adults with bipolar I disorder in the United States. Bipolar Disord. 2009;11:657–662. doi: 10.1111/j.1399-5618.2009.00735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cassidy F, Ahearn E, Carroll BJ. Elevated frequency of diabetes mellitus in hospitalized manic-depressive patients. Am J Psychiatry. 1999;156:1417–1420. doi: 10.1176/ajp.156.9.1417. [DOI] [PubMed] [Google Scholar]
- 10.Fagiolini A, Frank E, Scott JA, Turkin S, Kupfer DJ. Metabolic syndrome in bipolar disorder: findings from the Bipolar Disorder Center for Pennsylvanians. Bipolar Disord. 2005;7:424–430. doi: 10.1111/j.1399-5618.2005.00234.x. [DOI] [PubMed] [Google Scholar]
- 11.Nakao M, Yano E. Relationship between major depression and high serum cholesterol in Japanese men. Tohoku J Exp Med. 2004;204:273–287. doi: 10.1620/tjem.204.273. [DOI] [PubMed] [Google Scholar]
- 12.Nakao M, Ando K, Momura S, Kuboki T, Uehara Y, Toyooka T, et al. Depressive mood accompanies hypercholesterolemia in young Japanese adults. Jpn Heart J. 2001;42:739–748. doi: 10.1536/jhj.42.739. [DOI] [PubMed] [Google Scholar]
- 13.Takeuchi T, Nakao M, Nomura K, Yano E. Association of metabolic syndrome with depression and anxiety in Japanese men. Diabetes Metab. 2009;35:32–36. doi: 10.1016/j.diabet.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Shiotani I, Sato H, Nakatani D, Mizuno H, Ohnishi Y, Hishida E, et al. Depression symptoms predict 12-month prognosis in elderly patients with acute myocardial infarction. J Cardiovasc Risk. 2002;9:153–160. doi: 10.1097/00043798-200206000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Kilian R, Becker T, Krüger K, Schmid S, Frasch K. Health behavior in psychiatric in-patients compared with a German general population sample. Acta Psychiatr Scand. 2006;114:242–248. doi: 10.1111/j.1600-0447.2006.00850.x. [DOI] [PubMed] [Google Scholar]
- 16.Chuang HT, Mansell C, Patten SB. Lifestyle characteristics of psychiatric out patients. Can J Psychiatry. 2008;53:260–266. doi: 10.1177/070674370805300407. [DOI] [PubMed] [Google Scholar]
- 17.Vanable PA, Carey MP, Carey KB, Carey KB, Maisto SA. Smoking among psychiatric outpatients: relationship to substance use, diagnosis and illness severity. Psychol Addict Behav. 2003;17:259–265. doi: 10.1037/0893-164X.17.4.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diaz FJ, James D, Botts S, Maw L, Susce MT, Leon J. Tobacco smoking behaviors in bipolar disorder; a comparison of the general population, schizophrenia, and major depression. Bipolar Disord. 2009;11:154–165. doi: 10.1111/j.1399-5618.2009.00664.x. [DOI] [PubMed] [Google Scholar]
- 19.Mutsert R, Jager KJ, Zoccali C, Dekker FW. The effect of joint exposures: examining the presence of interaction. Kidney Int. 2009;75(7):677–681. doi: 10.1038/ki.2008.645. [DOI] [PubMed] [Google Scholar]
- 20.Akbartabartoori M, Lean ME, Hankey CR. Smoking combined with overweight or obesity markedly elevates cardiovascular factors. Eur J Cardiovasc Prev Rehabil. 2006;13:938–946. doi: 10.1097/01.hjr.0000214613.29608.f5. [DOI] [PubMed] [Google Scholar]
- 21.Kimijima M, Nishiyama M, Muto T. The combination of smoking and overweight is associated with dyslipidemia among inpatients and hypertension among outpatients with schizophrenia. Dokkyo J Med Sci. 2011;38:1–8. [Google Scholar]
- 22.World Health Organization, International Society of Hypertension Writing. 2003 World Health Organization (WHO)/ International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens 2003;21:1983–92. [DOI] [PubMed]
- 23.Japanese Diabetes Society The report by committee for clinical criteria of diabetes. Diabetes Mellitus. 1999;42(5):385. [Google Scholar]
- 24.Teramoto T, Sasaki J, Ueshima H, Egusa G, Kinoshita M, Shimamoto K et al. Diagnostic criteria for dyslipedemia—executive summary of Japan Atherosclerosis Society (JAS) guideline for diagnosis and prevention of atherosclerotic cardiovascular diseases for Japanese. J Atheroscler Thromb 2007;14:155–8. [DOI] [PubMed]
- 25.World Health Organization (WHO). International statistical classification of disease and related health problems. 10th revision, version for 2007, ICD 10 online: http://www.who.int/ classifications/apps/icd/.
- 26.Ministry of Health, Labour, and Welfare of Japan Outline of first longitudinal survey of middle and elderly persons. J Health Welf Stat. 2007;54(4):41–49. [Google Scholar]
- 27.Katon WJ. Epidemiology and treatment of depression in patients with chronic medical illness. Dialogues Clin Neurosci. 2011;13(1):7–23. doi: 10.31887/DCNS.2011.13.1/wkaton. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagata A, Watanabe K. The association of lifestyle-related diseases and depressive disorders. J Adult Dis. 2010;40:1122–1126. [Google Scholar]
- 29.The National Health and Nutrition Survey in Japan 2007. Tokyo: Daiichi Shuppan; 2009. [Google Scholar]
- 30.Kawai A, Abe H. The smoking situation of patients and staff in a psychiatry hospital. The smoking issue in psychiatry can be considered a neglected problem. Nippon Koshu Eisei Zasshi. 2007;54:626–632. [PubMed] [Google Scholar]
- 31.Fujiwara H, The Japanese Circulation Society Anti-smoking declaration—a message from the Japanese Circulation Society. Circ J. 2003;67:1–2. doi: 10.1253/circj.67.1. [DOI] [PubMed] [Google Scholar]
- 32.Katano S, Nakamura Y, Nakamura A, Murakami Y, Tanaka T, Nakagawa H, et al. Relationship among physical activity, smoking, drinking and clustering of the metabolic syndrome diagnostic components. J Atheroscler Thromb. 2010;17:644–650. doi: 10.5551/jat.3699. [DOI] [PubMed] [Google Scholar]
- 33.Vogelzangs N, Seldenrijk A, Beekman ATF, Hout HPJ, Jonge P, Penninx BWJH. Cardiovascular disease in persons with depressive and anxiety disorders. J Affect Disord. 2010;125:241–248. doi: 10.1016/j.jad.2010.02.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pepino MY, Finkbeiner S, Mennella JA. Similarities in food cravings and mood states between obese women and women who smoke tobacco. Obesity. 2009;17:1158–1163. doi: 10.1038/oby.2009.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boettger S, Wetzig F, Puta C, Donath L, Müller HJ, Gabriel HH, Bär KJ. Physical fitness and heart rate recovery are decreased in major depressive disorder. Psychosom Med. 2009;71:519–523. doi: 10.1097/PSY.0b013e3181a55303. [DOI] [PubMed] [Google Scholar]
- 36.Ng F, Dodd S, Berk M. The effects of physical activity in the acute treatment of bipolar disorder: a pilot study. J Affect Disord. 2007;101:259–262. doi: 10.1016/j.jad.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 37.Faulkner G, Taylor A, Munro S, Selby P, Gee C. The acceptability of physical activity programming within a smoking cessation service for individuals with severe mental illness. Patient Educ Couns. 2007;66:123–126. doi: 10.1016/j.pec.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 38.Cosci F, Schruers KRJ, Pistelli F, Griez EJ. Negative affectivity in smokers applying to smoking cessation clinics: a case–control study. Depress Anxiety. 2009;26:824–830. doi: 10.1002/da.20473. [DOI] [PubMed] [Google Scholar]


