Abstract
To characterize the clinical presentation and pathophysiology of inhalational brucellosis, Balb/c mice were challenged with Brucella melitensis 16M in a nose-only aerosol exposure chamber. A low dose of 1000 cfu/animal of B. melitensis resulted in 45% of mice with tissue burdens eight weeks post-challenge. The natural history of brucellosis in mice challenged by higher aerosol doses was examined by serial euthanizing mice over an eight week period. Higher challenge doses of 1.00E+05 and 5.00E+05 cfu resulted in positive blood cultures 14 days post-challenge and bacterial burdens were observed in the lung, liver and/or spleens 14 days post-challenge. In addition, the progression of brucellosis was similar between mice challenged by the intranasal and aerosol routes. The results from this study support the use of the Balb/c aerosol nose-only brucellosis mouse model for the evaluation of therapeutics against inhalational brucellosis.
S Brucella species are Gram negative coccobacilli that can cause significant disease in humans and animals. Brucella melitensis, one of the major Brucella species that cause disease in humans and animals, is an etiologic agent for brucellosis. Humans are commonly infected following contact with infected animals and the ingestion of contaminated milk, milk products and meat. Brucellosis is one of the most common laboratory acquired infections, as well as the most common zoonosis1,2. While humans can be asymptomatic for weeks, months or years following infection, the typical symptoms associated with brucellosis include flu-like symptoms such as fever, night sweats, headache, depression and arthritis. Meningitis and endocarditis, although not common, are associated with chronic brucellosis. Brucellosis is usually not fatal (less than 5%), but most brucellosis-related deaths occur for patients with endocarditis3. In addition, despite appropriate antibiotic treatment, relapses can occur4,5,6,7,8,9. The relapse rate following antibiotic therapy depends upon the types of antibiotic used, the duration of treatment, and patient population5,9,10,11,12,13,14.
Brucella species are considered potential biological warfare agents due to high infectivity, ease of aerosol infection, ability to incapacitate infected people and the persistent nature of human disease13. Therefore, an appropriate small animal model is needed to evaluate the efficacy of novel vaccines and therapeutics against brucellosis.
Intraperitoneal (IP), intranasal (IN), or whole-body aerosol routes of challenge15,16,17,18 have been used to establish brucellosis in mice. For each of these routes of exposure, Brucella rapidly spread from the site of challenge to regional lymph nodes, then to the liver and spleen.
We have developed a nose-only aerosol exposure model to test the efficacy of antibiotics and vaccines against inhalational brucellosis under the Food and Drug Administration (FDA) “Animal Rule.” In contrast to whole-body exposure systems as previously described, the Balb/c the nose-only aerosol challenge system reduces infection of the oral mucosa from animals licking contaminated fur. This study characterized the nose-only chronic Balb/c inhalational brucellosis model over an eight-week time period in which seven aerosol challenge doses were examined to determine the optimal dose for future treatment efficacy studies. An IN challenge group was also included in this study to bridge our results with a previously published IN challenge study15.
The clinical presentation and pathophysiology of inhalational and IN brucellosis in mice were examined by measuring body temperature, clinical hematology, blood culture, tissue burden and splenomegaly during the progression of disease. Gross necropsies were performed at the scheduled euthanasia time points and tissues were collected and evaluated by a board-certified veterinary pathologist for microscopic lesions. This study provided data that further expands the Balb/c mouse model for chronic inhalational brucellosis and supports the use of the nose-only aerosol model for testing novel vaccine and therapeutics for the treatment of brucellosis.
Results
Characterization of the Nose-Only Aerosol Exposure System
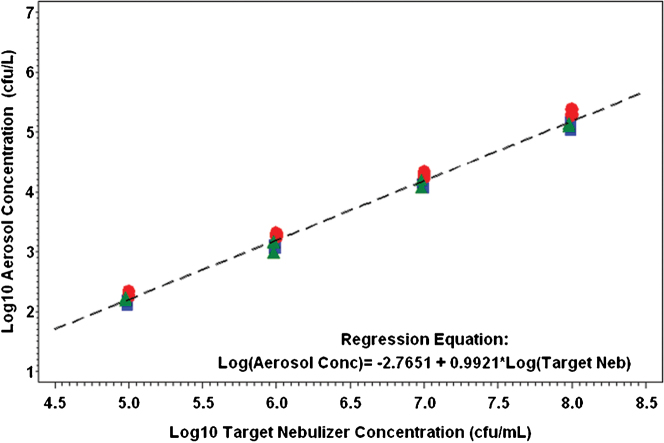
The relationship between the calculated aerosol concentration and the target nebulizer concentration was characterized by the model with a resulting slope of >0.99. The mean spray factor for each aerosol dissemination is shown in Table 1. The mean spray factor for all 12 aerosol disseminations was 1.53E-06 (95% confidence interval 8.68E-7, 2.68E-6).
Table 1. Geometric Mean for Aerosol Concentration and Spray Factor for Each Test Day.
| Aerosol Concentration (cfu/L) | Spray Factor | |||||||
|---|---|---|---|---|---|---|---|---|
| Target Nebulizer Concentration (cfu/mL) | Geometric Mean (11/16) | Geometric Mean (11/17) | Geometric Mean (11/18) | Geometric Mean Across Three Test Days | Geometric Mean (11/16) | Geometric Mean (11/17) | Geometric Mean (11/18) | Geometric Mean Across Three Test Days |
| 1.0 × 105 | 1.42 × 102 | 1.94 × 102 | 1.63 × 102 | 1.65 × 102 | 1.42 × 10−6 | 1.94 × 10−6 | 1.63 × 10−6 | 1.65 × 10−6 |
| 1.0 × 106 | 1.21 × 103 | 1.94 × 103 | 1.28 × 103 | 1.44 × 103 | 1.21 × 10−6 | 1.94 × 10−6 | 1.28 × 10−6 | 1.44 × 10−6 |
| 1.0 × 107 | 1.28 × 104 | 1.95 × 104 | 1.31 × 104 | 1.48 × 104 | 1.28 × 10−6 | 1.95 × 10−6 | 1.31 × 10−6 | 1.48 × 10−6 |
| 1.0 × 108 | 1.34 × 105 | 2.09 × 105 | 1.30 × 105 | 1.54 × 105 | 1.34 × 10−6 | 2.09 × 10−6 | 1.30 × 10−6 | 1.54 × 10−6 |
| All Data | N/A | N/A | N/A | 1.54 × 105 | 1.31 × 10−6 | 1.98 × 10−6 | 1.37 × 10−6 | 1.53 × 10−6 |
N/A = Not applicable.
The relationship between calculated aerosol concentration and the target nebulizer concentration indicates that the log10 aerosol concentration increases approximately 0.9921 log10 for each 1.0 log10 increase in nebulizer concentration (cfu/mL). The 95% confidence interval for the slope is (0.9746, 1.0095), which is significantly different from zero (p = < 0.0001). This model relationship between aerosol concentration and target nebulizer concentration is shown in Figure 1.
Figure 1. Relationship between aerosol concentration and target nebulizer concentration.
The enumerated aerosol concentration was plotted against the targeted nebulizer concentrations for all three spray factor test days. Blue square = Day 1; red circle = Day 2; Green triangle = Day 3; The mean spray factor for all 12 aerosol disseminations was 1.53E-06 (95% CI = 8.68E-07, 2.68E-6).
Low dose aerosol challenge study
In the low dose study, mice received an aerosol challenge dose of 2.30E+01 cfu (target of 3.00E+01 cfu), 7.40E+01 cfu (target of 1.00E+02 cfu), 2.63E+02 cfu (target of 3.00E+02 cfu) or 9.48E+02 cfu (target of 1.00E+03 cfu) B. melitensis (Table 2). The MMAD of B. melitensis was 3.41 µm, which is consistent with a particle size that will deposit within the lower respiratory tract19. Mice were euthanized eight weeks post-challenge, and the bacterial burden in the blood, lung, liver and spleen were determined for each mouse by qualitative (blood) or quantitative (tissue) plate count. The low-dose exposure of 30 cfu resulted in bacteria only being recovered from the lung, liver or spleen of one mouse, and there were no bacteria detected from the tissues of mice exposed to 100 cfu B. melitensis. A targeted challenge dose of 300 cfu resulted in a limited number of bacteria being recovered from the lung, liver and spleen in 2/20, 0/20 and 3/20, respectively, while the highest challenge dose of 1000 cfu resulted in 45% (9/20) of the mice having detectable levels of bacteria in the spleen (8/9 below limit of quantification). None of the mice challenged in the low-dose aerosol study were bacteremic by blood culture (data not shown).
Table 2. Summary of Challenge Data.
| Group | Number of Mice | Day of Scheduled Euthanasia (post-challenge) | Target challenge dosage (cfu/mouse) | Calculated inhaled dosage (cfu) |
|---|---|---|---|---|
| Aerosola | ||||
| Low Dose | ||||
| Aerosol | ||||
| Challenge Study | ||||
| 1 | 20 | Day 54 | 3.00E+01 | 2.30E+01 |
| 2 | 20 | Day 54 | 1.00E+02 | 7.40E+01 |
| 3 | 20 | Day 55 | 3.00E+02 | 2.63E+02 |
| 4 | 20 | Day 56 | 1.00E+03 | 9.48E+02 |
| 5 | 20 | Day 56 | 0.00E+00 | 0.00E+00 |
| Kinetics of | ||||
| Pathophysiology | ||||
| Study | ||||
| 6 | 11 | Day 14 | 1.00E+04 | 2.26E+04 |
| 7 | 11 | Day 28 | 1.00E+04 | 2.26E+04 |
| 8 | 11 | Day 42 | 1.00E+04 | 2.28E+04 |
| 9 | 12 | Day 56 | 1.00E+04 | 2.28E+04 |
| 10 | 12 | Day 14 | 1.00E+05 | 2.15E+05 |
| 11 | 12 | Day 28 | 1.00E+05 | 2.15E+05 |
| 12 | 11 | Day 42 | 1.00E+05 | 2.38E+05 |
| 13 | 11 | Day 56 | 1.00E+05 | 2.38E+05 |
| 14 | 12 | Day 14 | 5.00E+05 | 8.73E+05 |
| 15 | 11 | Day 28 | 5.00E+05 | 8.73E+05 |
| 16 | 12 | Day 42 | 5.00E+05 | 1.12E+06 |
| 17 | 12 | Day 56 | 5.00E+05 | 1.12E+06 |
| 18 | 12 | Day 56 | 0.00E+00 | 0.00E+00 |
| Intranasal | ||||
| 19 | 12 | Day 14 | 1.00E+04 | |
| 20 | 12 | Day 21 | 1.00E+04 | |
| 21 | 12 | Day 42 | 1.00E+04 | |
| 22 | 12 | Day 56 | 1.00E+04 |
aCalculated based on estimated concentration of B. melitensis 16 M in the exposure chamber and the total volume of air inhaled by the mice.
The aerosol concentration (cfu/L) was calculated as follows: AC = (C × V)/(S × T), where AC = Aerosol Concentration (cfu/L), C = AGI concentration (cfu/mL), V = AGI sampler volume (mL), S = sampling rate (∼6 L/min), and T = Exposure time (min).
Kinetics of the pathophysiology of inhalational brucellosis study
In order to achieve a higher infection rate, the targeted aerosol dose was increased for the kinetics of pathophysiology study. Mice were challenged with an aerosol dose of 2.26E+04 cfu to 2.28E+04 cfu (target of 1.0E+04 cfu; Groups 6–9), 2.15E+05 cfu to 2.38E+05 cfu (target of 1.0E+05 cfu; Groups 10–13) or 8.73E+05 cfu to 1.12E+06 cfu (target of 5.00E+05 cfu; Groups 14–17) B. melitensis 16 M (Table 2). In these aerosol challenges, the MMAD of B. melitensis was 2.96 µm, which is consistent with a particle size that will deposit within the lower respiratory tract. Mice were serially euthanized at 14, 28, 42 and 56 days post-challenge. The kinetics of inhalational brucellosis was followed by enriched blood culture and quantitative plate count of lung, liver and spleen tissue. In addition, the histopathology of disease was characterized. Samples of blood were analyzed for changes in blood hematology parameters.
Clinical observations and body temperature
None of the aerosol-challenged mice showed overt signs of infection due to brucellosis (i.e. hunched posture, ruffled fur). In addition, there were no changes in core body temperature associated with brucellosis infection. The lack of fever is consistent with the lack of physical signs observed in the Balb/c mice in this study.
Hematology
Overall, there were minimal changes in the hematology parameters for all the aerosol challenged Balb/c mice, which is similar to observations for the non-human primate (NHP) inhalational brucellosis model20 and human brucellosis patients4,20. Mild leucopenia with relative lymphocytosis, along with mild anemia and thrombocytopenia has been observed in human brucellosis patients21. In this study, trends in red blood cell parameters of challenged animals compared to unchallenged controls included mild decreases in red blood cells, hemoglobin, hematocrit, mean corpuscular volume (MCV) and slight increases in red cell distribution width (RDW) and corpuscular hemoglobin concentration mean (CHCM) (data not shown). While the group mean values were not severe enough to classify as anemia, these trends correlate to the mild anemia observed in humans4.
Splenomegaly
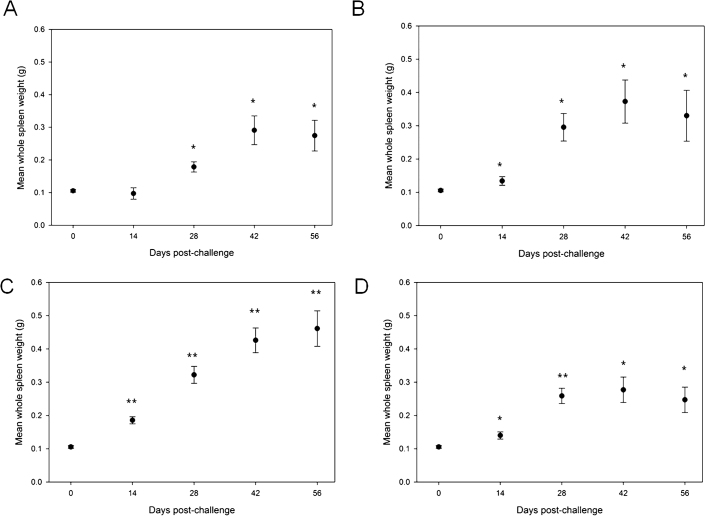
Spleen weights were measured for each animal on the day of scheduled euthanasia. As shown in Figure 2, on Day 14 post-challenge, there was a statistically significant difference (T-Test) in the spleen size of animals aerosol challenged with 1.00E+05 (p < 0.05) or 5.00E+05 cfu (p < 0.001) or with 1.00E+04 cfu (p < 0.05) by IN compared to control. By Day 28, splenomegaly was observed for all three aerosol challenge dose groups and the IN challenge dose group (statistically significant compared to control for all groups). The enlarged spleen size appears to peak on Day 42 post-challenge for the two lower aerosol-challenge doses and the IN dose, while the aerosol challenge with 5.0 x 105 cfu appears to result in a linear increase in splenomegaly compared to unchallenged spleens. The kinetics of splenomegaly were similar in mice challenged with 1.0E+04 cfu B. melitensis by either the aerosol or IN route.
Figure 2. The kinetics of spleen size in Balb/c mice challenged with aerosol doses of 1.00E+04 cfu (A), 1.00E+05 cfu (B) or 5.00E+05 cfu (C), or an intranasal dose of 1.00E+04 cfu (D) B. melitensis.
Average spleen sizes with standard error of the mean are illustrated at each scheduled euthanasia time point. The average unchallenged control spleen sizes are included on Day 0 in A, B, C, and D for comparison to the challenged animals. * = p < 0.05 compared to control; ** = p < 0.001 compared to control.
Blood culture
Blood samples were enriched prior to plating to improve the probability of detecting low levels of bacteria in whole blood samples. As shown in Table 3, mice challenged with 1.0E+04 cfu were negative for B. melitensis cultures until Day 42 post-challenge. At Day 14 post-challenge, 17% (2/12) and 42% (5/12) of animals aerosol challenged with 1.0E+05 cfu and 5.0E+05 cfu, respectively, yielded positive blood cultures. Positive blood cultures peaked on Day 42 post-challenge for all three aerosol-challenge dose groups and the IN challenge group. The kinetics of positive blood cultures was similar in mice challenged with 1.0E+04 cfu by the aerosol or IN route.
Table 3. Enriched Blood Culture Results.
| Group | Route of Challenge | Target dose (cfu/mouse) | Blood Culture Collection | Number of animals positive/Total number of animals in group |
|---|---|---|---|---|
| 6 | Aerosol | 1.00E+04 | Day 14 | 0/11 |
| 7 | Aerosol | 1.00E+04 | Day 28 | 0/11 |
| 8 | Aerosol | 1.00E+04 | Day 42 | 6/11* |
| 9 | Aerosol | 1.00E+04 | Day 56 | 4/12 |
| 10 | Aerosol | 1.00E+05 | Day 14 | 2/12 |
| 11 | Aerosol | 1.00E+05 | Day 28 | 2/12 |
| 12 | Aerosol | 1.00E+05 | Day 42 | 6/11 |
| 13 | Aerosol | 1.00E+05 | Day 56 | 4/11 |
| 14 | Aerosol | 5.00E+05 | Day 14 | 5/12** |
| 15 | Aerosol | 5.00E+05 | Day 28 | 7/11* |
| 16 | Aerosol | 5.00E+05 | Day 42 | 10/12* |
| 17 | Aerosol | 5.00E+05 | Day 56 | 7/12* |
| 18 | N/A | N/A | Day 56 | 0/12 |
| 19 | Intranasal | 1.00E+04 | Day 14 | 1/12 |
| 20 | Intranasal | 1.00E+04 | Day 28 | 2/12 |
| 21 | Intranasal | 1.00E+04 | Day 42 | 5/12* |
| 22 | Intranasal | 1.00E+04 | Day 56 | 2/12 |
N/A = not applicable.
* = Significantly greater than the number of positive blood cultures obtained from unchallenged mice.
**Significantly greater than the number of positive cultures obtained from unchallenged mice and mice challenged with 1.00 E+04 cfu on Day 14; Fisher's exact test p < 0.05.
There were significantly more mice in the highest aerosol-challenge group (5.0E+05 cfu) with positive blood cultures on Day 14 post-challenge than mice given an aerosol challenge of 1.0E+04 cfu. In addition, there were a significantly greater number of positive blood cultures for all of the groups of challenged mice compared to the unchallenged group on Day 42 and a significant difference between the highest aerosol-challenge group and the unchallenged group on Days 28 and 56. There were no statistically significant differences in the number of positive enriched blood cultures between any of the challenge groups on Days 42 and 56 post-challenge (Table 3).
Tissue burden
Bacterial burden was detected earlier than positive blood cultures for the lowest aerosol challenge group [lung (3/6), liver (2/6), and/or spleen (6/6)14 days post-challenge]. Notably, a bacterial burden in the lung (15/18), liver (12/18), and/or spleen (17/18) was detected for animals aerosol challenged with 1.0E+04 cfu, 1.0E+05 cfu, or 5.0E+05 cfu B. melitensis. There was a trend of an increasing percentage of animals with a bacterial burden in each tissue with increasing challenge dose (and a higher bacterial burden). In addition, the spleen tended to have higher bacterial counts at all time points than the liver or lungs for all three challenge doses (Figure 3). Mice challenged with 1.0E+04 cfu by the aerosol or IN route had similar spleen, lung and liver bacterial burden kinetics. Similar to the aerosol challenge, the IN challenge resulted in a bacterial burden in lungs and liver that peaked at 28 days post-challenge; the tissue burden in the spleen peaked at 42 days post-challenge.
Figure 3. Tissue burden in animals challenged with B. melitensis 16 M. Bacterial burden was measured from the spleen (A, B, C, J), lung (D, E, F,K) and liver (G,H,I,L) from animals aerosol challenged with 1.00E+04 cfu (A,D,G), 1.00E+05 cfu (B,E,H) or 5.00E+05 cfu (C,F,I), or administered an intranasal dose of 1.00E+04 cfu (J,K,L) B. melitensis.
Individual animal results are displayed.
Pathology
Splenomegaly was the only gross finding that was observed for animals in the pathophysiology study. On Day 56 post-challenge, 25% (3/12), 9% (1/11) and 25% (3/12) of animals challenged with targeted aerosol doses of 1.0E+04 cfu, 1.0E+05 cfu and 5.0E+05 cfu B. melitensis, respectively, were observed with enlarged spleens. Splenomegaly was also noted for one animal on Day 14 post-challenge for the 5.0E+05 cfu challenge group.
Table 4 shows the incidence of Brucella-related microscopic lesions, with average severity scores for animals by group. Microscopic lesions observed in the enlarged spleens were consistent with B. melitensis-induced splenic granulomatous inflammation [43% (9/21), 73% (16/22), 74% (17/23), and 50% (12/24) for aerosol doses 1.00E+04, 1.00E+05, 5.00E+05, and 1.00E+04 (IN), respectively] and/or increased extramedullary hematopoiesis [62% (13/21), 59% (13/22), 70% (16/23), and 38% (9/24) for aerosol doses 1.00E+04, 1.00E+05, 5.00E+05, and 1.00E+04 (IN), respectively]. No microscopic lesions were noted in the one enlarged spleen from the mouse given an IN challenge. Spleens from unchallenged control animals had normal histology.
Table 4. Incidence Summary of Brucella-related Microscopic Observations with Average Severity.
| Tissue/Observation | Number examineda (1.00E+04 cfu; aerosol) | Number observed | Average Severity | Number examineda (1.00E+05 cfu; aerosol) | Number observed | Average Severity | Number examineda (5.00E+05 cfu; aerosol) | Number observed | Average Severity | Number examineda (1.00E+04 cfu; intranasal) | Number observed | Average Severity |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Liver: Inflammation | 21 | 16 | 1.0 | 22 | 16 | 1.0 | 23 | 21 | 1.2 | 24 | 18 | 0.7 |
| Hematopoiesis | 21 | 5 | 0.3 | 22 | 5 | 0.3 | 23 | 2 | 0.1 | 24 | 2 | 0.1 |
| Infiltration of mononuclear cells | 21 | 2 | 0.1 | 22 | 0 | 0.0 | 23 | 0 | 0.0 | 24 | 0 | 0.0 |
| Lung: Inflammation | 21 | 11 | 0.5 | 22 | 9 | 0.4 | 23 | 15 | 0.7 | 24 | 8 | 0.4 |
| Mediastinal Lymph node: Infiltration of mononuclear cells, sinus | 20 | 5 | 0.3 | 16 | 4 | 0.3 | 20 | 8 | 0.7 | 21 | 1 | 0.0 |
| Hyperplasia, plasma cells | 20 | 6 | 0.4 | 16 | 4 | 0.2 | 20 | 10 | 0.6 | 21 | 3 | 0.0 |
| Spleen: Granulomatous inflammation | 21 | 9 | 0.6 | 22 | 16 | 1.0 | 23 | 17 | 1.1 | 24 | 12 | 0.7 |
| Increased extramedullary hematopoeisis | 21 | 13 | 0.6 | 22 | 13 | 0.8 | 23 | 16 | 0.9 | 24 | 9 | 0.4 |
| Heart: Epicardial inflammation | 21 | 1 | 0.1 | 22 | 3 | 0.2 | 23 | 6 | 0.3 | 24 | 4 | 0.2 |
| Kidney: Inflammation | 21 | 4 | 0.2 | 22 | 6 | 0.3 | 23 | 3 | 0.2 | 24 | 0 | 0.0 |
Lesions were graded on a scale of 1-4: 1, Minimal = least detectable lesion; 2, Mild, = Easily discemable lesion; 3, Moderate = Change affecting large area of represented tissues with potential to be relevant; 4, Marked = lesion that approached maximal.
In addition to the microscopic lesions in the spleen, prominent microscopic lesions were observed in other tissues in challenged mice. Chronic epicardial inflammation was characterized by accumulations of mononuclear cells and lymphocytes on the heart surface and was mostly in or near the base of the heart rather than the apex [5% (1/21), 14% (3/22), 26% (6/23), and 17% (4/24) for aerosol doses 1.00E+04, 1.00E+05, 5.00E+05, and 1.00E+04 (IN), respectively]. Perivascular or peribronchiolar mononuclear cell infiltration [0% (0/21), 14% (3/22), 17% (4/23), and 4% (1/24) for aerosol doses 1.00E+04, 1.00E+05, 5.00E+05, and 1.00E+04 (IN), respectively] and chronic inflammation of the pleura or mediastinum [52% (11/21), 41% (9/22), 65% (15/23), and 33% (8/24) for aerosol doses 1.00E+04, 1.00E+05, 5.00E+05, and 1.00E+04 (IN), respectively] was observed in the lungs. Plasma cell hyperplasia [30% (6/20), 25% (4/16), 50% (10/20), and 14% (3/21) for aerosol doses 1.00E+04, 1.00E+05, 5.00E+05, and 1.00E+04 (IN), respectively] and infiltration of sinuses by mononuclear cells [23% (5/21), 18% (4/22), 35% (8/23), and 4% (1/24) for aerosol doses 1.00E+04, 1.00E+05, 5.00E+05, and 1.00E+04 (IN), respectively] were noted in the mediastinal lymph nodes. Similar lesions were observed in the liver and spleen, including the granulomatous inflammation and increased extramedullary hematopoiesis. A few animals [19% (4/21), 27% (6/22), 13% (3/23), and 0% (0/24) for aerosol doses 1.00E+04, 1.00E+05, 5.00E+05, and 1.00E+04 (IN), respectively] had chronic inflammation in the kidneys, which was characterized by infiltration of mononuclear and polymorphonuclear inflammatory cells in the interstitial tissue of the kidney and nearby tubules or the renal pelvis.
Pathology results were similar regardless of the route of administration (aerosol or IN), or challenge dose. However, the incidence of chronic epicardial inflammation, chronic inflammation of the pleura or mediastinum, mediastinal lymph node plasma cell hyperplasia and splenic increased extramedullary hematopoiesis were highest in animals aerosol-challenged with 5.0 x 105 cfu B. melitensis.
Discussion
These studies present a small animal model for inhalational brucellosis by characterizing the spray factor for the nose-only multi-animal exposure system and then assessing the pathophysiology of inhalational brucellosis in Balb/c mice. In the spray factor study, the aerosol concentration was found to be nearly directly proportional to the targeted nebulizer concentration (statistically significant linear relationship), indicating the spray factor is a nearly constant value. The mouse nose-only aerosol system was characterized for brucellosis studies as a result of the consistent data obtained from the spray factor study and this aerosol system was subsequently used for Brucella mouse studies.
The optimal challenge dose to be used to examine the pathophysiology of inhalational brucellosis over an eight-week time period was determined based on the infection rate (tissue burden) in the low-dose aerosol study. Since only 45% of mice challenged with the aerosol dose of 1.00E+03 cfu had a bacterial tissue burden, it was concluded that a much higher dose should be used in the subsequent pathophysiology study to infect at least 95% of the challenged animals. Enrichment of blood samples was performed prior to plating to increase the probability of detecting bacteria, since it has been shown that positive results can vary when blood is directly plated20.
The kinetics of brucellosis showed modest changes in hematology parameters during the eight-week post-challenge period. The lack of physical changes and unremarkable hematology results for the aerosol and IN challenged mice is consistent with human brucellosis patients, in which constitutive symptoms were observed in only 26% of 100 studied human brucellosis cases, and hematology results were indistinct for the majority of these patients2,21,22,23,24,25. In addition, the mouse model is consistent with the NHP inhalational brucellosis model20,26, in which abnormal clinical observations were rarely noted and the clinical hematology results only involved minimal changes to anemia-related parameters20. Endocarditis was observed for 8% (14/186) of challenged mice, which is consistent with this rare complication being observed in human brucellosis patients24.
While the inhalational brucellosis mouse model appears consistent with human patients and other animal models in regards to non-specific clinical parameters, the mouse model does differ in regards to the effect of the infection on body temperature. Unlike the vast majority of human brucellosis patients and the NHP inhalational brucellosis model, the aerosol- and IN-challenged mice did not exhibit a fever at any point during the post-challenge period20,21. The lack of a fever in mice infected with brucellosis has been noted previously by Silva et al.18.
Despite the absence of overt clinical signs in challenged mice, these animals were infected with brucellosis as shown by detection of bacteria in the blood, lung, liver, and spleen in the pathophysiology study. Similar to human disease, positive blood cultures were variable as 22% (10/45), 30% (14/46) and 62% (29/47) of mice challenged with a Brucella aerosol dose of 1.0 x 104 cfu, 1.0 x 105 cfu, or 5.0 x 105 cfu B. melitensis, respectively, had positive cultures27. Therefore, a higher exposure dose of B. melitensis appears to increase the probability of detecting these bacteria in a blood sample. Notably, these positive cultures were observed after blood samples were enriched prior to plating on MTM agar. In addition, mice challenged by the IN route had a similar percentage of positive cultures (21%; 10/48) compared to the corresponding aerosol challenge dose group of 1.00E+04 cfu.
While blood samples were enriched to increase the probability of detecting positive cultures, bacterial burdens were readily identified in samples following direct plating on MTM agar. A bacterial burden was detected for 93% (67/72) and 96% (23/24) of aerosol and IN challenged animals, respectively, in the lung, liver and/or spleen at the scheduled euthanasia time points, indicating nearly all animals were infected with B. melitensis. In addition, the largest B. melitensis burdens were typically observed in the spleen (all three challenge doses), which is consistent with the NHP brucellosis model and other mouse brucellosis models (IP and aerosol whole body challenge routes)16,17,20,26. Although the spleen appears to be the primary target of Brucella in aerosol infection with B. melitensis 16 M in Balb/c mice, the recovery of Brucella from the lung and liver in this study was more variable and with lower burdens than the other published aerosol and IN challenges15,17,28,29. This difference could be due to differences in the aerosol system and the method used to determine the exposure dose, to normal variation in the infection rate and dissemination in the tissues, or to bacterial strain differences. In addition to the clinical parameters assessed, tissues from the animals not evaluated for tissue burden were assessed for microscopic lesions in order to define the pathology of B. melitensis-infected mice during the eight-week time period. Consistent with human disease and other brucellosis animal models (NHP, mouse), microscopic lesions associated with the lungs, lymph nodes, liver, kidney, and spleen were observed. In addition, endocarditis was observed for 8% (14/186) of challenged mice, and this infrequent observation has been noted for some human brucellosis patients24.
The results from this study suggest that this small animal model can be used to assess efficacy of vaccines, post-exposure prophylactics and therapeutics by monitoring splenomegaly, blood culture and bacterial burden in the lung, liver and spleen of treated and untreated animals. In addition, the data from this study suggest that an appropriate time to administer a therapeutic to a Balb/c mouse after aerosol or IN exposure to B. melitensis is two weeks post-challenge, based upon the vast majority of animals (78%) exhibiting a bacterial burden by this time point.
In summary, the BALB/c mouse aerosol model of brucellosis was developed that can be used to test the efficacy of antibiotic treatment regimens under the FDA Animal Rule. A clinically relevant end-point in this model would be assessment of bacterial burden in the spleen, liver, and lung of treated and untreated animals.
Methods
Brucella melitensis 16 M strain and preparation for aerosol challenge
Brucella melitensis 16 M biovar 1 ATCC 23456 was obtained from American Type Culture Collection (ATCC; Manassas, VA; original preparation, 1998). B. melitensis was prepared for aerosol challenge as previously described20.
Characterization of the nose-only aerosol exposure system for inhalational brucellosis
The aerosol exposure system used for characterization testing consisted of a nose-only exposure tower (CH Technologies, Inc., Westwood, NJ), which provides the ability to simultaneously challenge up to 30 mice. Aerosols were performed as previously described30. The Mass Median Aerodynamic Diameter (MMAD) of the aerosol was determined for at least one time point during each 10-minute test with an Aerodynamic Particle Sizer® Spectrometer (APS Model 3321 TSI, Inc., Shoreview, MN).
Four different targeted starting concentrations of B. melitensis (1.0E+05, 1.0E+06, 1.0E+07, and 1.0E+08 cfu/mL) were aerosolized for 10 minutes in triplicate and repeated over three days. A six-jet Collison nebulizer (BGI, Waltham, MA) with a precious fluid jar was used to generate a controlled delivery of aerosolized B. melitensis 16 M from a liquid suspension at the target starting concentrations into the exposure chamber (11 cm x 8 cm). Side-by-side aerosol samples were collected from the exposure chamber using glass impingers (model 7541 Ace Glass, Inc) to examine for spatial uniformity of the aerosol. The impinger contained approximately 20 ± 2 mL of Heart Infusion Broth (HIB) and anti-foam A, and was kept on ice during aerosol sampling. The impinger samples were serially diluted10-fold, and 0.1 mL samples of each dilution plated on tryptic soy agar plates containing 5% sheep blood. After 48–96 h incubation at 37°C the number of colonies on each plate were counted and results expressed as colony forming units per mL (cfu/mL). The temperature, relative humidity, and aerosol particle size were monitored for at least one time point during each 10-minute test at approximately 5 minutes into each test.
Animals
Balb/c mice (50% female, 50% male; Charles River Laboratories International, Inc., Wilmington, MA) were 6–8 weeks old at the time of receipt and weighed at least 16 g. For the clinical presentation and pathophysiology study, mice were implanted with a programmable temperature transponder chip (IPTT-300, BMDS, Seaford, DE) injected subcutaneously prior to challenge. Animals were anesthetized with xylazine (10–12 mg/kg IP) and ketamine (80–100 mg/kg IP) prior to injection of the temperature probe. All animals were in good health, free of malformations and exhibited no signs of clinical disease. The animal protocols were approved by Battelle's Institute Animal Care and Use Committee (IACUC) and the Department of Defense Animal Care and Use Review Office (ACURO) prior to the start of a study.
Preparation for IN challenge
Fresh B. melitensis 16 M suspensions were prepared as previously described20. The dosing material was diluted to a concentration of 3.33E+05 cfu B. melitensis/mL to reach target doses of 1.0E+04 cfu/animal. One milliliter of the challenge material was prepared for each group.
Aerosol challenge
The aerosol challenge was performed as described above, except for a single impinger was utilized to sample for viable organisms in the aerosol. Mice were exposed to the challenge dose for 10 minutes. In the low dose aerosol study, 20 mice (50% female, 50% male) were exposed to achieve targeted doses of 30 (Group 1), 100 (Group 2), 300 (Group 3) or 1000 (Group 4) cfu B. melitensis. To follow the kinetics of pathophysiology brucellosis, mice were serially euthanized following aerosol challenge. Twelve groups of 11 to 12 mice each were challenged via aerosol exposure on one study day, and one group of 12 mice were used as unchallenged controls (Group 18).
IN challenge
Four groups of 12 mice each (Groups 19–22) were challenged by the IN route. Mice were anesthetized IP with xylazine (10–12 mg/kg) and ketamine (80–100 mg/kg). A 30 µL volume containing 1.00E+04 B. melitensis cfu was administered with a micropipette dropwise into the external nares of each mouse.
Clinical Evaluation
In the pathophysiology study, twice-daily baseline temperatures were measured for 2.5 days prior to challenge (aerosol exposure) or 9.5 days prior to challenge (IN exposure). Temperatures were measured twice daily via the temperature chip following challenge.
In the low dose aerosol study, mice were euthanized eight weeks after challenge. In the pathophysiology study, mice were serially euthanized, according to Table 1. On the scheduled euthanasia day, mice were anesthetized with IP ketamine (99 mg/kg) and xylazine (11 mg/kg), and a terminal blood draw was performed by cardiac puncture into EDTA tubes for hematology (pathophysiology study) and blood culture analysis (low dose aerosol study and pathophysiology study). Hematology (Siemens Advia 120 hematology analyzer) included white blood cell count (WBC), differential leukocyte (% and absolute) count, neutrophil/lymphocyte ratio (N/L ratio), hemoglobin (HGB), hematocrit (HCT), red blood cell count (RBC), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), corpuscular hemoglobin concentration mean (CHCM), red cell distribution width (RDW), platelet count (PLT) and mean platelet volume (MPV).
Whole blood from EDTA tubes was qualitatively tested for bacteremia by culture by direct plating (low dose aerosol study) or enriched (pathophysiology study) methods. For the enrichment method, approximately 30–40 µL of each blood sample was placed in Brucella broth selectively supplemented with modified Brucella selective supplement (MBSS, Oxoid Limited, Cambridge, UK) as previously described20. Plates were examined for the presence or absence of B. melitensis.
Tissue burden was determined for samples of the lung, liver and spleen from all animals in the low dose aerosol study (Groups 1–5) and from half of the animals in each group from the pathophysiology study (Groups 6–21), as previously described20. Complete necropsies were performed on all mice in the pathophysiology study, but histopathology was only performed on the mice that were not assessed for tissue burden. The heart, lungs, liver, spleen, kidneys, brain, ovaries or testes as appropriate, and mediastinal lymph nodes were fixed in formalin, processed for routine hematoxylin and eosin paraffin sections and examined microscopically by a board-certified veterinary pathologist All microscopic findings were graded semi-quantitatively according to the following scale, with the associated numerical score used to calculate average severity grades for each lesion by group. Minimal (grade 1) represented the least detectable lesion; mild (grade 2) represented an easily discernable lesion; moderate (grade 3) represented a change affecting a large area of the represented tissue; and marked (grade 4) represented a lesion that approached maximal.
Statistical methods
Animal group randomizations were performed with SAS® statistical software Version 9.1.3 (SAS Institute, Cary, NC) utilizing the Plan procedure. No statistical differences in gender were observed in these studies and therefore gender is not discussed in the results.
Geometric means by day were generated for both aerosol concentration and spray factor for each level of target nebulizer concentration, as well as overall. Spray factors were calculated as aerosol concentration divided by targeted nebulizer concentration. The aerosol characterization data were examined under a number of potential statistical models to explain the relationship between aerosol concentration and target nebulizer concentration, and the final model selected was a regression model incorporating a random effect for test day. The model is the following: log10 (yijk) = ai + β * log10(x j(i)) + eijk. i = 3 test days; j(i) = 4 levels of target nebulizer concentration j within test day i; k = 3 replicates per test condition; log10 (yijk) = log10 transformed aerosol concentration for each of the k replicates with the jth level of target nebulizer concentration for the ith day; ai = intercept of the aerosol concentration versus target nebulizer concentration relationship for the ith day, treated as a random effect assumed to be distributed as N (α,σ2α); β = common slope of the aerosol concentration versus nebulizer concentration relationship treated as a fixed effect; x j(i) = known log10 transformed target nebulizer concentration values for the jth level; eijk = residual error left unexplained by the model, random effect assumed to be distributed as N (0, σ2) and independent of the ai. The model was fitted in SAS® (version 9.1) using PROC MIXED.
For aerosol-challenged animals in the pathophysiology study, a Fisher's exact test was used to compare the proportion of animals that were bacteremic by group at each scheduled euthanasia time point. For animals challenged by the IN route, Dunnett's multiple comparison procedure was used to compare the proportion of animals that were bacteremic in each group to the proportion of animals that were bacteremic in the control group. The spleen weights for each aerosol challenge group and IN challenge group of mice euthanized on days 14, 28, 42 and 56 post-challenge were compared to the control group by the T-test. Bacterial burdens in the lung, liver and spleen for each aerosol challenge group and the IN group were compared to the control group by T-test on ranks.
Author Contributions
Lisa Henning and Richard Warren wrote the manuscript. All authors reviewed the manuscript and were involved in the development of the figures and tables.
Acknowledgments
This work was supported by the Transformational Medical Technologies Program through CBRNIAC contract number SP0700-00-D-3180, Task CB-09-0018 Task 769 from the Department of Defense Chemical and Biological Defense program through the Defense Threat Reduction Agency (DTRA). We thank Dennis Pak, Amber Lindsay, Julie Lucas, Aaron Short, Phyllis Herr-Calomeni, and Dyana Fisher for their outstanding technical assistance.
References
- Boschiroli M. L., Foulongne V. & O'Callaghan D. Brucellosis: a worldwide zoonosis. Curr. Opin. Microbiol. 4, 58–64 (2001). [DOI] [PubMed] [Google Scholar]
- Demirdal T. & Demirturk N. Laboratory-acquired brucellosis. Ann. Acad. Med. Singapore 37, 86–87 (2008). [PubMed] [Google Scholar]
- Mohandas N., Balasubramanian R. & Prasad S. B. Can brucella endocarditis be treated successfully with medical therapy alone? Trop. Doct. 39, 123–124 (2009). [DOI] [PubMed] [Google Scholar]
- Bossi P. et al. Bichat guidelines for the clinical management of brucellosis and bioterrorism-related brucellosis. Euro. Surveill 9, E15–E16 (2004). [PubMed] [Google Scholar]
- Buzgan T. et al. Clinical manifestations and complications in 1028 cases of brucellosis: a retrospective evaluation and review of the literature. Int. J. Infect. Dis. 14, e469–e478 (2010). [DOI] [PubMed] [Google Scholar]
- Hall W. H. Modern chemotherapy for brucellosis in humans. Rev. Infect. Dis. 12, 1060–1099 (1990). [DOI] [PubMed] [Google Scholar]
- Kochar D. K. et al. Hospital-based case series of 175 cases of serologically confirmed brucellosis in Bikaner. J. Assoc. Physicians India 55, 271–275 (2007). [PubMed] [Google Scholar]
- Memish Z., Mah M. W., Al M. S., Al S. M. & Khan M. Y. Brucella bacteraemia: clinical and laboratory observations in 160 patients. J. Infect. 40, 59–63 (2000). [DOI] [PubMed] [Google Scholar]
- Solera J. et al. Doxycycline-rifampin versus doxycycline-streptomycin in treatment of human brucellosis due to Brucella melitensis. The GECMEI Group. Grupo de Estudio de Castilla-la Mancha de Enfermedades Infecciosas. Antimicrob. Agents Chemother. 39, 2061–2067 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almuneef M. et al. Brucella melitensis bacteremia in children: review of 62 cases. J. Chemother. 15, 76–80 (2003). [DOI] [PubMed] [Google Scholar]
- Cascio A. et al. Treatment of human brucellosis with rifampin plus minocycline. J. Chemother. 15, 248–252 (2003). [DOI] [PubMed] [Google Scholar]
- El Miedany Y. M., El G. M., Baddour M. & Ahmed I. Human brucellosis: do we need to revise our therapeutic policy? J. Rheumatol. 30, 2666–2672 (2003). [PubMed] [Google Scholar]
- Pappas G., Papadimitriou P., Christou L. & Akritidis N. Future trends in human brucellosis treatment. Expert. Opin. Investig. Drugs 15, 1141–1149 (2006). [DOI] [PubMed] [Google Scholar]
- Saltoglu N., Tasova Y., Inal A. S., Seki T. & Aksu H. S. Efficacy of rifampicin plus doxycycline versus rifampicin plus quinolone in the treatment of brucellosis. Saudi. Med. J. 23, 921–924 (2002). [PubMed] [Google Scholar]
- Mense M. G. et al. Bacteriologic and histologic features in mice after intranasal inoculation of Brucella melitensis. Am. J. Vet. Res. 62, 398–405 (2001). [DOI] [PubMed] [Google Scholar]
- Crawford R. M. et al. Deletion of purE attenuates Brucella melitensis infection in mice. Infect. Immun. 64, 2188–2192 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahl-McDonagh M. M., Arenas-Gamboa A. M. & Ficht T. A. Aerosol infection of BALB/c mice with Brucella melitensis and Brucella abortus and protective efficacy against aerosol challenge. Infect. Immun. 75, 4923–4932 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva T. M., Costa E. A., Paixao T. A., Tsolis R. M. & Santos R. L. Laboratory animal models for brucellosis research. J. Biomed. Biotechnol. 2011, 518323 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds W. C. Aerosol Technology: Properties, Behavior, and Measurement of Airborne Particles (Wiley-Interscience, John Wiley & Sons Inc.,January1999). [Google Scholar]
- Henning L. N. et al. Pathophysiology of the rhesus macaque model for inhalational brucellosis. Infect. Immun. 80, 298–310 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas G. et al. Brucellosis and the respiratory system. Clin. Infect. Dis. 37, e95–e99 (2003). [DOI] [PubMed] [Google Scholar]
- Cay S., Cagirci G., Maden O., Balbay Y. & Aydogdu S. Brucella endocarditis - a registry study. Kardiol. Pol. 67, 274–280 (2009). [PubMed] [Google Scholar]
- Cervantes F., Bruguera M., Carbonell J., Force L. & Webb S. Liver disease in brucellosis. A clinical and pathological study of 40 cases. Postgrad. Med. J. 58, 346–350 (1982). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt A. C. & Bothwell P. W. Histological findings in human brucellosis. J. Clin. Pathol. 20, 267–272 (1967). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yingst S. L., Huzella L. M., Chuvala L. & Wolcott M. A rhesus macaque (Macaca mulatta) model of aerosol-exposure brucellosis (Brucella suis): pathology and diagnostic implications. J. Med. Microbiol. 59, 724–730 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mense M. G., Borschel R. H., Wilhelmsen C. L., Pitt M. L. & Hoover D. L. Pathologic changes associated with brucellosis experimentally induced by aerosol exposure in rhesus macaques (Macaca mulatta). Am. J. Vet. Res. 65, 644–652 (2004). [DOI] [PubMed] [Google Scholar]
- Pappas G., Akritidis N., Bosilkovski M. & Tsianos E. Brucellosis. N. Engl. J. Med. 352, 2325–2336 (2005). [DOI] [PubMed] [Google Scholar]
- Olsen S. C., Waters W. R. & Stoffregen W. S. An aerosolized Brucella spp. challenge model for laboratory animals. Zoonoses. Public Health 54, 281–285 (2007). [DOI] [PubMed] [Google Scholar]
- Smither S. J. et al. Development and characterization of mouse models of infection with aerosolized Brucella melitensis and Brucella suis. Clin. Vaccine Immunol. 16, 779–783 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren R. et al. Cynomolgus macaque model for pneumonic plague. Microb. Pathog. 50, 12–22 (2011). [DOI] [PubMed] [Google Scholar]