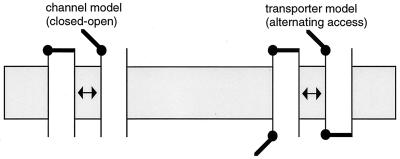
Physiologists say that ions and neutral solutes can cross biological membranes via “transporters” and “channels.” We tend to think about the difference between transporters and channels in terms of gating mechanisms. Ion channels exhibit a wide range of selectivity properties and permeation rates, but their gating at the most basic level can be thought of in terms of a single barrier or gate acting as a switch. When the gate is closed, ions can’t permeate; when the gate is opened, a permeation pathway for ions allows flux, often at very high rates (up to 108/sec). Transporters and ion pumps, on the other hand, mediate flux that can be explained better by the presence of two gates—one external and one internal (1). In this canonical transport scheme, the two gates are never open simultaneously. Instead they open sequentially to allow the cytoplasmic and extracellular compartments alternating access to the permeation pathway (Fig. 1). Unlike flux through an open ion channel, there must be a gating cycle every time solute is transported, so transporters generally mediate much slower rates of solute permeation (sometimes as slow as 1/sec). An alternating access model can explain how a neurotransmitter can be accumulated against its electrochemical gradient if other ions are stoichiometrically co- or countertransported down their gradients. The kinetic scheme is formally equivalent to that of a carrier (like valinomycin) that shuttles back and forth across the membrane, although the physical process is quite distinct.
Figure 1.
Switch (left) and alternating access- (right) gating models. Alternating access kinetic schemes can involve co- or countertransport of other ions with neurotransmitter in a transport cycle, effectively coupling their electrochemical gradients.
The switch model of channel gating is supported by numerous experiments including the direct observation of large ionic currents flowing through single ion channels. In contrast, the unitary ionic events in transporters are expected to involve only one or a few ions at a time, and these cannot be directly observed with the resolution of presently available instruments. Unexpectedly, recent studies on members of the Na+/Cl−-coupled neurotransmitter transporter family report sizeable unitary currents in membrane patches containing γ-aminobutyric acid (GABA), norepinephrine, and serotonin transporters (2–4). Channel-like current noise also has been associated with members of the other neurotransmitter transporter gene family, the Na+/H+/K+-coupled glutamate transporters (5, 6). These findings suggest that transporters may sometimes form a channel-like pore that inorganic ions can readily diffuse through, blurring the formal distinctions between the two classes of proteins. In this issue of the Proceedings, Galli et al. (7) show that in addition to inorganic ions, transmitter also can permeate the channels associated with norepinephrine transporters, blurring the distinctions still more.
Some early hints that neurotransmitter transporters did not behave according to perfect alternating access gating schemes came from studies comparing flux of labeled transmitter with electrical currents. These studies demonstrated that charge movement was not always stoichiometrically coupled to neurotransmitter flux in a straightforward way (8–13). For example, in voltage-clamped oocytes expressing rat serotonin transporters, serotonin was observed to activate macroscopic currents at least an order of magnitude larger than flux of [3H]serotonin, and large serotonin-independent currents also were observed that were blocked by selective transporter blockers (8). The microscopic basis of these anomalous currents was later elucidated with the direct observation of small unitary currents in cells transfected with serotonin transporters that displayed the same ion selectivity properties (2). Small conductance channels associated with rat GABA (3) and norepinephrine (4) transporters also have been observed in patches excised from transfected cells. Can we conclude that the transporters mediate these unitary currents? Probably so: channel open probability is affected by the presence of neurotransmitter or specific transporter blockers; furthermore, Lin et al. (2) have identified a point mutation in the serotonin transporter that increases the unitary conductance. In addition, for glutamate transporters, the channel kinetics are closely related to the transport kinetics of glutamate or glutamate analogs (5, 6).
Are these transporter-associated channels ion-selective? Neurotransmitter transport is generally highly selective for both the transmitter as well as the cotransported ions (e.g., Li+ is generally unable to substitute for Na+). However, the transporter and the associated channel often exhibit different selectivity: GABA transporter leakage currents are carried by Na+, Li+, or Cs+ (3) and serotonin transporter leakage currents by Na+, Li+, or K+, but not Cl− (8). Furthermore, the channel in the glutamate transporter is selective for Cl−, an ion not required for glutamate transport (12). Whether these differences indicate the presence of two distinct pores or a single pore that can undergo transient changes in selectivity remains to be determined. A related critical question concerns control of gating. For the GABA transporter, the probability of entering the channel mode appears to be very low because single channels were reported to be commonly observed in patches containing hundreds to thousands of transporters (3). A similar low frequency was observed for the serotonin transporter, suggesting that channel opening is not an obligatory event in a transport cycle (2). An important point remaining to be resolved is whether the transporters exhibit a uniformly very low open probability, or alternatively, a small fraction of transporters exhibit a correspondingly higher Po. If the latter is true, it might suggest that the channel and carrier modes are functionally independent and that although a molecule can switch from a transporter mode into a channel mode of activity, only a small fraction do so at any given time.
Perhaps it’s not surprising that a transporter with two gates acts like a channel occasionally. It is in fact expected, assuming that each gate has a finite open probability, as pointed out by Lester et al. (14). But a critical related issue concerns the thermodynamic demands of transporting neurotransmitter against an electrochemical gradient by coupling its translocation to co-or countertransport of other ions. Allowing significant uncoupled flux like that which occurs in a channel would severely undermine the transporter’s efficiency. However, channel openings would not necessarily interfere with flux coupling by a classical alternating access scheme, assuming that the channel is selective; i.e., a barrier for a neurotransmitter molecule may simply not be a barrier for another ion. For example, in the case of glutamate transport, glutamate does not measurably permeate the anion channel that is activated during transport (6), and flux coupling to the Na+, K+, and H+ gradients is highly efficient (15). This is particularly important since this excitatory neurotransmitter is present in millimolar concentrations inside neurons and its uncoupled efflux would be neurotoxic.
In the context of these thermodynamic considerations, the findings of Galli et al. (10) come as a surprise. In technically demanding experiments, they recorded currents in patches from cells expressing norepinephrine transporters while simultaneously monitoring norepinephrine flux using amperometry. Neurotransmitter was found to permeate in discrete bursts that were correlated with channel openings. With 4 mM extracellular norepinephrine present, the neurotransmitter carried 2% of the current, demonstrating that not only do inorganic ions rapidly permeate the open channel, but neurotransmitter does as well. These results raise many questions, but first and foremost, how can the transporter concentrate norepinephrine against an electrochemical gradient if there is an open channel that it can permeate? Galli et al. (7) suggest that because a large inward gradient of neurotransmitter is transiently present following release at synapses, channel permeation might be an efficient and important mechanism in the early stage of reuptake and clearance. But what happens when the extracellular neurotransmitter concentration declines and its electrochemical gradient diminishes or reverses? Critical issues that remain to be addressed include the channel selectivity and neurotransmitter concentration-dependence of channel opening and permeation. For example, is neurotransmitter flux through the channel still significant at ambient physiological concentrations of neurotransmitter? Different relationships between the norepinephrine concentration-dependence of transporter gating and channel gating and permeation could lead to complex behavior.
Many questions about neurotransmitter transporter structure and function remain, but evidence continues to accumulate suggesting that transporters and channels share some common fundamental features. We know that channels do not generally behave as simple, singly gated aqueous pores but instead may have multiple ion-binding sites corresponding to energy wells separated by barriers that can fluctuate in height (16). Recognizing that a high energy barrier is equivalent to a gate, one can imagine how the dynamics of channel state transitions could lead to a spectrum of kinetic behaviors. In the words of Peter Läuger (17): “Channel and carrier models should not be regarded as mutually exclusive possibilities but rather as limiting cases of a more general mechanism”. As neurotransmitter transporter studies progress, the logic of this idea becomes more and more compelling.
ABBREVIATION
- GABA
γ-aminobutyric acid
Footnotes
A commentary on this article begins on page 13260.
References
- 1. Jardetzky O. Nature (London) 1966;211:969–970. doi: 10.1038/211969a0. [DOI] [PubMed] [Google Scholar]
- 2.Lin F, Lester H A, Mager S. Biophys J. 1996;71:3126–3135. doi: 10.1016/S0006-3495(96)79506-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cammack J N, Schwartz E A. Proc Natl Acad Sci USA. 1996;93:723–727. doi: 10.1073/pnas.93.2.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galli A, Blakely R D, DeFelice L J. Proc Natl Acad Sci USA. 1996;93:8671–8676. doi: 10.1073/pnas.93.16.8671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Larsson H P, Picaud S A, Werblin F S, Lecar H. Biophys J. 1996;70:733–742. doi: 10.1016/S0006-3495(96)79613-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wadiche J I, Kavanaugh M P. J Neurosci. 1998;18:7650–7661. doi: 10.1523/JNEUROSCI.18-19-07650.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galli A, Blakely R D, DeFelice L J. Proc Natl Acad Sci USA. 1998;95:13260–13265. doi: 10.1073/pnas.95.22.13260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mager S, Min C, Henry D J, Chavkin C, Hoffman B J, Davidson N, Lester H A. Neuron. 1994;12:845–859. doi: 10.1016/0896-6273(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 9.Cammack J N, Rakhilin S V, Schwartz E A. Neuron. 1994;13:949–960. doi: 10.1016/0896-6273(94)90260-7. [DOI] [PubMed] [Google Scholar]
- 10.Galli A, DeFelice L J, Duke B J, Moore K R, Blakely R D. J Exp Biol. 1995;198:2197–2212. doi: 10.1242/jeb.198.10.2197. [DOI] [PubMed] [Google Scholar]
- 11.Wadiche J I, Arriza J L, Amara S G, Kavanaugh M P. Neuron. 1995;14:1019–1027. doi: 10.1016/0896-6273(95)90340-2. [DOI] [PubMed] [Google Scholar]
- 12.Fairman W A, Vandenberg R J, Arriza J L, Kavanaugh M P, Amara S G. Nature (London) 1995;375:599–603. doi: 10.1038/375599a0. [DOI] [PubMed] [Google Scholar]
- 13.Sonders M S, Zhu S J, Zahniser N R, Kavanaugh M P, Amara S G. J Neurosci. 1997;17:960–974. doi: 10.1523/JNEUROSCI.17-03-00960.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lester H A, Cao Y, Mager S. Neuron. 1996;17:807–810. doi: 10.1016/s0896-6273(00)80213-5. [DOI] [PubMed] [Google Scholar]
- 15.Zerangue N, Kavanaugh M P. Nature (London) 1996;383:634–637. doi: 10.1038/383634a0. [DOI] [PubMed] [Google Scholar]
- 16.Hille B. Ionic Channels of Excitable Membranes. Sunderland, MA: Sinauer; 1992. [Google Scholar]
- 17.Läuger P. J Membr Biol. 1980;57:163–178. doi: 10.1007/BF01869585. [DOI] [PubMed] [Google Scholar]