Background: Interstitial pulmonary fibrosis is caused by the excess production of extracellular matrix (ECM) by Fb in response to TGF-β1.
Results: The peptidyl-prolyl isomerase Pin1 modulates the production of many pro- and antifibrogenic cytokines and ECM after bleomycin injury.
Conclusion: Pin1 controls Smad6 function.
Significance: Pin1 may be a therapeutic target to prevent pathologic lung scarring.
Keywords: Lung Injury, Pulmonary Fibrosis, Signaling, Smad Transcription Factor, Transforming Growth Factor beta (TGFbeta), Pin1
Abstract
Interstitial pulmonary fibrosis is caused by the excess production of extracellular matrix (ECM) by Fb in response to TGF-β1. Here, we show that the peptidyl-prolyl isomerase Pin1 modulates the production of many pro- and antifibrogenic cytokines and ECM. After acute, bleomycin injury, Pin1−/− mice showed reduced, pulmonary expression of collagens, tissue inhibitors of metalloproteinases, and fibrogenic cytokines but increased matrix metalloproteinases, compared with WT mice, despite similar levels of inflammation. In primary fibroblasts, Pin1 was required for TGF-β-induced phosphorylation, nuclear translocation, and transcriptional activity of Smad3. In Pin1−/− cells, inhibitory Smad6 was found in the cytoplasm rather than nucleus. Smad6 knockdown in Pin1−/− fibroblasts restored TGF-β-induced Smad3 activation, translocation, and target gene expression. Therefore, Pin1 is essential for normal Smad6 function and ECM production in response to injury or TGF-β and thus may be an attractive therapeutic target to prevent excess scarring in diverse lung diseases.
Introduction
Normal pulmonary function requires precise balance between the amount and composition of the extracellular matrix (ECM).3 Despite robust production, net accumulation of ECM is opposed by continuous turnover, estimated at ∼10% per day (1). During the pathogenesis of lung fibrosis, a net increase in ECM content displaces epithelial cells and causes severe reductions of bronchial function. In both chronic asthma and idiopathic pulmonary fibrosis (IPF), bronchial and parenchymal inflammation and scarring stiffens the airways, reducing forced vital capacity, gas exchange, and overall pulmonary function. Unfortunately, effective therapies to reverse or stop the fibrotic process are lacking as is a complete understanding of the underlying molecular mechanisms leading to scar formation.
In normal lung, four interconnected processes dictate net ECM levels: 1) de novo synthesis and deposition of collagens I, III, and V; 2) degradation of existing ECM by matrix metalloproteinases (MMPs); 3) levels of anti-proteases, particularly the tissue inhibitors of MMPs (TIMPs); and 4) the amounts of soluble profibrotic mediators (e.g. IL-1β, TGF-β, FGF-1, PDGF, and CTGF). Of the latter, TGF-β1 is particularly important, directly stimulating parenchymal Fb to synthesize ECM. In bleomycin (BLM)-induced lung fibrosis, elevations in TGF-β1 precede increased expression and deposition of collagens (2). TGF-β1 is significantly increased and strongly correlated with airway and parenchymal fibrosis in patients with chronic asthma, IPF, and allograft rejection (3, 4).
Signaling by TGF-β1 is initiated by type I and II receptor-mediated phosphorylation (5). Activated TGF-β1 receptor I phosphorylates Smad2 (mothers against decapentaplegic homology 2) and Smad3 (R-Smads) at their C terminus, which is antagonized by inhibitory Smad6 and -7 (I-Smads). Following phosphorylation, R-Smads form complexes with Smad4 (Co-Smad), translocate to the nucleus, and activate ECM gene transcription. R-Smads are also multiply phosphorylated by MAPK, particularly on the linker region that bridges the N-terminal MH1 and C-terminal MH2 domains.
Phosphorylated serine or threonine N-terminal to proline (Ser/Thr-Pro) can be recognized by peptidyl-prolyl isomerases. Members of this family include cyclophilin A, FKBP (FK506-binding protein), and Pin1 (NIMA-interacting protein 1) (6, 7). The latter shows the narrowest target specificity binding only to and isomerizing phosphorylated Ser/Thr-Pro motifs. Isomerization has profound effects on target protein phosphorylation status, protein or RNA interactions, stability, and subcellular localization. Pin1 was originally implicated in the regulation of cell proliferation in part through control of cyclin D1 levels and stability. Recent data show Pin1 playing additional roles in immune responses, cytokine gene expression, and immune cell apoptosis. We have shown that Pin1 controls the expression of inflammatory cytokine and profibrotic growth factors by activated immune cells (8, 9). Pin1 blockade in vivo significantly reduced airway inflammation and pulmonary collagen deposition in animal models of asthma and lung transplantation (9, 10).
We now show that Pin1 regulates TGF-β1-mediated ECM deposition in the lung after experimental injury. Pin1−/− mice and explanted primary lung Fb expressed significantly less collagens and TIMPs but increased MMPs compared with wild type. Moreover, CTGF, IL-1β, and TGF-β1 were also significantly reduced. In WT cells, TGF-β1 induced the association of Pin1 with Smad6, prevented its nuclear export, and facilitated Smad3 cytoplasmic phosphorylation by TGF-β1 receptors. In the absence of Pin1, Smad6 was localized to the cytoplasm, leading to reduced Smad3 phosphorylation and attenuation of TGF-β-induced ECM gene expression. Our data suggest that Pin1 blockade can promote an antifibrogenic pulmonary milieu capable of reducing ECM deposition during pathological lung fibrosis.
EXPERIMENTAL PROCEDURES
Materials
Anti-MMP2, anti-TIMP1, TGF-β1 ELISA kit, and recombinant human TGF-β1 were purchased from R&D Systems. Bleomycin was from Sigma. Protease Inhibitor Mixture Set III and calf intestinal phosphatase were from Calbiochem. Antibody to active MAPK (pTEpY; V803A) and anti-Erk1/2 (V114A) were from Promega. Monoclonal anti-β-actin (Ab-1) was from Oncogene Research Products. Horseradish peroxidase-conjugated anti-rabbit (secondary antibody; NA934V) and the enhanced chemilumiscence ECL immunoblot detection system were from Amersham Biosciences. Monoclonal anti-collagen I was from Calbiochem. Anti-vimentin, anti-collagen III, and all anti-Smads (Smad2, -3, -4, -6, and -7) were from Abcam. TGF-β-specific Cignal-Lenti reporters were from SABiosience. SYBR Green PCR Master Mix was from Applied Biosystems. PCR primers (Table S1) were designed with Primer Express software and purchased from IDT, Inc.
Pin1−/− Mice
Pin1+/− and Pin1−/− mice on a C57BL/6J background have been described previously (9). All animal procedures conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the University of Wisconsin Animal Care and Use Committee.
BLM Treatment
Pulmonary fibrosis was induced by endotracheal BLM injection as described previously (2). Briefly, mice were injected with 0.08 units/25 g body weight of BLM diluted in sterile saline or the same volume of sterile saline alone. The day of BLM administration was designated as day 0. At day 14 post-BLM injection, mice were euthanized, and lungs were lavaged twice with saline (0.8 ml × 2) to collect bronchoalveolear lavage (BAL) cells. Inflammation and fibrosis were evaluated by histological and trichrome staining, hydroxyproline determination, and qPCR analysis.
Primary Lung Fb Preparation and Culture
Primary Fb were established from Pin1 mice (6–8 weeks old) as described previously (11). Briefly, whole lungs were removed from exsanguinated mice and transferred to DMEM supplemented with 10% fetal calf serum, 2 mm l-glutamine, and antibiotics; cut into 1-mm sections; and washed twice with the medium. The lung pieces were incubated at 37 °C in a humidified CO2 incubator. After Fb began growing, the tissue pieces were removed, and the Fb were allowed to grow until near confluence and were passaged following trypsinization. Cells were grown for 10–20 days and always used before passage 4. Before use, lung Fb were stained and immunoblotted for vimentin (marker for Fb), and α-smooth muscle actin (for myofibroblasts). >95% of cultured cells were Fb. For cytokine treatment, cultures were plated and grown to 70% confluence prior to serum starvation in DMEM containing antibiotics. After 2 days, the cells were stimulated by the exogenous addition of 1 ng/ml TGF-β1 for 10 min to 12 h prior to harvest of the supernatant and cells for assessment.
Reverse Transcription and Real-time PCR
RNA was extracted with TriReagent. cDNA quantitative PCR was performed in a SYBR PCR master. An ABI 7500 thermocycler (Applied Biosystems, Foster City, CA) was used for 45 cycles of PCR. ΔCT calculates the differences between target CT values and the normalizer (housekeeping gene) for each sample, ΔCT = CT (target) − CT (normalizer). The comparative ΔΔCT calculates the differences between each sample ΔCT value and the base-line ΔCT. The comparative expression level (-fold changes) was obtained, transforming the logarithmic values to absolute values using 2−ΔΔCT.
Immunoprecipitation and Immunoblots
Cell lysates were prepared in Nonidet P-40 buffer. For immunoprecipitation, 2–5 μg of antibody was added to each sample, followed by incubation for 2–4 h at 4 °C. Protein G-agarose beads (Sigma-Aldrich) were added, and incubation was continued overnight. Pellets were washed five times with lysis buffer, and beads were dissolved in SDS-PAGE loading buffer for immunoblot analysis. The proteins were transferred onto nitrocellulose membranes and probed with primary and secondary antibodies. Protein bands were detected using enhanced chemiluminescence (ECL). Signals were quantified by densitometry.
Zymography
18 μg of protein from BAL fluid was mixed with an equal volume of nonreducing sample buffer and electrophoresed in 8% polyacrylamide gels copolymerized with 1 mg/ml gelatin as substrate (12). After electrophoresis, the gels were washed twice for 30 min in 2.5% Triton X-100, rinsed briefly with water, and then incubated in developing buffer (50 mm Tris-HCl, pH 7.5, 10 mm CaCl2, and 0.02% Brij35) for 20 h at 37 °C. The MMP activities were visualized after staining with 0.3% Coomassie R-250 and destained in the same solution without dye. Active forms of MMP2 and MMP9 were detected using molecular weight standards and MMP standards.
Lung Hydroxyproline Determination
This assay was performed as described (2, 11). Briefly, lungs were frozen in liquid nitrogen, minced into a fine homogenous mixture, weighed, dried, and hydrolyzed in 10 n NaOH for 3 h at 135 °C. Results were expressed as hydroxyproline content per dry weight of lung tissue.
ELISA
Active and total TGF-β1 proteins in BAL fluid and lung tissue (lysates) were assessed using ELISA kits from R&D Systems. The sensitivity of detection was 7 pg/ml.
GST Pull-down Assay
GST-Pin1 and His-Smad6 (cDNA from Addgene) were expressed in bacteria, and the pull-down assay was performed as described previously (13).
Immunostaining and Confocal Analysis
For immunofluorescence analysis of α-SMA, vimentin, and Smad3/6, tissue sections or cells were permeabilized, blocked, and incubated with appropriate dilutions of primary antibodies followed by fluorescence-conjugated secondary antibodies. Nuclear staining was performed with DAPI, and images were collected by confocal laser microscopy (C1 Laser Scanning Confocal; Nikon).
Pin1 Activity Assay
Protease-coupled Pin1 activity was measured with 1 μg of protein as described (14).
RESULTS
Pin1 Is Required for BLM-induced Collagen Deposition
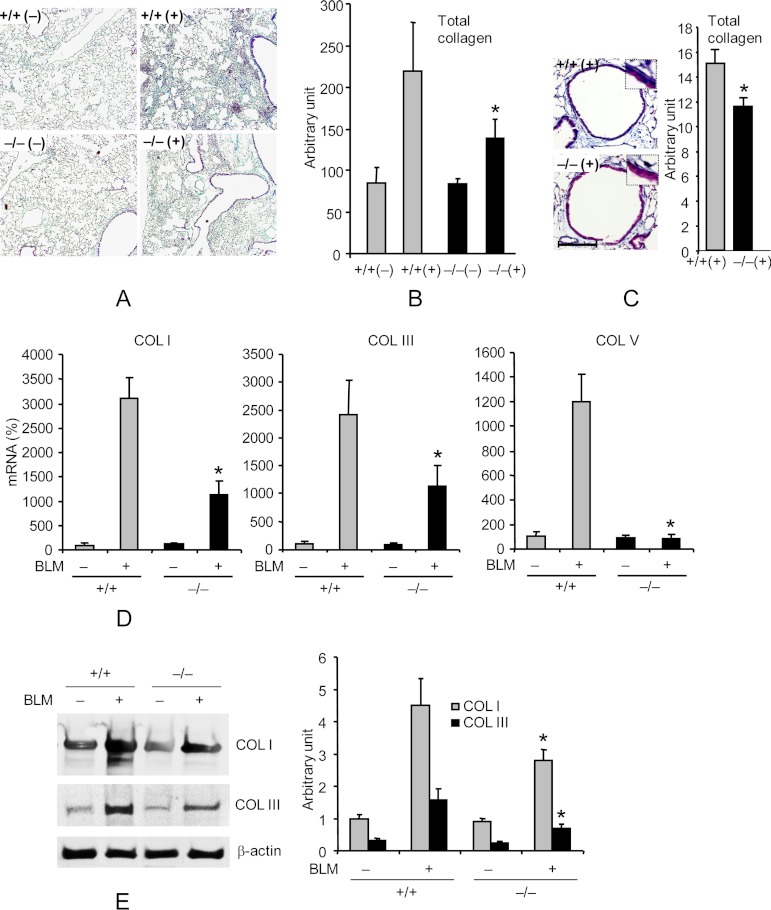
Recently, we showed significantly reduced collagen deposition in the airways or plura of Pin1 null rodents after the induction of allergic asthma or MHC-mismatched lung transplantation, respectively (9, 10). Therefore, we assessed if Pin1 was also important for interstitial fibrosis and thus assessed the effects of BLM, which models many features found in human IPF (15). BLM or saline control was introduced intratracheally (2) into Pin1−/− or wild-type mice (2–3 months old, all in pure C57/BL6 background). After 14 days, mice were euthanized and underwent BAL and lung removal. There were no significant differences in inflammatory cell counts in BAL between unchallenged Pin1−/− mice and wild-type controls except for a slight decrease in macrophages in knock-out animals (supplemental Fig. S1). BLM-challenged wild-type mice showed alveolar inflammatory cell infiltration and prominent, disorganized thickening of the alveolar septa (Fig. 1A) (data not shown), resulting in defacement of the normal architecture. However, Pin1−/− mice showed significantly less alveolar inflammatory cell infiltration and much more limited reactive changes compared with BLM treated, wild-type mice. Collagen accumulation as assessed by trichrome-staining (Fig. 1, B and C) and hydroxylproline content (supplemental Fig. S2) revealed significantly less fibrosis (>25%, p < 0.05) in Pin1−/− than wild-type mice with sparing of the alveoli and bronchioles (100–150 μm in diameter). Consistent with previous studies (2), BLM-induced injury dramatically increased the expression of type I, III, and V collagen mRNAs and proteins in wild-type mice, which was dramatically attenuated in Pin1−/− mice (Fig. 1, D and E). Interestingly, base-line collagen expression was comparable between wild-type and knock-out animals. These data show that Pin1 is required for the expression and deposition of multiple collagens during BLM-induced pulmonary fibrosis.
FIGURE 1.
Pin1−/− mice are resistant to BLM-induced lung collagen deposition. A, representative lung sections stained with trichrome for collagen deposition (blue) on day 14 after PBS (−) or BLM (+) challenge. +/+, Pin1 wild type; −/−, knockout. Images are shown at ×10 magnification. B, ImageJ quantification of total collagen staining shown in A. C, left, representative airway stained with trichrome for collagen deposition (blue). Scale bar, 100 μm. Right, ImageJ quantification of total collagen staining. *, p < 0.05 by Student's t test in a two-tailed analysis. No difference was identified between +/+ and −/− mice in base-line collagen levels after PBS treatment. D, qPCR analysis of collagens I, III, and V in lungs of control and BLM-challenged mice on day 14. The mRNA levels in untreated wild-type mice were set as 100% throughout unless otherwise specified. E, immunoblot (left) of collagens in lung of control and BLM-challenged mice on day 14. Right, ImageJ quantification of the immunoblots shown on the left. *, p < 0.05 between BLM-challenged wild type and knockout. No significant differences were identified between +/+ and −/− mice in base-line collagen mRNA and protein levels or after PBS treatment. Data shown are representative of three independent experiments and are expressed as the mean ± S.D. (error bars) of eight animals.
The Pulmonary Milieu of Pin1−/− Mice Is Antifibrogenic
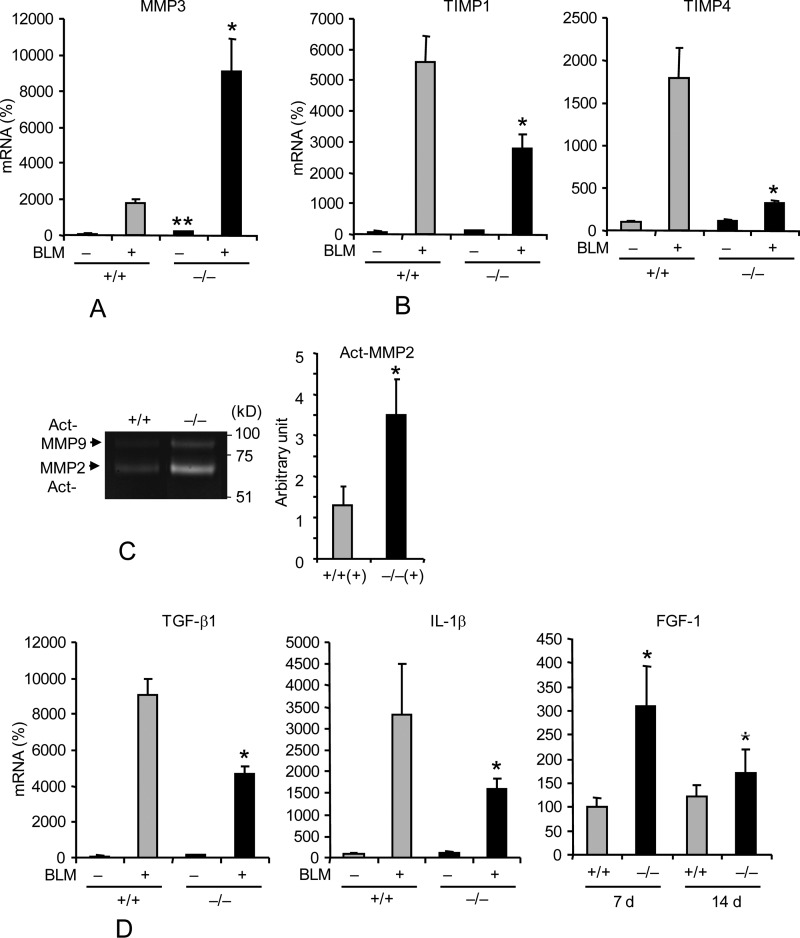
ECM accumulation ultimately reflects the dynamic equilibrium between secreted MMPs and TIMPs. MMP1, -2, -3, and -9 are directly activated by TGF-β1, IL-1β, and TNF-α but inhibited by interactions with TIMP1 and -4 (16–19). The lungs from Pin1 KO mice showed higher basal levels of MMP3 as well as significantly greater induction of MMP3 mRNA after BLM injury than WT (Fig. 2A). The basal levels of TIMP1 and -4 were similar between genotypes, but BLM injury failed to up-regulate TIMP1 and -4 mRNAs or proteins in Pin−/− compared with WT mice (Fig. 2B). Consistent with these results, gelatin zymography revealed significantly greater MMP2 and MMP9 activity (2–3-fold) in BAL fluid of Pin1−/− mice compared with wild type after BLM challenge (Fig. 2C). There was no difference in parenchymal cell death as assessed by TUNEL staining of lung sections after BLM injury (supplemental Fig. S3). Thus, we conclude that the fibrogenic response to BLM injury is substantially reduced in Pin1−/− mice. This probably reflects both a decreased production and increased catabolism of ECM compared with what is seen in wild-type mice.
FIGURE 2.
Pin1 regulates the expression of MMPs, TIMPs and fibrogenic growth factors in the lung. A, B, and D, qPCR analysis of ECM expression in the lung of control and BLM-challenged mice on day 14. **, p < 0.05 between vehicle-treated wild type and knockout. The FGF-1 mRNA data in D were all from BLM-treated mice (days 7 and 14). C, MMP2 (66 kDa) and MMP9 (84 kDa) bioactivity in BAL (n = 4–5) measured by zymography. NS, nonspecific bands. Right, ImageJ quantification of specific bands. *, p < 0.05 by Student's t test in a two-tailed analysis. Data shown are representative of three independent experiments and are expressed as the mean ± S.D. (error bars) of eight animals.
TGF-β1 and IL-1β are secreted by injured parenchyma as well as infiltrating, activated immune cells. Because Pin1 has been implicated TGF-β1 expression by immune cells (9), we measured profibrotic cytokine mRNAs and protein in the airway cells and lung tissue after injury. BLM challenge of wild-type mice dramatically increased TGF-β1 and IL-1β mRNAs (20), which were significantly attenuated in lungs from Pin1−/− mice (Fig. 2D). Interestingly, total TGF-β1 (latent and active forms) was similar in WT and Pin1−/− BAL fluid and tissue (supplemental Figs. S4–S7), suggesting that Pin1 participates in the regulation of TGF-β1 mRNA expression and possibly its translation (9). Because TGF-β1 was similar in WT and Pin1 KO, these results also implicate Pin1 in TGF-β receptor-mediated signaling in the lung. We observed similar differences in BAL cells (not shown) as for whole lung. FGF-1 mRNA was also significantly increased in lung tissue from knock-out mice compared with wild type 7 days after BLM and declined to near wild-type levels by day 14 (Fig. 2D). FGF-1 may prevent scarring by inducing Fb apoptosis and inhibition of myofibroblast differentiation (21).
Pin1 Is Required for Type III Collagen Production by Primary Lung Fb
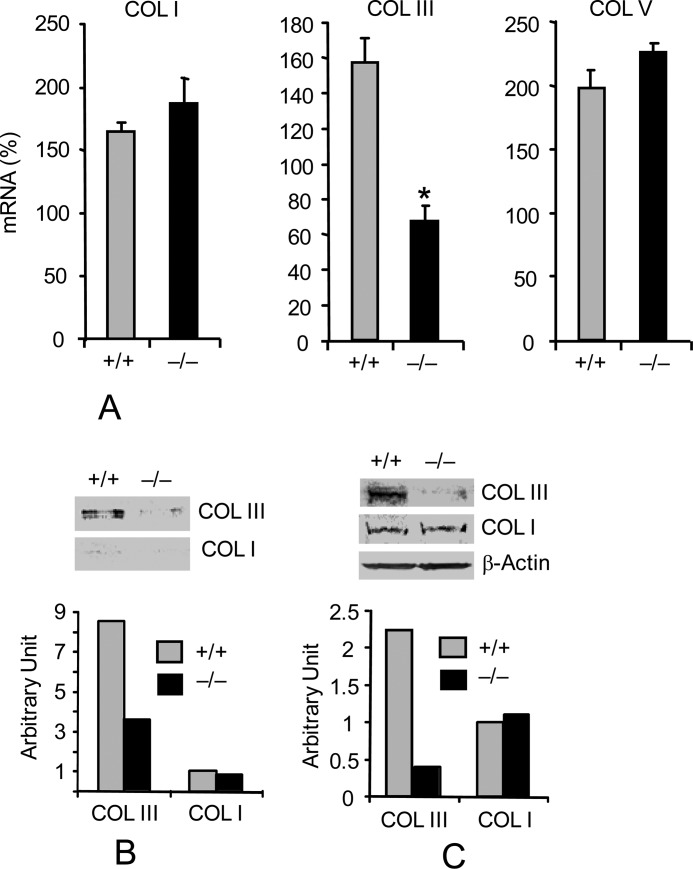
The reduced response to injury despite normal levels of profibrotic cytokines as seen in Pin1−/− mice suggests defects in intracellular signaling. The altered expression of MMPs and TIMPs suggests an involvement of Pin1 in TGF-β1 signaling and Fb gene regulation (10). Because resident Fb account for 30% of total lung cells and are the main producer of ECM (22), we established primary lung Fb cultures from wild-type and Pin1−/− mice. Purified cells expressed high levels of vimentin and low α-smooth muscle actin (α-SMA), consistent with Fb lineage (supplemental Fig. S8). Cells (3–4 divisions) were starved for 2 days to reduce serum effects before treatment with TGF-β1 for 12 h. qPCR analysis revealed constitutively high levels (c.t. 18–22; supplemental Fig. S9) of collagen I, III, and V in wild-type cells, which were significantly enhanced (50–100%) by TGF-β1 (Fig. 3A). However, the basal level of collagen III mRNA and protein were lower in untreated knock-out cells and nonresponsive to TGF-β1 (Fig. 3, A and C). Similarly, intracellular (Fig. 3C) and secreted (Fig. 3B) collagen III were lower in knock-out Fb than in WT. Juglone, a relatively specific Pin1 inhibitor (8, 9), also induced dose-dependent reductions of collagen III expression in human primary lung Fb (not shown). Therefore, Pin1 is required for TGF-β1-induced collagen III expression and secretion by lung Fb.
FIGURE 3.
Pin1 knockout decreases type III collagen expression in primary lung Fb. A, wild-type and Pin1−/− primary Fb were starved for 2 days before stimulation with TGF-β1 (1 ng/ml) for 12 h. Total RNA were subjected to qPCR analysis. Data are shown as increased mRNA (percentage) after TGF-β1 compared with the respective untreated control. Error bars, S.D. of 3–4 separate cultures in each group. Data are representative of at least three independent experiments. *, p < 0.05 by Student's t test in a two-tailed analysis. B, cells were treated as in A, and secreted collagens in the culture medium were immunoblotted (top) with anti-collagen I and III. ImageJ quantification (bottom) of the immunoblots from two independent experiments. C, cell lysates were immunoblotted (top) with anti-collagen antibodies shown. ImageJ quantification (bottom) of the immunoblots from two independent experiments.
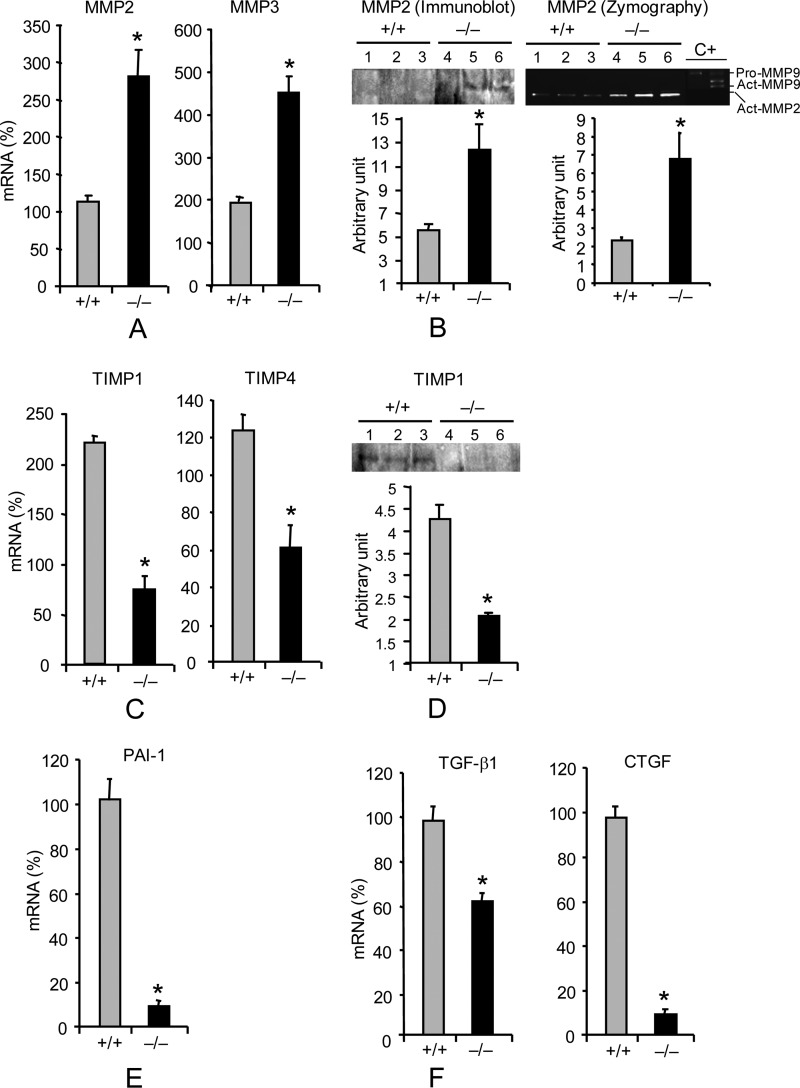
We next asked if the regulation of other ECM genes was altered in primary KO Fb. In wild-type cells, TGF-β1 selectively increased MMP3 mRNA expression by 2-fold without affecting other MMPs (Fig. 4A) (data not shown), whereas similarly treated Pin1−/− Fb showed a superinduction of MMP2 and -3, and other MMPs were unchanged (Fig. 4, A and B) (data not shown). TGF-β1 also failed to substantially alter TIMP expression by knock-out cells (Fig. 4, C and D). Consistent with these results, zymography revealed markedly increased MMP2 activity in the supernatant of KO cells (Fig. 4B). Because plasminogen activator inhibitor-I and CTGF have been implicated in the development of lung fibrosis and are themselves induced by TGF-β1, we evaluated their expression. TGF-β1 increased the level of plasminogen activator inhibitor-I and CTGF by 4–8-fold in wild-type cells, but the basal levels were significantly lower and non-responsive to cytokine treatment in Pin1−/− cells (Fig. 4, E and F) (data not shown). Somewhat unexpectedly, given the above data with whole lung, the production of TGF-β1 itself was also significantly reduced in knock-out cells. These data suggest that Pin1 regulates TGF-β1 signaling, leading to ECM production and TGF-β1 production by primary lung Fb. These results also suggest that the role of Pin1 in TGF-β1 signaling probably shows cell-specific differences.
FIGURE 4.
Pin1 regulates the expression of ECM and fibrogenic growth factors in primary lung Fb. Cells were treated as in Fig. 3A and analyzed for the expression of ECM and growth factors. A, mRNA for MMP2 and MMP3. Data are shown as increased mRNA (percentage) after TGF-β1 compared with respective untreated control. B, culture media from A were immunoblotted with anti-MMP-2, and the bioactivity was measured by zymography. C, mRNA for TIMP1 and TIMP4. Data are shown as increased mRNA (percentage) after TGF-β1 compared with respective untreated control. D, culture media from C were immunoblotted with anti-TIMP1. E and F, basal level of growth factor mRNA. Error bars, S.D. of 3–4 separate cultures in each group. Data are representative of at least five independent experiments *, p < 0.05 by Student's t test in a two-tailed analysis.
Pin1−/− Fb Are Defective in TGF-β/Smad Signaling
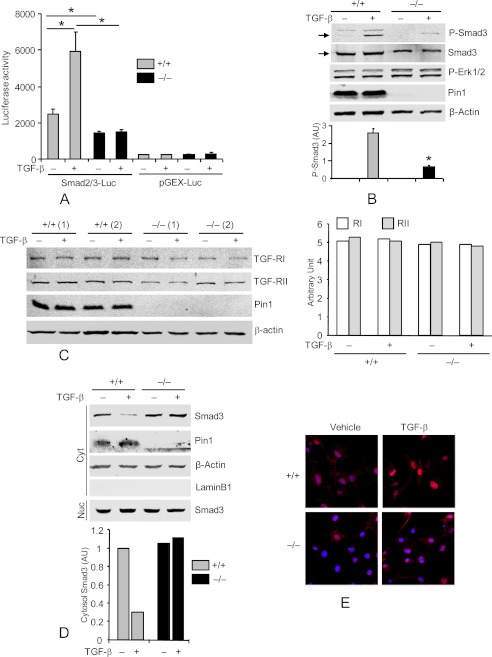
Smad2 and -3 mediate TGF-β1-induced ECM expression, play a critical role in Fb differentiation and tissue fibrosis (23), and show persistent activation in BLM-exposed lung tissue (24). Therefore, we evaluated Smad-dependent transcription 3 days after infecting primary wild-type and knock-out Fb with lentivirus containing a Smad2/3 response element upstream of a luciferase reporter. TGF-β1 stimulated Smad2/3 reporter activity by 2–3-fold in wild-type cells (25) (Fig. 5A) but had no effect on the pGEX-luc control reporters lacking Smad2/3 sites. Pin1−/− cells displayed significantly lower reporter activity under basal conditions and failed to respond to TGF-β1 stimulation. Therefore, wild-type and Pin1−/− cells were treated with diluent or TGF-β1 for 1 h before immunoblot of whole cell lysates. As shown (Fig. 5B), the level of phosphorylated Smad3 was ∼74% lower in knock-out than in wild-type cells following TGF-β1 stimulation. Neither TGF-β1 treatment nor Pin1 knockout significantly modified the level or phosphorylation of Erk1/2, β-actin, or type I/II TGF-β1 receptors (Fig. 5, B and C) (data not shown). Immunoblot of subcellular fractions showed the expected low cytosolic but high levels of nuclear Smad3 after TGF-β1 stimulation in wild type cells (Fig. 5D). KO cells, however, showed largely cytoplasmic Smad3 that closely resembled untreated, wild-type cells (Fig. 5D) (data not shown). Immunofluorescence analysis confirmed these results (Fig. 5E). Taken together, these data show that Pin1 is required for C-terminal Smad3 phosphorylation induced by TGF-β1 as well as subsequent nuclear translocation and target gene activation.
FIGURE 5.
Pin knockout suppresses TGF-β1-Smad signaling in primary lung Fb. A, cells were infected with lentiviral vectors containing Smad2/3-responsive luciferase reporter and Renilla-luciferase constitutive reporter. After 2 days, cultures were treated with vehicle or TGF-β1 (1 ng/ml) for an additional 2 days. Cell lysates were subjected to luciferase activity assay using the Dual-Luciferase Assay system and normalized to the Renilla-luciferase reporter. Lenti-pGEX-luc without Smad2/3 binding sites served as negative control. B, cells were starved for 2 days and treated with TGF-β1 for 1 h. Cell lysates were immunoblotted with antibodies to phospho-Smad3 (Ser534/536), total Smad3, phospho-Erk1/2, Pin1, and β-actin. The ratios of Smad3 phosphorylation to the total Smad3 were quantified by ImageJ (bottom). AU, arbitrary units. C, cells from two mice were treated as in B, and total cell lysates were immunoblotted with anti-type I and II TGF-β1 receptors. ImageJ quantification of the immunoblots is shown on the right. D, cells were treated as in B, and cytoplasmic (Cyt) and nuclear (Nuc) extracts were immunoblotted (top) with antibodies to total Smad3, Pin1, β-actin, and lamin B1 (nuclear marker) (Nuc). C+, positive control. Bottom, ImageJ quantification of the immunoblots from two independent experiments. E, cells were treated as in B and immunostained with anti-Smad3 (red) and TO-Pro (nuclear dye; blue). *, p < 0.05. Data shown are representative of five independent experiments and are expressed as the mean ± S.D.
Pin1 Associates with Smad3 and Smad6
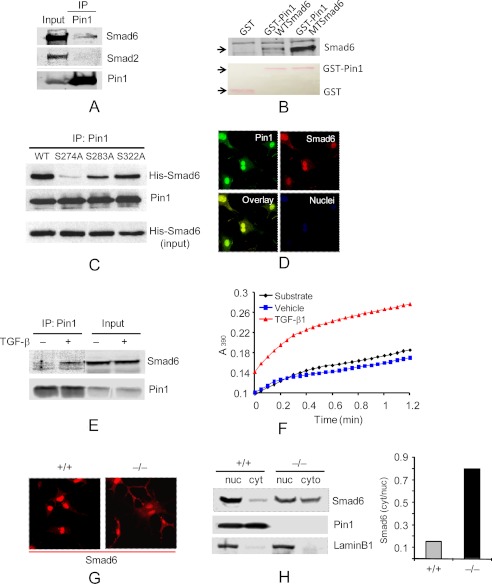
Smad6 and -7 block C-terminal, R-Smad phosphorylation and activation by interacting stably with the type I receptor (26, 27). R-Smad linkers are also phosphorylated at Ser-Pro and Thr-Pro sites by MAPKs. In tumor cells, these sites in Smad3 are targets for Pin1, although the physiologic consequences or occurrence in normal cells remains unclear (28, 29). To test this, lysates from primary Fb were immunoprecipitated with anti-Pin1 followed by immunoblot with anti-Smads. Anti-Pin1 reproducibly pulled down endogenous Smad6 from untreated cells (Fig. 6A) and variably pulled down Smad3. TGF-β increased the interactions of Pin1 with both Smad6 (Fig. 6E and supplemental Fig. S12) and Smad3 (not shown). However, normalized to Pin1, the amount of Smad6 in IP pellets, irrespective of TGF-β treatment, was ∼10-fold greater than the amount of Smad3 (supplemental Fig. S13). Confocal microscopy confirmed co-localization of Smad6 and Pin1 in both the nuclei and cytoplasm of WT cells (Fig. 6D). Therefore, we focused on characterizing the interactions between Pin1 and Smad6. GST-Pin1 pulled down recombinant Smad6 (Fig. 6B). Sequence analysis identified three potential Pin1 sites in the linker region (Ser274-Pro275, Ser283-Pro284, and Ser322-Pro323) and one in the MH2 domain (Ser414-Pro415) of Smad6. Phosphor mimic mutation of the four sites (all serine residues converted to glutamic acid) substantially increased the interaction of recombinant Smad6 with GST-Pin1 (Fig. 6B). Conversely, calf intestinal phosphatase treatment of cell lysates from control or TGF-β-treated fibroblasts abolished the Smad6-Pin1 interaction (supplemental Fig. S12). These data suggest that in cells, Smad6 phosphorylation on one or more of the four Ser-Pro sites enhanced the interaction with Pin1 (30). Despite these data, using generic anti-phosphoantibodies, we were unable to show increased Smad6 Ser (supplemental Fig. S11) or Thr (not shown) phosphorylation after TGF-β1 stimulation, similar to that seen previously (31). However, based on the above data, we produced Smad6 mutants with individual Pin1 sites converted to phosphor-null Ala and introduced recombinant Smad6 proteins into primary fibroblasts by TAT-mediated transduction. After TGF-β1, all Smad6 mutants (including WT) interacted strongly with endogenous Pin1 except for S274A (Fig. 6C). Therefore, we conclude that Pin1 interacts strongly with Smad6 at phosphorylated Ser274-Pro275 in primary fibroblasts.
FIGURE 6.
Pin1 directly interacts with Smad6 and modulates its localization. A, cell lysates were precleared with normal IgG and immunoprecipitated (IP) with anti-Pin1 prior to immunoblots with antibodies shown. 10% of lysates served as input. B, recombinant WT and mutant (MT) Smad6 with four potential Pin1 sites (Ser274-Pro, Ser283-Pro, Ser322-Pro, and Ser414-Pro) mutated from serine to glutamic acid were pulled down with GST and GST-Pin1 and probed with anti-Smad6. The inputs of control GST and GST-Pin1 were stained by Ponceau S (red) and are shown at the bottom. C, Pin1 interacts with the Ser274-Pro motif in Smad6. TAT-WT-Smad6 and TAT mutant Smad6 (S274A, S283A, and S322A) were added to the Fb culture before treatment of the cells with TGF-β1 (1 ng/ml) for 1 h. Cell lysates were pulled down with anti-Pin1 followed by immunoblot with anti-Pin1 and anti-His (Smad6). D, starved, wild-type cells (2 days) were treated with TGF-β1 for 1 h followed by immunostaining with anti-Pin1 (green), Smad6 (red), and TO-Pro (blue). E, cells were treated as in D and analyzed as in A. F, cells were treated with TGF-β1 for 10 min, and cytosolic protein was subjected to a Pin1 isomerase assay as described under “Experimental Procedures.” The products were measured at 390 nm. G, cells were treated as in D and immunostained with anti-Smad6. H, cells were treated as in D, and cytoplasmic (cyt) and nuclear extracts (nuc) were immunoblotted with antibody to Smad6, Pin1, and lamin B1 (nuclear marker). Data shown are representative of five independent experiments.
In order to determine if Pin1 could act on Smad6, we measured total cytoplasmic, isomerase activity after brief exposure (10 min) to diluent or TGF-β1. Pin1 activity was nearly undetectable in untreated Fb but significantly elevated by TGF-β1 (Fig. 6F), without affecting Pin1 protein levels (data not shown). Thus, TGF-β1 induces an interaction of active Pin1 with phospho-Smad6, probably through Ser274-Pro275 located in the linker region.
Pin1 isomerization can alter target protein localization. Thus, we asked if Smad6 showed altered intracellular location in the absence of Pin1. Therefore, we treated wild-type or KO cells with TGF-β1 and determined the location of Smad6 by confocal microscopy. Pin1−/− cells displayed far higher levels of cytoplasmic Smad6 than wild-type cells (Fig. 6G). Immunoblotting of subcellular fractions showed that ∼10% of Smad6 was in the cytoplasmic fraction and 90% in the nucleus of wild-type cells, whereas 40% was cytoplasmic and 60% nuclear in knock-out cells (Fig. 6H). These results were also observed on tissue sections from BLM-treated mice after double staining with anti-vimentin, a marker for Fb and myofibroblasts, along with anti-Smad6 and DAPI. In lung parenchyma from wild-type mice, most Smad6 was nuclear. In Pin1 KO, Smad6 was diffuse and found throughout the nucleus and cytoplasm (supplemental Fig. S10). These data strongly suggest that Pin1 is required for the nuclear localization of Smad6 both in vivo and in vitro. Because Smad6 antagonizes Smad3 function, we hypothesize excess cytoplasmic Smad6 suppresses Smad3 signaling and downstream ECM production observed in Pin1−/− mice or cells after TGF-β1.
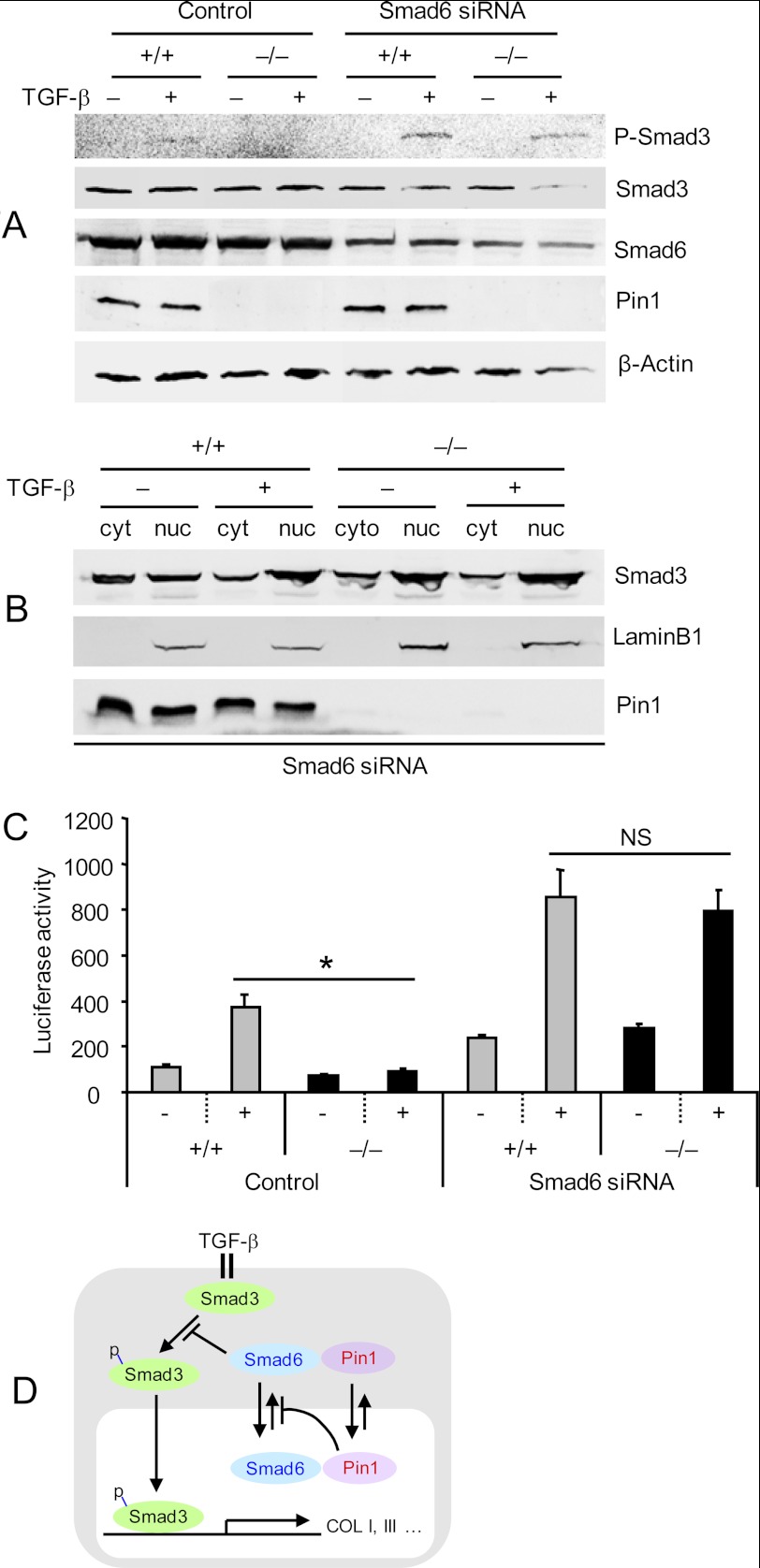
In order to support this hypothesis, we knocked down Smad6 using siRNA in Pin1−/− Fb. Cells transfected with specific siRNAs showed a 40–70% reduction in Smad6, whereas control siRNAs had no effect (Fig. 7A). Despite the absence of Pin1, Smad6-deficient Fb responded essentially normally to TGF-β1 stimulation as measured by C-terminal Smad3 phosphorylation (Fig. 7A) as well as nuclear translocation (Fig. 7B). Finally, we interrogated the trans-activation potential of nuclear, phospho-Smad3 in these cells by measuring luciferase reporter production after TGF-β1 treatment (see Fig. 5A). Consistent with the above results, Smad6 knockdown restored TGF-β1-induced, Smad3-luciferase reporter expression in Pin1 null cells to wild-type levels (Fig. 7C). Therefore, Pin1 modulates TGF-β1 signaling in primary murine Fb through its effects on Smad6 localization (Fig. 7D).
FIGURE 7.
Smad6 knockdown restores TGF-β signaling in Pin1 null cells. A, primary lung Fb transfected with control or Smad6-specific siRNA (50 nm) were treated with TGF-β1 for 1 h. Whole cell lysates were immunoblotted with antibodies shown on the right. B, cells were treated as in A, and cytoplasmic (cyt) and nuclear extracts (nuc) were probed with antibodies shown on the right. Lamin B1 served as nuclear marker. C, cells were transfected with Smad6-specific siRNA and then infected with lentiviral vectors containing Smad2/3-responsive luciferase reporter and Renilla-luciferase constitutively active reporter. After 1 day, cultures were treated with vehicle or TGF-β1 (1 ng/ml) for an additional 2 days. Cell lysates were subjected to a luciferase activity assay using the Dual-Luciferase Assay system and normalized to the Renilla-luciferase reporter. Data shown are representative of at least five independent experiments and are expressed as the mean ± S.D. *, p < 0.05. NS, not significant. D, proposed model of Pin1 regulation of TGF-β/Smad signaling (for details, see “Results”).
DISCUSSION
Here we investigate the role of Pin1 in BLM-induced pulmonary fibrosis as well as in primary lung Fb stimulated by TGF-β1. Pin1 coordinates the production and accumulation of ECM by regulating intracellular, Smad-dependent signaling. Both Pin1 PPIase activity and its interactions with Smad6 and Smad3 are modulated by TGF-β1 signaling. These observations support and expand on our previous observations that Pin1 controls collagen accumulation and airway remodeling in animal models of asthma. Therefore, our findings are of considerable importance for understanding and possibly treating fibrotic lung disease, such as asthma and IPF.
BLM-induced lung fibrosis is the most commonly used rodent model of IPF. Despite some limitations, BLM induces robust but self-limited inflammation, profibrotic cytokine expression, and pathologic interstitial collagen production and deposition (20). Pin1 has been implicated in allergic airway inflammation and remodeling through the regulation of eosinophilic inflammation and TGF-β1 production (9). As after allergic stimuli, Pin1 was required for BLM-dependent in vivo increases in collagen I, III, and V and TIMP1 and -4. In the absence of Pin1, the aggregate balance shifted from a fibrotic to a fibrolytic environment with reduced ECM and TIMPs and far more MMP expression. Explanted primary Fb recapitulated many, but not all, of these phenotypes. Although Pin1 knockout suppressed collagen I, III, and V in vivo, only reductions in collagen III were seen with Fb in vitro. This suggests that Pin1 also participates in the regulation of ECM by other cells in the lung, most likely epithelium and inflammatory cells. Indeed, we observed reduced collagen I, III, and V in BAL cells (composed primarily of neutrophils and T cells) from BLM-challenged knock-out mice (data not shown) as well as in primary murine lung epithelial cells co-treated with TGF-β1 and Pin1 inhibitors (supplemental Fig. S15). Epithelial cells also showed reduced pSmad3 after Pin1 inhibition (supplemental Fig. S14). In patients with IPF and asthma, collagens, TIMPs, and profibrogenic cytokines are increased (32, 33) whereas the levels of MMPs vary, depending on the stage of the disease (16). Therefore, Pin1 inhibitors, several of which show nanomolar Ki and favorable cell permeability (34), may be effective to treat fibrotic diseases of the lung.
Although TGF-β1 usually increases collagen synthesis, its effects on MMPs and TIMPs are less consistent and more poorly understood. In immortalized Fb, the expression of MMP13 increased whereas MMP1 decreased in response to TGF-β1 (35). Here, TGF-β1 significantly increased MMP3, TIMP1, TIMP3, and TIMP4 and decreased TIMP2 but had no effect on MMP2, MMP8, and MMP9 in primary wild-type Fb. In contrast to other ECM proteins, MMP2 and -3 were overexpressed by TGF-β1-treated Pin1 KO Fb. These data suggest that Pin1 is a crucial signaling molecule for the coordinated regulation of proteinases and anti-proteinases in the lung. Given the observed data, Pin1 must both positively and negatively modulate ECM gene expression in response to profibrotic cytokines. This suggests that Pin1 can influence the expression of subsets of ECM genes (e.g. MMP2 and -3). Somewhat similar results have been seen for relaxin, a reproductive hormone/cytokine that enhanced ECM turnover by Fb by decreasing the synthesis of collagen and TIMP1 but stimulated MMP1 expression (36). Whether this molecule is linked physiologically or genetically to Pin1 is unknown.
Previous studies in transformed human keratinocytes or prostate cancer cell lines have revealed TGF-β1-induced interactions between Pin1 and phosphorylated Thr179-Pro180 in the linker region of Smad3 (28, 29). In addition, Pin1 knockdown had no effect on TGF-β1-responsive genes in transformed keratinocytes but did suppress the migration and invasion of prostate cancer cell lines (29). Although we have also observed Smad3-Pin1 interactions, we now report TGF-β1-induced interactions between Smad6 and Pin1 as well. In addition, we note multiple and profound defects in TGF-β1 signaling in primary Fb and probably Pin1 KO mice that led to reduced ECM production in vitro and in vivo. These data suggest that Pin1-mediated regulation of TGF-β1 is cell- and possibly species-specific as well as subject to alteration after cell transformation. As such, more study exploring how Pin1 interfaces with and modulates TGF-β1 signaling is necessary.
I-Smads associate with type 1 receptors and prevent their phosphorylation of R-Smad2/3 (24, 27). In addition, Smad6 recruits the E3 ubiquitin ligase Smurf1 to the type 1 receptor and enhances receptor catabolism (37), although we did not observe significant reductions in type 1 or 2 receptor levels in Pin1−/− cells (Fig. 5C). After TGF-β1, Smad6 was predominantly nuclear in wild-type cells but remained cytoplasmic in knock-out cells. Smad6 knockout enhanced TGF-β1-mediated signaling and Smad2/3 phosphorylation in cancer cells (38). Similarly, we have shown that Smad6 knockdown reconstituted TGF-β1 signaling in primary, Pin1 null Fb. Thus, excess cytoplasmic Smad6 in Pin1−/− Fb most likely accounted for the observed decreases in Smad3 phosphorylation, nuclear localization, and gene activation despite TGF-β1 treatment. These results also imply that Pin1 was required for localization and/or nuclear retention of Smad6. The cytokine-induced nuclear localization of the Pin1 binding partner, p65 Rel, was prevented by Pin1 inhibitors (39). Therefore, Pin1-mediated isomerization can regulate intracellular location. We have shown that Smad6 is a bone fide Pin1 ligand, suggesting that it may be regulated as p65 Rel. Consistent with this mechanism, Fb treated briefly with TGF-β1 showed significantly increased isomerase activity and Pin1 binding to Smad6 (Fig. 6, D and E). Therefore, we propose that TGF-β induces Pin1 activity and enhances Pin1 binding to and isomerization of Smad6, which assumes a nuclear location. In the absence of Pin1, Smad6 remains predominantly cytoplasmic where it attenuates TGF-β1 signaling (Fig. 7D).
Typically, the phosphorylation of Ser-Pro or Thr-Pro motifs facilitates Pin1 binding. We were unable to detect significant changes in serine or threonine Smad6 phosphorylation after TGF-β, although phorbol esters induced the phosphorylation of Ser435-Leu436 by PrKX in HL60 cells (40). However, because calf intestinal phosphatase treatment abolished intracellular Pin1-Smad6 interactions, and substitution of all Ser at Pin1 sites with Glu increased Smad6-Pin1 interactions, we infer that target phosphorylation indeed is essential in cells. Based on mutational analysis, phosphorylation of Ser274-Pro275 is most likely required for maximal Pin1-Smad6 interactions. Although the MH2 domain is responsible for the interaction with TGF-β1 receptors, the biological function of the linker region is largely unknown. Pin1-mediated isomerization of this site may alter the function of the nuclear localization signal and/or nuclear export signal, contributing to enhanced cytoplasmic localization of Smad6 in Pin1 KO cells.
Overall, our data provide additional insight into how Pin1 regulates Smad signaling in Fb and strengthens the justification to employ Pin1 inhibitors for the treatment of pulmonary fibrotic disease, including IPF or chronic asthma.
Acknowledgments
We thank all of the members of the laboratory as well as the University of Wisconsin Asthma Biology group for helpful suggestions and criticism.
This work was supported, in whole or in part, by National Institutes of Health Grants R01HL087950, P01HL088594, and P30HD03352 (to J. S. M.).

This article contains supplemental Table S1 and Figs. S1–S15.
- ECM
- extracellular matrix
- TIMP
- tissue inhibitor of metalloproteinase
- MMP
- metalloproteinase
- IPF
- idiopathic pulmonary fibrosis
- Fb
- fibroblasts
- CTGF
- connective tissue growth factor
- BLM
- bleomycin
- BAL
- bronchoalveolar lavage
- SMA
- smooth muscle actin
- qPCR
- quantitative PCR.
REFERENCES
- 1. Laurent G. J. (1987) Dynamic state of collagen: pathways of collagen degradation in vivo and their possible role in regulation of collagen mass. Am. J. Physiol. 252, C1–C9 [DOI] [PubMed] [Google Scholar]
- 2. Wilson M. S., Madala S. K., Ramalingam T. R., Gochuico B. R., Rosas I. O., Cheever A. W., Wynn T. A. (2010) Bleomycin and IL-1β-mediated pulmonary fibrosis is IL-17A-dependent. J. Exp. Med. 207, 535–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Uhal B. D., Kim J. K., Li X., Molina-Molina M. (2007) Angiotensin-TGF-β1 cross-talk in human idiopathic pulmonary fibrosis. Autocrine mechanisms in myofibroblasts and macrophages. Curr. Pharm. Des. 13, 1247–1256 [DOI] [PubMed] [Google Scholar]
- 4. Zheng L., Walters E. H., Wang N., Whitford H., Orsida B., Levvey B., Bailey M., Williams T. J., Snell G. I. (2004) Effect of inhaled fluticasone propionate on BAL TGF-β1 and bFGF concentrations in clinically stable lung transplant recipients. J. Heart Lung Transplant. 23, 446–455 [DOI] [PubMed] [Google Scholar]
- 5. Halwani R., Al-Muhsen S., Al-Jahdali H., Hamid Q. (2011) Role of transforming growth factor-β in airway remodeling in asthma. Am. J. Respir. Cell Mol. Biol. 44, 127–133 [DOI] [PubMed] [Google Scholar]
- 6. Lu K. P., Zhou X. Z. (2007) The prolyl isomerase PIN1. A pivotal new twist in phosphorylation signaling and disease. Nat. Rev. Mol. Cell Biol. 8, 904–916 [DOI] [PubMed] [Google Scholar]
- 7. Lu K. P., Finn G., Lee T. H., Nicholson L. K. (2007) Prolyl cis-trans isomerization as a molecular timer. Nat. Chem. Biol. 3, 619–629 [DOI] [PubMed] [Google Scholar]
- 8. Shen Z. J., Esnault S., Malter J. S. (2005) The peptidyl-prolyl isomerase Pin1 regulates the stability of granulocyte-macrophage colony-stimulating factor mRNA in activated eosinophils. Nat. Immunol. 6, 1280–1287 [DOI] [PubMed] [Google Scholar]
- 9. Shen Z. J., Esnault S., Rosenthal L. A., Szakaly R. J., Sorkness R. L., Westmark P. R., Sandor M., Malter J. S. (2008) Pin1 regulates TGF-β1 production by activated human and murine eosinophils and contributes to allergic lung fibrosis. J. Clin. Invest. 118, 479–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Esnault S., Braun R. K., Shen Z. J., Xiang Z., Heninger E., Love R. B., Sandor M., Malter J. S. (2007) Pin1 modulates the type 1 immune response. PLoS One 2, e226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Avcuoglu S., Wygrecka M., Marsh L. M., Günther A., Seeger W., Weissmann N., Fink L., Morty R. E., Kwapiszewska G. (2011) Neurotrophic tyrosine kinase receptor B/neurotrophin 4 signaling axis is perturbed in clinical and experimental pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 45, 768–780 [DOI] [PubMed] [Google Scholar]
- 12. Mitra R. S., Goto M., Lee J. S., Maldonado D., Taylor J. M., Pan Q., Carey T. E., Bradford C. R., Prince M. E., Cordell K. G., Kirkwood K. L., D'Silva N. J. (2008) Rap1GAP promotes invasion via induction of matrix metalloproteinase 9 secretion, which is associated with poor survival in low N-stage squamous cell carcinoma. Cancer Res. 68, 3959–3969 [DOI] [PubMed] [Google Scholar]
- 13. Izquierdo J. M. (2010) Heterogeneous ribonucleoprotein C displays a repressor activity mediated by T-cell intracellular antigen-1-related/like protein to modulate Fas exon 6 splicing through a mechanism involving Hu antigen R. Nucleic Acids Res. 38, 8001–8014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shen Z. J., Esnault S., Schinzel A., Borner C., Malter J. S. (2009) The peptidyl-prolyl isomerase Pin1 facilitates cytokine-induced survival of eosinophils by suppressing Bax activation. Nat. Immunol. 10, 257–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moeller A., Ask K., Warburton D., Gauldie J., Kolb M. (2008) The bleomycin animal model. A useful tool to investigate treatment options for idiopathic pulmonary fibrosis? Int. J. Biochem. Cell Biol. 40, 362–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cataldo D. D., Gueders M. M., Rocks N., Sounni N. E., Evrard B., Bartsch P., Louis R., Noel A., Foidart J. M. (2003) Pathogenic role of matrix metalloproteases and their inhibitors in asthma and chronic obstructive pulmonary disease and therapeutic relevance of matrix metalloproteases inhibitors. Cell Mol. Biol. 49, 875–884 [PubMed] [Google Scholar]
- 17. Fang Q., Liu X., Al-Mugotir M., Kobayashi T., Abe S., Kohyama T., Rennard S. I. (2006) Thrombin and TNF-α/IL-1β synergistically induce fibroblast-mediated collagen gel degradation. Am. J. Respir. Cell Mol. Biol. 35, 714–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Todorova L., Gürcan E., Westergren-Thorsson G., Miller-Larsson A. (2009) Budesonide/formoterol effects on metalloproteolytic balance in TGFβ-activated human lung fibroblasts. Respir. Med. 103, 1755–1763 [DOI] [PubMed] [Google Scholar]
- 19. Jiang Y., Goldberg I. D., Shi Y. E. (2002) Complex roles of tissue inhibitors of metalloproteinases in cancer. Oncogene 21, 2245–2252 [DOI] [PubMed] [Google Scholar]
- 20. Gasse P., Mary C., Guenon I., Noulin N., Charron S., Schnyder-Candrian S., Schnyder B., Akira S., Quesniaux V. F., Lagente V., Ryffel B., Couillin I. (2007) IL-1R1/MyD88 signaling and the inflammasome are essential in pulmonary inflammation and fibrosis in mice. J. Clin. Invest. 117, 3786–3799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ramos C., Montaño M., Becerril C., Cisneros-Lira J., Barrera L., Ruíz V., Pardo A., Selman M. (2006) Acidic fibroblast growth factor decreases α-smooth muscle actin expression and induces apoptosis in human normal lung fibroblasts. Am. J. Physiol. Lung Cell Mol. Physiol. 291, L871–L879 [DOI] [PubMed] [Google Scholar]
- 22. Tompa A., Langenbach R. (1979) Culture of adult rat lung cells. Benzo(a)pyrene metabolism and mutagenesis. In Vitro 15, 569–578 [DOI] [PubMed] [Google Scholar]
- 23. Le A. V., Cho J. Y., Miller M., McElwain S., Golgotiu K., Broide D. H. (2007) Inhibition of allergen-induced airway remodeling in Smad 3-deficient mice. J. Immunol. 178, 7310–7316 [DOI] [PubMed] [Google Scholar]
- 24. Venkatesan N., Pini L., Ludwig M. S. (2004) Changes in Smad expression and subcellular localization in bleomycin-induced pulmonary fibrosis. Am. J. Physiol. Lung Cell Mol. Physiol. 287, L1342–L1347 [DOI] [PubMed] [Google Scholar]
- 25. Hayashida T., Decaestecker M., Schnaper H. W. (2003) Cross-talk between ERK MAP kinase and Smad signaling pathways enhances TGF-β-dependent responses in human mesangial cells. FASEB J. 17, 1576–1578 [DOI] [PubMed] [Google Scholar]
- 26. Hayashi H., Abdollah S., Qiu Y., Cai J., Xu Y. Y., Grinnell B. W., Richardson M. A., Topper J. N., Gimbrone M. A., Jr., Wrana J. L., Falb D. (1997) The MAD-related protein Smad7 associates with the TGFβ receptor and functions as an antagonist of TGFβ signaling. Cell 89, 1165–1173 [DOI] [PubMed] [Google Scholar]
- 27. Nakao A., Afrakhte M., Morén A., Nakayama T., Christian J. L., Heuchel R., Itoh S., Kawabata M., Heldin N. E., Heldin C. H., ten Dijke P. (1997) Identification of Smad7, a TGF-β-inducible antagonist of TGF-β signaling. Nature 389, 631–635 [DOI] [PubMed] [Google Scholar]
- 28. Nakano A., Koinuma D., Miyazawa K., Uchida T., Saitoh M., Kawabata M., Hanai J., Akiyama H., Abe M., Miyazono K., Matsumoto T., Imamura T. (2009) Pin1 down-regulates transforming growth factor-β (TGF-β) signaling by inducing degradation of Smad proteins. J. Biol. Chem. 284, 6109–6115 [DOI] [PubMed] [Google Scholar]
- 29. Matsuura I., Chiang K. N., Lai C. Y., He D., Wang G., Ramkumar R., Uchida T., Ryo A., Lu K., Liu F. (2010) Pin1 promotes transforming growth factor-β-induced migration and invasion. J. Biol. Chem. 285, 1754–1764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Boussetta T., Gougerot-Pocidalo M. A., Hayem G., Ciappelloni S., Raad H., Arabi Derkawi R., Bournier O., Kroviarski Y., Zhou X. Z., Malter J. S., Lu P. K., Bartegi A., Dang P. M., El-Benna J. (2010) The prolyl isomerase Pin1 acts as a novel molecular switch for TNF-α-induced priming of the NADPH oxidase in human neutrophils. Blood. 116, 5795–5802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hata A., Lagna G., Massagué J., Hemmati-Brivanlou A. (1998) Smad6 inhibits BMP/Smad1 signaling by specifically competing with the Smad4 tumor suppressor. Genes Dev. 12, 186–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ramos C., Montaño M., García-Alvarez J., Ruiz V., Uhal B. D., Selman M., Pardo A. (2001) Fibroblasts from idiopathic pulmonary fibrosis and normal lungs differ in growth rate, apoptosis, and tissue inhibitor of metalloproteinases expression. Am. J. Respir. Cell Mol. Biol. 24, 591–598 [DOI] [PubMed] [Google Scholar]
- 33. Chiappara G., Gagliardo R., Siena A., Bonsignore M. R., Bousquet J., Bonsignore G., Vignola A. M. (2001) Airway remodeling in the pathogenesis of asthma. Curr. Opin. Allergy Clin. Immunol. 1, 85–93 [DOI] [PubMed] [Google Scholar]
- 34. Guo C., Hou X., Dong L., Dagostino E., Greasley S., Ferre R., Marakovits J., Johnson M. C., Matthews D., Mroczkowski B., Parge H., Vanarsdale T., Popoff I., Piraino J., Margosiak S., Thomson J., Los G., Murray B. W. (2009) Structure-based design of novel human Pin1 inhibitors (I). Bioorg. Med. Chem. Lett. 19, 5613–5616 [DOI] [PubMed] [Google Scholar]
- 35. Uría J. A., Jiménez M. G., Balbín M., Freije J. M., López-Otín C. (1998) Differential effects of transforming growth factor-β on the expression of collagenase-1 and collagenase-3 in human fibroblasts. J. Biol. Chem. 273, 9769–9777 [DOI] [PubMed] [Google Scholar]
- 36. Unemori E. N., Pickford L. B., Salles A. L., Piercy C. E., Grove B. H., Erikson M. E., Amento E. P. (1996) Relaxin induces an extracellular matrix-degrading phenotype in human lung fibroblasts in vitro and inhibits lung fibrosis in a murine model in vivo. J. Clin. Invest. 98, 2739–2745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Murakami G., Watabe T., Takaoka K., Miyazono K., Imamura T. (2003) Cooperative inhibition of bone morphogenetic protein signaling by Smurf1 and inhibitory Smads. Mol. Biol. Cell 14, 2809–2817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jeon H. S., Dracheva T., Yang S. H., Meerzaman D., Fukuoka J., Shakoori A., Shilo K., Travis W. D., Jen J. (2008) SMAD6 contributes to patient survival in non-small cell lung cancer and its knockdown reestablishes TGF-β homeostasis in lung cancer cells. Cancer Res. 68, 9686–9692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fan G., Fan Y., Gupta N., Matsuura I., Liu F., Zhou X. Z., Lu K. P., Gélinas C. (2009) Peptidyl-prolyl isomerase Pin1 markedly enhances the oncogenic activity of the Rel proteins in the nuclear factor-κB family. Cancer Res. 69, 4589–4597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Glesne D., Huberman E. (2006) Smad6 is a protein kinase X phosphorylation substrate and is required for HL-60 cell differentiation. Oncogene 25, 4086–4098 [DOI] [PubMed] [Google Scholar]