Background: Regulatory mechanisms of adult neurogenesis are not clearly defined.
Results: Extracellular signal-regulated Kinase 5 is specifically expressed in adult neurogenic regions, and is critical for adult hippocampal neurogenesis.
Conclusion: ERK5 signaling regulates adult hippocampal neurogenesis, a process that may be mediated through Neurogenin 2.
Significance: Identification of signaling pathways involved in adult neurogenesis contributes toward delineating the molecular mechanisms regulating adult neurogenesis.
Keywords: Cell Signaling, ERK, MAP Kinases (MAPKs), Neurogenesis, Signal Transduction, Adult Neurogenesis
Abstract
Recent studies have led to the exciting idea that adult-born neurons in the dentate gyrus of the hippocampus may play a role in hippocampus-dependent memory formation. However, signaling mechanisms that regulate adult hippocampal neurogenesis are not well defined. Here we report that extracellular signal-regulated kinase 5 (ERK5), a member of the mitogen-activated protein kinase family, is selectively expressed in the neurogenic regions of the adult mouse brain. We present evidence that shRNA suppression of ERK5 in adult hippocampal neural stem/progenitor cells (aNPCs) reduces the number of neurons while increasing the number of cells expressing markers for stem/progenitor cells or proliferation. Furthermore, shERK5 attenuates both transcription and neuronal differentiation mediated by Neurogenin 2, a transcription factor expressed in adult hippocampal neural progenitor cells. By contrast, ectopic activation of endogenous ERK5 signaling via expression of constitutive active MEK5, an upstream activating kinase for ERK5, promotes neurogenesis in cultured aNPCs and in the dentate gyrus of the mouse brain. Moreover, neurotrophins including NT3 activate ERK5 and stimulate neuronal differentiation in aNPCs in an ERK5-dependent manner. Finally, inducible and conditional deletion of ERK5 specifically in the neurogenic regions of the adult mouse brain delays the normal progression of neuronal differentiation and attenuates adult neurogenesis in vivo. These data suggest ERK5 signaling as a critical regulator of adult hippocampal neurogenesis.
Introduction
Adult neurogenesis occurs in the dentate gyrus of mammalian brains, including the human brain (1–4). Adult-born neurons functionally integrate into the hippocampal circuitry (5–11), suggesting that adult neurogenesis may contribute to neuroplasticity. This idea is supported by the observation that hippocampus-dependent, but not hippocampus-independent learning increases the number of adult-born neurons in the dentate gyrus (12–14). Despite the interest in the physiological roles of adult-born neurons, mechanisms regulating adult neurogenesis have not been fully elucidated.
ERK5 is a member of the mitogen-activated protein (MAP)2 kinase family that includes ERK1/2, p38, and JNK (15, 16). It is specifically phosphorylated and activated by MEK5 (15, 17). MEK5 is specific for ERK5 and does not phosphorylate ERK1/2, JNK, or p38 even when overexpressed (15, 17). ERK5 is activated by neurotrophins (NT) through MEK5, which promotes the survival of newborn neurons during embryonic development (18–23). Furthermore, ERK5 specifies cortical stem/progenitor cells toward a neuronal lineage during development by phosphorylating and modulating the activity of neurogenin (Neurog) 1 (24, 25). ERK5 expression in the brain is developmentally regulated; it is high during early embryonic development but declines postnatally as the brain matures (21). Interestingly, although there is very little ERK5 expression throughout the adult brain (26), upon closer examination we report here that ERK5 is prominently expressed in the two adult neurogenic regions: the subgranular zone (SGZ) of the dentate gyrus and the subventricular zone (SVZ) along the lateral ventricles. This unique pattern of expression suggests a fundamentally important role for ERK5 in regulating adult neurogenesis.
In this study, we have characterized the cell types expressing ERK5 along the SGZ of the dentate gyrus. To investigate a role for ERK5 in the regulation of adult neurogenesis, we utilized RNAi and transgenic mouse technologies to inhibit ERK5 expression as well as retroviral expression of a constitutively active (ca) MEK5 to stimulate ERK5 both in vitro and in vivo. Our data suggest a critical role for ERK5 in the regulation of adult hippocampal neurogenesis.
EXPERIMENTAL PROCEDURES
Animals
The generation of Nestin-CreERTM (27) mice, ERK5loxP/loxP (28) mice, and Nestin-CreERTM/ERK5loxP/loxP mice (29) have been described. A small cohort of Nestin-CreERTM mice were also bred with Gt(ROSA)26Sor-YFP (R26-YFP) mice (30) to yield Nestin-CreERTM/R26-YFPloxP/loxP mice. All animal experiments were performed with identically treated littermate controls. Animals were housed under standard conditions (12 h light/dark cycle) with food and water provided ad libitum. All experimental procedures were approved by the University of Washington Institutional Animal Care and Use Committee.
Reagents
The following plasmids have been described. The NeuroD2-Luc reporter (pCS2-NeuroD2-Luc) was obtained from Dr. Jim Olson (31), and cDNA sequence for mouse Neurog2 in pcDNA3 from Dr. Jane Johnson (University of Texas at Southwestern Medical Center). A FLAG sequence was inserted at the N terminus of the Neurog2 cDNA. The following primary antibodies and dilutions were used for immunohistochemistry: rat monoclonal anti-BrdU (1:500, AbD Serotec); mouse monoclonal antibodies against PCNA (1:500, Millipore), Sox2 (1:200, R&D Systems), GFAP (1:500, Millipore), NCAM (1:200, Developmental Studies Hybridoma Bank), NeuN (1:500, Millipore), Calretinin (1:200, Abcam), and Calbindin (1:200, Abcam); goat polyclonal antibodies against NeuroD (1:200, Santa Cruz Biotechnology Inc.) and DCX (1:200, Santa Cruz Biotechnology Inc.); and rabbit polyclonal antibody against GFP (1:500, Invitrogen). Rabbit polyclonal ERK5 antibody (1:500 dilution) was generated previously (18) and affinity purified using recombinant MBP-ERK5 protein. The following primary antibodies and dilutions were used for immunocytochemistry: mouse monoclonal antibodies against GFP (1:5,000, Invitrogen), Nestin (1:500, Developmental Studies Hybridoma Bank), Sox2 (1:500, R&D Systems), and β-III tubulin (1:500, Promega); and rabbit polyclonal antibodies against PCNA (1:500, Millipore) and GFP (1:5,000, Invitrogen). The following primary antibodies and dilutions were used for Western blot analysis: rabbit polyclonal ERK5 antiserum (1:1,000), rabbit polyclonal MEK5 antibody (1:500, Santa Cruz Biotechnology Inc.), rabbit polyclonal ERK1/2 (1:10,000, Millipore), rabbit polyclonal p-ERK5 antibody (1:1,000, Cell Signaling), and mouse monoclonal β-actin antibody (1:10,000, Sigma). Secondary antibodies were rabbit polyclonal horseradish peroxidase (HRP) antibody (1:10,000, Calbiochem) and mouse monoclonal HRP antibody (1:20,000, Calbiochem).
BrdU and Tamoxifen Administration
Mice were treated with 100 mg/kg of BrdU (Sigma) by intraperitoneal injection 5 times (every 2 h for 10 h) in 1 day followed by sacrifice 4 weeks later to identify BrdU-retaining, adult-born cells. Tamoxifen (Sigma) was made fresh daily and dissolved in 2% glacial acetic acid in corn oil solution (Sigma). To activate Cre-mediated recombination, 5 mg of pre-warmed tamoxifen was administered orally to 10–12-week-old male mice daily for 7 days (Fig. 9, A and B) or once per day for 4 day in each cycle, for 3 cycles with 2-week inter-cycle intervals (Fig. 9, C–M).
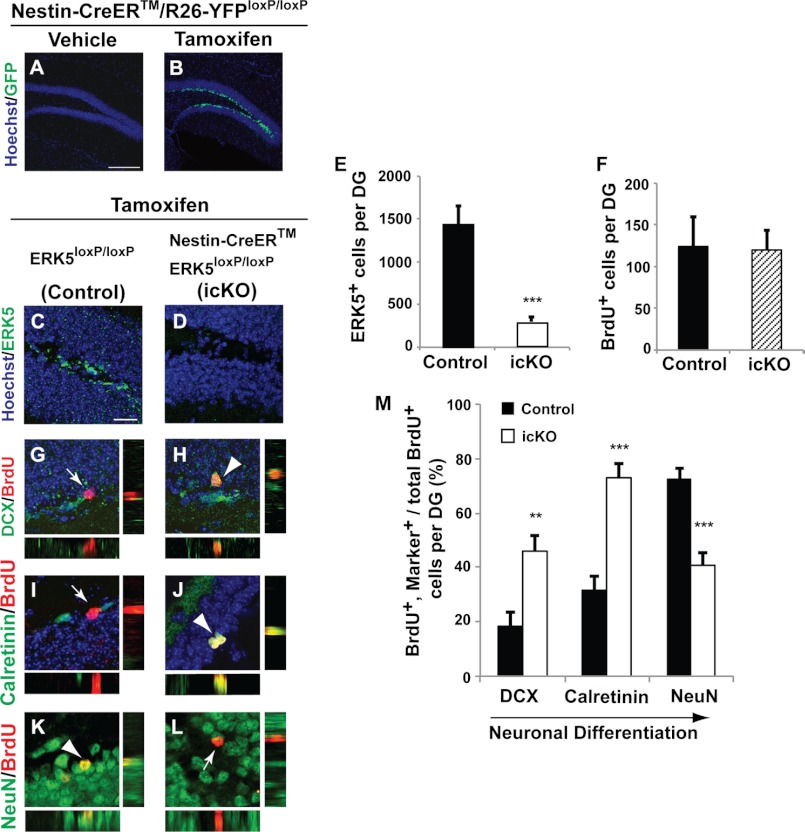
FIGURE 9.
Conditional deletion of ERK5 in adult neurogenic regions attenuates neuronal differentiation and SGZ neurogenesis. A and B, YFP immunostaining of Nestin-CreER/R26-YFPloxP/loxP reporter animals treated with tamoxifen or vehicle control, demonstrating the specificity and effectiveness of Nestin-Cre-ER-mediated recombination. C and D, representative confocal images of ERK5 immunostaining (green) in the SGZ for tamoxifen-treated control (ERK5loxP/loxP) or ERK5 inducible and conditional knock-out (icKO) mice (Nestin-CreER/ERK5loxP/loxP). Hoechst staining (blue) was used to visualize all nuclei. E, quantification of total ERK5+ cells per dentate gyrus. The number of ERK5+ cells in the SGZ was reduced in ERK5 icKO mice. F, quantification of total BrdU+ cells per dentate gyrus 4 weeks following BrdU administration. G–L, representative confocal images of immunostaining in the SGZ of control and ERK5 icKO mice for BrdU (red) and DCX (green) (G and H), Calretinin (green) (I and J), or NeuN (green) (K and L). Cells co-labeled with BrdU and DCX, Calretinin, or NeuN are yellow (arrowheads). Side panels to G–L are orthographic views of the corresponding cells in the main panels. Arrows point to BrdU+ cells that are negative for DCX, Calretinin, or NeuN. M, ERK5 gene deletion increases the total number of immature adult-born neurons (DCX and BrdU double-positive; Calretinin and BrdU double-positive cells among total BrdU+ cells) while concomitantly decreasing the total number of adult-born mature neurons (BrdU and NeuN double-positive cells among total BrdU+ population) along the SGZ. Scale bar in panel A represents 100 μm and applies to B, whereas the scale bar in panel C represents 25 μm and applies to D–L.
Immunohistochemistry
Brains were post-fixed in 4% paraformaldehyde in PBS overnight at 4 °C after standard intracardial perfusion procedures. Brains were then placed in 30% (w/v) sucrose in PBS at 4 °C until brains sunk and the brains were immediately frozen at −80 °C. Immunohistochemistry was performed on 30-μm thick coronal brain sections using a free-floating antibody staining method as described (29).
Immunocytochemistry
Cells were fixed in PBS containing 4% paraformaldehyde and 4% sucrose at room temperature for 30 min. Fixed cells were washed 3 × 5 min in PBS, 5 min in 1% SDS, and washed again 3 × 5 min in PBS. Cells were then incubated in blocking buffer consisting of 5% bovine serum albumin (BSA) in PBST (PBS + 0.1% Triton X-100) for 2 h, followed by incubation with primary antibodies overnight at 4 °C. Cells were then washed 3 × 10 min in PBST, followed by incubation with secondary antibodies at 1:5,000 dilution (Alexa Fluor-488) or 1:2,000 dilution (Alexa Fluor-594) for 2 h in blocking buffer. Cells were then washed 3 × 10 min in PBST followed by a 10-min incubation in Hoechst 33342 for nuclei visualization and a final wash of 10 min in PBST prior to mounting onto slides using anti-fade Aqua Poly/Mount solution. Unless otherwise stated, all steps were carried out at room temperature.
Confocal Imaging and Analysis
All images were captured with an Olympus Fluoview-1000 laser scanning confocal microscope with numerical aperture 0.75, ×20 lens or numerical aperture 1.3, ×40 oil immersion lens. Optical Z-sections (0.5–1 μm) were collected and processed using ImageJ software (NIH). Images were uniformly adjusted for color, brightness, and contrast with Adobe Photoshop CS4 (Adobe Systems Inc).
Quantification of Immunostained Cells
Greater than 100 immunopositive cells per coverslip per experiment were quantified using an inverted fluorescence microscope (Leitz DMIRB, Leica) with a ×40 objective (Leica) following immunocytochemistry. A modified optical fractionator method was used as an unbiased stereological method for obtaining an estimation of total cell counts per SGZ following immunohistochemistry (32–34). The method for in vivo cellular quantification and co-localization analysis per SGZ was as described (29).
SGZ-derived Adult Neural Progenitor Cell (aNPCs) Cultures
Primary cell cultures were prepared as described (35, 36). Briefly, tissue samples containing the dentate gyrus were micro-dissected and enzymatically digested with 0.1% trypsin-EDTA (Invitrogen) for 7 min at 37 °C followed by incubation with equal volume of 0.014% trypsin inhibitor (Invitrogen). Tissue samples were then spun down and resuspended in culture media consisting of DMEM/F-12 (Invitrogen), 1× N2 supplement (Invitrogen), 1× B27 supplement without retinoic acid (Invitrogen), 100 units/ml of penicillin/streptomycin (Invitrogen), 2 mm l-glutamine (Invitrogen), 2 μg/ml of heparin (Sigma), 20 ng/ml of EGF (EMD Chemicals), and 10 ng/ml of bFGF (Millipore). Culture medium for adult neural progenitor cells always contain EGF and bFGF unless otherwise specified. Tissue was mechanically triturated and filtered through a 40-μm cell sieve and plated in Petri dishes and cultured for 10–14 days until neurospheres are formed. Growth factors were replenished every 3 days during this period. Following primary passage, neurospheres were isolated, dissociated into single-cell suspension enzymatically and mechanically, and replated at low density and cultured for secondary neurosphere formation. Spheres collected from secondary passage were dissociated and plated as a monolayer culture on poly-d-lysine/laminin- (BD Biosciences) or poly-l-ornithine/fibronectin (BD Biosciences)-coated aclar coverslips (Electron Microscopy Sciences) for experiments.
Retrovirus Construction and Production
The ERK5 shRNA and control nonspecific shRNA (shNS) retroviruses have been previously described (25). Briefly, shNS directed toward the dsRED sequence (agttccagtacggctccaa), and shERK5 directed toward the murine ERK5 sequence (amino acids 106–111: acacttcaaacacgacaat), were subcloned into pSIE retroviral vector (37). cDNA sequences encoding wild-type ERK5 or constitutively active MEK5 (24, 38) were subcloned into the SalI/XhoI restriction sites within the multiple-cloning site of an oncoretroviral expression vector, which contains an IRES-GFP sequence and was described in Ref. 39. High-titer VSV-G pseudotyped retroviral stocks were produced as described (37).
Characterization of Retroviral ERK5 or caMEK5
NIH-3T3 cells were plated in 6-well tissue culture-treated plates at 5 × 104 cells per well in DMEM (Invitrogen) containing 10% fetal bovine serum and 100 units/ml of penicillin/streptomycin. Following overnight plating, protamine sulfate (Invitrogen) was added to culture media at a final concentration of 8 μg/ml and 8 μl of 1 × 109 infection units per ml (IU/ml) of retroviruses were added to each well and allowed to transduce cells for 24 h. Where co-transduction was required, a 1:1 ratio of retroviral ERK5 and caMEK5 were added to each well and cells were transduced for 24 h. Following a 24-h transduction, media was refreshed and cells were cultured for an additional 3 days before processing for Western blot analysis as described (21).
Viral Transduction of aNPCs
For Western blot analysis of shERK5 specificity, aNPCs were plated as a monolayer culture on poly-l-ornithine/fibronectin-coated, 12-well tissue culture plates at a density of 3 × 105 cells per well in culture media as described above. Twenty-four hours after plating, protamine sulfate was added to the culture media at a final concentration of 8 μg/ml, and cells were infected with 20 μl of 1 × 109 IU/ml of shNS and shERK5 retroviruses. Four days following retrovirus infection, cells were lysed for Western blot analysis as described (21). For immunocytochemistry studies, aNPCs were plated as a monolayer culture on poly-d-lysine/laminin- or poly-l-ornithine/fibronectin-coated aclar coverslips in 24-well plates at a density of 1 × 105 cells per well in culture media. Twenty-four hours after plating, protamine sulfate was added to culture media at a final concentration of 8 μg/ml and 6–8 μl of 1 × 109 IU/ml of retroviruses were added to each well and allowed to transduce cells for 24 h. In cases where co-transduction with retrovirus and lentivirus were needed (Fig. 6), aNPCs were first transduced with retrovirus for 10 h followed by transduction with lentivirus for an additional 24 h at a ratio of 3:1, respectively. Following transduction, culture media was changed and cells were cultured for an additional 5 days before being processed for immunocytochemistry.
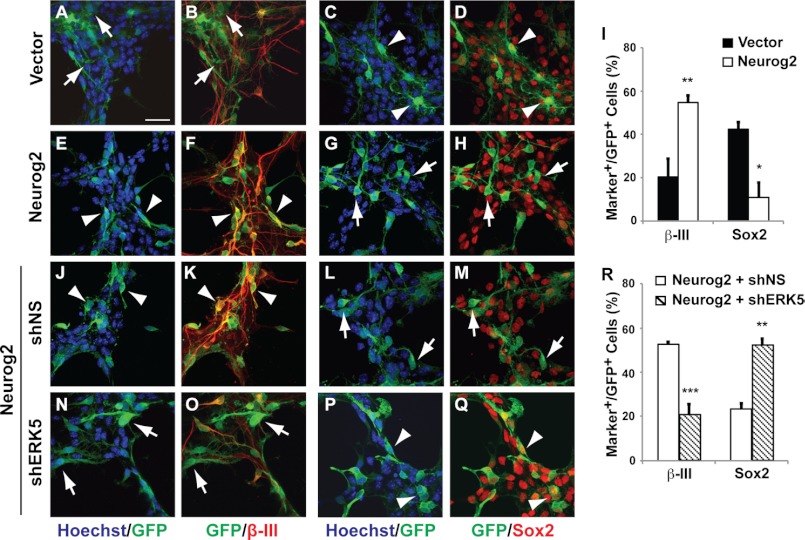
FIGURE 6.
Neurog2 confers ERK5-dependent pro-neural activity in SGZ-derived aNPCs. SGZ-derived aNPCs were infected with control retroviral and lentiviral vectors expressing eGFP only (A–D), lentiviral Neurog2 only (E–H), and lentiviral Neurog2 together with control retroviral shNS (J–M) or shERK5 (N–Q). One day after virus infection, cells were washed and then maintained in regular EGF- and bFGF-containing medium for 5 days before co-immunostaining for GFP (green) and various cellular markers (red) including β-III tubulin or Sox2 as indicated. The percentage of GFP+ cells that also express β-III tubulin or Sox2 were quantified (I and R). Scale bar in A represents 25 μm and applies to all images. Arrowheads point to GFP+ co-labeled cells, whereas arrows point to GFP+ cells that are not co-labeled with cell-type specific markers.
Neurotrophin Treatment
For neurotrophin activation of ERK5, aNPCs were plated as a monolayer on poly-l-ornithine/fibronectin-coated 12-well plates at a density of 7 × 105 cells per well. Forty-eight hours after plating, cells were switched into culture medium free of EGF and bFGF overnight before treatment with brain-derived neurotrophic factor (BDNF) (50 ng/ml, Alomone Labs) or NT3 (100 ng/ml, Millipore). For neurotrophin stimulation of aNPC neuronal differentiation, cells were plated as a monolayer on poly-l-ornithine/fibronectin-coated aclar coverslips at a density of 1 × 105 cells per well. One day after plating, cells were transduced with 8 μl of shNS or shERK5 retrovirus in the presence of 8 μg/ml of protamine sulfate. Twenty-four hours later, the virus-containing medium was removed and cells were cultured with fresh medium for 3 days to allow retroviral expression. Cells were then incubated for 3 days in fresh medium containing 100 ng/ml of NT3, or 1 μg/ml of BSA as a control. Finally, EGF and bFGF were removed from the culture medium and cells were incubated for an additional 5 days in the continued presence of NT3 or BSA to allow neuronal differentiation.
To examine the effect of shERK5 on more differentiated aNPCs, cells were plated as a monolayer at a density of 1 × 105 cells per well on poly-l-ornithine/fibronectin-coated aclar coverslips in medium containing 50 ng/ml of BDNF or 100 ng/ml of NT3 for 3 days. EGF and bFGF were then removed from the culture medium and cells were incubated for an additional 3 days in the continued presence of BDNF or NT3. Cells were then transduced with shNS or shERK5 retroviruses as above and incubated for an additional 5 days in culture medium free of EGF and bFGF but in the continued presence of BDNF or NT3.
Lentiviral Neurog2 Transfer Construct (pRRL-cPPT-CMV-Neurog2-PRE-SIN-IRES-EGFP)
The FLAG-Neurog2 cDNA sequence was inserted into a multiple cloning site of lentiviral transfer vector pRRL-cPPT-CMV-X-PRE-SIN-IRES-EGFP, described in Ref. 24, upstream from the IRES-directed marker protein eGFP (enhanced green fluorescent protein). High-titer lentiviral stocks were produced as described (24).
NeuroD2-Luciferase Reporter Gene Assay
Primary cortical neurons were prepared from embryonic day 15 (E15) Sprague-Dawley rats (Charles River Laboratories) and cultured in Petri dishes for 5 h before transfection. Cells were transiently transfected with Nucleofector® Transfection Reagent (Amaxa Biosystems, Inc.) as previously described (25). Briefly, E15 cortical neurons were collected and resuspended in Rat Neural Stem Cell Nucleofector® Transfection Reagent at a density of 6 × 106 cells/100 μl. For each transfection, 6 × 106 cells were transfected with 5 μg of NeuroD2-Luc reporter, 100 ng of pRL Renilla Luc reporter (Promega), 1 μg of FLAG-Neurog2 expression construct or pCDNA3 control plasmid, and 4 μg of shERK5 retroviral plasmid or shNS control plasmid using the Amaxa Nucleofector® with A31 protocol. Immediately following Nucleofection, cells were resuspended in pre-warmed (37 °C) regular culture medium and incubated at 37 °C for 20 min. Cells were then resuspended in regular culture medium (Neurobasal Medium (Invitrogen), 2% B27 without retinoic acid, 10 ng/ml of bFGF) and plated onto 12-well plates coated with poly-d-lysine/laminin. After 72 h in culture, cells from each well were lysed with 100 μl of passive lysis buffer and 20 μl of lysates were applied for dual luciferase assay per the manufacturer's protocol (Promega).
Stereotaxic Surgery
The stereotaxic procedure was performed on adult C57/BL6 male mice (8–10 weeks old, Charles River Laboratories) as described (40, 41). Mice were anesthetized by intraperitoneal injection (21–23 μl/g of body weight) of ketamine (7.0 mg/ml) and xylazine (0.44 mg/ml) dissolved in 0.9% bacteriostatic saline (Hospira, Inc.). One microliter of retrovirus (109–1010 IU/ml) was injected at a rate of 0.25 ml/min bilaterally into the dentate gyrus with the following coordinates relative to Bregma: 1.65 mm posterior, ±1.62 mm medial-lateral, and 2.30 mm ventral.
Statistical Analysis
All of the in vitro cell culture data were from at least two independent experiments with duplicates or triplicates each (total n ≥ 5 for each data point). In vivo cellular quantification data were from at least two independent experiments with n ≥ 12 for data in Fig. 8 and n ≥ 6 for data in Fig. 9. Pairwise comparison of the means was analyzed by Student's t test, two-tailed analysis for data presented in Figs. 3, 4, 6, 7, and 9. One-way analysis of variance with Fisher's LSD post hoc analysis was performed to analyze data presented in Figs. 5 and 8. Data represent mean ± S.E., n.s. not significant; *, p < 0.05; **, p < 0.01; ***, p < 0.001.
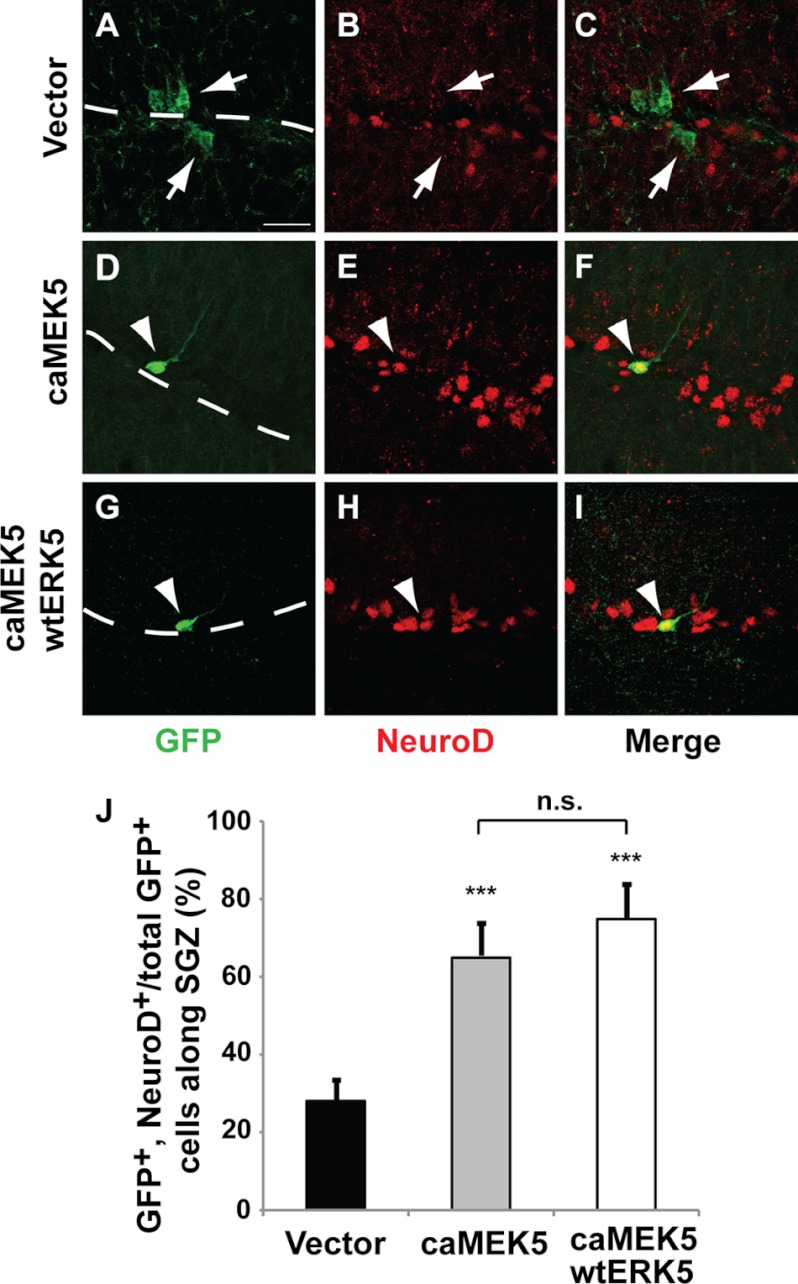
FIGURE 8.
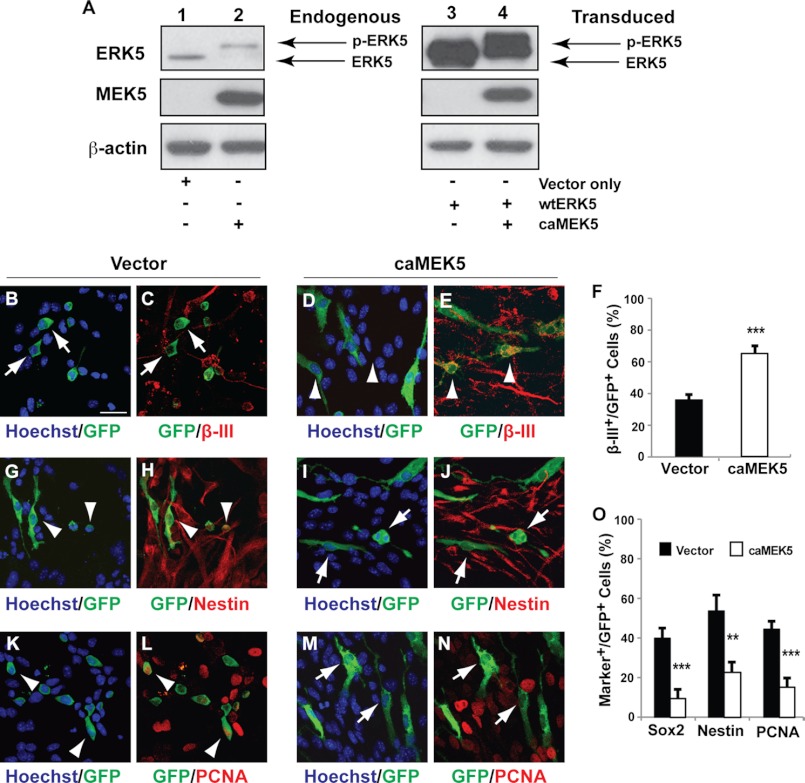
Ectopic activation of ERK5 promotes SGZ neurogenesis in vivo. Retroviral eGFP control (vector) (A–C), caMEK5-IRES-eGFP alone (D–F), or together with wtERK5-IRES-eGFP (G–I) was stereotaxically injected into the dentate gyrus of 8–10-week-old mice. Mice were sacrificed 2 weeks later and brain sections were immunostained for GFP (green) or NeuroD (red). Scale bar in A represents 25 μm and applies to all images. Dashed lines outline the SGZ layer of the dentate gyrus. Arrowheads point to GFP+/NeuroD+ co-labeled cells, whereas arrows point to GFP+ cells that are negative for NeuroD. J, the percentage of GFP+ and NeuroD+ co-labeled cells along the SGZ was quantified. Expression of caMEK5 alone or together with wtERK5 greatly increases the number of NeuroD+ cells in the total GFP+ population.
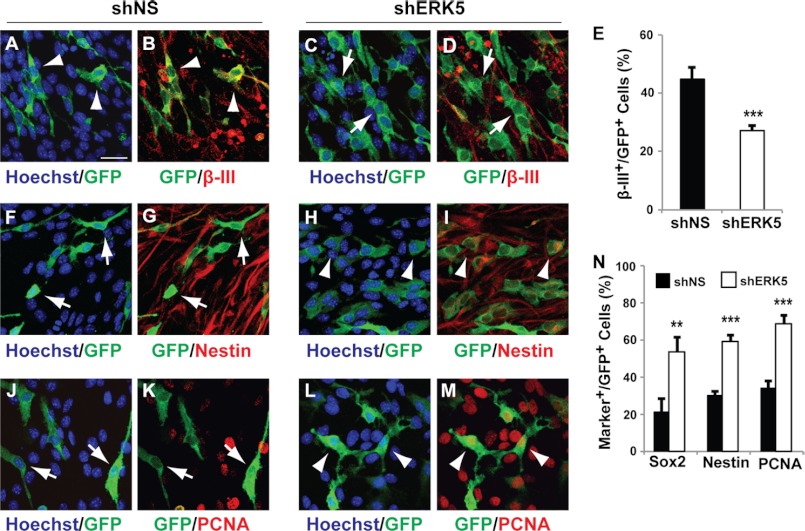
FIGURE 3.
ERK5 signaling is necessary for promoting neurogenesis of SGZ-derived aNPCs in culture. aNPCs were infected with nonspecific shRNA control retroviral vector (shNS) or shRNA to ERK5 retrovirus as indicated. Both retroviral vectors encode eGFP marker protein under a bicistronic promoter. One day after virus infection, cells were washed and then incubated in culture medium free of EGF and bFGF for 5 days to allow spontaneous differentiation. Cells were then fixed for immunocytochemistry and co-stained for GFP to identify virus-infected cells (green) and various cell-type specific markers (red). A–D, co-staining of GFP with β-III tubulin. E, quantification of the percentage of GFP+ cells that are also β-III tubulin+. F–I, immunostaining of GFP+-infected cells and Nestin+ neural stem cells. J–M, immunostaining of GFP+-infected cells and PCNA+ proliferating cells. N, quantification of the percentage of GFP+ cells that also express Sox2, Nestin, or PCNA. Scale bar in A represents 25 μm and applies to all images. Arrowheads point to GFP+ cells co-labeled with various cell markers, whereas arrows point to GFP+ cells that are marker negative.
FIGURE 4.
Activation of endogenous ERK5 signaling is sufficient to promote neurogenesis of dentate gyrus-derived aNPCs in culture. A, anti-ERK5 and MEK5 Western analysis of NIH-3T3 cells infected with retroviruses expressing caMEK5 and/or wild-type (wt−) ERK5. Cells infected with GFP retrovirus (vector only) were used as controls. β-Actin was used as a loading control. Expression of caMEK5 caused reduced electrophoretic mobility of endogenous ERK5 (lanes 1 and 2) as well as the co-infected wtERK5 (lanes 3 and 4), indicative of ERK5 phosphorylation (p-ERK5) and activation. These data ascertain the constitutive active nature of the caMEK5 virus. B–O, aNPCs were infected with control retroviral vector expressing eGFP only or expressing caMEK5-IRES-eGFP. One day after virus infection, cells were washed and then maintained in regular EGF- and bFGF-containing medium for 5 days before co-immunostaining for GFP (green) and various cellular markers (red) including β-III tubulin (B–E), Nestin (G–J), and PCNA (K–N). The percentage of GFP+ cells that also express β-III tubulin (F), Sox2, Nestin, or PCNA (O) was quantified. Scale bar in B represents 25 μm and applies to all images. Arrowheads point to GFP+ cells co-labeled with various cell markers, whereas arrows point to GFP+ cells that are marker negative.
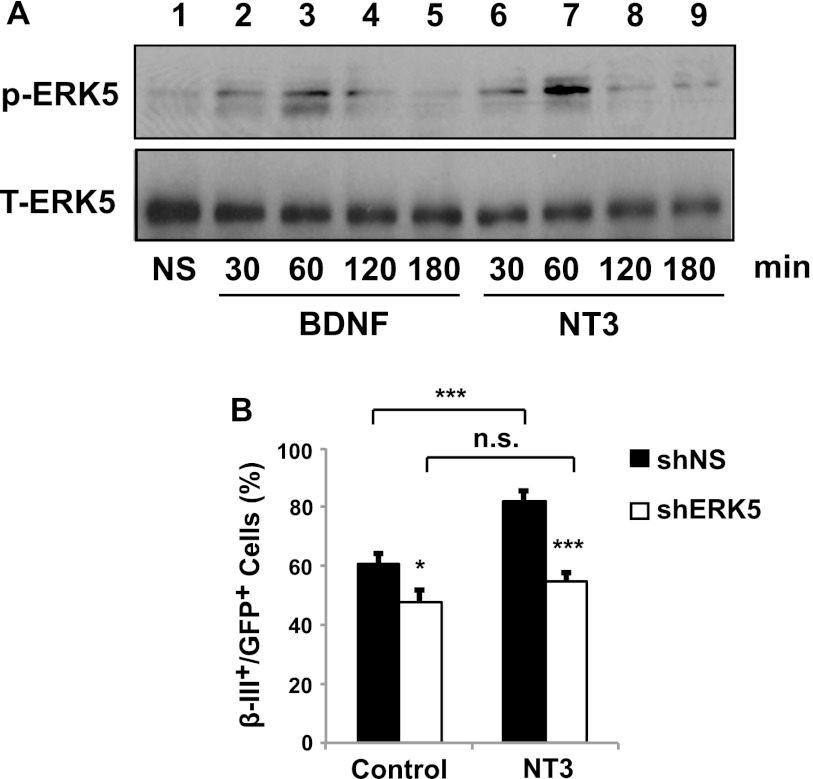
FIGURE 7.
NT3 activates ERK5 and stimulates neuronal differentiation of SGZ-derived aNPCs through ERK5. A, BDNF and NT3 induce ERK5 phosphorylation, indicative of ERK5 activation. SGZ-derived aNPCs were treated with vehicle control (NS, lane 1), 50 ng/ml of BDNF (lanes 2–5), or 100 ng/ml of NT3 (lanes 6–9) for the indicated times. Total ERK5 (T-ERK5) was used as a loading control. B, NT3 stimulates neuronal differentiation of SGZ-derived aNPCs through a process that requires ERK5. SGZ-derived aNPCs were infected with retroviruses encoding a nonspecific control (shNS) or shERK5. Cells were then incubated in EGF/bFGF-free medium and treated with NT3 (100 ng/ml) for 5 days to induce neuronal differentiation. Cells treated with BSA (1 μg/ml) were used as a control.
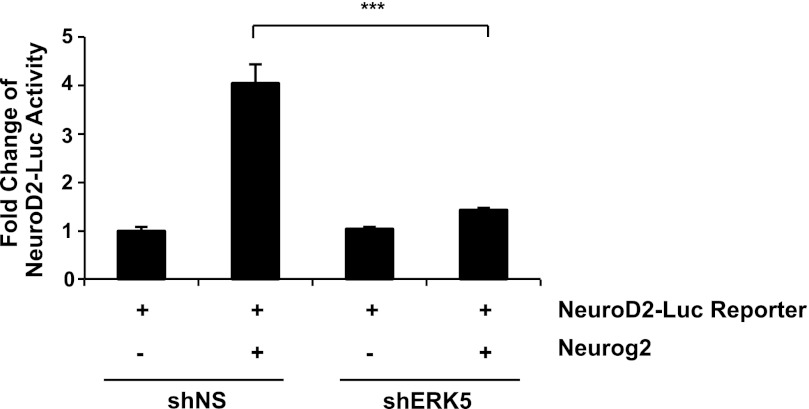
FIGURE 5.
Neurog2 transcriptional activity requires ERK5 activity. Rat embryonic day 15 primary cortical cells were transfected with plasmids for NeuroD2-Luc reporter only, or together with a Neurog2 expression vector in pcDNA, shERK5 retroviral plasmid or shNS control plasmid.
RESULTS
ERK5 Expression in the Adult Mouse Brain Is Specific to the Neurogenic Regions
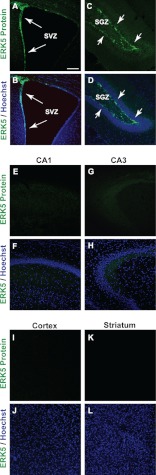
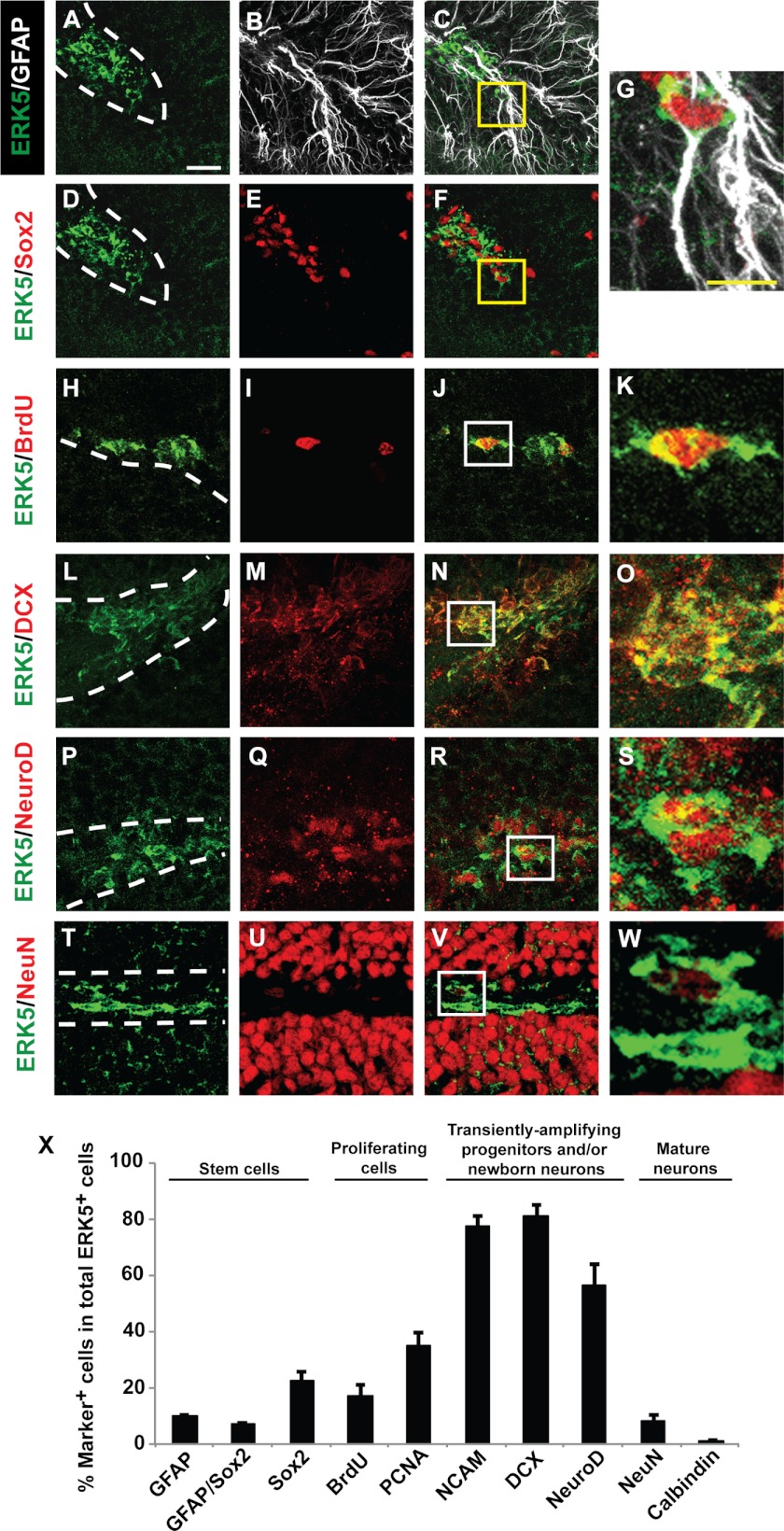
ERK5 expression in the adult mouse brain was examined by immunohistochemistry using an affinity-purified ERK5-specific antibody directed against the unique C-terminal tail of ERK5 protein (18). We found no ERK5 protein in cornu ammonis (CA) 1 and CA3 regions of the hippocampal formation, or most other areas of adult brain including the cortex and striatum (Fig. 1), consistent with other reports (26). However, ERK5 protein was specifically expressed in the SVZ (Fig. 1, A and B) and along the SGZ of the dentate gyrus in the hippocampal formation (Fig. 1, C and D). Specifically, ERK5 was expressed in SGZ cells co-labeled with markers for stem/progenitor cells (Sox2, GFAP), proliferation (BrdU, PCNA), transiently amplifying progenitors and/or newborn neurons (PSA-NCAM, DCX, and NeuroD) (Fig. 2, A–S and X). Some of the ERK5+ cells were also positive for both GFAP and Sox2, suggesting ERK5 expression in radial glia-like stem cells (Fig. 2, A–G and X). However, very few ERK5+ cells co-expressed NeuN, a marker for mature neurons. Of the few NeuN+ cells that were also ERK5+, the NeuN staining intensity was much lower than that in NeuN+/ERK5− cells (Fig. 2, T–W). Thus, these ERK5+/NeuN+ cells likely represent cells just beginning to express NeuN and are still in the early stage of terminal differentiation. This is consistent with the fact that none of the ERK5+ cells co-express calbindin, a marker for mature granule neurons (Fig. 2X). Finally, almost 80% of ERK5+ cells in the SGZ were also PSA-NCAM+ and DCX+. The specific expression of ERK5 in adult neurogenic regions is quite unique and interesting, and suggests an important function for ERK5 in regulating adult neurogenesis, particularly for the regulation of the cell fate of transiently amplifying progenitors and/or newborn neuron populations.
FIGURE 1.
ERK5 MAPK expression in the adult mouse brain is restricted to the adult neurogenic regions. Images are representative immunostaining of coronal sections of adult mouse brain tissue showing ERK5 protein expression (green) primarily in the SVZ (A and B) and SGZ (C and D) but not in CA1 or CA3 of the hippocampal formation (E–H), the cortex (I and J), or striatum (K and L). Hoechst staining (blue) was used to identify all cell nuclei (B, D, F, H, J, and L). Scale bar in A represents 100 μm and applies to all panels.
FIGURE 2.
ERK5 is primarily expressed in transiently amplifying progenitors and/or newborn neurons in the SGZ. A–W, representative confocal images of brain sections immunostained for ERK5 (green), GFAP (A–C and G) (white), and various other cellular markers (red) including Sox2 (D–G), BrdU (H–K), doublecortin (DCX) (L–O), NeuroD (P–S), and NeuN (T–W). Panel G represents the enlarged boxed areas in panels C and F. Similarly, images in panels K, O, S, and W are enlarged images of their corresponding boxed areas in panels J, N, R, and V. Scale bar in A represents 25 μm and applies to all images except the enlarged ones. Scale bar in G represents 10 μm and applies to K, O, S, and W. Dashed lines outline the SGZ layer of the dentate gyrus. X, quantification of marker-positive cells in total ERK5+ cell population along the SGZ. Data represent mean percentage of double- or triple-labeled cells.
ERK5 Signaling Contributes to Neuronal Differentiation of SGZ-derived aNPCs in Culture
SGZ-derived aNPCs were prepared from the dentate gyrus of 8–10-week-old adult mice as described (35, 42). Western analysis confirmed ERK5 expression in these cells (supplemental Fig. S1). When aNPCs were allowed to differentiate in culture by removing bFGF and EGF from the culture media, retroviral infection of ERK5 shRNA, which specifically suppresses the expression of endogenous ERK5 but not the closely related ERK1/2 (Ref. 25 and supplemental Fig. S1), significantly decreased the number of cells expressing β-III tubulin, a marker for newborn neurons (Fig. 3, A–E). Concomitantly, shRNA to ERK5 increased the number of cells expressing markers for stem/progenitor cells (Sox2, Nestin) and proliferation (PCNA) (Fig. 3, F–N). However, it did not promote glial differentiation (33 versus 26% cells co-expressed glial marker GFAP in shNS- or shERK5-infected cells, respectively, p > 0.5).
To activate endogenous ERK5 signaling, wild type (wt) ERK5 or caMEK5 were subcloned into a retroviral expression vector, upstream from an IRES-directed marker protein eGFP. Retroviral transduction of caMEK5 specifically activated endogenous ERK5 (Fig. 4A) and was sufficient to decrease the number of cells positive for Sox2, Nestin, and PCNA, whereas simultaneously increasing the pool of β-III tubulin+ neurons even in the presence of mitogens bFGF and EGF (Fig. 4, B–O). These data suggest a critical role for ERK5 in promoting neurogenesis in cultured aNPCs. Furthermore, aNPCs that did not differentiate into neurons upon ERK5 inhibition remained in the proliferating and stem/progenitor stage rather than undergoing precocious glial differentiation (data not shown).
Neurog2 May Be a Downstream Target of ERK5 in Adult Neurogenesis
To elucidate downstream mechanisms mediating the neurogenic effect of ERK5 in SGZ cells, we investigated if ERK5 regulates the transcriptional activity of Neurog2, a basic helix-loop-helix transcription factor expressed in SGZ progenitors (43, 44). A dual luciferase reporter assay was performed to measure Neurog2-stimulated transcription initiated from the NeuroD2-Luc reporter (25). Expression of Neurog2 alone stimulated NeuroD2-Luc activity 4-fold; this transcription was suppressed by co-transfection of shERK5 (Fig. 5). Neurog2 is essential for neurogenesis in the dentate gyrus during development (45); however, its function in adult hippocampal neurogenesis has not been elucidated. Consequently, we examined if ectopic expression of Neurog2 is sufficient to promote neuronal differentiation of SGZ cells and if this is regulated by ERK5 signaling. SGZ-derived aNPCs were infected with lentiviruses expressing Neurog2-IRES-GFP; lentiviral-GFP was used as a control. Expression of Neurog2 was sufficient to increase the number of β-III tubulin+ neurons even in the presence of bFGF and EGF (Fig. 6, A–I). In contrast, Neurog2 decreased the number of Sox2+ neural stem cells. Significantly, co-infection of shERK5 retroviruses blocked the effect of Neurog2 on neuronal differentiation (Fig. 6, J–R). These data suggest that Neurog2 exhibits pro-neural activity in SGZ cells and may act as a downstream target of ERK5 during adult hippocampal neurogenesis.
NT3 Promotes Neuronal Differentiation of SGZ-derived aNPCs in an ERK5-dependent Manner
To begin to identify upstream activators of ERK5 signaling that regulate SGZ adult neurogenesis, we determined if ERK5 is activated by neurotrophins in aNPCs. Treatment with either BDNF or NT3 induced phosphorylation of ERK5 (p-ERK5) in SGZ-derived aNPCs (Fig. 7A), indicative of ERK5 activation. NT3 also increased the number of β-III tubulin+ neurons (Fig. 7B), suggesting that NT3 stimulates neuronal differentiation of these cells. Significantly, the effect of NT3 on neuronal differentiation was completely blocked by shERK5. Additionally, shERK5 inhibited neuronal differentiation when aNPCs were pretreated with BDNF or NT3 to prime neuronal differentiation (supplemental Fig. S2). These data suggest that NT3 stimulates hippocampal neurogenesis by activating the ERK5 signaling pathway.
Activation of Endogenous ERK5 Promotes SGZ Neurogenesis in Vivo
To investigate if activation of ERK5 promotes adult neurogenesis in vivo, we delivered retroviruses encoding caMEK5-IRES-eGFP and/or wtERK5-IRES-eGFP as well as the vector-control retrovirus to the dentate gyrus of adult mice using a stereotaxic surgery protocol (41). Two weeks following stereotaxic surgery, mice were sacrificed and their brains processed for immunohistochemistry to identify GFP+-infected cells and cells expressing NeuroD (Fig. 8, A–I). Retroviral expression of caMEK5 alone or together with wtERK5 in vivo significantly increased the number of NeuroD+ cells in the retrovirus-infected cell population (GFP+) along the SGZ (Fig. 8J). The fact that co-expression of wtERK5 with caMEK5 did not further increase the number of NeuroD+ cells relative to caMEK5 alone suggests that caMEK5 is sufficient to activate enough endogenous ERK5 to stimulate neurogenesis in vivo.
Inducible and Conditional Deletion of ERK5 in Adult Neurogenic Regions Reduces Adult Hippocampal Neurogenesis by Attenuating Neuronal Differentiation
To determine whether ERK5 signaling is required for adult neurogenesis in vivo, we utilized the inducible and conditional ERK5 knock-out (icKO) mice we recently generated (29). Tamoxifen administration into adult Nestin-CreER/ERK5loxP/loxP mice specifically deletes the erk5 gene in Nestin-expressing neural stem cells, thereby avoiding potential deleterious effects on the entire brain during embryonic development. Male Nestin-CreERTM/ERK5loxP/loxP and female ERK5loxP/loxP mice were mated to generate experimental animals (Nestin-CreERTM/ERK5loxP/loxP and ERK5loxP/loxP mice). The general health and overall appearance were not different among littermates before or after tamoxifen treatment.
To confirm the specificity of tamoxifen-induced, Nestin-CreER-mediated recombination, we crossed Nestin-CreERTM with R26-YFP reporter mice where Cre-mediated recombination removes a transcriptional STOP to allow YFP expression (30). Tamoxifen or vehicle was administered to Nestin-CreERTM/R26-YFPloxP/loxP mice, and animals were sacrificed 10 days after the last dose of tamoxifen. We observed no YFP expression in the mouse brain of vehicle control treated mice (Fig. 9A). In contrast, there were abundant YFP+ cells along the SGZ of tamoxifen-treated mouse brains (Fig. 9B). Furthermore, YFP+ cells in tamoxifen-treated mice were restricted to the adult neurogenic regions only (data not shown). These data suggest that Cre-ER-mediated recombination is specific to adult neurogenic regions and there is no discernible leakiness of the Cre-recombinase from the Nestin-CreERTM driver.
Tamoxifen was administered to male Nestin-CreERTM/ERK5loxP/loxP (ERK5 icKO) and ERK5loxP/loxP (control) mice to induce Cre-mediated recombination of erk5 in Nestin-expressing neural stem cells. To label adult-born cells, BrdU was administered 7 days after the last dose of tamoxifen treatment and mice were sacrificed 4 weeks later. Treatment with tamoxifen effectively decreased the number of ERK5+ cells in the SGZ of ERK5 icKO mice by 75% compared with control animals (Fig. 9, C–E). There was no difference in the total number of BrdU+ cells in the dentate gyrus of control versus ERK5 icKO mice (Fig. 9F). However, deletion of the erk5 gene reduced the number of adult-born, mature neurons (NeuN and BrdU double-positive cells among total BrdU+ population) in the dentate gyrus (Fig. 9, K–M). Concomitantly, there was an increase in the number of BrdU+ cells co-labeled with DCX or Calretinin, a marker for immature granular neurons (Fig. 9, G–J and M). This suggests that although ERK5 deletion does not change the total number of adult-born cells in the SGZ, it reduces the total number of adult-born mature neurons by delaying neuronal differentiation.
DISCUSSION
New neurons are continuously born in the adult dentate gyrus of the hippocampus. Although these adult-born neurons have been characterized at the cellular level, signaling mechanisms regulating adult hippocampal neurogenesis are not well defined. The goal of this study was to investigate the role of the ERK5 MAP kinase in the regulation of adult hippocampal neurogenesis.
Despite its abundant presence in the developing brain, ERK5 expression declines as the brain matures (21) and it is generally thought to be absent in the adult brain (see Ref. 26 and Allen Brain Atlas). We report here, that although ERK5 expression is generally absent in most areas of the adult brain, it is prominently expressed in the two neurogenic regions. The expression of ERK5 MAP kinase is quite unique and distinct from other signaling molecules implicated in adult neurogenesis, such as NeuroD, sonic hedgehog, Wnt, PI3K-Akt, and BDNF, which are more widely expressed in the brain (46–54). It also suggests that ERK5 may be critical in the regulation of adult neurogenesis. Indeed, shRNA knockdown of ERK5 in cultured aNPCs or conditional deletion of the erk5 gene specifically in the neurogenic regions of the adult mouse brain reduces neurogenesis in vitro and in vivo, respectively. By contrast, ectopic activation of endogenous ERK5 signaling via expression of caMEK5 promotes neurogenesis in cultured aNPCs as well as in the dentate gyrus of mouse brains. These data suggest that ERK5 signaling is an important regulator of adult neurogenesis in SGZ cells both in vitro and in vivo.
Although ERK5 expression in the SGZ of the adult mouse brain is found in cells expressing markers for neural stem/progenitor cells, actively proliferating cells, transiently amplifying progenitors and/or newborn neurons, the majority of ERK5-positive cells express markers for transiently amplifying progenitors and/or newborn neurons. These data indicate that ERK5 may primarily regulate adult hippocampal neurogenesis through its action on these cell populations, including affecting neuronal differentiation and maturation. Indeed, shRNA knockdown of ERK5 in cultured aNPCs reduces the number of newborn neurons while simultaneously increasing the number of proliferating cells and progenitor cells. These data, coupled with the fact that shERK5 did not increase the number of GFAP+ astrocytes suggest that inhibition of ERK5 attenuates neuronal differentiation without causing precocious glial differentiation. Using transgenic mouse technology, we conditionally deleted the erk5 gene specifically in Nestin-expressing neural stem cells in the adult brain. Although this inducible and conditional gene targeting of erk5 did not affect the total number of adult-born cells (BrdU+) in the dentate gyrus in vivo, it reduced the number of adult-born mature neurons (BrdU+ and NeuN+) while concomitantly increasing the number of cells expressing immature neuron markers DCX and Calretinin. These data suggest that loss of ERK5 causes a delay in the normal progression of neuronal differentiation and maturation during adult neurogenesis.
Neural progenitors in the SGZ express a cascade of transcription factors including Neurog2 (43, 44). Neurog2 belongs to a family of basic helix-loop-helix transcription factors that also includes Neurog1 and are critical for neuronal fate specification during development (55). Neurog2 is essential for neurogenesis in the dentate gyrus during development (45). However, its role in adult SGZ neurogenesis has not been demonstrated. Here we report that ectopic expression of Neurog2 is sufficient to promote neuronal differentiation of SGZ-derived aNPCs in culture, providing evidence that Neurog2 may confer pro-neural activity during adult hippocampal neurogenesis. We previously reported that ERK5 regulates both the transcriptional and pro-neural activities of Neurog1 during cortical development (25). This prompted us to investigate if ERK5 regulates adult hippocampal neurogenesis through Neurog2. Indeed, shRNA suppression of ERK5 signaling inhibited both the transcriptional and pro-neural activities of Neurog2 in cultured SGZ-derived aNPCs. Although it is possible that ERK5 could act downstream, in parallel, or upstream of Neurog2, the fact that ERK5 activity is required for Neurog2-stimulated transcription favors the interpretation that ERK5 is an upstream regulator of Neurog2.
What are the upstream extracellular signals that ERK5 responds to in regulating adult hippocampal neurogenesis? We have published evidence that ERK5 is activated by neurotrophins including brain-derived neurotrophic factor (BDNF) and NT3 in neurons (18). Interestingly, BDNF/TrkB signaling has been implicated in regulating adult hippocampal neurogenesis both under normal physiological conditions and after experimental manipulations such as voluntary exercise and chronic treatment with antidepressants (56–67). However, its downstream mechanisms have not been elucidated. We hypothesized that neurotrophins may activate ERK5 in SGZ-derived aNPCs and promote neuronal differentiation through ERK5. Indeed, ERK5 is activated by both BDNF and NT3 in these cells, and shRNA inhibition of ERK5 signaling suppresses the neuronal differentiation effect of NT3.
In summary, findings in this study identify ERK5 MAP kinase as a novel signaling pathway regulating adult hippocampal neurogenesis, especially in neuronal differentiation. Furthermore, Neurog2 may be a downstream target, whereas NT3 may be an upstream activator of ERK5 in this process. Because erk5 gene deletion is temporally and spatially regulated and specific to adult neural stem cells in the brain of the Nestin-CreERTM/ERK5loxP/loxP mouse, this ERK5 icKO mouse strain provides a unique and powerful tool to investigate the relationship between adult neurogenesis and hippocampus-dependent learning and memory.
Acknowledgments
We thank Drs. Cathy Tournier and Bradford C. Berk for the transfer of ERK5loxP/loxP mice, Dr. Hongjun Song for pXIE and pSIE retroviral vectors, Dr. Jane Johnson for Neurog2 cDNA expression vector, Dr. Jim Olson for the NeuroD2-Luc reporter (pCS2-NeuroD2-Luc), members of the Xia laboratory for critical reading of the manuscript, and Glen MacDonald for technical assistance on confocal imaging.
This work was supported, in whole or in part, by National Institutes of Health Grants AG19193 (to Z. X.), NS20498 (to D. R. S.), T32HD007183 and F31DC011216 (to Y. W. P) and Grant P30 HD02274 from the NICHD.

This article contains supplemental Figs. S1 and S2.
- MAP
- mitogen-activated protein
- ERK5
- extracellular signal-regulated kinase 5
- SGZ
- subgranular zone
- SVZ
- subventricular zone
- aNPC
- adult neural progenitor cell
- Neurog2
- neurogenin 2
- icKO
- induced-conditional knock-out
- NT
- neurotrophins
- PCNA
- proliferating cell nuclear antigen
- shNS
- nonspecific shRNA
- eGFP
- enhanced green fluorescent protein
- BDNF
- brain-derived neurotrophic factor
- IRES
- internal ribosome entry site.
REFERENCES
- 1. Altman J., Das G. D. (1965) Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J. Comp. Neurol. 124, 319–335 [DOI] [PubMed] [Google Scholar]
- 2. Alvarez-Buylla A., Theelen M., Nottebohm F. (1988) Birth of projection neurons in the higher vocal center of the canary forebrain before, during, and after song learning. Proc. Natl. Acad. Sci. U.S.A. 85, 8722–8726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Deng W., Aimone J. B., Gage F. H. (2010) New neurons and new memories. How does adult hippocampal neurogenesis affect learning and memory? Nat. Rev. Neurosci. 11, 339–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ming G. L., Song H. (2005) Adult neurogenesis in the mammalian central nervous system. Annu. Rev. Neurosci. 28, 223–250 [DOI] [PubMed] [Google Scholar]
- 5. van Praag H., Schinder A. F., Christie B. R., Toni N., Palmer T. D., Gage F. H. (2002) Functional neurogenesis in the adult hippocampus. Nature 415, 1030–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Song H. J., Stevens C. F., Gage F. H. (2002) Neural stem cells from adult hippocampus develop essential properties of functional CNS neurons. Nat. Neurosci. 5, 438–445 [DOI] [PubMed] [Google Scholar]
- 7. Toni N., Teng E. M., Bushong E. A., Aimone J. B., Zhao C., Consiglio A., van Praag H., Martone M. E., Ellisman M. H., Gage F. H. (2007) Synapse formation on neurons born in the adult hippocampus. Nat. Neurosci. 10, 727–734 [DOI] [PubMed] [Google Scholar]
- 8. Ge S., Yang C. H., Hsu K. S., Ming G. L., Song H. (2007) A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron 54, 559–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schmidt-Hieber C., Jonas P., Bischofberger J. (2004) Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature 429, 184–187 [DOI] [PubMed] [Google Scholar]
- 10. Kee N., Teixeira C. M., Wang A. H., Frankland P. W. (2007) Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat. Neurosci. 10, 355–362 [DOI] [PubMed] [Google Scholar]
- 11. Ramirez-Amaya V., Marrone D. F., Gage F. H., Worley P. F., Barnes C. A. (2006) Integration of new neurons into functional neural networks. J. Neurosci. 26, 12237–12241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gould E., Beylin A., Tanapat P., Reeves A., Shors T. J. (1999) Learning enhances adult neurogenesis in the hippocampal formation. Nat. Neurosci. 2, 260–265 [DOI] [PubMed] [Google Scholar]
- 13. Epp J. R., Spritzer M. D., Galea L. A. (2007) Hippocampus-dependent learning promotes survival of new neurons in the dentate gyrus at a specific time during cell maturation. Neuroscience 149, 273–285 [DOI] [PubMed] [Google Scholar]
- 14. Leuner B., Mendolia-Loffredo S., Kozorovitskiy Y., Samburg D., Gould E., Shors T. J. (2004) Learning enhances the survival of new neurons beyond the time when the hippocampus is required for memory. J. Neurosci. 24, 7477–7481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhou G., Bao Z. Q., Dixon J. E. (1995) Components of a new human protein kinase signal transduction pathway. J. Biol. Chem. 270, 12665–12669 [DOI] [PubMed] [Google Scholar]
- 16. Lee J. D., Ulevitch R. J., Han J. (1995) Primary structure of BMK1. A new mammalian map kinase. Biochem. Biophys. Res. Commun. 213, 715–724 [DOI] [PubMed] [Google Scholar]
- 17. English J. M., Vanderbilt C. A., Xu S., Marcus S., Cobb M. H. (1995) Isolation of MEK5 and differential expression of alternatively spliced forms. J. Biol. Chem. 270, 28897–28902 [DOI] [PubMed] [Google Scholar]
- 18. Cavanaugh J. E., Ham J., Hetman M., Poser S., Yan C., Xia Z. (2001) Differential regulation of mitogen-activated protein kinases ERK1/2 and ERK5 by neurotrophins, neuronal activity, and cAMP in neurons. J. Neurosci. 21, 434–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Watson F. L., Heerssen H. M., Bhattacharyya A., Klesse L., Lin M. Z., Segal R. A. (2001) Neurotrophins use the Erk5 pathway to mediate a retrograde survival response. Nat. Neurosci. 4, 981–988 [DOI] [PubMed] [Google Scholar]
- 20. Shalizi A., Lehtinen M., Gaudilliere B., Donovan N., Han J., Konishi Y., Bonni A. (2003) Characterization of a neurotrophin signaling mechanism that mediates neuron survival in a temporally specific pattern. J. Neurosci. 23, 7326–7336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu L., Cavanaugh J. E., Wang Y., Sakagami H., Mao Z., Xia Z. (2003) ERK5 activation of MEF2-mediated gene expression plays a critical role in BDNF-promoted survival of developing but not mature cortical neurons. Proc. Natl. Acad. Sci. U.S.A. 100, 8532–8537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang Y., Su B., Xia Z. (2006) Brain-derived neurotrophic factor activates ERK5 in cortical neurons via a Rap1-MEKK2 signaling cascade. J. Biol. Chem. 281, 35965–35974 [DOI] [PubMed] [Google Scholar]
- 23. Finegan K. G., Wang X., Lee E. J., Robinson A. C., Tournier C. (2009) Regulation of neuronal survival by the extracellular signal-regulated protein kinase 5. Cell Death Differ. 16, 674–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu L., Cundiff P., Abel G., Wang Y., Faigle R., Sakagami H., Xu M., Xia Z. (2006) Extracellular signal-regulated kinase (ERK) 5 is necessary and sufficient to specify cortical neuronal fate. Proc. Natl. Acad. Sci. U.S.A. 103, 9697–9702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cundiff P., Liu L., Wang Y., Zou J., Pan Y. W., Abel G., Duan X., Ming G. L., Englund C., Hevner R., Xia Z. (2009) ERK5 MAP kinase regulates neurogenin1 during cortical neurogenesis. PLoS ONE 4, e5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Di Benedetto B., Hitz C., Hölter S. M., Kühn R., Vogt Weisenhorn D. M., Wurst W. (2007) Differential mRNA distribution of components of the ERK/MAPK signalling cascade in the adult mouse brain. J. Comp. Neurol. 500, 542–556 [DOI] [PubMed] [Google Scholar]
- 27. Kuo C. T., Mirzadeh Z., Soriano-Navarro M., Rasin M., Wang D., Shen J., Sestan N., Garcia-Verdugo J., Alvarez-Buylla A., Jan L. Y., Jan Y. N. (2006) Postnatal deletion of Numb/Numblike reveals repair and remodeling capacity in the subventricular neurogenic niche. Cell 127, 1253–1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang X., Merritt A. J., Seyfried J., Guo C., Papadakis E. S., Finegan K. G., Kayahara M., Dixon J., Boot-Handford R. P., Cartwright E. J., Mayer U., Tournier C. (2005) Targeted deletion of mek5 causes early embryonic death and defects in the extracellular signal-regulated kinase 5/myocyte enhancer factor 2 cell survival pathway. Mol. Cell Biol. 25, 336–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pan Y. W., Chan G. C., Kuo C. T., Storm D. R., Xia Z. (2012) Inhibition of adult neurogenesis by inducible and targeted deletion of ERK5 mitogen-activated protein kinase specifically in adult neurogenic regions impairs contextual fear extinction and remote fear memory. J. Neurosci. 32, 6444–6455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Srinivas S., Watanabe T., Lin C. S., William C. M., Tanabe Y., Jessell T. M., Costantini F. (2001) Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 1, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McCormick M. B., Tamimi R. M., Snider L., Asakura A., Bergstrom D., Tapscott S. J. (1996) NeuroD2 and neuroD3. Distinct expression patterns and transcriptional activation potentials within the neuroD gene family. Mol. Cell Biol. 16, 5792–5800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. West M. J., Slomianka L., Gundersen H. J. (1991) Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anat. Rec. 231, 482–497 [DOI] [PubMed] [Google Scholar]
- 33. Malberg J. E., Eisch A. J., Nestler E. J., Duman R. S. (2000) Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J. Neurosci. 20, 9104–9110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kempermann G., Kuhn H. G., Gage F. H. (1997) Genetic influence on neurogenesis in the dentate gyrus of adult mice. Proc. Natl. Acad. Sci. U.S.A. 94, 10409–10414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rietze R. L., Reynolds B. A. (2006) Neural stem cell isolation and characterization. Methods Enzymol. 419, 3–23 [DOI] [PubMed] [Google Scholar]
- 36. Bull N. D., Bartlett P. F. (2005) The adult mouse hippocampal progenitor is neurogenic but not a stem cell. J. Neurosci. 25, 10815–10821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Duan X., Chang J. H., Ge S., Faulkner R. L., Kim J. Y., Kitabatake Y., Liu X. B., Yang C. H., Jordan J. D., Ma D. K., Liu C. Y., Ganesan S., Cheng H. J., Ming G. L., Lu B., Song H. (2007) Disrupted-In-Schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell 130, 1146–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kato Y., Kravchenko V. V., Tapping R. I., Han J., Ulevitch R. J., Lee J. D. (1997) BMK1/ERK5 regulates serum-induced early gene expression through transcription factor MEF2C. EMBO J. 16, 7054–7066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kim J. Y., Duan X., Liu C. Y., Jang M. H., Guo J. U., Pow-anpongkul N., Kang E., Song H., Ming G. L. (2009) DISC1 regulates new neuron development in the adult brain via modulation of AKT-mTOR signaling through KIAA1212. Neuron 63, 761–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wong S. T., Trinh K., Hacker B., Chan G. C., Lowe G., Gaggar A., Xia Z., Gold G. H., Storm D. R. (2000) Disruption of the type III adenylyl cyclase gene leads to peripheral and behavioral anosmia in transgenic mice. Neuron 27, 487–497 [DOI] [PubMed] [Google Scholar]
- 41. Athos J., Storm D. R. (2001) High precision stereotaxic surgery in mice. Curr. Protocols Neurosci. Appendix 4A [DOI] [PubMed] [Google Scholar]
- 42. Reynolds B. A., Weiss S. (1992) Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science 255, 1707–1710 [DOI] [PubMed] [Google Scholar]
- 43. Ozen I., Galichet C., Watts C., Parras C., Guillemot F., Raineteau O. (2007) Proliferating neuronal progenitors in the postnatal hippocampus transiently express the proneural gene Ngn2. Eur. J. Neurosci. 25, 2591–2603 [DOI] [PubMed] [Google Scholar]
- 44. Hodge R. D., Kowalczyk T. D., Wolf S. A., Encinas J. M., Rippey C., Enikolopov G., Kempermann G., Hevner R. F. (2008) Intermediate progenitors in adult hippocampal neurogenesis. Tbr2 expression and coordinate regulation of neuronal output. J. Neurosci. 28, 3707–3717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Galichet C., Guillemot F., Parras C. M. (2008) Neurogenin 2 has an essential role in development of the dentate gyrus. Development 135, 2031–2041 [DOI] [PubMed] [Google Scholar]
- 46. Gao Z., Ure K., Ables J. L., Lagace D. C., Nave K. A., Goebbels S., Eisch A. J., Hsieh J. (2009) Neurod1 is essential for the survival and maturation of adult-born neurons. Nat. Neurosci. 12, 1090–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kuwabara T., Hsieh J., Muotri A., Yeo G., Warashina M., Lie D. C., Moore L., Nakashima K., Asashima M., Gage F. H. (2009) Wnt-mediated activation of NeuroD1 and retro-elements during adult neurogenesis. Nat. Neurosci. 12, 1097–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jiao J., Chen D. F. (2008) Induction of neurogenesis in nonconventional neurogenic regions of the adult central nervous system by niche astrocyte-produced signals. Stem Cells 26, 1221–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kenney A. M., Widlund H. R., Rowitch D. H. (2004) Hedgehog and PI-3 kinase signaling converge on Nmyc1 to promote cell cycle progression in cerebellar neuronal precursors. Development 131, 217–228 [DOI] [PubMed] [Google Scholar]
- 50. Lie D. C., Colamarino S. A., Song H. J., Désiré L., Mira H., Consiglio A., Lein E. S., Jessberger S., Lansford H., Dearie A. R., Gage F. H. (2005) Wnt signaling regulates adult hippocampal neurogenesis. Nature 437, 1370–1375 [DOI] [PubMed] [Google Scholar]
- 51. Ma D. K., Ponnusamy K., Song M. R., Ming G. L., Song H. (2009) Molecular genetic analysis of FGFR1 signalling reveals distinct roles of MAPK and PLCγ1 activation for self-renewal of adult neural stem cells. Mol. Brain 2, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jessberger S., Clark R. E., Broadbent N. J., Clemenson G. D., Jr., Consiglio A., Lie D. C., Squire L. R., Gage F. H. (2009) Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn Mem. 16, 147–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bruel-Jungerman E., Veyrac A., Dufour F., Horwood J., Laroche S., Davis S. (2009) Inhibition of PI3K-Akt signaling blocks exercise-mediated enhancement of adult neurogenesis and synaptic plasticity in the dentate gyrus. PLoS One 4, e7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Suh H., Deng W., Gage F. H. (2009) Signaling in adult neurogenesis. Annu. Rev. Cell Dev. Biol. 25, 253–275 [DOI] [PubMed] [Google Scholar]
- 55. Ross S. E., Greenberg M. E., Stiles C. D. (2003) Basic helix-loop-helix factors in cortical development. Neuron 39, 13–25 [DOI] [PubMed] [Google Scholar]
- 56. Takahashi J., Palmer T. D., Gage F. H. (1999) Retinoic acid and neurotrophins collaborate to regulate neurogenesis in adult-derived neural stem cell cultures. J. Neurobiol. 38, 65–81 [PubMed] [Google Scholar]
- 57. Li Y., Luikart B. W., Birnbaum S., Chen J., Kwon C. H., Kernie S. G., Bassel-Duby R., Parada L. F. (2008) TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron 59, 399–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lee J., Seroogy K. B., Mattson M. P. (2002) Dietary restriction enhances neurotrophin expression and neurogenesis in the hippocampus of adult mice. J. Neurochem. 80, 539–547 [DOI] [PubMed] [Google Scholar]
- 59. Duman R. S., Monteggia L. M. (2006) A neurotrophic model for stress-related mood disorders. Biol. Psychiatry 59, 1116–1127 [DOI] [PubMed] [Google Scholar]
- 60. Warner-Schmidt J. L., Duman R. S. (2006) Hippocampal neurogenesis. Opposing effects of stress and antidepressant treatment. Hippocampus 16, 239–249 [DOI] [PubMed] [Google Scholar]
- 61. Rossi C., Angelucci A., Costantin L., Braschi C., Mazzantini M., Babbini F., Fabbri M. E., Tessarollo L., Maffei L., Berardi N., Caleo M. (2006) Brain-derived neurotrophic factor (BDNF) is required for the enhancement of hippocampal neurogenesis following environmental enrichment. Eur. J. Neurosci. 24, 1850–1856 [DOI] [PubMed] [Google Scholar]
- 62. Russo-Neustadt A. A., Alejandre H., Garcia C., Ivy A. S., Chen M. J. (2004) Hippocampal brain-derived neurotrophic factor expression following treatment with reboxetine, citalopram, and physical exercise. Neuropsychopharmacology 29, 2189–2199 [DOI] [PubMed] [Google Scholar]
- 63. Scharfman H., Goodman J., Macleod A., Phani S., Antonelli C., Croll S. (2005) Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp. Neurol. 192, 348–356 [DOI] [PubMed] [Google Scholar]
- 64. Shirayama Y., Chen A. C., Nakagawa S., Russell D. S., Duman R. S. (2002) Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J. Neurosci. 22, 3251–3261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sairanen M., Lucas G., Ernfors P., Castrén M., Castrén E. (2005) Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation, and survival in the adult dentate gyrus. J. Neurosci. 25, 1089–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bergami M., Rimondini R., Santi S., Blum R., Götz M., Canossa M. (2008) Deletion of TrkB in adult progenitors alters newborn neuron integration into hippocampal circuits and increases anxiety-like behavior. Proc. Natl. Acad. Sci. U.S.A. 105, 15570–15575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chan J. P., Cordeira J., Calderon G. A., Iyer L. K., Rios M. (2008) Depletion of central BDNF in mice impedes terminal differentiation of new granule neurons in the adult hippocampus. Mol. Cell. Neurosci. 39, 372–383 [DOI] [PMC free article] [PubMed] [Google Scholar]