Background: Pesticin is a protein toxin that is formed by Yersinia pestis to kill related strains.
Results: The crystal structure and functional analyses revealed a receptor binding, a translocation, and an activity domain.
Conclusion: Folding of the activity domain is very similar to folding of phage T4 lysozyme.
Significance: This is the first case that an activity domain is derived from a known enzyme.
Keywords: Bacterial Toxins, Crystal Structure, Fusion Protein, Protein Secretion, Transport, Active Site, Import, Pesticin, Pesticin-Lysozyme Hybrid, T4 Lysozyme
Abstract
Yersinia pestis produces and secretes a toxin named pesticin that kills related bacteria of the same niche. Uptake of the bacteriocin is required for activity in the periplasm leading to hydrolysis of peptidoglycan. To understand the uptake mechanism and to investigate the function of pesticin, we combined crystal structures of the wild type enzyme, active site mutants, and a chimera protein with in vivo and in vitro activity assays. Wild type pesticin comprises an elongated N-terminal translocation domain, the intermediate receptor binding domain, and a C-terminal activity domain with structural analogy to lysozyme homologs. The full-length protein is toxic to bacteria when taken up to the target site via the outer or the inner membrane. Uptake studies of deletion mutants in the translocation domain demonstrate their critical size for import. To further test the plasticity of pesticin during uptake into bacterial cells, the activity domain was replaced by T4 lysozyme. Surprisingly, this replacement resulted in an active chimera protein that is not inhibited by the immunity protein Pim. Activity of pesticin and the chimera protein was blocked through introduction of disulfide bonds, which suggests unfolding as the prerequisite to gain access to the periplasm. Pesticin, a muramidase, was characterized by active site mutations demonstrating a similar but not identical residue pattern in comparison with T4 lysozyme.
Introduction
Bacteriocins are proteinaceous toxins produced by many Gram-negative bacteria to inhibit the growth of closely related bacterial strains. Pesticin (Pst)3 from Yersinia pestis is a toxin that kills Y. pestis, Yersinia enterocolitica, and certain Escherichia coli strains (1). It belongs to the large group of bacteriocins from Gram-negative bacteria of which the colicins derived from E. coli strains are the largest and best studied class (1). Nearly half of the natural E. coli isolates produce colicins (1). Although Y. pestis includes only one plasmid-encoded toxin, E. coli can encode a variety of up to 21 colicins (1). Bacteria within natural populations losing the bacteriocin-coding plasmid may be killed by the surrounding bacteria that produce bacteriocins. Bacteriocin-producing cells are protected from suicide after re-uptake of toxins by expression of small immunity proteins.
The large class of colicins displays a variety of enzymatic functions among which are nucleases for the degradation of DNA, rRNA, or tRNA (1). A second class of colicins forms pores in the inner membrane that dissipate the electrochemical potential (1). Currently, only two bacteriocins are known to develop activity in the periplasm. Colicin M inhibits peptidoglycan (PG) biosynthesis (2), whereas pesticin is a muramidase that degrades PG (3, 4). The genetic organization and the principal structural architecture of Pst resemble colicins. The pst operon encodes Pst and the Pim protein, an antagonist to pesticin also known as the immunity protein that confers pesticin resistance to Pst-producing cells. Pim resides in the periplasm where the PG substrate of Pst is located (5).
Colicins are the only proteins actively imported by E. coli cells, which make them particularly attractive for studies of bacterial protein import. Colicin activity requires at least three steps as follows: 1) binding on the cell surface via receptor recognition; 2) energy-dependent uptake into the compartment of activity, and 3) destruction of the cellular target. Colicins denoted as group A colicins are imported across the outer membrane by the Tol system, whereas group B colicins use the Ton system for import. Colicins are typically composed of three domains as follows: the N-terminal translocation domain (T domain) comprising the TonB box segment that is important for binding to TonB and subsequent uptake across the outer membrane. Uptake requires energy that is generated by the protonmotive force of the cytoplasmic membrane. The energy-coupling machinery transforming electrochemical energy into the pulling force for colicin import consists of the TonB, ExbB, and ExbD proteins that are all anchored in the cytoplasmic membrane (1). This complex recognizes via TonB N-terminal TonB box sequences of outer membrane receptor proteins and bacteriocins, which results in import of the receptor-bound ligands into the periplasm.
The central receptor binding domain (R domain) is essential for the intimate contact with the outer membrane protein receptor, and the C-terminal activity domain (A domain) includes the deathly activity. Crystal structures of colicin R domains bound to receptors have been solved for colicins E2 and E3 bound to the BtuB receptor (6, 7) and for colicin Ia bound to the Cir receptor (8). Binding of the E3 colicin to BtuB induces local unfolding in the R domain, which is believed to be transmitted to the T domain (6). The receptor-bound R domain of the Ia structure does not differ from the R domain in the structure of the isolated complete Ia. Structural changes in both receptors do not open the pore within the β-barrel. After the first intimate contact of colicins E2 and E3 with BtuB, the bacterial porin OmpF is recruited to form a ternary complex through which the colicins are imported (6, 8). In contrast, colicin Ia first binds to Cir and then to a second Cir for translocation across the outer membrane (9). Despite detailed structural and functional information, the translocation mechanism through receptors remains unknown.
FyuA is an energy-coupled receptor in the outer membrane of Y. pestis through which the iron siderophore yersiniabactin and Pst are taken up into the periplasm. Some E. coli strains also express FyuA and are killed by Pst (10). After binding to FyuA, Pst uptake proceeds by a direct contact of the FyuA TonB box with TonB with subsequent translocation into the periplasm. In the bacterial periplasm Pst hydrolyzes PG by cleaving the bond between the C1 of N-acetylmuramic acid and C4 of N-acetylglucosamine (lysozyme activity) (4). In the absence of an intact PG layer, the cytoplasmic membrane no longer withstands the cytoplasmic osmotic pressure, and cells undergo lysis. Pst slowly hydrolyzes PG, which results in the formation of osmotically stable spheroplast-like forms. The spheroplast-like structures are obtained in the absence of sucrose to adapt the external osmotic pressure to the internal osmotic pressure. Nevertheless, addition of Pst to exponentially growing cells results in immediate dose-dependent loss of the ability to form colonies (3).
Very little is known about the mechanisms by which bacteriocins are translocated across the outer membrane. In particular, the transient steps for successive structural unfolding during translocation lack experimental tracing. To extract geometrical parameters influential for bacteriocin uptake, we chose the PG-degrading enzyme Pst and extended our previous functional studies (4, 5) toward a mechanistic understanding. To this end, we determined crystal structures of pesticin, Pst mutants, and a pesticin-T4 lysozyme chimera, and these structural studies were supported by functional studies regarding Pst uptake and activity. Together, these experiments allowed us to dissect the role of the R, T, and A domains in uptake and unfolding during translocation. Moreover, by mutational analysis we tested putative active site residues that we found to be overlapping but not identical to related lysozyme proteins. Taken together, these studies provide a structural view of Pst and advance knowledge of the uptake and plasticity of this bacteriocin.
EXPERIMENTAL PROCEDURES
Recombinant DNA Techniques
Plasmids were manipulated according to the methods of Sambrook and Russell (11). Plasmids were isolated and purified on a silica matrix with PureYield columns (Promega, Mannheim, Germany). Oligonucleotides were synthesized by Eurofins MWG Operon (Ebersberg, Germany). PCRs were performed with a Mastercycler ep gradient S (Eppendorf, Hamburg, Germany) and the Phusion DNA polymerase (Finnzymes, Espoo, Finland). DNA sequencing was carried out with the BigDye terminator version 3.1 cycle sequencing kit (Applied Biosystems, Foster City, CA). Samples were run on a 3730xl DNA analyzer (Applied Biosystems), a service provided by the Max Planck Institute for Developmental Biology.
Construction of Plasmids and Strains
The E. coli strains and plasmids used in this work are given in Table 1. For construction of plasmids, overhanging DNA ends were made blunt prior to ligation. Vectors were dephosphorylated with calf intestine alkaline phosphatase (Fermentas, St. Leon-Rot, Germany), and PCR products were phosphorylated by T4 polynucleotide kinase (Fermentas). The ligation products were introduced into E. coli DH5α (Stratagene, Heidelberg, Germany). The construct sequences were confirmed by DNA sequencing.
TABLE 1.
E. coli strains and plasmids used in this work
| Strain, plasmid | Genotype or description | Ref. or source |
|---|---|---|
| E. coli strains | ||
| BL21(DE3) | hsdS (rB−mB−) gal dcm ompT λ(DE3), T7 RNA polymerase gene under control of the lacUV5 promoter | 51 |
| C41 | Derivative of BL21 (DE3) | 52 |
| SIP1332 | C41 pSP130/29 | This study |
| SIP1453 | As SIP1332 but tonB | This study |
| SIP1461 | As SIP1332 but fhuA | This study |
| Plasmids | ||
| pHM10 | pACYC184 encoding FyuA | 53 |
| pSP130/29 | pET25b encoding FyuA with His tag at N terminus of mature protein | This study |
| pSP130/33 | pET22b encoding Pim-His6 | This study |
| pSP130/64 | pET22b encoding Pst(1–167)-His6 (PstRT) | This study |
| pSP130/66 | pET22b encoding Pst(164–357)-His6 (PstA) | This study |
| pSP130/70 | pET22b encoding Pst(1–357)His6 (Pst) | This study |
| pSP130/73 | pET22b encoding PstQ301A-His6 | This study |
| pSP130/88 | pET22b encoding PstP187A-His6 | This study |
| pSP130/92 | pET22b encoding PstT201A-His6 | This study |
| pSP130/110 | pET22b encoding PstE178A-His6 | This study |
| pSP130/467 | pET22b encoding PstS89C/S285C-His6 | This study |
| pSP130/480 | pET22b encoding PstD338A-His6 | This study |
| pSP130/486 | pET22b encoding PstD207A-His6 | This study |
| pSP130/258 | pET22b encoding PstΔ10–15-His6 | This study |
| pSP130/262 | pET22b encoding PstΔ10–20-His6 | This study |
| pSP130/375 | pET22b encoding T4L-His6; T4L refers to LYS_BPT4 Swiss-Prot P00720 with replacement of the two intrinsic cysteine residues (C221T/C264A) | This study |
| pSP130/187 | pET24d encoding Pst-T4L-His6 | This study |
| pSP130/432 | pET24d encoding Pst-T4LD294C/R321C-His6 | This study |
| pSP130/379 | pET24d encoding Pst-T4LI176C/R321C-His6 | This study |
| pSP130/441 | pET24d encoding Pst-T4LI176C/L331C-His6 | This study |
| pSP130/452 | pET24d encoding Pst-T4LS257C/Q289C-His6 | This study |
| pSP130/151 | Encodes MalE (1–26)-Pst with a C-terminal His tag (MalE′-Pst) controlled by the araBAD promoter, araC, Ampr | This study |
| pSP130/152 | Encodes MalE (1–26)-Pst (164–357) with a C-terminal His tag (MalE′-PstA) under control of the araBAD promoter, araC, Ampr | This study |
| pSP130/176 | Encodes MalE′-PstE178A with a C-terminal His tag under control of the araBAD promoter, araC, Ampr | This study |
| pSP130/178 | Encodes MalE′-PstA (E178A) with a C-terminal His tag under control of the araBAD promoter, araC, Ampr | This study |
Pst Constructs
The genes encoding Pst, PstRT, and PstA with a C-terminal His tag were cloned by PCR amplification from pUH64 (4) with the primer pairs TGTCAGATACAATGGTAGTGAATGGTTC and GTTTTAACAATCCACTATCGATATCTTTTTGCAC, TGTCAGATACAATGGTAGTGAATGGTTC and GAACAATATTTACCGAACGACGTAAAAATTCC, and TGGTAAATATTGTTGACCACGATATTTTCTC and GTTTTAACAATCCACTATCGATATCTTTTTGCAC and ligation into the NdeI/XhoI cut expression vector pET22b (Novagen, Darmstadt, Germany) resulting in plasmids pSP130/70, pSP130/64, and pSP130/66, respectively. Pst was fused to a signal sequence on pUH64 that was amplified by PCR with the primers TGTCAGATACAATGGTAGTGAATGGTTC and GTTTTAACAATCCACTATCGATATCTTTTTGCAC, ligated to a sequence encoding a C-terminal His tag, and cloned downstream of malE′ of pMA-RQ MalE′-Cma1–130 synthesized by Geneart (Regensburg, Germany) as described previously (12), replacing cma1–130. The resulting plasmid pSP130/151 encodes the signal sequence MalE(1–26) (Swissprot P0AEX9), Pst, and the C-terminal His tag LAHHHHHH under control of the araBAD promoter and contains araC. The plasmid encoding the activity domain PstA equipped with a signal sequence was cloned accordingly with the primers TGGTAAATATTGTTGACCACGATATTTTCTC and GTTTTAACAATCCACTATCGATATCTTTTTGCAC resulting in pSP130/152.
Pst-T4L Chimera Constructs
The gene encoding the T4 lysozyme derivative T4L (C221T and C264A) was amplified by PCR with the primers TGAATATATTTGAAATGTTACGTATAGATGAAGG and ATAGATTTTTATACGCGTCCCAAGTGCC from plasmid T4L, which was kindly provided by Joachim E. Schultz, University of Tübingen, Germany. The PCR product was cloned into the NdeI/XhoI cut expression vector pET22b yielding pSP130/375 and into pPstT7Lys (Trenzyme Biotechnology, Konstanz, Germany) replacing the T7 lysozyme gene yielding pSP130/187.
N-terminal Pst Deletion Constructs
The deletion encoding PstΔ10–15 was generated by PCR amplification of pSP130/70 with the primers ACCATTCACTACCATTGTATCTGACATAAAA and TTTCTCTTTTCCGGAAGTACATTAAGCAG and subsequent ligation, resulting in plasmid pSP130/258. For PstΔ10–20 plasmid pSP130/262 was generated accordingly with the primers ACCATTCACTACCATTGTATCTGACATAAAA and AGTACATTAAGCAGTTACAGACCAAATTTTG.
Active Site Mutants of Pst
Site-directed mutagenesis was performed by PCR with two complementary primers containing the mutation (primer sequences are available upon request) and treatment with DpnI (Fermentas) prior to transformation of DH5α (13).
FyuA Construct
fyuA, including the stop codon, was amplified by PCR from pHM10 and cloned into the NdeI/XhoI cut vector pET25b (Novagen). The sequence CATCATCACCATCATGCGGCCGCACTC encoding His5-Ala-Ala-Ala-Leu was inserted between the sequence encoding the signal peptide and the sequence encoding mature FyuA, yielding plasmid pSP130/29.
Pim Construct
Plasmid pSP130/33 was constructed by amplification of pim from pUH64 by PCR with the primers TGATCTCAAAGTTATTTTGCTTGGCTCTC and AATGATAATATACAGTGCAACCAGGG and ligation of the product into the NdeI/XhoI cut vector pET22b.
Mutant Strains
The chromosomal tonB mutant SIP1453 was obtained by selecting for Cma-resistant colonies of SIP1332. Sequencing revealed a point mutation resulting in a TonB109Stop mutation. The chromosomal fhuA mutant SIP1461 was gained by selecting for bacteriophage T5-resistant colonies of SIP1332. Sequencing revealed a point mutation resulting in an FhuA253Stop mutation.
Protein Overproduction and Purification
Pst without His tag was purified using a modified version of a protocol described previously (4). Briefly, E. coli BL21 (DE3) RIL (Stratagene) was transformed by electroporation with plasmid pUH64 that encodes the pesticin gene pst and the pesticin immunity gene pim downstream of gene 10 promoter of bacteriophage T7. Cells were grown at 37 °C in Luria Bertani (LB) medium supplemented with ampicillin (100 μg/ml) and chloramphenicol (25 μg/ml). When the A600 value reached 0.8, isopropyl β-d-1-thiogalactopyranoside was added to a final concentration of 1 mm. After 4 h of cultivation, cells were harvested and resuspended in 50 mm sodium phosphate buffer, pH 8.1. Cells were disrupted with a French press, and solid (NH4)2SO4 was added to the soluble fraction to 50% saturation at which pesticin precipitated. The precipitate was solubilized in 20 mm Tris-HCl, pH 8.4, and dialyzed against this buffer. The solution was loaded on a Mono Q column (GE Healthcare) equilibrated with the Tris-HCl buffer (see above), and Pst eluted by applying a NaCl gradient in Tris-HCl. Fractions containing pesticin were pooled, dialyzed against 10 mm HEPES, pH 7.5, 50 mm NaCl, 3 mm MgCl2, and the protein was concentrated to 41 mg/ml. The SeMet derivative was obtained by the same method with the exception that cells were grown in M9 salt minimal medium supplemented with the amino acids Leu, Ile, Phe, Thr, Lys, and Val (14).
His tag versions of PstWT, PstE178A, PstP187A, PstT201A, PstD207A, PstQ301A, PstD338A, PstS89C/S285C, PstRT, PstA, PstΔ10–15, and PstΔ10–20 were purified from transformants of E. coli BL21(DE3) harboring the recombinant plasmids pSP130/70, pSP130/110, pSP130/88, pSP130/92, pSP130/486, pSP130/73, pSP130/480, pSP130/467, pSP130/64, pSP130/66, pSP130/258, and pSP130/262, respectively, on Ni-NTA-agarose (Qiagen) according to the manufacturer's protocol, taking advantage of the C-terminal His tag on the proteins. E. coli BL21(DE3) was not killed by Pst because it does not express the receptor FyuA. For synthesis of PstRT and PstA, cells were grown at 28 °C, because under these conditions approximately half of the protein was found in the soluble fraction, whereas virtually all protein was found in inclusion bodies when grown at 37 °C. Pst and the other Pst derivatives did not form inclusion bodies in significant amounts, and cells were grown at 37 °C.
Because Pst-T4L in high amounts is toxic for E. coli strains due to the T4 lysozyme activity, T4L, Pst-T4L, and its derivatives Pst-T4LD294C/R321C, Pst-T4LI176C/R321C, Pst-T4LI176C/L331C, and Pst-T4LS257C/Q289C were overexpressed from pSP130/375, pSP130/187, pSP130/432, pSP130/379, pSP130/441, and pSP130/452, respectively, in E. coli C41 that carries mutations in the lacUV5 promoter controlling the T7 RNA polymerase, which results in reduced T7 RNA polymerase levels. The proteins were purified on Ni-NTA-agarose.
Crystallization of Pst and Pst Derivatives
Initial sitting-drop crystallization trials of Pst were performed with the Honeybee 961 robot (Genomic solutions) using 13 commercial high throughput screens on 3:1 Corning 3550 96-well plates. Drops mixed from 0.4 μl of protein solution and 0.4 μl of reservoir solution were equilibrated against 75 μl of precipitant solution at 20 °C. Crystals of different shape appeared under a variety of conditions that were further reproduced under hanging-drop conditions. Needle-shaped crystals suitable for the x-ray data collection were obtained with a solution containing 18% PEG 8000, 0.2 m MgCl2, and 0.1 m HEPES, pH 7.5. The Pst-SeMet construct was crystallized under the same conditions as Pst without the His tag. PstT201A and PstE178A were crystallized in a hanging-drop setup by mixing 1 μl of protein solution and 1 μl of ground solution, which contained 20% PEG 3350 and 0.2 m NaNO3. Pst-T4L was crystallized in a hanging-drop setup by mixing 1.2 μl of protein solution and 0.8 μl of ground solution, which contained 20% PEG 3350 and 0.15 m K2SO4. For crystallization of PstD207A, the reservoir solution contained 20% PEG 3350 and 0.2 m NaI.
Structural Solution of SeMet-Pst, Pst, and Pst Mutants
The structure of Pst was solved by single anomalous diffraction methods using a SeMet-labeled Pst protein crystal. Protein crystals were flash-frozen in the presence of 15% glycerol for Pst, PstT201A, and Pst-T4L, 17% glycerol for PstE178A, and 20% PEG 400 for PstD207A added to the solution of crystallization conditions mentioned above. All x-ray crystallographic data were collected at beamline PX10 at the Swiss Light Source (SLS, Villigen, Switzerland). The crystals were kept at 100 K during data collection, and a 360° dataset was collected. Data were processed with the XDS/XSCALE (15) program package, and the structure was solved using the SHARP/autoSHARP program package (16, 17). Initial model building was performed in Arp/Warp and Buccaneer (18, 19). Subsequent improvement of models was achieved using the PHENIX program package in combination with Coot for the construction of the models (20, 21). The structure was analyzed using PROCHECK (22), and the figures were prepared using the PyMOL program package.
CD Spectroscopy
CD measurements were performed on a JASCO J-810 spectropolarimeter (Jasco, Gross-Umstadt, Germany) equipped with a thermoelectric temperature controller. All spectra were recorded with a 1-mm quartz cuvette at 190–250 nm using a bandwidth of 1 nm, a scanning speed of 100 nm min−1, a data pitch of 0.2 nm, a response time of 1 s, and an accumulation of at least five spectra for averaging. Thermal denaturation was monitored at 222 nm between 10 and 90 °C with a temperature ramp of 1 °C min−1 and a data pitch of 0.5 °C. For data processing, subtracting the buffer base line, calculation of the melting temperature, and prediction of secondary structure, the Spectra Manager 2 software was used. Measurements were performed in 25 mm NaCl and 25 mm Tris-HCl, pH 7.5.
In Vivo Pesticin Activity Tests (Plaque Assay)
In vivo activity was tested using the sensitivity of an E. coli strain harboring a fyuA plasmid. LB plates (10 g of bacto tryptone, 5 g of yeast extract, 5 g of NaCl, 15 g of agar per liter) were overlaid with 3 ml of top agar seeded with 109 cells of the strain to be tested. 6 μl of 10-fold dilutions of pesticin solutions were spotted onto these plates. Following incubation overnight at 37 °C, the diameter of the lysis zones was measured. Cystine bridges were reduced by addition of 10 mm DTT. Cysteines were oxidized by 0.2 mm Cu2+ in complex with 1,10-phenanthroline.
In Vitro Lysozyme Assay
The sample was incubated in 50 mm NaCl and 50 mm Tris-HCl, pH 7.5, with fluorescein-labeled cell walls of Micrococcus lysodeikticus (Invitrogen) at 37 °C. Hydrolysis of the cell walls relieves fluorescence quenching, yielding a strong increase in fluorescence, which was measured with an excitation wavelength of 485 nm and an emission wavelength of 530 nm. The background fluorescence without sample was subtracted from each value.
Activity Assay for Secreted Pst
Cells synthesizing Pst and PstA fused to the MalE signal sequence were grown in LB medium containing ampicillin at 30 and 37 °C, respectively. It was not possible to grow E. coli containing pSP130/151 (encoding MalE′-Pst) at 37 °C even in the presence of glucose, because cells lysed. Because Pst is less toxic for E. coli cells at lower temperatures, this problem could be overcome by growth at 30 °C. In the exponential growth phase, arabinose was added to the final concentrations indicated. Cell growth and lysis were monitored by measuring the absorbance at 578 nm.
RESULTS
Crystallization and Structural Analysis of Pst, Pst Mutants, and Pesticin-T4 Lysozyme (Pst-T4L) Chimera
Pesticin was overproduced in E. coli cells harboring the plasmid encoding the wild type enzyme that was purified by previously established methods (4). The highly expressed protein devoid of any purification tag was purified by conventional methods to finally achieve electrophoretic homogeneity (supplemental Fig. S1A). Crystals of the wild type protein were obtained that diffracted to a resolution of 1.98 Å (Table 2). The structure of Pst was solved by the single anomalous diffraction method using a single selenomethionine-labeled protein crystal. Additional Pst constructs (mutants and chimera) with C-terminal His tags were purified by Ni-NTA chromatography and subjected to crystallization. All structures were solved by molecular replacement using wild type Pst or the structure of T4L as search models. In total, we determined six crystal structures as follows: wild type Pst, a Pst cysteine double mutant (PstS89C/S285C), a Pst (residues 1–164)-T4L chimera (Pst-T4L), and three Pst active site mutants, PstE178A, PstT201A, and PstD207A. A summary of all refined structures, crystallization conditions, and data collection statistics is given in Table 2.
TABLE 2.
Data collection and refinement statistics
| PstWT | PstS89C/S285C | PstT201A | PstD207A | PstE178A | Pst-T4L | |
|---|---|---|---|---|---|---|
| Data collectiona | ||||||
| Space group | P21 | P21 | P21 | P22121 | P212121 | C2 |
| Cell dimensions | ||||||
| a, b, c | 36.55, 86.78, 122.27 Å | 35.96, 86.12, 122.02 Å | 65.53, 43.21, 133.20 Å | 35.32, 92.62, 225.92 Å | 44.64, 127.83, 158.36 Å | 194.87, 51.5, 107.92 Å |
| α, β, γ | 90, 96.14, 90° | 90, 97.48, 90° | 90, 99.37, 90° | 90° | 90° | 90, 91.01, 90° |
| Resolution | 30 to 1.98 Å (2.02 to 1.98 Å) | 36 to 2.6 Å (2.66 to 2.6 Å) | 44 to 2.0 Å (2.03 to 2.0 Å) | 58 to 2 Å (2.05 to 2.0 Å) | 79 to 2.3 Å (2.34 to 2.3 Å) | 73 to 2.6 Å (2.66 to 2.6 Å) |
| Rsym or Rmerge | 0.1 (0.65) | 0.04 (0.21) | 0.09 (0.42) | 0.06 (0.48) | 0.13 (0.81) | 0.08 (0.48) |
| I/σI | 12.3 (2.0) | 12.9 (2.2) | 10.1 (2.8) | 15.3 (2.5) | 10.3 (2.1) | 7.8 (1.8) |
| Completeness | 99.2% (98.1%) | 97.7% (97.8%) | 99.2% (98.7%) | 97.8% (91.0%) | 99.6% (97.7%) | 95.2% (91.8%) |
| Redundancy | 4.7 (4.5) | 2.2 (2.2) | 3.1 (3.1) | 5.4 (4.8) | 7.7 (7.3) | 2.1 (2.0) |
| Refinementa | ||||||
| Resolution | 30 to 1.98 Å (2.02 to 1.98 Å) | 36 to 2.6 Å (2.66 to 2.6 Å) | 44 to 2.0 Å (2.03–2.0 Å) | 58 to 2.0 Å (2.05 to 2.0 Å) | 79 to 2.3 Å (2.34 to 2.3 Å) | 73 to 2.6 Å (2.66 to 2.6 Å) |
| No. of reflections | 52,797 (2683) | 22,615 (2668) | 50,034 (1783) | 50,456 (3038) | 41,466 (2449) | 33,200 (2367) |
| Rwork/Rfree | 0.19/0.24 (0.32/0.35) | 0.23/0.26 (0.32/0.37) | 0.18/0.21 (0.24/0.29) | 0.19/0.24 (0.28/0.31) | 0.21/0.25 (0.31/0.35) | 0.19/0.24 (0.26/0.30) |
| No. of atoms (all) | 5802 | 5522 | 6226 | 5776 | 5701 | 5198 |
| Protein (chains/residues) | 2/678 | 2/679 | 2/693 | 2/689 | 2/680 | 2/635 |
| Ligands (sulfate) | 5 | |||||
| Water | 385 | 115 | 667 | 283 | 304 | 78 |
| B-factors | ||||||
| Protein | 47 | 65 | 31 | 34 | 55 | 58 |
| Ligands (sulfate) | 62 | |||||
| Water | 46 | 45 | 35 | 41 | 42 | 43 |
| Root mean square deviations | ||||||
| Bond lengths | 0.008 Å | 0.01 Å | 0.004 Å | 0.019 Å | 0.021 Å | 0.018 Å |
| Bond angles | 1.1° | 1.46° | 0.82° | 1.2° | 1.2° | 1.5° |
| Ramachandran statistics | ||||||
| Residues in favored regions | 648 (96.7%) | 657 (97.8%) | 669 (97.1%) | 669 (98%) | 650 (97%) | 612 (97.6%) |
| Residues in allowed region | 18 (2.7%) | 12 (1.8%) | 20 (2.9%) | 14 (2%) | 19 (2.8%) | 15 (2.4%) |
| Residues in outlier region | 4 (0.6%) | 2 (0.3%) | 0 (0%) | 0 (0%) | 1 (0.1%) | 0 (0%) |
| Crystallization conditions | 18% PEG 8000 | 18% PEG 8000 | 20% PEG 3350 | 20% PEG 3350 | 20% PEG 3350 | 20% PEG 3350 |
| 0.2 m MgCl2 | 0.2 m MgCl2 | 0.2 m NaNO3 | 0.2 m NaI | 0.2 m NaNO3 | 0.15 m K2SO4 | |
| 0.1 m HEPES, pH 7.5 | 0.1 m HEPES, pH 7 | |||||
| Cryo protectant added | 15% glycerol | 15% glycerol | 15% glycerol | 20% PEG 400 | 17% glycerol | 15% glycerol |
| PDB code | 4AQN | 4ARQ | 4ARM | 4ARL | 4ARP | 4ARJ |
a Values in parentheses are for highest resolution shell.
Pesticin, Three Domain Organization, and a Novel Phage Lysozyme Homolog
All bacteriocins consist of three domains as follows: the translocation domain, the receptor binding domain, and the activity domain. Accordingly, Pst includes a small and partially unfolded random coil translocation domain (residues 1 to ∼40; PstT) with the eight N-terminal residues composing the TonB box element not visible in the electron density map (Fig. 1A). The elongated T domain with a molecular interface of 875 Å2 to the R domain and high B-factors is rich in glycines (4), prolines (3), and serines (7), two of which (Ser-19 and Ser-21) are important for the alignment to the R domain surface. The FyuA receptor binding domain includes residues 41–166 (PstR) and is formed by a mixed α/β-fold. The β-sheet of seven anti-parallel β-strands (β1–β7) together with three helices displays the topology of β1/β2/β3/β4/β5/α1/α2/β6/β7/α3 (Fig. 1A). β-Strands 1, 2, and 6, 7 are aligned almost parallel, and strands 3–5 are bent in a different angle (Fig. 1A for a topology scheme). This R domain does not display structural homology to any known domain of the PDB database and represents a novel fold (23). Notably, neither PstT nor PstR shows significant sequence homologies to the sequences in the Protein Data Bank. Therefore, the fold appears to have specifically adapted to bind to and to be taken up by the FyuA receptor.
FIGURE 1.
Structural model of pesticin from Y. pestis. A, Pst structure in schematic representation is displayed from two different views related to each other by a rotation of 180° around the y axis. The N-terminal (NT) 12 residues, including the TonB box (TBB) and residues 30–34, are not visible in the crystal structure due to disorder. Pst consists of three domains as follow: the translocation domain (T), marked in red; the receptor binding domain (R), marked in blue, and the activity domain (A), marked in orange. The secondary structure assignment is given for β-strands (β1–β8) and α-helices (α1–α11). Two independent PstAD1 and PstAD2 domains connected by α7 helix form the basis of the subdomains of the T4 lysozyme-like structure. The interface residues between the R (blue) and A domain (orange) are highlighted in the box below the structure. One particular hydrogen bond used for the construction of PstS89C/S285C is marked with dashed lines. The R domain includes a mixed α/β-fold, and the A domain is α-helical with three short conserved β-strands (β8–β10, see also Fig. 2A). The R domain displays a new fold that is drawn schematically below the structure (β-strands in blue and α-helices in red). The domain composition together with domain residue boundaries is given in the schematic bar below the structures. B, surface charge representation of Pst illustrated from the same orientations as in A. The active site (marked by a black star) is flanked by two patches of positively charged residues that are further surrounded by extended patches of negatively charged residues. C, activity domain in the same orientation as A is displayed from two sides related by a rotation of 180° around the y axis to illustrate conservation of residues. The structure is shown as a schematic model together with the surface representation of conserved (color coded in blue) and highly conserved residues (in yellow) (see also the alignment in supplemental Fig. S6B for comparison). Single residues lining the active site are more strongly conserved and marked with residue type and number. Residues Glu-178, Thr-201, and Asp-207 are particularly important as they form part of the active site (these residues are marked with large yellow dots).
The R and A domains are interconnected and stabilized relative to each other by 10 H-bonds forming a small interface of 530 Å2 (see Fig. 1A for details). The activity domain includes residues 167–357 (PstA) and is an almost entirely α-helical domain (α4–α11) with three small β-strands (β8–β10) important for maintaining the active site architecture (Fig. 2). B-Factors of the active site vicinity are particularly low. The entire domain can be subdivided into two largely separated domains D1 (PstAD1; residues 167–266) and D2 (PstAD2; residues 267–357) interconnected by the long α-helical segment α7 forming the active site cleft for substrate binding (Figs. 1A and 2A). This cleft has an approximate diameter of 1 nm and is sufficiently large to accommodate the PG substrate. Both areas facing the entry of this cleft show a strong positive charge profile with conserved arginine residues for substrate binding (Fig. 1, B and C). Although the T and R domains are unique in sequence, there are homologous sequences to PstA among the Pst-like proteins that after alignment indicate a particularly strong conservation of the active site residues Glu-178, Asp-207, and Thr-201 (see below) and more distant residues Gln-301, Arg-339, Arg-340, and Lys-341 (Fig. 1C and supplemental Fig. S6B). Moreover, additional residues in the backbone core of the lysozyme-like fold are conserved, some of which surround the active site and others extend from there to presumably stabilize this fold.
FIGURE 2.
Comparison of the Pst activity domain with lysozyme topology structures. In the upper row the crystal structures of Pst structure alone and structure superpositions (A) with the domain of gp5 of bacteriophage T4 (PDB code 1WTH) (B), bacteriophage 21 lysozyme (PDB code 3HDF) (C), bacteriophage T4 lysozyme (PDB code 148L) (D), and HEWL (PDB code 1H6M) (E) are shown. In the bottom row, the secondary structural topology of the five proteins (based on the DSSP algorithm) are given with β-strands marked in blue and α-helices in red. A, two subdomains forming the activity domain of Pst are encircled by dashed lines. B, general structure motif of the lysozyme-like molecules excluding HEWL is marked by dashed lines. D, active site residues of the T4 lysozyme are marked in green (Glu-11, Asp-20, and Thr-26). One additional structurally related residue of Pst and T4L mutated in this work is shown.
Pesticin, New Member of the Small Galaxy of Lysozyme Folds
The activity domain structure of Pst is unique and resembles the fold of a protein class of similar fold but of a biologically unrelated species. Similar sequences are classified into five large groups of T4-like lysozyme homologs as follows: 1) phage-related lysozymes, 2) a group of VgrG proteins comprising lysozyme domains, and 3) three smaller subclusters with pesticin as one representative (supplemental Fig. S2). Few structures of the clustering sequences have been solved, including the unrelated hen egg white lysozyme (HEWL), phage T4 lysozyme, the phage 21 endolysin domain, and a lysozyme-like fold as part of the gp5 protein (24). gp5 is part of the needle-like supramolecular punctuation structure of phage T4. Although all of these enzymes cleave PG, their individual fold may be quite diverse (Fig. 2). In comparison, Pst contains the largest domain with additional secondary structural elements not present in the related structures, in particular two helices located in the AD1 domain (Fig. 2A). The smallest microbial lysozyme is the p21 endolysin containing only three anti-parallel β-strands of the PstAD1 domain and the AD2 domain. The PstAD1-like domains in T4 lysozyme and gp5 are essentially identical as they both derive from the same phage but serve different biological functions (Fig. 2, B and D). Although the three phage structures and the Pst largely coincide in the connection between AD1 and AD2 and their common four helix fold forming PstAD2, HEWL contains an entirely different fold of AD1 and AD2. Here, both domains include a different number of secondary structural elements, a strongly shortened connecting helix, whereas almost the entire four helix bundle of AD2 is missing (Fig. 2E). Notably, all structures compared in Fig. 2 contain the unique fingerprint of the three β-sheets located in AD1. In T4 lysozyme and HEWL, these sheets contain the most important residues for activity (25, 26). As T4 lysozyme, Pst hydrolyzes PG by cleaving the glycosidic bond between C1 of N-acetylmuramic acid and C4 of N-acetylglucosamine (muramidase activity) (4). The fold of the active centers of both enzymes is similar, but the residues of T4 lysozyme involved in binding and hydrolysis of the substrate differ in part (see below).
Binding and Translocation Studies of Pst, Importance of the R and T Domain
Pesticin is translocated across the outer membrane through the FyuA receptor that is coupled to the energy-delivering machinery of the cytoplasmic membrane formed by the proteins TonB, ExbB, and ExbD. Residues 3–7 of Pst represent the conserved TonB box motif (DTMVV) by which pesticin most likely interacts with TonB, as has been previously shown for colicins B (27), D (28), M (5), and Ia (8). It is unknown why most group B colicins in addition to their receptors need to interact with TonB. Translocation domains in bacteriocin structures often include an approximate length of ∼40 N-terminal residues formed as random coil arrangement attached to the R domain. Examples are pesticin, colicin M (29), or colicin B (30), which show such elongated elements (Fig. 3, A and B).
FIGURE 3.
Structure and organization of the translocation domain in bacteriocins. A, surface representation of Pst shown in sand color with the T domain shown in schematic form as partially random coil in magenta with the N terminus (NT) marked. B, for comparison, the surface representation of colicin M (Cma) in the same schematic drawing as Pst showing the T domain wrapped around the R domain. C, model of the translocation of Pst through the FyuA receptor (the structure of FhuA, PDB code 1BY3, was taken as model for FyuA due to the similarity). The distance of the entire membrane-spanning protein is ∼9 nm, although the diameter of the membrane spanning part is 5–6 nm. Assuming the T domains of Pst are extended by an artificial force, the terminus could be elongated to ∼12 nm. If this terminus is reduced by five residues (∼1.5 nm), it would be sufficient to span the membrane and to attach to the periplasmic domain of TonB. A further reduction in length of this domain by another 10 residues (∼3 nm) would presumably not allow binding of the bacteriocin to TonB and thereby abolish uptake and in vivo activity. The model illustrates the effect of truncation of the T domain on Pst uptake. A schematic representation of the Pst constructs used to determine the importance of the T domain in uptake and activity is given on the right side. AA, amino acids; TBB, TonB box.
To examine whether the RT domain PstRT is sufficient for binding to the FyuA receptor, competition of PstRT with killing by Pst-T4L (see below) was determined. An excess of PstRT over Pst-T4L inhibited the lytic activity of Pst-T4L in liquid culture (supplemental Fig. S3) as well as in plaque assays (Table 3). This indicates that PstRT competes with Pst-T4L for binding to the FyuA receptor. PstA was used as a negative control in the competition assay of which a surplus did not affect the lysis zones formed by Pst-T4L (Table 3).
TABLE 3.
Binding of PstRT, PstD207A, and PstΔ10–20 proteins to the FyuA receptor determined by competition with Pst-T4L
PstA was used as a negative control. The numbers indicate the diameters of lysis zones (in mm) that were formed in a plaque assay on LB agar plates seeded with E. coli SIP1332 (fyuA+) onto which 6 μl of the protein mixture with the molar ratio indicated was applied. The concentration of Pst-T4L was 10 μm. – indicates no lysis zone; turbid lysis zones are given in parentheses, very turbid lysis zones in double parentheses; ND means not determined.
| Protein or ratio Pst-T4L:Pst | PstRT | PstD207A | PstΔ10–20 | PstA |
|---|---|---|---|---|
| Pst-T4L, no Pst derivative | 11 | 11 | 11 | 11 |
| 1:2 | (8) | (7.5) | ND | 11 |
| 1:5 | ((8)) | ((7.5)) | – | 11 |
| 1:10 | – | – | – | 11 |
| 1:20 | – | – | – | 11 |
| 1:50 | – | – | – | 11 |
To study whether the total extension of the T domain has an influence on uptake of Pst, we generated deletions in the T domain adjacent to the TonB box of Pst and tested whether these mutants are still able to interact with the Ton system (Fig. 3C). The PstΔ10–15 mutant missing residues 10–15 displayed a low activity in the plaque assay forming turbid lysis zones up to a dilution of 1:104 (wild type 1:105), whereas PstΔ10–20 missing residues 10–20 was inactive and formed no lysis zones. To examine whether these mutants still bind to FyuA, competition of PstΔ10–20 with killing by Pst-T4L was determined. PstΔ10–15 showed a residual activity and was therefore unsuitable for the competition assay. In plaque assays, a 5-fold molar excess of PstΔ10–20 over Pst-T4L completely prevented formation of a lysis zone by Pst-T4L under conditions by which Pst-T4L gave a clear lysis zone of 11 mm (Table 3). Competition with the same concentration of PstRT or inactive PstD207A (see below) resulted in turbid lysis zones (Table 3). This suggests that PstΔ10–20 remains bound to the FyuA receptor, whereas PstRT and PstD207A are taken up, and FyuA is cleared. Therefore, higher concentrations of PstRT and PstD207A compared with PstΔ10–20 are necessary to completely block the FyuA receptor for Pst-T4L. The deletion mutant PstΔ10–20 still binds to FyuA, which can also be inferred for PstΔ10–15. Assuming the length of the T domain to span the outer membrane receptor plays an important role for the translocation event, we delineated a model on the basis of the Pst, PstΔ10–15, and PstΔ10–20 and FhuA (resembling the FyuA) receptor structures (Fig. 3C). Assuming that ∼33 residues (the T domain reduced by the TonB box residues) are part of the T domain, this elongated protein chain has an approximate extension of 11 nm. Deletion of five residues (PstΔ10–15) would shorten this construct by 2 nm to 9 nm, whereas deletion of 10 residues would result in an elongated peptide of ∼7 nm (Fig. 3C). The PstΔ10–20 mutant has probably been shortened too much to be able to reach the periplasmic TonB. Taking these results together, it appears plausible that the length of the translocation domain is critical for the activity of bacteriocins imported through Ton-dependent uptake receptors.
Plasticity in Bacteriocins, Exchange of the A Domain by T4 Lysozyme Results in an Active Chimera
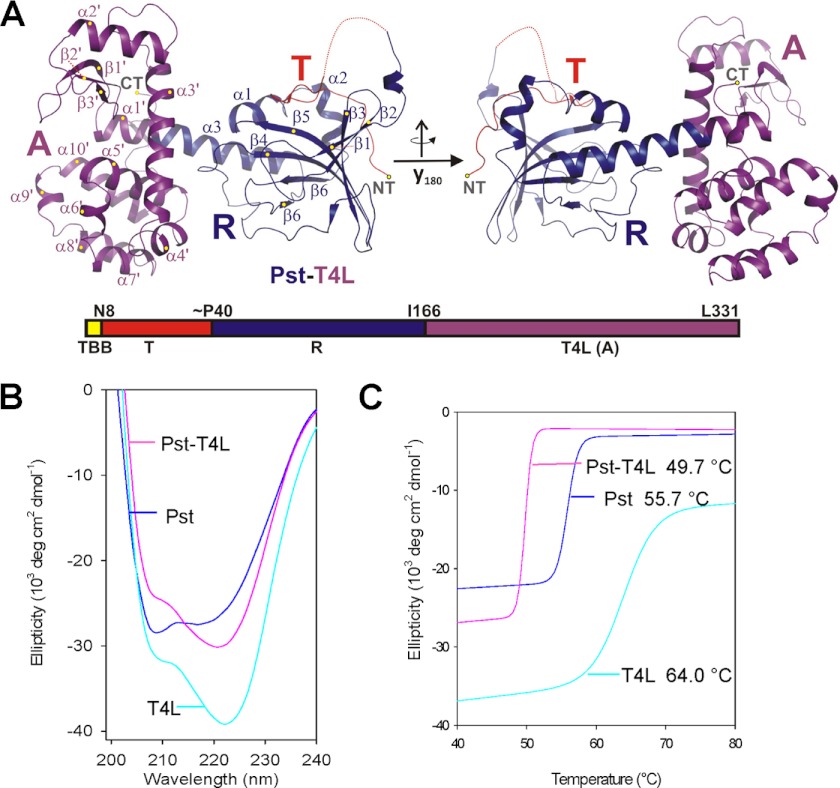
One of the common features of colicins is their modular architecture composed of T, R, and A building blocks. To examine whether an enzyme that is not derived from bacteriocins can be linked to the RT domain of Pst in a functionally active form and is carried into cells by the RT domain, we replaced PstA by T4L, which assumes a similar architecture. If the sequence was decisive in translocation across the outer membrane, we expected that Pst-T4L was not transferred into the periplasm because sequence identity to Pst is only 13%. The crystal structure of the fusion protein was solved and showed only small variations to the T and R domain of Pst (Fig. 4A and supplemental Fig. S4 for variations). The A domain of Pst-T4L is structurally identical to the open form of T4 lysozyme (PDB code 168L, supplemental Fig. S4). Unexpectedly, the C-terminal helix of PstRT and the N-terminal helix of T4L form a long continuous connecting helix (Fig. 4A). We also collected the CD spectra of Pst, Pst-T4L, and T4L to show that the chimera protein was properly folded in solution (Fig. 4B). The melting temperature (Tm) of Pst-T4L (49.7 °C) was significantly lower than that for wild type Pst (55.7 °C) and for T4L (64.0 °C) (Fig. 4C). The interface between the R and A domains in Pst is stabilized by 10 hydrogen bonds, whereas this interface is destroyed in the chimera protein. The R and A domain are aligned relative to each other mostly by the long α-helix and a small contact interface. Although PstRT and T4L form separate domains in the chimera, the Tm of T4L alone is apparently lowered in the presence of the Pst domain.
FIGURE 4.
Structure and stability of the Pst-T4L chimera protein. A, chimera structure of the three-domain protein formed by the Pst N-terminal translocation and receptor binding domains (T and R, in red and blue) and the C-terminal domain, which represents the T4L protein (A in magenta). The structure is displayed from two points of view related by a rotation of 180° around the y axis. The secondary structure assignment essentially follows Fig. 1 for Pst, but the structural elements are individually assigned for the T/R domain (α1–α3 and β1–β6) and the A domain (α1′–α10′ and β1′–β3′). The domain organization (TBB, TonB box) also including the boundary residues are marked with numbers and represented in the bar-like scheme beyond the structure. B, CD spectra; C, melting curves of Pst (260 μg/ml), T4L (200 μg/ml), and Pst-T4L (260 μg/ml). The melting temperatures (Tm values) are indicated.
To test if the chimera protein was translocated into E. coli, we performed plaque assays using genetically constructed E. coli strains harboring the FyuA uptake receptor on the surface. Pst-T4L formed a lysis zone on E. coli SIP1332 fyuA+ and SIP1461 fyuA+ fhuA−, and no lysis zone on E. coli C41 fyuA− and SIP1453 fyuA+ tonB− (Table 4). The lysis zones of Pst-T4L were clear compared with slightly turbid lysis zones of Pst. To examine whether Pst-T4L is inhibited by Pim, strain SIP1332 could not be used because the pim-plasmid pSP130/33 was not compatible with the fyuA-plasmid pSP130/29. Therefore, C41 pHM10 fyuA+ was used as an indicator strain. On this strain Pst and Pst-T4L formed lysis zones (Table 4). When the immunity protein Pim was synthesized (C41 pHM10 pSP130/33), only Pst-T4L but not Pst formed a lysis zone, indicating that Pim only protects against Pst and not against Pst-T4L. Although folding of T4L is very similar to that of PstA, the different sequence prevents Pim from recognizing T4L. Compared with wild type Pst, Pst-T4L exhibited ∼20% in vivo activity, because an ∼6-fold higher concentration of Pst-T4L than Pst was necessary to give rise to lysis zones of the same size. The results listed in Table 4 show that the chimera protein was imported into the periplasm, and import required the presence of both the FyuA receptor and TonB. The helix connecting PstRT with T4L may affect different functions, binding to FyuA, unfolding during translocation, refolding in the periplasm, and activity. In fact, Pst-T4L hydrolyzed isolated fluorescein-labeled Micrococcus lysodeikticus cell walls with ∼10% of the rate of T4L (supplemental Fig. S5).
TABLE 4.
In vivo activities of Pst-T4L, Pst and T4L
The numbers indicate the diameters of lysis zones (in mm) that were formed on LB agar plates seeded with the E. coli strain indicated onto which 6 μl of 6 μm Pst-T4L, ∼1 μm Pst, or 30 μm T4L were spotted. – indicates no lysis zone.
| Strain | Protein |
||
|---|---|---|---|
| Pst-T4L | Pst | T4L | |
| C41 (fyuA−) | – | – | – |
| SIP1332 (fyuA+) | 11 | 11 | – |
| SIP1453 (fyuA+ tonB−) | – | – | – |
| SIP1461 (fyuA+ fhuA−) | 11 | 11 | – |
| C41 pHM10 (fyuA+) | 11 | 11 | – |
| C41 pHM10 pSP130/33 (fyuA+ pim+) | 11 | – | – |
Disulfide Cross-linking of Structural Motifs Prevents Unfolding and Pesticin Uptake
To test whether unfolding is necessary for uptake of Pst, domains and secondary structural motifs were cross-linked by disulfide bonds. First, we exchanged a serine located in the R domain (S89C) and in close proximity to a serine in the A domain (S285C) by cysteine. These residues are also part of the interface between the R and A domains (Fig. 1A and supplemental Fig. S6A). Disulfide bond formation was tested by SDS-PAGE whereby the oxidized form has a higher electrophoretic mobility than the reduced form due to the more compact structure. The mutant protein became oxidized during purification, and disulfide bonds were spontaneously formed (Fig. 5C). When the cysteine bridges were reduced with DTT, the protein showed a slower electrophoretic mobility (Fig. 5C). The crystal structure of the oxidized form determined at 2.6 Å resolution was identical to the wild type protein with a small deviation between main chain atoms of 0.8 Å root mean square deviation (Fig. 5A). In the oxidized form, the protein was inactive in the plaque assay and did not form lysis zones (Table 5). The reduced protein displayed the same activity as wild type Pst. Lysis zones up to a 105-fold dilution were observed on agar plates seeded with E. coli SIP1332 fyuA+ (Table 5).
FIGURE 5.
Structure of the covalently linked disulfide derivative of PstS89C/S285C, location of cysteine mutations of Pst-T4L derivatives, and determination of redox state by SDS-PAGE. A, superposition of Pst (orange) and the double Cys mutant PstS89C/S285C in the color coding red (T domain), blue (R domain), and green (A domain). The root mean square deviation between the two structures is 0.55 Å. The left panel shows a zoom-in into the structure with the disulfide bond highlighted that connects PstR with PstA. B, location of the residues mutated to cysteines that were used for studies of the redox-dependent uptake of Pst-T4L. Disulfide bonds are displayed as dashed lines in the structure of the Pst-T4L activity domain (Pst-T4LA). C and D, SDS-PAGE of purified double cysteine derivatives of Pst and Pst-T4L, respectively. Standard proteins were used as molecular weight markers (St; Fermentas). The gels were stained with Coomassie Blue. C, PstS89C/S285C oxidized with Cu2+ (ox, lane 3), reduced with 10 mm DTT (red, lane 1) or without addition (lane 2). D, Pst-T4LI176C/L331C (lanes 1–3), Pst-T4LS257C/Q289C (lanes 4–6), Pst-T4LD294C/R321C (lanes 7–9), and Pst-T4LI176C/R321C (lanes 10–12), oxidized with Cu2+ (ox, lanes 2, 5, 8, and 11), reduced with 10 mm DTT (red, lanes 3, 6, 9, and 12), or without addition (lanes 1, 4, 7, and 10).
TABLE 5.
In vivo activities of oxidized and reduced Pst-T4L and Pst disulfide derivatives
The numbers indicate the diameters of lysis zones (turbid lysis zones in parentheses) that were formed on LB agar plates seeded with the pesticin-sensitive E. coli strain SIP1322 fyuA+ onto which 6 μl of serial 101- to 105-fold dilutions of pesticin solutions were placed. The concentration of the stock solution was 10 μm for Pst-T4L wild type and derivatives and 2 mm for Pst wild type and derivatives. – indicates no lysis zone.
| Protein | Dilution |
||||
|---|---|---|---|---|---|
| 1:101 | 1:102 | 1:103 | 1:104 | 1:105 | |
| Pst-T4L wild type | 11 | 9 | 6.5 | (5) | – |
| Pst-T4L wild type + DTT | 11 | 9 | 6.5 | (5) | – |
| Pst-T4L wild type + Cu2+ | 11 | 9 | 6 | (5) | – |
| Pst-T4LI176C/L331C | – | – | – | – | – |
| Pst-T4LI176C/L331C + DTT | 11 | 9.5 | 7.5 | (5) | – |
| Pst-T4LS257C/Q289C | – | – | – | – | – |
| Pst-T4LS257C/Q289C + DTT | 10 | 8 | (8) | – | – |
| Pst-T4LD294C/R321C | 10 | 8 | 6 | (5) | – |
| Pst-T4LD294C/R321C + DTT | 10 | 8 | 6 | (5) | – |
| Pst-T4LD294C/R321C + Cu2+ | (10) | – | – | – | – |
| Pst-T4LI176C/R321C | 9 | (7.5) | – | – | – |
| Pst-T4LI176C/R321C + DTT | 9 | 7.5 | (6) | – | – |
| Pst wild type | 16 | 14.5 | 12 | 10 | 7 |
| PstS89C/S285C | – | – | – | – | – |
| PstS89C/S285C + DTT | 16.5 | 15 | 13 | 10.5 | 8 |
To examine whether small subdomains (e.g. two α-helices) must unfold for Pst uptake, intra-domain cysteine mutant pairs were prepared. For these studies the T4L domain of the Pst-T4L chimera was selected, because in vitro hydrolysis of PG by the disulfide mutant proteins could be tested, which cannot be done with Pst, which in vitro is inactive. In addition, we could rely on the structure and stability information of disulfide mutants of T4 lysozyme previously determined in the group of Matthews and co-workers (31). Like Pst, the Pst-T4L construct does not contain cysteines (the cysteines of T4 lysozyme were mutated to alanine and threonine). To examine whether disulfide bonds were formed, the Pst-T4L mutant proteins were isolated, purified, and applied to SDS-PAGE (Fig. 5D). The two cysteine residues in Pst-T4LI176C/L331C are localized at the N and C termini of the T4L domain, and the cysteine residues in Pst-T4LS257C/Q289C are located at the C-terminal ends of helices α4 and α7 (Fig. 5B). Isolated Pst-T4LI176C/L331C and Pst-T4LS257C/Q289C showed an electrophoretic mobility that was not altered upon incubation with Cu2+ to oxidize cysteines. After incubation with DTT and reduction of the disulfide bond, the proteins showed a lower electrophoretic mobility (Fig. 5D). These data indicate that the two proteins quantitatively formed disulfide bonds during isolation. In contrast, Pst-T4LD294C/R321C with the cysteines in two parallel helices α8 and α10 moved into the position of the reduced form and transformed into the oxidized form upon incubation with Cu2+. After incubation with DTT, the protein shifted back to the open form with retarded mobility. This protein did not spontaneously form disulfide bridges, but the cysteines were located sufficiently close enough that Cu2+ induced disulfide bond formation. A fourth double mutant Pst-T4LI176C/R321C was constructed as control with both mutated residues located far apart from each other, and intramolecular disulfide bond formation was not expected. In SDS-PAGE, the protein was primarily located at the position of the reduced form, but a small portion moved slower at the position corresponding to the cross-linked protein dimer (Fig. 5D). The formation of a stable structure after cross-linking was examined by determination of the melting temperatures. Although the Pst-T4L chimera showed a transition state temperature of 49.7 °C both under reducing and oxidizing conditions, Pst-T4LI176C/L331C showed unfolding at 53.4 °C in the oxidized form and at 49.4 °C in the reduced form. In comparison, Pst-T4LS257C/Q289C was unfolded at 50.0 °C in the oxidized form and at 47.6 °C in the reduced form (supplemental Fig. S7).
To examine the activities of the disulfide derivatives, 10-fold serially diluted protein solutions were spotted onto nutrient agar plates seeded with E. coli SIP1332 fyuA+. As expected, both oxidized Pst-T4LI176C/L331C and Pst-T4LS257C/Q289C constructs did not inhibit cell growth (Table 5). Upon reduction with DTT, Pst-T4LI176C/L331C expressed the same activity as wild type Pst-T4L, whereas Pst-T4LS257C/Q289C showed a 10-fold lower but still significant antimicrobial activity (Table 5). The constructs harboring disulfide bonds were inactive in the oxidized form and became active after reduction to cysteines. In contrast, Pst-T4LD294C/R321C showed the same activity as wild type Pst-T4L but became inactive upon incubation with Cu2+. This mutant did not spontaneously form disulfide bridges, and oxidation by Cu2+ was required. In the three mutant proteins tested, disulfide bond formation hampered unfolding of the A domain, which rendered the cross-linked mutants inactive. Pst-T4LI176C/R321C showed residual activity which upon reduction was 10-fold increased. Introduction of the cysteines led to an aberrant form (dimer), and the wild type structure was not fully restored upon reduction.
To examine whether introduction of cysteine residues and formation of disulfide bonds did not influence the activity of the T4L domain, the in vitro activity of oxidized Pst-T4LI176C/L331C and Pst-T4LS257C/Q289C was tested with fluorescently marked cell walls of M. lysodeikticus. Both derivatives showed activities in the range of Pst-T4L activity (shown in supplemental Fig. S5 for Pst-T4LI176C/L331C). The data clearly show an unaltered enzymatic activity of the disulfide-linked Pst-T4L proteins, which excluded that disulfide bond formation inactivated their active sites. The data indicate that cross-linking of the R domain with the A domain and cross-linking within the A domain prevents unfolding and as a consequence uptake of Pst and Pst-T4L into the periplasm.
Pst and the Pst Activity Domain Kill Cells if Imported from the Cytoplasm
Bacteriocins like colicin M and Pst predominantly remain in the cytoplasm because their operons do not encode lysis proteins for release of the bacteriocins. For colicin M it has been shown that the colicin must be imported to kill cells (32). To test if Pst when secreted from the cytoplasm into the periplasm by the Sec machinery can degrade PG, we fused Pst and its activity domain PstA to the signal peptide of the MalE periplasmic binding protein. The genes were cloned downstream of the ara promoter that tightly controls transcription of downstream genes. In addition, glucose represses transcription. This was important for cloning because noninduced basal levels of pst transcription might have resulted in the failure to obtain transformants because of cell lysis. In addition, the level of expression can be adjusted with arabinose up to 1200-fold (33). As controls, we chose the proteins without signal sequence and the in vivo inactive PstE178A mutant. Cells were grown either in the presence of 0.2% glucose or with various concentrations of arabinose to induce transcription. Lysis was determined by quantification of the absorbance of growing cultures. Because MalE′-Pst lysed cells already without induction at 37 °C, cells were cultured at 30 °C in the presence of 0.2% glucose. Under these conditions, cells stopped growing but did not lyse (Fig. 6A). Expression of MalE′-Pst and induction of cell lysis were differentially induced and monitored through addition of 0.0001 to 0.1% arabinose. Cells lysed already when grown in the presence of 0.001% arabinose (Fig. 6A). A qualitatively similar result was obtained with MalE′-PstA, which lysed cells in the presence of 0.1% arabinose; 0.01% arabinose stopped growth and 0.001% retarded growth. MalE′-PstA secreted via the Sec system into the periplasm killed cells but was less effective than MalE′-Pst (Fig. 6B). Compared with MalE′-Pst, less of the synthesized MalE′-PstA is processed (MalE signal sequence cut off) and exported into the periplasm as shown by Western blotting of MalE′-PstE178A and MalE′-PstA E178A, which do not lyse cells (see below), and immunodetection with a monoclonal anti-His tag antibody (data not shown). This might be due to a higher propensity of PstA than Pst to form inclusion bodies in the cytoplasm (see under “Experimental Procedures”). The controls, Pst and PstA without MalE signal sequence (data not shown) as well as MalE′-PstE178A and MalE′-PstA E178A carrying an active site mutation, did not lyse cells (Fig. 6, A and B). The A domain apparently folds in the periplasm to an active domain, and folding does not require the RT domain. Import across the outer membrane via FyuA, TonB, ExbB, and ExbD is not required.
FIGURE 6.

A, growth curves of E. coli DH5α transformed with plasmids that encode wild type Pst fused to the MalE signal sequence at 30 °C. Transcription was induced with 0.1% arabinose (■) and 0.001% arabinose (▾) or repressed by 0.2% glucose (glc) (●). Cells transformed with the mutated MalE′-PstE178A plasmid were not inhibited after induction with 0.1% arabinose (□). B, growth curves of E. coli SIP1332 fyuA+ transformed with plasmids that encode PstA fused to the MalE signal sequence at 37 °C. Transcription was induced with 0.1% arabinose (■), 0.01% arabinose (▴), or repressed by 0.2% glucose (●). Cells transformed with the mutated MalE′-PstA(E178A) plasmid were not lysed after induction with 0.1% arabinose (□) and 0.01% arabinose (Δ).
Investigation of the Active Site of Pesticin
PstA and T4 lysozyme show a very similar fold and the same enzymatic activity. To further characterize the active center of Pst, we employed structural analysis, sequence comparison (Fig. 7A), and mutant analysis. Based on the extensive studies by Matthews and co-workers (34, 35) who identified Glu-11, Asp-20, and Thr-26 as key residues for enzymatic activity, we selected equivalent residues in Pst. Pairwise sequence and structural alignment of the two proteins only partially matched due to the low sequence identity of the proteins (13%). Mutants based on the alignments of Pst with Pst homologs (supplemental Fig. S6B) seemed to be more reliable for active site determination. Glu-178, Pro-187, and Thr-201 were selected for alanine substitutions because they are located in the predicted active center (Fig. 7A). Asp-207 was selected due to its proximity to the active site (Fig. 7, A and B) (35), and Gln-301, although distant from the active site, corresponds to Gln-105 of T4L. Asp-207 in Pst has no functionally conserved residue in T4L but may resemble Asp-20 of T4L because it is the only Asp residue in proximity to Glu-178 in Pst. Finally, PstD338A was constructed as a negative control because this residue is an Asp residue apart from the active site (Fig. 7B). Mutant proteins were purified to electrophoretic homogeneity (supplemental Fig. S1B), and the activities of the resulting derivatives were tested in vivo. 10-Fold serial dilutions of the purified proteins were spotted on nutrient agar plates seeded with E. coli SIP1322 carrying the plasmid-encoded fyuA gene that conferred sensitivity to Pst. The wild type Pst and PstD338A solution could be diluted up to 105-fold to yield clear lysis zones (Table 6). In contrast, PstE178A, PstT201A, and PstD207A were completely inactive, whereas PstP187A and PstQ301A displayed 10% of wild type activity. The mutations did not alter the overall structure of the mutant proteins, as revealed by the CD spectra that were identical to wild type Pst (supplemental Fig. S8B) and by determination of the crystal structures (supplemental Fig. S8A). The melting temperatures Tm of PstE178A, PstP187A, PstT201A, PstQ301A, and PstD338A were similar to the Tm of wild type Pst (supplemental Fig. S8C), whereas the Tm value for PstD207A was ∼4 °C lower. To verify that the inactive proteins were not impaired in binding to FyuA, competition with killing by Pst-T4L was determined for PstD207A as an example. At a 10-fold molar excess over Pst-T4L, PstD207A completely prevented lysis zone formation (Table 3), indicating that it binds FyuA.
FIGURE 7.
Tracing the active center of Pst using point mutants. A, sequence and structural alignment of the activity domain of Pst and T4 lysozyme. In the sequence alignment (lines 1–3), the identical residues are marked with asterisk, strongly conserved residues are marked with colon, and weakly conserved residues are marked with period. In the structural alignment, only the identical positions (within a root mean square deviation of 3.5 Å between Cα positions) are marked (*). Residues mutated in Pst for activity assays are marked in green. Active site residues in T4 lysozyme are highlighted in red. B, superposition of the structures of PstA and T4 lysozyme (PDB code 168L) in ribbon representation in two orientations (left and right). Residues chosen for mutagenesis according to the structure and sequence alignment were mutated into alanines (see also A). These residues are marked as sticks together with residue and number for Pst and compared with identical T4 lysozyme positions (T4 lysozyme/Pst). Additional residues mutated in Pst are marked by sticks and residue numbers (Asp-207, Gln-301, and Asp-338).
TABLE 6.
Activities of Pst and its predicted active site derivatives
The numbers indicate the diameters of lysis zones (in mm) that were formed on LB agar plates seeded with the pesticin-sensitive E. coli strain SIP1322 fyuA+ onto which serial 20 μl of 101- to 106-fold dilutions of pesticin solutions (2 mm) were placed. – indicates no lysis zone.
| Protein | Dilution |
|||||
|---|---|---|---|---|---|---|
| 1:101 | 1:102 | 1:103 | 1:104 | 1:105 | 1:106 | |
| PstWT | 20 | 17 | 15 | 14 | 10 | – |
| PstE178A | – | – | – | – | – | – |
| PstP187A | 14 | 14 | 11 | 10 | – | – |
| PstT201A | – | – | – | – | – | – |
| PstD207A | – | – | – | – | – | – |
| PstQ301A | 16 | 15 | 14 | 12 | – | – |
| PstD338A | 20 | 17 | 15 | 14 | 10 | – |
PG hydrolysis of the Pst mutant proteins could not be tested in vitro by hydrolysis of E. coli spheroplasts, M. lysodeikticus cells, and fluorescein-labeled M. lysodeikticus cell walls, because Pst was inactive in these assays. However, these substrates were rapidly hydrolyzed by HEWL and isolated T4L (see above for fluorescein-labeled M. lysodeikticus cell walls). The failure to hydrolyze these substrates agrees with the known low enzymatic activity of pesticin (3, 4).
DISCUSSION
Bacteria compete with closely related bacteria of the same niche by secretion of toxins into the external space. In natural E. coli cultures, a significant number of roughly 50% of bacteria harbor plasmids that encode a variable number of colicins, immunity proteins, and lysis proteins produced to allow for the improved release into the external medium (1). Although bacteriocins of the colicin type have been intensively studied for many years, related proteins from pathogenic bacteria remained enigmatic, although protein toxins from many pathogenic bacteria, including Vibrio cholerae, Pseudomonas aeruginosa, Salmonella typhimurium, and Y. pestis, for example, have been described in the literature. Recently, the application of engineered E. coli strains secreting pyocin was shown to inhibit the formation of biofilms, and killing of pathogenic P. aeruginosa was demonstrated (36). Further possible applications of bacteriocins in targeting pathogenic bacteria also require studies as to the mechanisms by which these molecules become active to improve targeting strategies in the future.
Yersinia bacteria harbor small plasmids that encode pesticin as the unique anti-microbial weapon. These anti-bacterial proteins are similar in function to other toxins or antibiotics but act more specific against a subset of closely related bacteria. By contrast to small anti-microbial agents (peptides and chemicals), bacteriocins misuse existing transport systems for substrates via outer membrane receptors coupled either to the Ton or Tol systems to kill bacteria in their vicinity. To understand the mechanism by which pesticin from Y. pestis mechanistically acts on related bacteria, we have analyzed its crystal structure and found a similar domain organization as known for colicins. Three structurally separated domains, the N-terminal translocation domain, the receptor binding domain, and the C-terminal activity domain, are present. This architecture is archetypical for most bacteriocins.
The structure of pesticin revealed several unexpected features. Subdivision of colicins into domains is sometimes apparent from the structural or sequence analysis. In Pst, the RT domain forms one entity and the A domain another compact structured domain that are linked by a short unstructured linker. In many colicin structures the A domains are well separated from the R and T domains, although this rule does not apply to colicin M, for example. In some colicins, e.g. E3 and Ia, the R and T domains form separate folds, whereas in colicins B, M, and S4 (37), the T domains are less structured and form flexible regions that are prone to unfold during import across the outer membrane (1). In a structural homology search, domains similar to Pst appeared only for the A domain that shows a structure similar to phage proteins (Fig. 2). Despite the low sequence identity of 13% between Pst and T4 lysozyme, the Pst A domain folds similarly into two domains (AD1 and AD2, equivalent to the N- and C-terminal domain in T4 lysozyme) with small structural deviations due to insertions in the sequence. So far, none of the bacteriocin A domains (DNases, RNases, or phosphatases) displayed an overall structure similarity to existing enzymes. Although the sequence identity to T4 lysozyme is low, there is a clear sequence relationship between the Pst A domain and the related lysozymes. Therefore, it is tempting to speculate that one of the phage lysozymes formed the evolutionary precursor of Pst (supplemental Fig. S2). Although shuffling of DNA fragments encoding R, T, or A domains is a major driving force for the evolution of colicins (38–40), the exchange of DNA fragments was observed among colicins but not with chromosomal genes, phage genes, and plasmid-encoded genes outside colicin loci. Pst may be the first representative of this kind. By contrast to the A domain, neither the T nor the R domain show any significant sequence relationship to known proteins and may have specifically evolved to bind to the import receptor. This is typical for several colicins, including colicin M, for which related activity domains exist but the T and R domains are unique (29).
Pesticin is unique for additional reasons, one of which is degradation of PG. This periplasmic activity of pesticin is shared so far only with colicin M from E. coli, although this protein acts as phosphatase on the lipid-linked biosynthetic precursor of PG. Both bacteriocins bind and translocate via iron siderophore receptors, FhuA for colicin M and FyuA for pesticin. In analogy to colicin M, Pst harbors an elongated and unstructured T domain with an approximate length of 40 residues. Another example is colicin B, which is recognized and translocated through the FepA iron receptor (30). This colicin also comprises a long unfolded and partially unstructured N-terminal domain of ∼60 residues, which is slightly longer than that of Pst or colicin M. The T domains are firmly attached to the protein and wrap around the R domain of the bacteriocin species (Fig. 3, A and B). The length of these extensions if elongated is sufficient to span the entire receptor of ∼ 8 nm and to attach to the periplasmic TonB protein. If the length of the Pst N terminus is reduced by 5 or 10 residues, while keeping the TonB box motif intact as prerequisite for translocation, the activity of the protein is reduced or abolished.
To test the plasticity of Pst, we exchanged the structurally well separated A domain and replaced it by T7 and T4 lysozymes to examine whether these chimera killed E. coli cells. Although overexpressed Pst-T7 lysozyme was inactive, the Pst-T4L fusion was active, and import was demonstrated. The Pst-T4L hybrid protein caused clear lysis zones on nutrient agar plates seeded with a pesticin-sensitive E. coli strain and lysed cells in liquid culture. The high activity of Pst-T4L indicated the efficient uptake into the periplasmic space via the RT domain and the FyuA receptor. Given the low sequence similarities between T4 lysozyme and PstA, the uptake of the A domain across the outer membrane apparently has no or little sequence specificity. Nevertheless, it appears plausible that for the directed import into the periplasm, involving unfolding and refolding, the structure of the protein may need to resemble the structure of PstA, as is the case for T4L. Both domains unfold already under relatively moderate conditions (Tm ∼50 °C), which may be a prerequisite for their successful import via the Pst RT domain. Pst-T4L killed cells less effective than Pst (size of the lysis zones in plaque assays). The lower in vivo activity may be caused by a less efficient translocation across the outer membrane for which PstA was optimized during evolution but not T4L. In particular, the unfolding rate may be reduced by the α-helix that connects the Pst RT domain with T4L. In contrast to T4L, pesticin did not hydrolyze isolated fluorescein-labeled PG of M. lysodeikticus. This in vitro assay can be used to discriminate between T4L and Pst activity, and the data argue for a difference in the active site of T4L and Pst. We also tried this assay at pH 4.7, which is reported to be the optimum for Pst activity (41), and with addition of Ca2+, but Pst did not hydrolyze the substrate. It might be that the difference in peptide side chains of PG of M. lysodeikticus compared with PG of E. coli or a steric hindrance due to the fluorescein labeling of amino groups account for this. However, also E. coli PG is hydrolyzed only slowly. In former experiments, radiolabeled E. coli PG was incubated up to 5 h with a 6-fold higher concentration than HEWL to identify the cleavage site of Pst (4). The low PG hydrolase activity seems to be sufficient to kill E. coli cells. We consider it as less likely that in addition to PG hydrolysis Pst disrupts PG biosynthesis by binding to PG or that an additional factor in the periplasm enhances the Pst hydrolysis rate. Rapid killing of cells despite formation of stable spheroplasts in the absence of osmotically stabilizing sucrose indicates a special mode of action based on slow PG hydrolysis.
Although T4L folds like PstA and has the same enzymatic activity, it is not recognized by the Pst immunity protein Pim. The sequences of Pst and T4L are not sufficiently similar that Pim binds to T4L. Lack of inactivation shows that Pim binds preferentially to PstA and that the RT domain plays no major role. However, the helix connecting PstRT with T4L may also interfere with Pim binding.
Proteins typically need to unfold during their passage across membranes. This has been demonstrated for the bacterial inner membrane and the outer membrane of mitochondria. However, the extent of unfolding has not been clearly determined, and it has been reported that the TOM complex of the mitochondrial membrane allows for the passage of small and folded secondary structural elements. To examine to what extent Pst needs to be unfolded, we introduced disulfide bridges between domains of Pst and between secondary structural elements of the activity domain of the Pst-T4L chimera and monitored the redox-dependent activity in vivo and in vitro. The oxidized forms of these proteins were completely inactive, but activity could be recovered upon reduction through reducing agents. To exclude that the cross-links inactivated the lysozyme domain, two of the oxidized and reduced forms were incubated in vitro with PG. Both forms displayed activities similar to wild type Pst-T4L. Inactivity of the disulfide cross-linked derivatives was not caused by inactivation of the lysozyme. Rather, the cross-linked derivatives were impaired in uptake because unfolding was prevented. Evidence for unfolding of colicins during import has been obtained by various techniques, such as disulfide cross-linking of colicins (42–44), FRET analysis of labeled colicin (45), competition between colicins (46), and identification of the released A domain (nuclease) in the cytoplasm (47). Thus, interpretation of the cross-linking data of Pst-T4L is in agreement with previous results on related colicins (see also Discussion in Ref 48). Although we expected incremental differences in activity due to distinct unfolding properties of the disulfide mutants, the total lack of activity indicates that the uptake system does not tolerate cross-linking of domains and subdomains, even if structurally neighboring helices are concerned. Presumably, the diameter of the uptake receptor barrel restricts the simultaneous translocation of (partially) folded secondary structural elements. This does not exclude that helices remain folded during their passage through the uptake channel.
Colicins that are co-expressed with a lysis protein are not released from cells by specific export systems but by quasi-lysis of the cell membranes. Colicins without this lysis protein remain inside cells (1). The lack of a lysis protein retains more than 90% of Pst inside producer cells. We asked whether Pst and its A domain equipped with a signal sequence were secreted by the Sec apparatus from the cytoplasm into the periplasm. Unfolded proteins are secreted by Sec and must refold in the periplasm to be active in this compartment. We tested cell lysis at various protein expression levels. Pst and the Pst A domain lysed cell, which means that they were secreted and refolded. Secretion across the cytoplasmic membrane supports the ability of Pst to unfold during import across the outer membrane. The Pst activity domain folds in the periplasm without the help of the RT domain.
Although the same bond in PG is hydrolyzed by Pst and T4L, the catalytic mechanism may be slightly distinct because residues in the predicted active center of Pst differ from residues known to contribute to PG binding and hydrolysis of T4L (Fig. 7B) (49, 50). Five residues of Pst were replaced by alanine, and the activity of the resulting proteins was determined by inspecting lysis of cells on nutrient agar plates. Three of the proteins (PstE178A, PstT201A, and PstD207A) were inactive. Two of the residues (Glu-178 and Thr-201) are structurally conserved between Pst and T4 lysozyme. Two mutants (PstP187A and PstQ301A) showed a 10-fold reduced activity. Apart from Gly, Ala, and Leu, Glu-178, Pro-187, Thr-201, Asp-207, and Gln-301 are the only residues that are strictly conserved throughout all sequences in an alignment of Pst with Pst homologs (supplemental Fig. S6B). The inactive mutants may define residues involved in hydrolysis, whereas the partially active mutants may affect residues that bind the substrate or affect folding.
CONCLUSIONS
This paper describes for the first time the structure of a bacteriocin that kills cells by degrading PG. Determination of the crystal structure of Pst revealed a similar fold to T4 lysozyme and lysozyme homologs. This finding is somewhat surprising as lysozymes have very different sequences, and no colicin up to now resembled the structure of another known enzyme fold. We showed that the length of the T domain is important for uptake. Furthermore, the replacement of PstA by T4L yielded a functional toxin that was taken up across the outer membrane by the RT domain. This result allows a number of future studies with respect to the general plasticity of import mechanisms (e.g. domain size, stability) and may in principle allow the import of other bacterial and nonbacterial proteins. The cross-linking data indicate that Pst and Pst-T4L need to unfold during import into the periplasm. Moreover, unfolded Pst and PstA fused to a signal sequence are secreted from the cytoplasm into the periplasm by the Sec translocase and fold in the periplasm to an active toxin. Translocation across the outer membrane differs from translocation across the cytoplasmic membrane. Folding of the A domain to the active toxin occurs in the periplasm regardless of whether the RT domain is present or not. Both Pst and PstA did not hydrolyze M. lysodeikticus cell walls. This low in vitro hydrolysis is an intrinsic property of Pst. Finally, we identified the active site residues of Pst that are similar but not identical to those of T4 lysozyme, and we revealed additional amino acids previously not identified that influence Pst activity.
Acknowledgments
V. B. and S. I. P. thank Andrei N. Lupas for generous hospitality and discussion. Thomas Ellinghaus and Niklas Hoffmann made initial contributions to this work. We thank the entire beamline staff of PXII at the Swiss Light Source (SLS) for beamline maintenance and continuous support.
This work was supported by the Max Planck Society and the Fonds der Chemischen Industrie (to V. B.).

This article contains supplemental Figs. S1–S8 and additional references.
- Pst
- pesticin
- SeMet
- selenomethionine
- PDB
- Protein Data Bank
- HEWL
- hen egg white lysozyme
- PG
- peptidoglycan
- Ni-NTA
- nickel-nitrilotriacetic acid
- PEG
- polyethylene glycol.
REFERENCES
- 1. Cascales E., Buchanan S. K., Duché D., Kleanthous C., Lloubès R., Postle K., Riley M., Slatin S., Cavard D. (2007) Colicin biology. Microbiol. Mol. Biol. Rev. 71, 158–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schaller K., Höltje J. V., Braun V. (1982) Colicin M is an inhibitor of murein biosynthesis. J. Bacteriol. 152, 994–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hall P. J., Brubaker R. R. (1978) Pesticin-dependent generation of somotically stable spheroplast-like structures. J. Bacteriol. 136, 786–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vollmer W., Pilsl H., Hantke K., Höltje J. V., Braun V. (1997) Pesticin displays muramidase activity. J. Bacteriol. 179, 1580–1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pilsl H., Killmann H., Hantke K., Braun V. (1996) Periplasmic location of the pesticin immunity protein suggests inactivation of pesticin in the periplasm. J. Bacteriol. 178, 2431–2435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kurisu G., Zakharov S. D., Zhalnina M. V., Bano S., Eroukova V. Y., Rokitskaya T. I., Antonenko Y. N., Wiener M. C., Cramer W. A. (2003) The structure of BtuB with bound colicin E3 R domain implies a translocon. Nat. Struct. Biol. 10, 948–954 [DOI] [PubMed] [Google Scholar]
- 7. Sharma O., Yamashita E., Zhalnina M. V., Zakharov S. D., Datsenko K. A., Wanner B. L., Cramer W. A. (2007) Structure of the complex of the colicin E2 R domain and its BtuB receptor. The outer membrane colicin translocon. J. Biol. Chem. 282, 23163–23170 [DOI] [PubMed] [Google Scholar]
- 8. Buchanan S. K., Lukacik P., Grizot S., Ghirlando R., Ali M. M., Barnard T. J., Jakes K. S., Kienker P. K., Esser L. (2007) Structure of colicin I receptor bound to the R-domain of colicin Ia: implications for protein import. EMBO J. 26, 2594–2604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jakes K. S., Finkelstein A. (2010) The colicin Ia receptor, Cir, is also the translocator for colicin Ia. Mol. Microbiol. 75, 567–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rakin A., Boolgakowa E., Heesemann J. (1996) Structural and functional organization of the Yersinia pestis bacteriocin pesticin gene cluster. Microbiology 142, 3415–3424 [DOI] [PubMed] [Google Scholar]
- 11. Sambrook J., Russell D. W. (2001) Molecular Cloning: A Laboratory Manual, 3 Ed., Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 12. Helbig S., Patzer S. I., Schiene-Fischer C., Zeth K., Braun V. (2011) Activation of colicin M by the FkpA prolyl cis-trans isomerase/chaperone. J. Biol. Chem. 286, 6280–6290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Papworth C., Bauer J. C., Braman J., Wright D. A. (1996) Site-directed mutagenesis in 1 day with >80% efficiency. Strategies 9, 3–4 [Google Scholar]
- 14. Hanoulle X., Rollet E., Clantin B., Landrieu I., Odberg-Ferragut C., Lippens G., Bohin J. P., Villeret V. (2004) Structural analysis of Escherichia coli OpgG, a protein required for the biosynthesis of osmoregulated periplasmic glucans. J. Mol. Biol. 342, 195–205 [DOI] [PubMed] [Google Scholar]
- 15. Kabsch W. (2010) Integration, scaling, space-group assignment and post-refinement. Acta Crystallogr. D Biol. Crystallogr. 66, 133–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vonrhein C., Blanc E., Roversi P., Bricogne G. (2007) Automated structure solution with autoSHARP. Methods Mol. Biol. 364, 215–230 [DOI] [PubMed] [Google Scholar]
- 17. Sheldrick G. M. (2010) Experimental phasing with SHELXC/D/E. Combining chain tracing with density modification. Acta Crystallogr. D Biol. Crystallogr. 66, 479–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Langer G., Cohen S. X., Lamzin V. S., Perrakis A. (2008) Automated macromolecular model building for x-ray crystallography using ARP/wARP version 7. Nat. Protoc. 3, 1171–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cowtan K. (2006) The Buccaneer software for automated model building. 1. Tracing protein chains. Acta Crystallogr. D Biol. Crystallogr. 62, 1002–1011 [DOI] [PubMed] [Google Scholar]
- 20. Zwart P. H., Afonine P. V., Grosse-Kunstleve R. W., Hung L. W., Ioerger T. R., McCoy A. J., McKee E., Moriarty N. W., Read R. J., Sacchettini J. C., Sauter N. K., Storoni L. C., Terwilliger T. C., Adams P. D. (2008) Automated structure solution with the PHENIX suite. Methods Mol. Biol. 426, 419–435 [DOI] [PubMed] [Google Scholar]
- 21. Emsley P., Lohkamp B., Scott W. G., Cowtan K. (2010) Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Laskowski R. A., MacArthur M. W., Moss D. S., Thornton J. M. (1993) PROCHECK. A program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 26, 283–291 [Google Scholar]
- 23. Holm L., Kääriäinen S., Rosenström P., Schenkel A. (2008) Searching protein structure databases with DaliLite version 3. Bioinformatics 24, 2780–2781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kanamaru S., Ishiwata Y., Suzuki T., Rossmann M. G., Arisaka F. (2005) Control of bacteriophage T4 tail lysozyme activity during the infection process. J. Mol. Biol. 346, 1013–1020 [DOI] [PubMed] [Google Scholar]
- 25. Weaver L. H., Grütter M. G., Matthews B. W. (1995) The refined structures of goose lysozyme and its complex with a bound trisaccharide show that the “goose-type” lysozymes lack a catalytic aspartate residue. J. Mol. Biol. 245, 54–68 [DOI] [PubMed] [Google Scholar]
- 26. Vocadlo D. J., Davies G. J., Laine R., Withers S. G. (2001) Catalysis by hen egg-white lysozyme proceeds via a covalent intermediate. Nature 412, 835–838 [DOI] [PubMed] [Google Scholar]
- 27. Mende J., Braun V. (1990) Import-defective colicin B derivatives mutated in the TonB box. Mol. Microbiol. 4, 1523–1533 [DOI] [PubMed] [Google Scholar]
- 28. Mora L., Diaz N., Buckingham R. H., de Zamaroczy M. (2005) Import of the transfer RNase colicin D requires site-specific interaction with the energy-transducing protein TonB. J. Bacteriol. 187, 2693–2697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zeth K., Römer C., Patzer S. I., Braun V. (2008) Crystal structure of colicin M, a novel phosphatase specifically imported by Escherichia coli. J. Biol. Chem. 283, 25324–25331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hilsenbeck J. L., Park H., Chen G., Youn B., Postle K., Kang C. (2004) Crystal structure of the cytotoxic bacterial protein colicin B at 2.5 Å resolution. Mol. Microbiol. 51, 711–720 [DOI] [PubMed] [Google Scholar]
- 31. Matsumura M., Becktel W. J., Levitt M., Matthews B. W. (1989) Stabilization of phage T4 lysozyme by engineered disulfide bonds. Proc. Natl. Acad. Sci. U.S.A. 86, 6562–6566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Harkness R. E., Braun V. (1990) Colicin M is only bactericidal when provided from outside the cell. Mol. Gen. Genet. 222, 37–40 [DOI] [PubMed] [Google Scholar]
- 33. Guzman L. M., Belin D., Carson M. J., Beckwith J. (1995) Tight regulation, modulation, and high level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177, 4121–4130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Baase W. A., Liu L., Tronrud D. E., Matthews B. W. (2010) Lessons from the lysozyme of phage T4. Protein Sci. 19, 631–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kuroki R., Weaver L. H., Matthews B. W. (1993) A covalent enzyme-substrate intermediate with saccharide distortion in a mutant T4 lysozyme. Science 262, 2030–2033 [DOI] [PubMed] [Google Scholar]
- 36. Saeidi N., Wong C. K., Lo T. M., Nguyen H. X., Ling H., Leong S. S., Poh C. L., Chang M. W. (2011) Engineering microbes to sense and eradicate Pseudomonas aeruginosa, a human pathogen. Mol. Syst. Biol. 7, 521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Arnold T., Zeth K., Linke D. (2009) Structure and function of colicin S4, a colicin with a duplicated receptor-binding domain. J. Biol. Chem. 284, 6403–6413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Roos U., Harkness R. E., Braun V. (1989) Assembly of colicin genes from a few DNA fragments. Nucleotide sequence of colicin D. Mol. Microbiol. 3, 891–902 [DOI] [PubMed] [Google Scholar]
- 39. Riley M. A., Wertz J. E. (2002) Bacteriocin diversity. Ecological and evolutionary perspectives. Biochimie 84, 357–364 [DOI] [PubMed] [Google Scholar]
- 40. Braun V., Patzer S. I., Hantke K. (2002) Ton-dependent colicins and microcins. Modular design and evolution. Biochimie 84, 365–380 [DOI] [PubMed] [Google Scholar]
- 41. Ferber D. M., Brubaker R. R. (1979) Mode of action of pesticin. N-Acetylglucosaminidase activity. J. Bacteriol. 139, 495–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Penfold C. N., Healy B., Housden N. G., Boetzel R., Vankemmelbeke M., Moore G. R., Kleanthous C., James R. (2004) Flexibility in the receptor-binding domain of the enzymatic colicin E9 is required for toxicity against Escherichia coli cells. J. Bacteriol. 186, 4520–4527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Duché D., Baty D., Chartier M., Letellier L. (1994) Unfolding of colicin A during its translocation through the Escherichia coli envelope as demonstrated by disulfide bond engineering. J. Biol. Chem. 269, 24820–24825 [PubMed] [Google Scholar]
- 44. Zhang Y., Vankemmelbeke M. N., Holland L. E., Walker D. C., James R., Penfold C. N. (2008) Investigating early events in receptor binding and translocation of colicin E9 using synchronized cell killing and proteolytic cleavage. J. Bacteriol. 190, 4342–4350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zakharov S. D., Sharma O., Zhalnina M. V., Cramer W. A. (2008) Primary events in the colicin translocon. FRET analysis of colicin unfolding initiated by binding to BtuB and OmpF. Biochemistry 47, 12802–12809 [DOI] [PubMed] [Google Scholar]
- 46. Duché D. (2007) Colicin E2 is still in contact with its receptor and import machinery when its nuclease domain enters the cytoplasm. J. Bacteriol. 189, 4217–4222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chauleau M., Mora L., Serba J., de Zamaroczy M. (2011) FtsH-dependent processing of RNase colicins D and E3 means that only the cytotoxic domains are imported into the cytoplasm. J. Biol. Chem. 286, 29397–29407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kleanthous C. (2010) Swimming against the tide. Progress and challenges in our understanding of colicin translocation. Nat. Rev. Microbiol. 8, 843–848 [DOI] [PubMed] [Google Scholar]
- 49. Anderson W. F., Grütter M. G., Remington S. J., Weaver L. H., Matthews B. W. (1981) Crystallographic determination of the mode of binding of oligosaccharides to T4 bacteriophage lysozyme. Implications for the mechanism of catalysis. J. Mol. Biol. 147, 523–543 [DOI] [PubMed] [Google Scholar]
- 50. Tsugita A. (1971) in The Enzymes (Boyer P. D., ed) 3rd Ed., pp. 343–411, Academic Press, New York [Google Scholar]
- 51. Studier F. W., Moffatt B. A. (1986) Use of bacteriophage T7 RNA polymerase to direct selective high level expression of cloned genes. J. Mol. Biol. 189, 113–130 [DOI] [PubMed] [Google Scholar]
- 52. Miroux B., Walker J. E. (1996) Overproduction of proteins in Escherichia coli. Mutant hosts that allow synthesis of some membrane proteins and globular proteins at high levels. J. Mol. Biol. 260, 289–298 [DOI] [PubMed] [Google Scholar]
- 53. Rakin A., Saken E., Harmsen D., Heesemann J. (1994) The pesticin receptor of Yersinia enterocolitica. A novel virulence factor with dual function. Mol. Microbiol. 13, 253–263 [DOI] [PubMed] [Google Scholar]