Background: Contractions activate the sodium pump, Na+,K+-ATPase, and the energy sensor, AMP-activated protein kinase (AMPK), in skeletal muscle.
Results: AMPK activation increases Na+,K+-ATPase activity in skeletal muscle cells.
Conclusion: Activation of Na+,K+-ATPase in skeletal muscle cells is AMPK-dependent.
Significance: AMPK activation of the sodium pump may be crucial during exercise to maintain ion homeostasis and delay muscle fatigue.
Keywords: AMP-activated Kinase (AMPK); Na,K-ATPase; Potassium Transport; PP2A; Skeletal Muscle
Abstract
Contraction stimulates Na+,K+-ATPase and AMP-activated protein kinase (AMPK) activity in skeletal muscle. Whether AMPK activation affects Na+,K+-ATPase activity in skeletal muscle remains to be determined. Short term stimulation of rat L6 myotubes with the AMPK activator 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR), activates AMPK and promotes translocation of the Na+,K+-ATPase α1-subunit to the plasma membrane and increases Na+,K+-ATPase activity as assessed by ouabain-sensitive 86Rb+ uptake. Cyanide-induced artificial anoxia, as well as a direct AMPK activator (A-769662) also increase AMPK phosphorylation and Na+,K+-ATPase activity. Thus, different stimuli that target AMPK concomitantly increase Na+,K+-ATPase activity. The effect of AICAR on Na+,K+-ATPase in L6 myotubes was attenuated by Compound C, an AMPK inhibitor, as well as siRNA-mediated AMPK silencing. The effects of AICAR on Na+,K+-ATPase were completely abolished in cultured primary mouse muscle cells lacking AMPK α-subunits. AMPK stimulation leads to Na+,K+-ATPase α1-subunit dephosphorylation at Ser18, which may prevent endocytosis of the sodium pump. AICAR stimulation leads to methylation and dephosphorylation of the catalytic subunit of the protein phosphatase (PP) 2A in L6 myotubes. Moreover, AICAR-triggered dephosphorylation of the Na+,K+-ATPase was prevented in L6 myotubes deficient in PP2A-specific protein phosphatase methylesterase-1 (PME-1), indicating a role for the PP2A·PME-1 complex in AMPK-mediated regulation of Na+,K+-ATPase. Thus contrary to the common paradigm, we report AMPK-dependent activation of an energy-consuming ion pumping process. This activation may be a potential mechanism by which exercise and metabolic stress activate the sodium pump in skeletal muscle.
Introduction
Na+,K+-ATPase is a transmembrane heterodimer ion pump that exchanges intracellular Na+ for extracellular K+ (1). The minimal functional pump consists of a catalytic α-subunit and a β-subunit, which controls the assembly of the heterodimer and the insertion into the membrane. Several isoforms of these subunits exist, with four α- and three β-subunits identified (2). Skeletal muscle expresses α1, α2, β1, β2, and β3 isoforms (3–5). Na+,K+-ATPase is critically involved in the maintenance of the intracellular Na+ and K+ concentrations and participates in the maintenance of cell volume and electrochemical gradients. Na+,K+-ATPase also promotes membrane repolarization and re-uptake of extracellular K+ in excitable cells, including skeletal muscle (6, 7). During exercise, K+ and Na+ are displaced across the skeletal muscle sarcolemma, which is counteracted by an increase in intrinsic activity of Na+,K+-ATPase and its abundance at the plasma membrane (7, 8). Contraction-induced up-regulation of Na+,K+-ATPase activity in skeletal muscle is therefore important for the prevention of fatigue during exercise (6, 7).
The AMP-activated protein kinase (AMPK)3 is a major metabolic sensor, which maintains cellular energy homeostasis under energy-deprived conditions (9, 10). AMPK, activated by an increase in the intracellular AMP/ATP ratio, facilitates a switch in cellular metabolism from an anabolic state, where nutrients are taken up and stored, to a catabolic state, where they are oxidized, which promotes resynthesis (11, 12).
A number of pharmacological agents activate AMPK. The most commonly used AMPK activator is the adenosine analog 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR). AICAR is metabolized to the monophosphorylated nucleotide, ZMP by adenosine kinase, and mimics the effect of AMP on AMPK activation (13). The A-769662 compound is another AMPK activator (14). Using L6 muscle cells, we have previously shown that A-769662 promotes AMPK phosphorylation, whereas simultaneously directly inhibiting the activity of the purified Na+,K+-ATPase in a manner analogous to ouabain treatment (15).
AMPK acts as a metabolic sensor and regulates ion transport by inhibiting several transport proteins including the epithelial Na+ channel (16–18), the cystic fibrosis transmembrane conductance regulator Cl− channel (19, 20), and the renal-specific Na+,K+-2Cl− co-transporter 2 (21). AMPK inhibits Na+,K+-ATPase in lung epithelial cells (H441) (22, 23). Moreover, AMPK activation decreases pump activity and stimulates endocytosis in primary alveolar epithelial cells (24). Collectively, these studies provide evidence to suggest that most of the energy consuming processes in the cell, including Na+,K+-ATPase activity (25), are switched-off in response to AMPK activation.
Contrary to the common view that AMPK activation inhibits all energy consuming processes, in MDCK cultured renal epithelial cells, Na+,K+-ATPase is activated via an AMPK-dependent mechanism (26). Moreover, in lung epithelium of mice exposed to ozone, AMPK phosphorylation is increased, concomitant with appearance of the sodium pump at the cell surface (27). Additionally AICAR infusion in rodents increases AMPK phosphorylation, concomitant with an acute decrease in plasma [K+], without increasing urinary excretion of [K+] (28). The effect of AICAR on AMPK activation and plasma [K+] is blunted in mice overexpressing a kinase-dead form of AMPK (28). Thus, AICAR may increase either Na+,K+-ATPase or potassium channel activity in skeletal muscle. In parallel with Na+,K+-ATPase activation, exercise increases AMPK phosphorylation and activity. However, the direct effect of AMPK on Na+,K+-ATPase activity in skeletal muscle remains to be determined. Thus, the aim of the present study was to determine the effect of AMPK activation on Na+,K+-ATPase activity in skeletal muscle cells.
EXPERIMENTAL PROCEDURES
Antibodies and Reagents
Specific anti-Na+,K+-ATPase α1-subunit monoclonal antibodies were obtained from Dr. M. Caplan (Yale University, New Haven, CT). The McK1 antibody was a generous gift by Dr. K. Sweadner (Massachusetts Central Hospital, MA). Immunoprecipitation of total Na+,K+-ATPase α-subunit was performed using the polyclonal antibody, anti-NK1, raised against purified rat kidney holoenzyme (29). The antibodies against phospho-Akt Ser(P)473, phospho-ERK1/2 Thr(P)202/Tyr(P)204, phospho-AMPK Thr(P)172, phospho-PKCζ/λ Thr(P)410/403, phospho(Ser/Thr)-Akt substrate (PAS) and phospho-ACC Ser(P)79 were from Cell Signaling (Beverly, MA). The antibodies against methyl-PP2Aα Leu309 and phospho-PP2Aα Tyr(P)307 were from Abcam (Cambridge, UK). Antibody against PME-1 was from Millipore (Billerica, MA). Cyanide, kinase inhibitors Compound C, GF109203X, and the PKC activator, phorbol 12-myristate 13-acetate (PMA), were from Calbiochem (La Jolla, CA). Streptavidin-agarose beads and EZ-link Sulfo-NHS-SS-biotin were from Pierce Chemical Co. (Rockford, IL). Cell culture media and reagents were from Invitrogen. Human insulin (Actrapid) was from Novo Nordisk AS (Copenhagen, Denmark). Dimethyl sulfoxide (Calbiochem) was used as a solvent for the protein kinase and phosphatase inhibitors. A-769662 was a generous gift of Dr. Kei Sakomoto (University of Dundee, Dundee, UK). All other reagents were of analytical grade (Sigma).
L6 Muscle Cells
Rat L6 muscle cells (provided by Dr. Amira Klip, The Hospital for Sick Children, Toronto, Canada) were grown in αMEM supplemented with 10% FBS, 1% penicillin-streptomycin (100 units/ml of penicillin and 100 μg/ml of streptomycin), and 1% Fungizone (Amphotericin B) in 5% CO2, 95% O2 humidified air at 37 °C. L6 cells were differentiated into myotubes for 6 days in αMEM supplemented with 2% FBS. Myotubes were serum-starved 18 h prior to the experiment. Differentiated L6 myotubes express only the α1-subunit isoform of Na+,K+-ATPase (30).
AMPKα Knock-out Cells
Primary muscle cell cultures were derived from gastrocnemius and tibialis anterior muscles of 4-week-old wild-type and AMPKα1−/−; AMPKα2fl/fl mice as previously described (31). Targeted disruption of the AMPKα2 loci was performed by infection of myoblasts with an adenovirus expressing the Cre recombinase (32). Cells were grown with DMEM/F-12 containing 1% penicillin-streptomycin, 0.2% Fungizone, 1% glutamine (Invitrogen), 10% FBS, and 2% Ultroser G (Pall Life Sciences) in 5% CO2, 95% O2 humidified air at 37 °C. The cells were grown on plates coated with 0.02% gelatin. Cells were differentiated into myotubes for 4 days in DMEM/F-12, 1% penicillin-streptomycin, 0.2% Fungizone (Amphotericin B), 1% glutamine, and 2% horse serum (Invitrogen) on plates coated with Matrigel (BD Biosciences). On the day of the experiment, cells were serum starved for 4–5 h.
siRNA Transfection of Myotubes
The siRNA sequences used to silence rat AMPKα1, AMPKα2, and PME-1 were: AMPKα1, GCA UAU GCU GCA GGU AGA UdTdT; AMPKα2, CGU CAU UGA UGA UGA GGC UdTdT (Qiagen, Hilden, Germany) (33); and PME-1, CAA GGA UAC UUU UCG AGU UdTdT (Ambion, Carlsbad, CA). A scrambled siRNA was used as a control in all experiments. L6 cells (seeded and grown as described above) were transfected with 100 nm siRNA using calcium phosphate (CellPhect Transfection kit; GE Healthcare) on days 2 and 4 of differentiation. siRNA-calcium phosphate precipitates were removed 16–18 h after addition of siRNA. Experiments were performed 48 h after the last transfection.
Cell Incubation
For the protein phosphorylation analysis, myotubes, differentiated for 6–7 days, were incubated for 40 min with 0.1% dimethyl sulfoxide, AICAR (1 mm), or cyanide (100 μm). L6 cells were incubated for 1 h with 100 μm A-769662. Thereafter the medium was removed and the cells were incubated with starvation medium (serum-free αMEM) for an additional 1, 2, 3, or 4 h. For treatment with Compound C, cells were preincubated in serum-free αMEM with 0.1% dimethyl sulfoxide or 40 μm AMPK inhibitor Compound C for 10 min at 37 °C. Cells were then stimulated with AICAR (1 mm for 40 min) in the presence of Compound C for 40 min. Cells were washed twice with ice-cold PBS, and harvested by scraping cells into ice-cold lysis buffer A (20 mm Tris, pH 8.0, 135 mm NaCl, 1 mm MgCl2, 2.7 mm KCl, 10 mm Na4P2O7, 0.5 mm Na3VO4, 10 mm NaF, 1 μm okadaic acid, 1% Triton X-100, 10% (v/v) glycerol, 0.2 mm PMSF, 10 μg/ml of leupeptin, and 10 μg/ml of aprotinin). Cells were lysed by repeated pipetting, and lysates were agitated for 60 min at 4 °C and subjected to centrifugation (12,000 × g for 10 min at 4 °C). Protein concentration was determined using a bicinchoninic acid (BCA) protein assay kit (Pierce Chemical Co.). Lysates were stored at −80 °C before subsequent Western blot analysis.
Cell Surface Biotinylation
Cells were washed 3 times with ice-cold PBS, and thereafter exposed to EZ-link Sulfo-NHS-SS-biotin (Pierce Chemical Co.) at a final concentration of 1.5 mg/ml in PBS at 4 °C for 60 min with gentle shaking. Cell surface biotinylation was performed as described (34). Cells were then harvested and lysed in ice-cold buffer A as described above, and cell lysates were subjected to streptavidin precipitation. Samples were analyzed by SDS-PAGE with subsequent Western blot.
Measurement of Ouabain-sensitive 86Rb+ Uptake
Na+,K+-ATPase transport activity was measured as ouabain-sensitive uptake of 86Rb+, under conditions of the initial rate, as previously described (34). Myotubes were incubated in the presence or absence of ouabain (2 mm) and AMPK activators and/or inhibitor for 15 min, as indicated. Na+,K+-ATPase transport activity was determined after the addition of 50 μl of medium containing tracer amounts of 86RbCl (100 nCi/sample; GE Healthcare) for 10 min. Reactions were stopped by cooling on ice and cells were washed three times with an ice-cold washing solution containing 150 mm choline chloride, 1.2 mm MgSO4, 1.2 mm CaCl2, 2 mm BaCl2, and 5 mm HEPES, pH 7.4. Cells were lysed in 500 μl of 0.4 m NaOH and the radioactivity was measured by liquid scintillation. Protein content was determined in parallel using the BCA protein assay. Ouabain-sensitive 86Rb+ uptake was calculated as the difference between the mean values, measured in triplicate samples, incubated without or with 2 mm ouabain. Basal ouabain-sensitive 86Rb+ uptake in L6 myotubes was 7.8 ± 0.2 pmol of Rb/μg of protein per minute.
Metabolic Labeling of Myotubes with 32Pi
32Pi metabolic labeling was performed (34) to investigate in vitro phosphorylation of α-subunits of Na+,K+-ATPase. Myotubes (day 6 of differentiation) grown on 60-mm dishes were incubated for 3 h at 37 °C in serum-free αMEM containing 32Pi (1 mCi/ml). AICAR (1 mm) or PKC activator PMA (500 nm) were added during the last 40 min of the incubation time. The incubation was terminated by cooling the culture dishes on ice and washing the cells with ice-cold PBS. Myotubes were lysed in 0.5 ml of ice-cold lysis buffer A. Insoluble material was removed by centrifugation (12,000 × g for 10 min at 4 °C). Aliquots of supernatant (300 μg of protein) were immunoprecipitated overnight at 4 °C with 50 μl of polyclonal anti-NK1 rabbit antibodies. Immunoprecipitates were collected on protein G-agarose beads (Invitrogen; Dynal). Beads were washed four times in lysis buffer A and twice in PBS. Pellets were resuspended in Laemmli sample buffer (60 μl) (62.5 mm Tris-HCl, 2% SDS, 10% glycerol, and 10 mm DTT), separated by SDS-PAGE, and transferred to polyvinylidene difluoride (PVDF) membranes (Millipore). Phosphoproteins were analyzed using Bio-Imaging Analyzer BAS-1800II (Fuji Photo Film Co., Ltd., Japan), and quantification was performed using the Image Gauge software, version 3.4 (Fuji Photo Film Co., Ltd., Japan). In each experiment, the amount of radioactivity incorporated into the α-subunit was corrected for the α-subunit protein content detected by Western blot analysis. The quantitative data are reported as arbitrary units.
Western Blot Analysis
Aliquots of cell lysate (30 μg of protein) or immunoprecipitates were resuspended in Laemmli sample buffer. Proteins were then separated by SDS-PAGE, transferred to PVDF membranes, blocked with 7.5% nonfat milk, washed with TBST (10 mm Tris-HCl, 100 mm NaCl, 0.02% Tween 20), and incubated with the appropriate primary antibodies overnight at 4 °C. Membranes were washed with TBST and incubated with an appropriate secondary antibody. Proteins were visualized by enhanced chemiluminescence and quantified by densitometry.
Statistics
Data are presented as mean ± S.E. Comparisons between groups were performed using one-way analysis of variance or Student's t test when only two groups were compared. Significance was established at p < 0.05.
RESULTS
AMPK Activation Increases Transport Activity and Cell Surface Abundance of Na+,K+-ATPase in L6 Muscle Cells
Ouabain-sensitive 86Rb+ uptake and cell surface biotinylation was measured to assess the activity and abundance of the sodium pump in the plasma membrane in the presence or absence of AMPK activators (AICAR or cyanide). Both AICAR and cyanide increased AMPK phosphorylation at Thr172 in L6 cells (Fig. 1A), concomitant with increased cell surface expression of α1-subunit of the sodium pump (Fig. 1B) and pump activity (Fig. 1C). These data provide evidence to suggest that AMPK activation increases expression of the α1-subunit Na+,K+-ATPaseat the plasma membrane, as well as the activity of the pump in L6 cells.
FIGURE 1.
AICAR and cyanide increase the cell surface abundance and transport activity of Na+,K+-ATPase in L6 cells. L6 cells were incubated with either AICAR (1 mm for 40 min) or cyanide (NaCN; 100 μm for 40 min). Cells were biotinylated on the cell surface. Biotinylated proteins were recovered from cell extracts by precipitation with streptavidin-agarose, and Na+,K+-ATPase α1-subunit on the cell surface was detected by Western blot analysis using Na+,K+-ATPase α1-subunit antibody (B). An aliquot of the total cell extract from each sample was also processed by a parallel SDS-PAGE and subjected to immunoblot analysis to determine AMPK Thr172 phosphorylation (A). Results are mean ± S.E. n = 3–5. *, p < 0.05 versus basal. C, 86Rb+ uptake assay was performed as described under “Experimental Procedures.” Oubain (2 mm) was used to inhibit Na+,K+-ATPase. Data are mean ± S.E. n = 5. *, p < 0.05 versus basal.
A-769662 Increases the Activity and Cell Surface Expression of Na+,K+-ATPase α1-Subunit after Washout
A-769662 is a potent direct AMPK activator (14). However, A-769662 directly inhibits the purified Na+,K+-ATPase α1-isoform isolated from kidney cells (15). A washout experiment was performed to eliminate the direct effect of A-769662 on the sodium pump. L6 cells were incubated for 1 h with A-769662 (Fig. 2). Thereafter, the medium was removed, cells were washed and incubated in A-769662-free medium for an additional 1, 2, 3, or 4 h. A 1-h incubation with A-769662 transiently increased AMPK phosphorylation at Thr172, which returned to the basal level during the washout period (1–4 h; Fig. 2A). ACC phosphorylation at Ser79 was increased after incubation with A-769662 and remained elevated for 3 h after removal of A-769662 (Fig. 2B). Consistent with our previous observation (15), the cell surface expression of the Na+,K+-ATPase α1-subunit in L6 myotubes was decreased after a 1-h exposure to A-769662 (Fig. 2C). However, cell surface expression of the Na+,K+-ATPase α1-subunit was quickly normalized during the washout and was markedly increased after a 2-h incubation with starvation medium, which was followed by return to the basal level (Fig. 2C). Sodium pump activity decreased after a 1-h incubation with A-769662, but then increased 2 h following removal of A-769662 (Fig. 2D), concomitant with an increase in the Na+,K+-ATPase α1-subunit cell surface expression.
FIGURE 2.
AMPK activation by A-769662 after the compound washout increases the transport activity and cell surface abundance of Na+,K+-ATPase in L6 cells. Differentiated L6 cells were preincubated in the presence or absence of 100 μm A-769662 for 1 h. L6 cells were lysed or the medium containing A-769662 was removed and replaced by starvation medium and incubated for 1, 2, 3, or 4 h and then lysed. Cell lysates were processed as described under “Experimental Procedures.” Samples were separated on a 7.5–20% SDS-PAGE, and subjected to immunoblot analysis for pAMPK at Thr172 (A) and pACC at Ser79 (B). A representative Western blot is shown in the upper panel. Densitometric analysis of different proteins is expressed in arbitrary units. Results are mean ± S.E. for n = 8. *, p < 0.05 versus basal. C, after incubation, the cell surface was biotinylated as specified in the legend to Fig. 1A. Cell surface Na+,K+-ATPase α1-subunit was detected by Western blot analysis. Densitometric analysis of the protein is shown in arbitrary units. Results are mean ± S.E. for n = 3–6. *, p < 0.05 versus basal. D, ouabain-sensitive 86Rb+ uptake assay was performed and results represent mean ± S.E. n = 3–5. *, p < 0.05 versus basal.
The AMPK Inhibitor Compound C Abolishes the Effect of AICAR on Na+,K+-ATPase in L6 Muscle Cells
Exposure of L6 myotubes to a chemical AMPK inhibitor, Compound C, was without effect on basal AMPK and ACC phosphorylation (Fig. 3, A and B). Furthermore, basal cell surface expression of the Na+,K+-ATPase α1-subunit (Fig. 3C) and pump activity (Fig. 3D) were unaltered by Compound C. However, Compound C abolished the effect of AICAR on AMPK and ACC phosphorylation (Fig. 3, A and B). Similarly, Compound C blocked the effect of AICAR on expression of the Na+,K+-ATPase α1-subunit at the plasma membrane, as well as pump activity (Fig. 3, C and D). These data further support a role for AMPK in activation of the sodium pump.
FIGURE 3.
Compound C blocks the effect of AICAR on cell surface abundance and transport activity of Na+,K+-ATPase in L6 cells. L6 cells were incubated with AICAR (1 mm for 40 min) and Compound C (40 μm for 50 min) or with AICAR or Compound C alone, and a cell surface biotinylation assay was performed. An aliquot of the total cell extract was processed by a parallel SDS-PAGE. Representative blots and densitometric analysis of: pAMPK at Thr172 (A), pACC at Ser79 (B), and cell surface α1 Na+,K+-ATPase (C). Densitometric analysis of different proteins is shown in arbitrary units. Results are mean ± S.E. for n = 3–6. *, p < 0.05 versus basal. D, 86Rb+ uptake assay was performed as described under “Experimental Procedures.” The values are the mean ± S.E., n = 3–5. *, p < 0.05 versus basal. Ouabain (2 mm) was used to inhibit Na+,K+-ATPase.
The AICAR Effect on the Na+,K+-ATPase Transport Activity and Cell Surface Abundance Requires AMPK α-Subunits
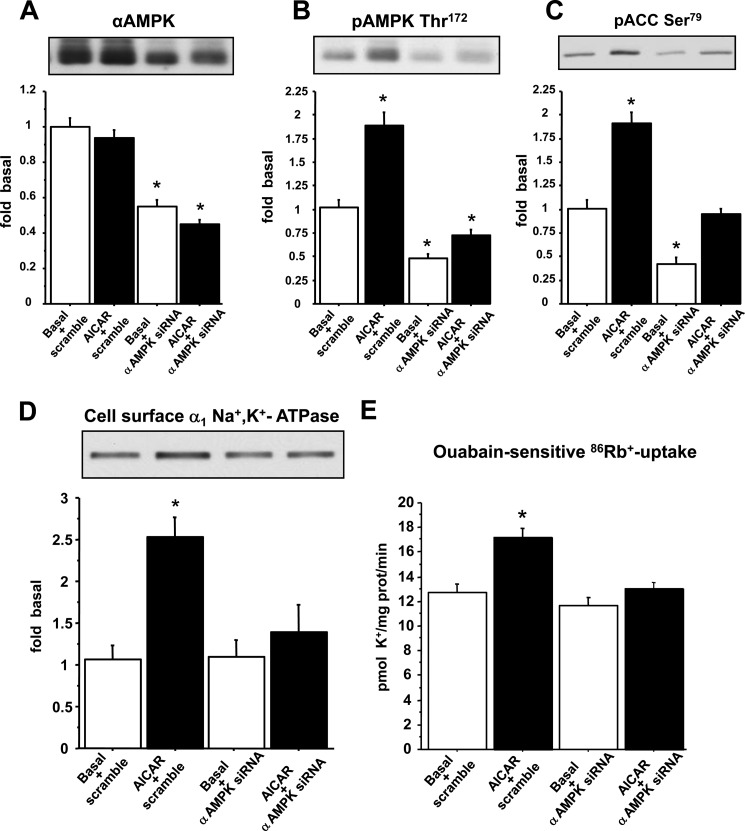
To determine whether AMPK α-subunits are required for the AICAR-stimulated increase in Na+,K+-ATPase transport activity and cell surface abundance, we silenced AMPK using siRNA against AMPKα1 and AMPKα2. A 50% decrease in AMPK α-subunit expression (Fig. 4A) substantially reduced the AICAR-stimulated AMPK phosphorylation at Thr172 (Fig. 4B) and ACC phosphorylation at Ser79 (Fig. 4C). The effect of AICAR on cell surface expression and activity of the sodium pump was prevented in cells following a 50% decrease in AMPK α-subunit expression (Fig. 4, D and E). Because we were unable to completely knock-down AMPK α-subunit expression in L6 cells by siRNA, we further tested our hypothesis in primary mouse muscle cells deficient in the α1- and α2-subunits of AMPK. In wild-type cells, where both α-subunits of AMPK are endogenously expressed, AICAR increased the phosphorylation of AMPK at Thr172 and ACC at Ser79 (Fig. 5, A–C). Conversely, in AMPK α-subunit-deficient cells basal and AICAR-stimulated phosphorylation of AMPK and ACC could not be detected (Fig. 5, A–C). Wild-type and AMPKα knock-out cells expressed a similar amount of the α1-subunit of Na+,K+-ATPase (Fig. 5D). Thus, we next assessed the effects of AICAR on Na+,K+-ATPase in AMPKα knock-out cells. AICAR was without effect on either transport activity or cell surface abundance of Na+,K+-ATPase in AMPKα knock-out cells (Fig. 6, A and B). These data provide evidence that the AMPK α-subunits are required for AICAR-induced effects on Na+,K+-ATPase.
FIGURE 4.
AMPKα siRNA abolishes the AICAR-induced increase in transport activity and cell surface abundance of Na+,K+-ATPase in L6 cells. L6 cells were transfected with siRNA against AMPKα or a scramble sequence as described under “Experimental Procedures.” Transfected cells were incubated in the presence or absence of 1 mm AICAR for 30 min. L6 cells were biotinylated with the cleavable biotinylation reagent sulfo-NHS-SS-biotin. An aliquot of the total cell extract was processed by a parallel SDS-PAGE. Biotinylated or total proteins were subjected to immunoblot analysis of: total AMPKα (A), pAMPK at Thr172 (B), pACC at Ser79 (C), and cell surface α1 Na+,K+-ATPase (D). Results are mean ± S.E. in arbitrary units, n = 7–10. *, p < 0.05 versus basal scrambled. E, ouabain-sensitive 86Rb+ uptake assay was performed as described under “Experimental Procedures.” Results are mean ± S.E. n = 9–15. *, p < 0.05 versus basal. 2 mm ouabain was used to totally inhibit Na+,K+-ATPase.
FIGURE 5.
AMPKα knock-out cells express Na+,K+-ATPase but not the AMPK α-subunits. Wild-type and AMPKα knock-out (KO) cells were incubated with AICAR (1 mm for 20 min). Cell lysates were subjected to immunoblot analysis of total AMPKα (A), pAMPK Thr172 (B), pACC at Ser79 (C), and Na+,K+-ATPase α1-subunit (D). Densitometric analysis of different proteins is shown as fold of basal. Results are mean ± S.E., n = 3–6. *, p < 0.05 versus basal.
FIGURE 6.
The increase in the transport activity and cell surface abundance of Na+,K+-ATPase is blocked in AMPKα knock-out cells. Wild-type and AMPKα knock-out (KO) cells were incubated with AICAR (1 mm for 20 min). A, the ouabain-sensitive 86Rb+ uptake assay was performed as described under “Experimental Procedures.” Results are mean ± S.E. n = 9–15. *, p < 0.05 versus wild-type basal. B, biotinylated proteins were recovered from cell extracts by precipitation on streptavidin-agarose. Na+,K+-ATPase α1-subunit abundance on the cell surface was detected by Western blot analysis using an antibody against the Na+,K+-ATPase α1-subunit. Representative blots and densitometric analysis of cell surface Na+,K+-ATPase α1-subunit are shown. Results are mean ± S.E., n = 7–10. *, p < 0.05 versus wild-type basal.
Phosphorylation of Na+,K+-ATPase α-Subunit in L6 Muscle Cells in Response to AICAR
Phosphorylation of the α-subunit of Na+,K+-ATPase triggers signals controlling Na+,K+-ATPase endocytosis and exocytosis (34–36). To determine whether AICAR promotes phosphorylation of Na+,K+-ATPase, myotubes were metabolically labeled with 32Pi for 3 h. Thereafter, cells were incubated for 40 min with AICAR, or the PKC activator PMA, as a positive control. AICAR was without effect on the pattern of total protein phosphorylation, whereas PMA notably increased protein phosphorylation (Fig. 7A). Next, we precipitated the α1-subunit of Na+,K+-ATPase with the NK1 antibody. Total phosphorylation of the Na+,K+-ATPase α1-subunit was decreased by AICAR exposure, whereas PMA increased the total phosphorylation of the Na+,K+-ATPase α1-subunit (Fig. 7B). To further explore the potential role of AICAR in Na+,K+-ATPase α1-subunit phosphorylation, we utilized the McK1 antibody that specifically recognizes the nonphosphorylated form of the Na+,K+-ATPase on Ser18, a residue that is a target for phosphorylation by protein kinase C (PKC) (35–37). Phosphorylation of the α1-subunit on Ser18 is essential for Na+,K+-ATPase endocytosis (35–38). The use of McK1 as a tool to investigate phosphorylation of the α1-subunit has been validated earlier (39, 40). Incubation with AICAR decreased phosphorylation of the Na+,K+-ATPase α1-subunit on Ser18, whereas PMA decreased McK1 immunoreactivity (Fig. 7C), indicating PKC-dependent phosphorylation on Ser18. In alveolar epithelial cells, the protein phosphatase 2A catalytic subunit (PP2A) directly dephosphorylates the Na+,K+-ATPase α1-subunit, thereby promoting translocation to the plasma membrane (41). We assessed whether AICAR altered PP2A methylation and phosphorylation. AICAR stimulation resulted in dephosphorylation (Fig. 7D) and methylation (Fig. 7E) of PP2A in L6 myotubes, which promotes activation of the phosphatase (42, 43) and may subsequently cause dephosphorylation and activation of the Na+,K+-ATPase. These data suggest that the increase in Na+,K+-ATPase abundance in response to AICAR is dependent on phosphorylation of the α-subunit of the sodium pump on Ser18.
FIGURE 7.
AICAR decreases the phosphorylation of Na+,K+-ATPase. Cells were pretreated with dimethyl sulfoxide (Basal), AICAR (1 mm for 30 min), or PMA (500 nm for 40 min). A, an autoradiogram of 32P-labeled total proteins after SDS-PAGE and electrophoretic transfer is shown. B, Na+,K+-ATPase α1-subunits were immunoprecipitated using Na+,K+-ATPase α1-subunit antibody as described under “Experimental Procedures.” Immunoprecipitates (IP) were then processed by SDS-PAGE and subjected to immunoblot analysis using a Na+,K+-ATPase α1-antibody. C, total proteins were subjected to immunoblot analysis with the McK1 antibody. The McK1 antibody recognizes the unphosphorylated α1-subunits (on Ser18). Activation of PP2A was assessed by measurement of phosphorylation at Tyr307 (D) and methylation at Leu309 (E). Representative blots and densitometric analysis are shown. Results are mean ± S.E., n = 4–5. *, p < 0.05 versus basal. WB, Western blot.
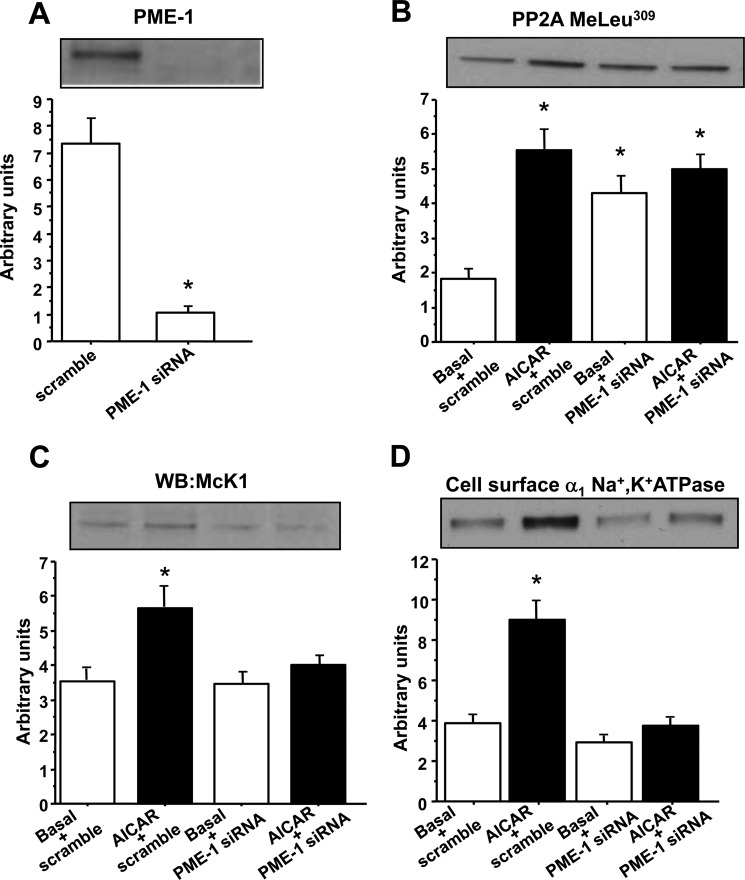
PME-1 Is Required for AICAR-stimulated Na+,K+-ATPase α1-Subunit Dephosphorylation and Increased Cell Surface Abundance
PP2A and a PP2A-specific protein phosphatase methylesterase-1 (PME-1) constitutively associate with Na+,K+-ATPase α-subunit (44). PME-1 is a negative regulator of PP2A and its dissociation from the complex triggers Na+,K+-ATPase dephosphorylation in kidney cells (44). To determine whether PME-1 is involved in AMPK-mediated Na+,K+-ATPase regulation in skeletal muscle, L6 muscle cells were transfected with siRNA against PME-1, which resulted in a markedly reduced expression of PME-1 protein (Fig. 8A). As expected, methylation of PP2A at Leu309 was increased in PME-1-deficient muscle cells, but incubation with AICAR had no additional effect (Fig. 8B). Next, the role of PME-1 in regulation of Na+,K+-ATPase phosphorylation and cell surface expression was assessed. PME-1 silencing was without effect under basal conditions, but completely abolished AICAR-stimulated dephosphorylation of the Na+,K+-ATPase α1-subunit (Fig. 8C) and translocation to the cell membrane (Fig. 8D). These data indicate that PME-1 is required for AMPK-mediated effects on Na+,K+-ATPase.
FIGURE 8.
PME-1 siRNA prevents AICAR-stimulated Na+,K+-ATPase dephosphorylation and increased cell surface abundance in L6 cells. L6 cells were transfected with siRNA against PME-1 or a scramble sequence as described under “Experimental Procedures.” Transfected cells were incubated in the presence or absence of 1 mm AICAR for 30 min. L6 cells were biotinylated with the cleavable biotinylation reagent sulfo-NHS-SS-biotin. An aliquot of the total cell extract was processed by a parallel SDS-PAGE. Biotinylated or total proteins were subjected to immunoblot analysis of total PME-1 (A), methylated PP2A at Leu309 (B), unphosphorylated Na+,K+-ATPase α1-subunit (on Ser18), as assessed by McK1 antibody (C) and cell surface α1 Na+,K+-ATPase (D). Data are mean ± S.E. in arbitrary units, n = 6. *, p < 0.05 versus basal. WB, Western blot.
Phosphorylation of PKCζ and AS160 in Response to AICAR in L6 Muscle Cells
In rat alveolar epithelial cells, activation of AMPK by hypoxia phosphorylates PKCζ and promotes endocytosis of Na+,K+-ATPase (45). We tested the hypothesis that atypical PKC could be activated by AMPK concomitant with an increased Na+,K+-ATPase α1-subunit translocation and pump activity in L6 muscle cells. PKCζ/λ Thr410/403 phosphorylation, a marker of PKC activity, was unaltered after AICAR or A-769662 exposure in L6 cells (Fig. 9A), but insulin increased PKCζ/λ Thr410/403 phosphorylation (Fig. 9B). Recently, the Rab-GTPase activating protein AS160 (TBC1D4) has been suggested to play a role in Na+,K+-ATPase translocation (26, 46). In MDCK-cultured renal epithelial cells, inhibition of AMPK resulted in an AS160-dependent intracellular accumulation of Na+,K+-ATPase (26). We determined AS160 phosphorylation in the presence or absence of AICAR (Fig. 9, C and D). As expected, insulin increased AS160 phosphorylation in L6 cells (Fig. 9D). However, exposure to either of the AMPK activators (AICAR or A-769662) was without effect on AS160 phosphorylation in L6 cells (Fig. 9C). Our data provide evidence to suggest that neither atypical PKCs nor AS160 are involved in the AMPK-dependent activation on Na+,K+-ATPase in L6 muscle cells.
FIGURE 9.
AICAR has no effect on PKCζ and AS160 activation in L6 cells. L6 cells were incubated with insulin (20 min at 120 nm), AICAR (40 min at 1 mm), A-769662 (40 min at 100 μm), or both AICAR and insulin. Cell lysates were separated on 7.5–20% SDS-PAGE, and subjected to immunoblot analysis for pPKCζ/λ Thr410/403 (A and B) and PAS (C and D). Representative blots and densitometric analysis are shown. Results are mean ± S.E., n = 8. *, p < 0.05 versus basal.
DISCUSSION
The Na+,K+-ATPase plays a critical role in regulating fluid and electrolyte transport in a variety of tissues and different physiological stimuli can promote endocytosis or translocation of the pump to the plasma membrane (35, 47, 48). Contractile activity stimulates Na+,K+-ATPase activity in skeletal muscle, at least partially via translocation of the pump to the plasma membrane from intracellular stores (8). The molecular mechanism underpinning this phenomenon is incompletely resolved.
In skeletal muscle, contraction and metabolic stress are potent activators of AMPK (49). AMPK regulates Na+,K+-ATPase cell surface expression and activity in various cell lines (22–24). However, the effect of AMPK on Na+,K+-ATPase activity in skeletal muscle cells and the molecular mechanism behind this effect are unknown. Here we provide evidence that the AMPK activator AICAR increases Na+,K+-ATPase activity and abundance at the cell surface in L6 skeletal muscle cells with a magnitude similar to that of insulin. This effect is likely to be due to the dephosphorylation of the Na+,K+-ATPase on Ser18 in response to activation of the Ser/Thr protein phosphatase (PP) 2A. Our data provide evidence to suggest that AMPK activation modifies Na+,K+-ATPase activity, partly by increasing cell surface abundance of Na+,K+-ATPase. Collectively, these findings suggest that AICAR-induced activation of Na+,K+-ATPase is dependent on AMPK activation.
The Na+,K+-ATPase is a highly energy-dependent pump and it seems counterintuitive that AMPK, which generally turns-off ATP-consuming processes (49), would sustain or even increase Na+,K+-ATPase activity by prohibiting its internalization. AMPK activation promotes internalization of Na+,K+-ATPase in a lung epithelial cell line (H441) (22) and alveolar epithelial cells (24). Conversely, in rat L6 muscle cells, AICAR-induced AMPK activation did not promote Na+,K+-ATPase internalization. This observation is consistent with studies in MDCK epithelial cells, where AMPK inhibition by Compound C induces Na+,K+-ATPase internalization (26). Furthermore, in ATP-depleted MDCK cells, metformin prevents redistribution of Na+,K+-ATPase from the basolateral membrane to the intracellular vesicular compartment (50). These data indicate that AMPK is not a simple energy-sensor, which automatically turns-off energy-consuming processes regardless of specific tissue and cellular functions. There are substantial differences in energy requirements and demands of major energy-consuming tissues (51). Under standard resting conditions, 19–28% of whole body ATP is consumed by Na+,K+-ATPase, 25–30% by protein synthesis, 4–8% by Ca2+-ATPase, 2–8% by actinomyosin ATPase, 7–10% by gluconeogenesis, 3% by ureagenesis, and the remaining percentage by RNA synthesis and substrate cycling (51). However, Na+,K+-ATPase contributes to only 5–10% of oxygen consumption in skeletal muscle and even less in heart (1–5%), compared with 40–70% in kidney, 60% in the gastrointestinal tract, and 50–60% in brain (7, 51–53). Even during muscle contraction, the fractional energy consumption by Na+,K+-ATPase appears to remain constant (5–10%), whereas actinomyosin ATPase (up to 80%) and Ca2+-ATPase (10–50%) are by far the major consumers of ATP (7, 51, 54). Inconsistent results regarding AMPK-mediated effects on Na+,K+-ATPase activity and/or abundance obtained in different cells and tissues may therefore reflect these fundamental differences in the fractional energy requirements of Na+,K+-ATPase.
AICAR infusion and concomitant AMPK phosphorylation, is associated with an acute fall in plasma [K+], without increasing K+ urinary excretion. This effect of AICAR on plasma [K+] was blunted in mice overexpressing a kinase-dead form of AMPK in skeletal muscle (28). Moreover, Na+,K+-ATPase expression at the cell surface was unaltered, but only the Na+,K+-ATPase α2-subunit was measured in the plasma membrane fraction. In mice exposed to ozone, AMPK phosphorylation and Na+,K+-ATPase expression at the plasma membrane of lung epithelium is increased. The effect of ozone on Na+,K+-ATPase cell surface abundance was not observed in AMPKα1-deficient mice (27). Thus, in skeletal muscle, the Na+,K+-ATPase may not be a primary target of energy-conserving measures. Activation of the energy sensing AMPK may be a potential mechanism for contraction-induced activation of the sodium pump.
To dissect the molecular mechanism behind the increase of Na+,K+-ATPase activity following AMPK activation, we determined whether Na+,K+-ATPase α1-subunit phosphorylation is altered in response to AMPK activation by AICAR in L6 cells. Total phosphorylation of the Na+,K+-ATPase α1-subunit, as well as phosphorylation on Ser18 was decreased following AICAR treatment, possibly explaining the increased cell surface Na+,K+-ATPase abundance. Indeed, endocytosis of rodent Na+,K+-ATPase requires Ser18 phosphorylation of the α1-subunit (35–38). Thus, AMPK activation decreases Na+,K+-ATPase phosphorylation on Ser18 and prevents endocytosis of Na+,K+-ATPase. PKCζ phosphorylates the Na+,K+-ATPase α1-subunit on Ser18 and triggers Na+,K+-ATPase endocytosis in polarized epithelial cells from lung (24, 37). Here we report that PKCζ phosphorylation, a marker of PKCζ activity was unchanged after AICAR stimulation, consistent with the decrease of Na+,K+-ATPase α1-subunit Ser18 phosphorylation after AICAR stimulation. This result provides evidence that AMPK stimulation of the sodium pump occurs via a mechanism independent of PKCζ activation.
The Rab-GTPase activating protein AS160 (TBC1D4) plays a role in regulating the sodium pump. In alveolar epithelial cells, AS160 phosphorylation by Akt is required for Na+,K+-ATPase trafficking to the cell surface (46). AS160 interacts directly with the α-subunit of the Na+,K+-ATPase in MDCK-cultured renal epithelial cells. Inhibition of AMPK by Compound C decreased cell surface expression of the Na+,K+-ATPase and enhanced the interaction of the pump with AS160 (26). Here we investigated the role of AS160 in the regulation of Na+,K+-ATPase in L6 myotubes, however, AS160 phosphorylation in response to AICAR was unaltered. Of note, the antibody used in this study recognizes phosphorylation of AS160 and another closely related Rab-GTPase activating protein TBC1D1, thus the integrated phosphorylation signal from these two proteins was measured. Therefore, we cannot exclude the possibility that TBC1D1 phosphorylation was modified. Additional experiments are required to further explore this question.
The FXYD1 protein (phospholemman) is one of the major substrates for protein kinases in the sarcolemma and is an important regulator of Na+,K+-ATPase activity (55). Therefore, we speculated that FXYD1 may be a candidate protein for the regulation of Na+,K+-ATPase by AMPK. However, in L6 muscle cells, the FXYD1 protein was undetectable and the level of FXYD1 mRNA expression was at the limit of detection (data not shown). Moreover, an acute exposure of isolated mouse muscles to AICAR was without effect on FXYD1 phosphorylation,4 which therefore excludes FXYD1 as a regulator of the sodium pump in L6 myotubes.
The observed decrease in Na+,K+-ATPase phosphorylation on Ser18 following AMPK activation appears to involve Ser/Thr phosphatase PP2A. The catalytic subunit PP2Ac regulates Na+,K+-ATPase activity (56, 57). In alveolar epithelial cells, Na+,K+-ATPase recruitment to the plasma membrane is regulated by dephosphorylation of the Na+,K+-ATPase α1-subunit at the Ser18 residue by PP2Ac and it is mediated by a direct interaction between the proteins (41). However, PP2Ac directly dephosphorylates and inactivates AMPK phosphorylation in mammals (58, 59). Here we observed that AMPK activation increased pump activity and Na+,K+-ATPase α1-subunit expression at the cell surface concomitant with dephosphorylation and increased methylation of PP2Ac. Salt-inducible kinase 1 (SIK1), a member of the sucrose-nonfermenting 1 protein kinase (SNF1)/AMPK family of the Ser/Thr protein kinases, activates PP2Ac, thereby promoting the dephosphorylation of the Na+,K+-ATPase α-subunits and increasing its catalytic activity (44). Therefore, increased PP2Ac activity is not always coincident with AMPK inhibition.
AICAR-stimulated dephosphorylation of Na+,K+-ATPase α1-subunit at Ser18 in skeletal muscle cells was dependent on the PP2A-specific methylesterase PME-1. Upon activation, PP2A directly interacts with the Na+,K+-ATPase α1-subunit, dephosphorylates Ser18, and stimulates its recruitment to the plasma membrane (41). Because PME-1 acts as a negative regulator of PP2A (60), increased activity at Ser18 could therefore be expected in PME-1-deficient muscle cells. However, PP2A and PME-1 constitutively form a complex, which associates with the Na+,K+-ATPase α-subunit and regulates its phosphorylation and activity (44). Disruption of the PP2A·PME-1 complex in PME-1-deficient cells may therefore affect PP2A targeting to the Na+,K+-ATPase α1-subunit and prevent dephosphorylation of Ser18 upon activation of PP2A. Our result is consistent with an earlier observation showing that PP2A Thr phosphatase activity is reduced in PME-1−/− mice despite increased methylation of PP2Ac (61). Thus, PME-1 seems to be particularly important for PP2A Ser/Thr phosphatase activity. Indeed, deletion of yeast methylesterase PPE1 was associated with increased methylation of PPH21 (PP2A homolog), but decreased phosphatase activity (62). Our results therefore provide evidence to suggest that AMPK may act on PP2Ac to regulate Na+,K+-ATPase in a PME-1-dependent manner.
In conclusion, AMPK activation increases activity and cell surface abundance of the sodium pump in skeletal muscle cells. This effect appears to be dependent on the activation of the phosphatase PP2Ac. Based on these findings, we propose the following mechanism: AMPK activation directly or indirectly stimulates phosphatase PP2Ac, which subsequently dephosphorylates the Na+,K+-ATPase α1-subunit on Ser18, thereby preventing endocytosis and increasing cell surface abundance and activity of the sodium pump. The increase in sodium pump activity by AMPK activation may be crucial under physiological conditions, such as physical exercise, to maintain ion homeostasis and transmembrane currents and thereby delay muscle fatigue.
Acknowledgment
We thank Professor Anna Krook, Karolinska Institutet, for support and discussion.
This work was supported by grants from the Swedish Research Council, the Novo Nordisk Foundation, and the European Foundation for the Study of Diabetes and Commission of the European Communities Contracts LSHM-CT-2004-512013 EUGENEHEART and LSHM-CT-2004-005272 EXGENESIS.
B. Benziane, M. Björnholm, S. Pirkmajer, R. L. Austin, O. Kotova, B. Viollet, J. R. Zierath, and A. V. Chibalin, unpublished observations.
- AMPK
- AMP-activated protein kinase
- AICAR
- 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside
- MDCK
- Madin-Darby canine kidney cells
- PME-1
- protein phosphatase methylesterase-1
- αMEM
- α-minimal essential medium
- PMA
- phorbol 12-myristate 13-acetate.
REFERENCES
- 1. Skou J. C. (1988) The Na,K-pump. Methods Enzymol. 156, 1–25 [DOI] [PubMed] [Google Scholar]
- 2. Blanco G., Mercer R. W. (1998) Isozymes of the Na,K-ATPase. Heterogeneity in structure, diversity in function. Am. J. Physiol. Renal Physiol. 275, F633–F650 [DOI] [PubMed] [Google Scholar]
- 3. Hundal H. S., Maxwell D. L., Ahmed A., Darakhshan F., Mitsumoto Y., Klip A. (1994) Subcellular distribution and immunocytochemical localization of Na,K-ATPase subunit isoforms in human skeletal muscle. Mol. Membr. Biol. 11, 255–262 [DOI] [PubMed] [Google Scholar]
- 4. Juel C., Nielsen J. J., Bangsbo J. (2000) Exercise-induced translocation of Na+,K+ pump subunits to the plasma membrane in human skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 278, R1107–R1110 [DOI] [PubMed] [Google Scholar]
- 5. Murphy K. T., Snow R. J., Petersen A. C., Murphy R. M., Mollica J., Lee J. S., Garnham A. P., Aughey R. J., Leppik J. A., Medved I., Cameron-Smith D., McKenna M. J. (2004) Intense exercise up-regulates Na+,K+-ATPase isoform mRNA, but not protein expression in human skeletal muscle. J. Physiol. 556, 507–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nielsen O. B., Clausen T. (2000) The Na+/K+ pump protects muscle excitability and contractility during exercise. Exerc. Sport Sci. Rev. 28, 159–164 [PubMed] [Google Scholar]
- 7. Clausen T. (2003) Na+,K+ pump regulation and skeletal muscle contractility. Physiol. Rev. 83, 1269–1324 [DOI] [PubMed] [Google Scholar]
- 8. Benziane B., Chibalin A. V. (2008) Frontiers. Skeletal muscle sodium pump regulation. A translocation paradigm. Am. J. Physiol. Endocrinol. Metab. 295, E553–558 [DOI] [PubMed] [Google Scholar]
- 9. Hardie D. G. (2003) Minireview. The AMP-activated protein kinase cascade. The key sensor of cellular energy status. Endocrinology 144, 5179–5183 [DOI] [PubMed] [Google Scholar]
- 10. Kemp B. E., Stapleton D., Campbell D. J., Chen Z. P., Murthy S., Walter M., Gupta A., Adams J. J., Katsis F., van Denderen B., Jennings I. G., Iseli T., Michell B. J., Witters L. A. (2003) AMP-activated protein kinase, super metabolic regulator. Biochem. Soc. Trans. 31, 162–168 [DOI] [PubMed] [Google Scholar]
- 11. Hardie D. G. (2004) AMP-activated protein kinase. A key system mediating metabolic responses to exercise. Med. Sci. Sports Exerc. 36, 28–34 [DOI] [PubMed] [Google Scholar]
- 12. Hardie D. G., Sakamoto K. (2006) AMPK. A key sensor of fuel and energy status in skeletal muscle. Physiology 21, 48–60 [DOI] [PubMed] [Google Scholar]
- 13. Corton J. M., Gillespie J. G., Hawley S. A., Hardie D. G. (1995) 5-Aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur. J. Biochem. 229, 558–565 [DOI] [PubMed] [Google Scholar]
- 14. Cool B., Zinker B., Chiou W., Kifle L., Cao N., Perham M., Dickinson R., Adler A., Gagne G., Iyengar R., Zhao G., Marsh K., Kym P., Jung P., Camp H. S., Frevert E. (2006) Identification and characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome. Cell Metab. 3, 403–416 [DOI] [PubMed] [Google Scholar]
- 15. Benziane B., Bjornholm M., Lantier L., Viollet B., Zierath J. R., Chibalin A. V. (2009) AMP-activated protein kinase activator A-769662 is an inhibitor of the Na+,K+-ATPase. Am. J. Physiol. Cell Physiol. 297, 1554–1566 [DOI] [PubMed] [Google Scholar]
- 16. Almaça J., Kongsuphol P., Hieke B., Ousingsawat J., Viollet B., Schreiber R., Amaral M. D., Kunzelmann K. (2009) AMPK controls epithelial Na+ channels through Nedd4-2 and causes an epithelial phenotype when mutated. Pflugers Arch. 458, 713–721 [DOI] [PubMed] [Google Scholar]
- 17. Bhalla V., Oyster N. M., Fitch A. C., Wijngaarden M. A., Neumann D., Schlattner U., Pearce D., Hallows K. R. (2006) AMP-activated kinase inhibits the epithelial Na+ channel through functional regulation of the ubiquitin ligase Nedd4-2. J. Biol. Chem. 281, 26159–26169 [DOI] [PubMed] [Google Scholar]
- 18. Carattino M. D., Edinger R. S., Grieser H. J., Wise R., Neumann D., Schlattner U., Johnson J. P., Kleyman T. R., Hallows K. R. (2005) Epithelial sodium channel inhibition by AMP-activated protein kinase in oocytes and polarized renal epithelial cells. J. Biol. Chem. 280, 17608–17616 [DOI] [PubMed] [Google Scholar]
- 19. Hallows K. R., Raghuram V., Kemp B. E., Witters L. A., Foskett J. K. (2000) Inhibition of cystic fibrosis transmembrane conductance regulator by novel interaction with the metabolic sensor AMP-activated protein kinase. J. Clin. Invest. 105, 1711–1721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kongsuphol P., Hieke B., Ousingsawat J., Almaca J., Viollet B., Schreiber R., Kunzelmann K. (2009) Regulation of Cl− secretion by AMPK in vivo. Pflugers Arch. 457, 1071–1078 [DOI] [PubMed] [Google Scholar]
- 21. Fraser S. A., Gimenez I., Cook N., Jennings I., Katerelos M., Katsis F., Levidiotis V., Kemp B. E., Power D. A. (2007) Regulation of the renal-specific Na+,K+-2Cl− co-transporter NKCC2 by AMP-activated protein kinase (AMPK). Biochem. J. 405, 85–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Woollhead A. M., Scott J. W., Hardie D. G., Baines D. L. (2005) Phenformin and 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR) activation of AMP-activated protein kinase inhibits transepithelial Na+ transport across H441 lung cells. J. Physiol. 566, 781–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Woollhead A. M., Sivagnanasundaram J., Kalsi K. K., Pucovsky V., Pellatt L. J., Scott J. W., Mustard K. J., Hardie D. G., Baines D. L. (2007) Pharmacological activators of AMP-activated protein kinase have different effects on Na+ transport processes across human lung epithelial cells. Br. J. Pharmacol. 151, 1204–1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vadász I., Dada L. A., Briva A., Trejo H. E., Welch L. C., Chen J., Tóth P. T., Lecuona E., Witters L. A., Schumacker P. T., Chandel N. S., Seeger W., Sznajder J. I. (2008) AMP-activated protein kinase regulates CO2-induced alveolar epithelial dysfunction in rats and human cells by promoting Na,K-ATPase endocytosis. J. Clin. Invest. 118, 752–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lingrel J. B., Kuntzweiler T. (1994) Na+,K+-ATPase. J. Biol. Chem. 269, 19659–19662 [PubMed] [Google Scholar]
- 26. Alves D. S., Farr G. A., Seo-Mayer P., Caplan M. J. (2010) AS160 associates with the Na+,K+-ATPase and mediates the adenosine monophosphate-stimulated protein kinase-dependent regulation of sodium pump surface expression. Mol. Biol. Cell 21, 4400–4408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hulo S., Tiesset H., Lancel S., Edmé J. L., Viollet B., Sobaszek A., Nevière R. (2011) AMP-activated protein kinase deficiency reduces ozone-induced lung injury and oxidative stress in mice. Respir. Res. 12, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zheng D., Perianayagam A., Lee D. H., Brannan M. D., Yang L. E., Tellalian D., Chen P., Lemieux K., Marette A., Youn J. H., McDonough A. A. (2008) AMPK activation with AICAR provokes an acute fall in plasma [K+]. Am. J. Physiol. Cell Physiol. 294, C126–C135 [DOI] [PubMed] [Google Scholar]
- 29. Chibalin A. V., Kovalenko M. V., Ryder J. W., Féraille E., Wallberg-Henriksson H., Zierath J. R. (2001) Insulin- and glucose-induced phosphorylation of the Na+,K+-adenosine triphosphatase α-subunits in rat skeletal muscle. Endocrinology 142, 3474–3482 [DOI] [PubMed] [Google Scholar]
- 30. Ragolia L., Cherpalis B., Srinivasan M., Begum N. (1997) Role of serine/threonine protein phosphatases in insulin regulation of Na+,K+-ATPase activity in cultured rat skeletal muscle cells. J. Biol. Chem. 272, 23653–23658 [DOI] [PubMed] [Google Scholar]
- 31. Mounier R., Lantier L., Leclerc J., Sotiropoulos A., Pende M., Daegelen D., Sakamoto K., Foretz M., Viollet B. (2009) Important role for AMPKα1 in limiting skeletal muscle cell hypertrophy. FASEB J. 23, 2264–2273 [DOI] [PubMed] [Google Scholar]
- 32. Lantier L., Mounier R., Leclerc J., Pende M., Foretz M., Viollet B. (2010) Coordinated maintenance of muscle cell size control by AMP-activated protein kinase. FASEB J. 24, 3555–3561 [DOI] [PubMed] [Google Scholar]
- 33. Konrad D., Rudich A., Bilan P. J., Patel N., Richardson C., Witters L. A., Klip A. (2005) Troglitazone causes acute mitochondrial membrane depolarization and an AMPK-mediated increase in glucose phosphorylation in muscle cells. Diabetologia 48, 954–966 [DOI] [PubMed] [Google Scholar]
- 34. Al-Khalili L., Kotova O., Tsuchida H., Ehrén I., Féraille E., Krook A., Chibalin A. V. (2004) ERK1/2 mediates insulin stimulation of Na+,K+-ATPase by phosphorylation of the α-subunit in human skeletal muscle cells. J. Biol. Chem. 279, 25211–25218 [DOI] [PubMed] [Google Scholar]
- 35. Chibalin A. V., Ogimoto G., Pedemonte C. H., Pressley T. A., Katz A. I., Féraille E., Berggren P. O., Bertorello A. M. (1999) Dopamine-induced endocytosis of Na+,K+-ATPase is initiated by phosphorylation of Ser18 in the rat α-subunit and is responsible for the decreased activity in epithelial cells. J. Biol. Chem. 274, 1920–1927 [DOI] [PubMed] [Google Scholar]
- 36. Chibalin A. V., Pedemonte C. H., Katz A. I., Féraille E., Berggren P. O., Bertorello A. M. (1998) Phosphorylation of the catalyic α-subunit constitutes a triggering signal for Na+,K+-ATPase endocytosis. J. Biol. Chem. 273, 8814–8819 [DOI] [PubMed] [Google Scholar]
- 37. Dada L. A., Chandel N. S., Ridge K. M., Pedemonte C., Bertorello A. M., Sznajder J. I. (2003) Hypoxia-induced endocytosis of Na,K-ATPase in alveolar epithelial cells is mediated by mitochondrial reactive oxygen species and PKC-ζ. J. Clin. Invest. 111, 1057–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Doné S. C., Leibiger I. B., Efendiev R., Katz A. I., Leibiger B., Berggren P. O., Pedemonte C. H., Bertorello A. M. (2002) Tyrosine 537 within the Na+,K+-ATPase α-subunit is essential for AP-2 binding and clathrin-dependent endocytosis. J. Biol. Chem. 277, 17108–17111 [DOI] [PubMed] [Google Scholar]
- 39. Cheng S. X., Aizman O., Nairn A. C., Greengard P., Aperia A. (1999) [Ca2+]i determines the effects of protein kinases A and C on activity of rat renal Na+,K+-ATPase. J. Physiol. 518, 37–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sweeney G., Niu W., Canfield V. A., Levenson R., Klip A. (2001) Insulin increases plasma membrane content and reduces phosphorylation of Na+-K+ pump α1-subunit in HEK-293 cells. Am. J. Physiol. Cell Physiol. 281, C1797–1803 [DOI] [PubMed] [Google Scholar]
- 41. Lecuona E., Dada L. A., Sun H., Butti M. L., Zhou G., Chew T. L., Sznajder J. I. (2006) Na,K-ATPase α1-subunit dephosphorylation by protein phosphatase 2A is necessary for its recruitment to the plasma membrane. FASEB J. 20, 2618–2620 [DOI] [PubMed] [Google Scholar]
- 42. Fellner T., Lackner D. H., Hombauer H., Piribauer P., Mudrak I., Zaragoza K., Juno C., Ogris E. (2003) A novel and essential mechanism determining specificity and activity of protein phosphatase 2A (PP2A) in vivo. Genes Dev. 17, 2138–2150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kim K. Y., Baek A., Hwang J. E., Choi Y. A., Jeong J., Lee M. S., Cho D. H., Lim J. S., Kim K. I., Yang Y. (2009) Adiponectin-activated AMPK stimulates dephosphorylation of AKT through protein phosphatase 2A activation. Cancer Res. 69, 4018–4026 [DOI] [PubMed] [Google Scholar]
- 44. Sjöström M., Stenström K., Eneling K., Zwiller J., Katz A. I., Takemori H., Bertorello A. M. (2007) SIK1 is part of a cell sodium-sensing network that regulates active sodium transport through a calcium-dependent process. Proc. Natl. Acad. Sci. U.S.A. 104, 16922–16927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gusarova G. A., Dada L. A., Kelly A. M., Brodie C., Witters L. A., Chandel N. S., Sznajder J. I. (2009) α1-AMP-activated protein kinase regulates hypoxia-induced Na,K-ATPase endocytosis via direct phosphorylation of protein kinase Cζ. Mol. Cell. Biol. 29, 3455–3464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Comellas A. P., Kelly A. M., Trejo H. E., Briva A., Lee J., Sznajder J. I., Dada L. A. (2010) Insulin regulates alveolar epithelial function by inducing Na+,K+-ATPase translocation to the plasma membrane in a process mediated by the action of Akt. J. Cell Sci. 123, 1343–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Barlet-Bas C., Khadouri C., Marsy S., Doucet A. (1990) Enhanced intracellular sodium concentration in kidney cells recruits a latent pool of Na,K-ATPase whose size is modulated by corticosteroids. J. Biol. Chem. 265, 7799–7803 [PubMed] [Google Scholar]
- 48. Efendiev R., Das-Panja K., Cinelli A. R., Bertorello A. M., Pedemonte C. H. (2007) Localization of intracellular compartments that exchange Na,K-ATPase molecules with the plasma membrane in a hormone-dependent manner. Br. J. Pharmacol. 151, 1006–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hardie D. G. (2011) Energy sensing by the AMP-activated protein kinase and its effects on muscle metabolism. Proc. Nutr. Soc. 70, 92–99 [DOI] [PubMed] [Google Scholar]
- 50. Seo-Mayer P. W., Thulin G., Zhang L., Alves D. S., Ardito T., Kashgarian M., Caplan M. J. (2011) Preactivation of AMPK by metformin may ameliorate the epithelial cell damage caused by renal ischemia. Am. J. Physiol. Renal Physiol. 301, F1346–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rolfe D. F., Brown G. C. (1997) Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol. Rev. 77, 731–758 [DOI] [PubMed] [Google Scholar]
- 52. Erecińska M., Silver I. A. (1989) ATP and brain function. J. Cereb. Blood Flow Metab. 9, 2–19 [DOI] [PubMed] [Google Scholar]
- 53. Harris S. I., Balaban R. S., Barrett L., Mandel L. J. (1981) Mitochondrial respiratory capacity and Na+- and K+-dependent adenosine triphosphatase-mediated ion transport in the intact renal cell. J. Biol. Chem. 256, 10319–10328 [PubMed] [Google Scholar]
- 54. Clausen T., Van Hardeveld C., Everts M. E. (1991) Significance of cation transport in control of energy metabolism and thermogenesis. Physiol. Rev. 71, 733–774 [DOI] [PubMed] [Google Scholar]
- 55. Crambert G., Fuzesi M., Garty H., Karlish S., Geering K. (2002) Phospholemman (FXYD1) associates with Na,K-ATPase and regulates its transport properties. Proc. Natl. Acad. Sci. U.S.A. 99, 11476–11481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Blot-Chabaud M., Coutry N., Laplace M., Bonvalet J., Farman N. (1996) Role of protein phosphatase in the regulation of Na+,K+-ATPase by vasopressin in the cortical collecting duct. J. Membr. Biol. 153, 233–239 [DOI] [PubMed] [Google Scholar]
- 57. Lecuona E., Garcia A., Sznajder J. I. (2000) A novel role for protein phosphatase 2A in the dopaminergic regulation of Na,K-ATPase. FEBS Lett. 481, 217–220 [DOI] [PubMed] [Google Scholar]
- 58. Wang M. Y., Unger R. H. (2005) Role of PP2C in cardiac lipid accumulation in obese rodents and its prevention by troglitazone. Am. J. Physiol. Endocrinol. Metab. 288, E216–221 [DOI] [PubMed] [Google Scholar]
- 59. Wu Y., Song P., Xu J., Zhang M., Zou M. H. (2007) Activation of protein phosphatase 2A by palmitate inhibits AMP-activated protein kinase. J. Biol. Chem. 282, 9777–9788 [DOI] [PubMed] [Google Scholar]
- 60. Xing Y., Li Z., Chen Y., Stock J. B., Jeffrey P. D., Shi Y. (2008) Structural mechanism of demethylation and inactivation of protein phosphatase 2A. Cell 133, 154–163 [DOI] [PubMed] [Google Scholar]
- 61. Ortega-Gutiérrez S., Leung D., Ficarro S., Peters E. C., Cravatt B. F. (2008) Targeted disruption of the PME-1 gene causes loss of demethylated PP2A and perinatal lethality in mice. PLoS One 3, e2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hombauer H., Weismann D., Mudrak I., Stanzel C., Fellner T., Lackner D. H., Ogris E. (2007) Generation of active protein phosphatase 2A is coupled to holoenzyme assembly. PLoS Biol. 5, e155. [DOI] [PMC free article] [PubMed] [Google Scholar]