Background: We previously reported that bovine lactoferrin (bLF) reduces LPS-induced bone resorption. However, it was not clear how bLF inhibits LPS-mediated osteoclastogenesis through osteoblasts.
Results: bLF impaired LPS-mediated NFκB activation by interfering with TNF receptor-associated factor 6 (TRAF6) polyubiquitination.
Conclusion: bLF is a potent anti-inflammatory agent.
Significance: bLF may hold promise as a therapeutic agent for inflammatory diseases associated with bone destruction.
Keywords: Inflammation, Lipopolysaccharide (LPS), NFκB (NFκB), Periodontal Disease, Tumor Necrosis Factor (TNF), TRAF6, Lactoferrin
Abstract
Lactoferrin (LF) is an important modulator of the immune response and inflammation. It has also been implicated in the regulation of bone tissue. In our previous study we demonstrated that bovine LF (bLF) reduces LPS-induced bone resorption through a reduction of TNF-α production in vivo. However, it was not known how bLF inhibits LPS-mediated TNF-α and RANKL (receptor activator of nuclear factor κB ligand) production in osteoblasts. In this study we show that bLF impairs LPS-mediated TNF-α and RANKL production. bLF inhibited LPS-mediated osteoclastogenesis via osteoblasts in a co-culture system. Furthermore, bLF pretreatment inhibited LPS-induced NFκB DNA binding activity as well as IκBα and IKKβ (IκB kinase β) phosphorylation. MAP kinase activation was also inhibited by bLF pretreatment. However, bLF pretreatment failed to block the degradation of IRAK1 (interleukin-1 receptor-associated kinase 1), which is an essential event after its activation. Remarkably, we found that bLF pretreatment inhibited LPS-mediated Lys-63-linked polyubiquitination of TNF receptor-associated factor 6 (TRAF6). We also found that bLF is mainly endocytosed through LRP1 (lipoprotein receptor-related protein-1) and intracellular distributed bLF binds to endogenous TRAF6. In addition, bLF inhibited IL-1β- and flagellin-induced TRAF6-dependent activation of the NFκB signaling pathway. Collectively, our findings demonstrate that bLF inhibits NFκB and MAP kinase activation, which play critical roles in chronic inflammatory disease by interfering with the TRAF6 polyubiquitination process. Thus, bLF could be a potent therapeutic agent for inflammatory diseases associated with bone destruction, such as periodontitis and rheumatoid arthritis.
Introduction
Lactoferrin (LF)3 is an 80-kDa glycoprotein belonging to the transferrin family of iron-binding proteins. It has a wide spectrum of biological effects, including anti-bacterial, anti-viral, anti-cancer, immunomodulatory, and anti-inflammatory activities. In vitro studies have shown that LF inhibits the production of lipopolysaccharide (LPS)-induced proinflammatory cytokines such as TNF-α, IL-6, and IL-1β (1, 2). However, the molecular mechanisms that mediate its anti-inflammatory effects are not well known. LF is present in various secretory fluids, such as milk, saliva, tears, and nasal secretions. In particular, it is most abundant in human colostrum followed by human milk and cow milk, and it can be easily and safely purified from the latter. Therefore, there is growing interest in the therapeutic use of bovine LF (bLF) for treating inflammation associated with bone destruction, such as in chronic periodontitis and rheumatoid arthritis.
In our previous study we demonstrated that oral administration of liposomal bLF, which exhibits improved stability in the stomach and enhanced absorption by the intestinal tract, significantly reduces alveolar bone resorption by decreasing TNF-α production by host cells stimulated with LPS (3). In addition, our in vitro analysis showed that bLF pretreatment inhibits LPS-induced TNF-α and RANKL (receptor activator of nuclear factor κB ligand) expression in ST2 cells (a bone marrow-derived osteogenic cell line). It has been reported that the anti-inflammatory effects of bLF are partially due to its LPS-chelating properties and the ability to reduce binding of LPS to CD14 (4, 5). However, in our experiments ST2 cells were pretreated with bLF and stimulated by LPS in fresh medium containing 10% FBS only after carefully washing with PBS to avoid the inhibitory effects caused by direct binding between bLF and LPS. Thus, we hypothesized that bLF inhibits LPS-induced TNF-α expression through an unknown mechanism, perhaps by interfering with an intracellular signaling pathway.
It is well known that LPS induces TNF-α and RANKL expression via the TLR4 transcription factor in the nuclear factor κB (NFκB) pathway. NFκB is responsible for regulating a multitude of different processes, including cell proliferation, differentiation, and survival (6). It plays a particularly important role in the regulation of inflammation and inflammation-associated bone destruction (7, 8). In unstimulated cells, NFκB is retained in the cytoplasm through an interaction with inhibitory proteins known as IκBs. After stimulation by innate immune and proinflammatory stimuli, such as LPS, TNF-α, and IL-1β, IκBs are rapidly phosphorylated and ubiquitinated and are subsequently degraded by the proteasome complex (9). IκB phosphorylation is carried out by the IκB kinase (IKK), a complex composed of 3 subunits, IKKα, IKKβ, and IKKγ/NFκB essential modulator (NEMO) (10). In this process TRAF6-mediated Lys-63-linked polyubiquitination of IKKγ/NEMO is essential (11, 12). TRAF6 is a member of the TNF receptor-associated factor (TRAF) family of proteins. It mediates signaling not only by the members of the TNF receptor superfamily but also by the members of the Toll/IL-1 family. Signals from TLR4 and IL-1 have been shown to be mediated by TRAF6. The interaction of this protein with UBE2N/UBC13 and UBE2V1/UEV1A, which are ubiquitin conjugating enzymes catalyzing the formation of polyubiquitin chains, has been found to be required for IKK activation by this protein (13).
Numerous studies have been carried out on the anti-inflammatory effects of bLF; however, these investigations do not provide any data on the underlying molecular mechanisms. This study is the first to focus on the anti-inflammatory mechanism of bLF at the molecular level. In addition to clarifying the molecular biology of bLF function, our results suggest that this protein may hold promise as a therapeutic agent for several human inflammatory diseases.
EXPERIMENTAL PROCEDURES
Reagents
The bLF was purchased from Morinaga Milk Industry (Tokyo, Japan). LPS from Aggregatibacter actinomycetemcomitans (ATCC 29522) was kindly provided by Professor Tatsuji Nishihara of the Kyusyu Dental College. Monoclonal anti-pIκBα, polyclonal anti-IkBa, anti-TRAF6, and anti-TAK1 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Monoclonal anti-phospho-JNK, polyclonal anti- interleukin-1 receptor-associated kinase 1 (IRAK1), anti-JNK, anti-p38, anti-phospho-p38, anti-JNK, anti-IKKβ, and anti-phospho-IKKβ antibodies were obtained from Cell Signaling Technologies (Danvers, MA). Monoclonal anti-bLF was from HyCult Biotech (Uden, The Netherlands). Monoclonal anti-Lys-63 linkage-specific polyubiquitin was purchased from Enzo Life Sciences (Plymouth Meeting, PA). Polyclonal anti-p65 was from IMAGENEX Corp. (San Diego, CA). The dicumarol (JNK MAPK inhibitor), SB203580 (p38 MAPK inhibitor), and caffeic acid phenethyl ester (CAPE; NFκB inhibitor) were purchased from Sigma. The TLR4-specific inhibitor, CLI-095, was obtained from Invivogen (San Diego, CA). The IKKβ-specific inhibitor SC-514 was purchased from Calbiochem.
Cell Lines and Culture
Primary osteoblasts (OBs) from mouse calvarias were isolated from fetuses by sequential collagenase digestion as described previously (3). Briefly, calvariae were dissected free from loosely adherent connective tissues, minced, and sequentially digested in type I collagenase (Sigma) solution. OBs and ST2 (a bone-marrow-derived osteogenic cell line) cells were maintained in a α-MEM (Invitrogen) with 10 mm HEPES (pH 7.2), 10% FBS (Invitrogen), and 100 units/ml penicillin-streptomycin (Invitrogen) at 37 °C in a humidified atmosphere containing 5% CO2.
Gene Expression Experiments
OBs and ST2 cells were seeded in 6-well culture plates (3 × 105 cells/well) and cultured in α-MEM containing 10% FBS for 2 days. After a 4-h pretreatment with bLF (1, 10, or 100 μg/ml) or no bLF pretreatment, the culture plates were briefly washed 2 times with PBS. Then cells were cultured in fresh α-MEM containing 10% FBS with LPS (100 ng/ml) or mouse recombinant IL-1β (10 ng/ml). The cultured cells were collected 2 h after LPS or mouse IL-1β stimulation. Then total RNAs were isolated from cultures of confluent cells by using an RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. The preparations were quantified, and their purity was determined by standard spectrophotometric methods. cDNA was synthesized from 1 μg of total RNA using a ReverTra Ace kit (Toyobo Biochemicals, Tokyo, Japan). For real-time PCR, aliquots of total cDNA were amplified with the TaqMan Fast Universal PCR Master Mix (Applied Biosystems, Foster City, CA). Data acquisition and analysis were performed with a Step One Real-Time PCR System using Step One Software, Version 2.1 (Applied Biosystems). The PCR products were quantified using S18 as the reference gene. The primers and TaqMan probes were purchased from Applied Biosystems.
Determination of Cytokine Production by ELISA
ST2 cells were seeded in a 10-cm culture dish (3 × 106 cells/dish) and cultured as described above. After a 4-h pretreatment with bLF, the cells were incubated with LPS for 48 h. Then, culture media and cells were collected separately and concentrated using an Amicon Ultra Centrifugal Filter Device (Millipore, Billerica, MA) for ELISA assay. TNF-α protein levels in culture media and the RANKL protein levels in cell lysate, prepared by the Boabaid method (39), were measured by ELISA as recommended by the manufacturer. Results were normalized to total protein levels. The protein concentration was determined by the Bradford protein assay (Bio-Rad) with BSA (Sigma) as a standard.
Osteoclast Formation Assay
Bone marrow cells were collected from the femora and tibiae of 6–9-week-old male mice as previously described (3). OBs and bone marrow cells (2 × 105) were co-cultured for 5 days in α-MEM containing 10% FBS in 96-well tissue culture plates and incubated with LPS (1 μg/ml) for the final 3 days. Some co-cultures were pretreated with or without bLF (1 or 10 μg/ml) for 2 days before LPS treatment. Co-cultures were then fixed and stained for tartrate-resistant acid phosphatase (an osteoclast marker). All tartrate-resistant acid phosphatase-positive cells with more than three nuclei or with less than two nuclei in each well were observed under a microscope and designated as osteoclasts and preosteoclasts, respectively.
EMSA Assay
To obtain nuclear and cytoplasmic fractions, we used the nuclear/cytoplasmic fractionation kit (Pierce) as previously described (13). EMSA was performed according to the manufacturer's instructions (gel shift assay system; Promega, Madison, WI). The NFκB probe was labeled with [γ-32P]ATP using T4 polynucleotide kinase. Binding reaction mixtures containing 4 μg of nuclear extract protein and 32P-labeled probe were incubated for 30 min at room temperature. The reaction mixtures were analyzed by electrophoresis on native 4% polyacrylamide gels. The sequence of the NFκB probe was purchased from Promega.
Western Blot and Immunoprecipitation Analyses
Cells were lysed in ice-cold lysis buffer containing 50 mm Tris-HCl (pH 7.5), 250 mm NaCl, 0.1% Triton X-100 (Roche Applied Science), 1 mm EDTA, 50 mm NaF, 0.1 mm Na3VO4, 1 mm DTT, 0.1 mm leupeptin, 0.1 μg/ml soybean trypsin inhibitor, 10 μg/ml l-1-chlor-3-(4-tosylamido)-4 phenyl-2-butanon (TPCK), 10 μg/ml l-1 chlor-3-(4-tosylamido)-7-amino-2-heptanon hydrochloride (TLCK), 10 μg/ml aprotinin, and 50 μg/ml phenylmethylsulfonyl fluoride. Lysates were incubated on ice for 30 min and centrifuged at 15,000 rpm for 20 min at 4 °C. The supernatants were deemed whole lysates. To obtain nuclear and cytoplasmic fractions, we used the nuclear/cytoplasmic fractionation kit. Western blotting was carried out as described previously (14). Thirty micrograms of protein lysate were subjected to 10% polyacrylamide gel electrophoresis followed by electroblotting onto a nitrocellulose filter. For detection of the immunocomplex, an ECL Western blotting detection system (Amersham Biosciences) was used. The immunoprecipitates were also subjected to Western blotting. For detecting TRAF6 auto-polyubiquitination with Lys-63 and the TRAF6 complex, cell lysates were obtained by lysing the cell monolayer with 1% Brij 96 lysis buffer containing 1% Brij 96, 25 mm HEPES (pH 7.5), 150 mm NaCl, and 5 mm MgCl2.
siRNA Gene Knockdown
Lipoprotein receptor-related protein-1 (LRP1) siRNA oligo, Nucleolin siRNA oligo, CD14 siRNA oligo, and control siRNA oligo were purchased from Applied Biosystems. ST2 cells were incubated with Lipofectamine RNAiMAX (Invitrogen) according to the manufacturer's protocol. ST2 cells were carefully dissociated into single cell suspensions using 0.5 mm EDTA/phosphate-buffered saline (PBS; Invitrogen). The cells were then transferred to 6-well plates and cultured in α-MEM with 10% FBS. After 24 h, ST2 cells were transfected with siRNA when cells reached 30–50% confluence according to the manufacturer's protocol. Briefly, gene-specific siRNA oligomers (20 nm) were diluted in 100 μl of Opti-MEM I reduced serum medium (Invitrogen) and mixed with 3 μl of each transfection reagent (Invitrogen) pre-diluted in 97 μl of Opti-MEM. After a 20-min incubation at room temperature, the complexes were added to the cells in a final volume of 2 ml of medium. Cells were transfected again after 24 h.
Statistical Analysis
Statistical differences among experimental groups were evaluated by the Scheffe's test with the level of significance set at p < 0.05. Values are expressed as the mean ± S.D.
RESULTS
bLF Inhibits LPS-induced Osteoclastogenesis by Reducing TNF-α and RANKL Expression in Osteoblasts
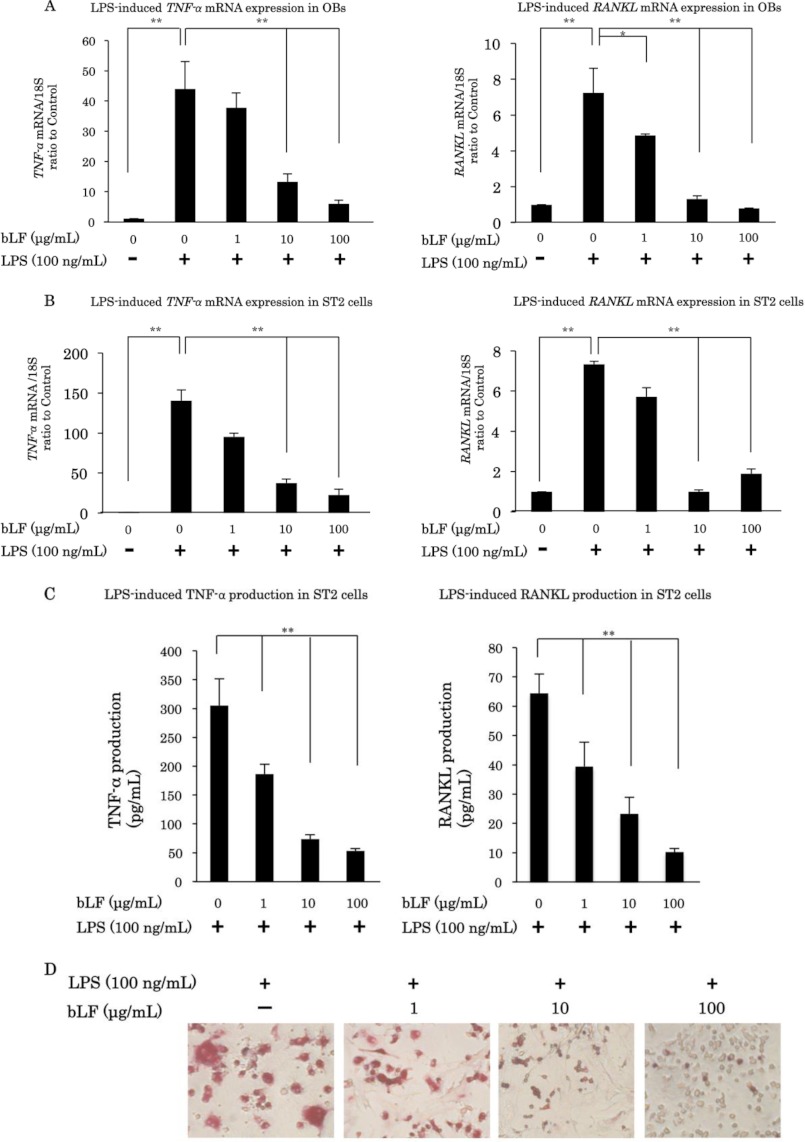
LPS is known to induce the expression of various cytokines, such as TNF-α and RANKL, in osteoblasts. Here, we demonstrate that LPS significantly up-regulates TNF-α mRNA expression at 2 h and RANKL mRNA expression at 12 h. However, pretreatment with bLF significantly blocked the LPS-induced up-regulation of TNF-α and RANKL mRNA expression in a dose-dependent manner in primary OBs (Fig. 1A). We also examined the inhibitory effects of bLF on LPS-induced cytokine expression using ST2 cells. bLF significantly suppressed the LPS-mediated up-regulation of TNF-α and RANKL mRNA levels in ST2 cells (Fig. 1B). Moreover, bLF inhibited the LPS-induced up-regulation of TNF-α and RANKL protein levels in ST2 cells (Fig. 1C). Furthermore, bLF inhibited the differentiation and maturation of osteoclasts, resulting in a reduction in the number of tartrate-resistant acid phosphatase-positive cells in co-culture of OBs and bone marrow cells stimulated with LPS (Fig. 1D). These results suggest that bLF inhibits LPS-induced osteoclastogenesis by reducing TNF-α and RANKL expression.
FIGURE 1.
bLF inhibits LPS-induced osteoclastogenesis by reducing TNF-α and RANKL expression in osteoblasts. Primary OBs (A) and ST2 cells (B) were preincubated with 1, 10, or 100 μg/ml bLF for 4 h before LPS (100 ng/ml) stimulation, and cells were carefully washed twice with PBS. Cells were then cultured in medium with or without LPS. TNF-α mRNA expression was analyzed 2 h after LPS stimulation, and RANKL mRNA expression was examined 12 h after LPS stimulation. C, bLF-pretreated ST2 cells were stimulated with LPS for 48 h, and TNF-α and RANKL production were measured with ELISA assay. D, OBs and bone marrow cells (2 × 105) were co-cultured for 5 days. Co-cultures were precultured with bLF for 2 days. After 2 days of bLF exposure, co-cultures were washed twice and cultured in the presence of LPS (1 μg/ml) for the final 3 days. *, p < 0.05; **, p < 0.01.
LPS Up-regulates TNF-α and RANKL mRNA Expression Mainly through NFκB Pathway
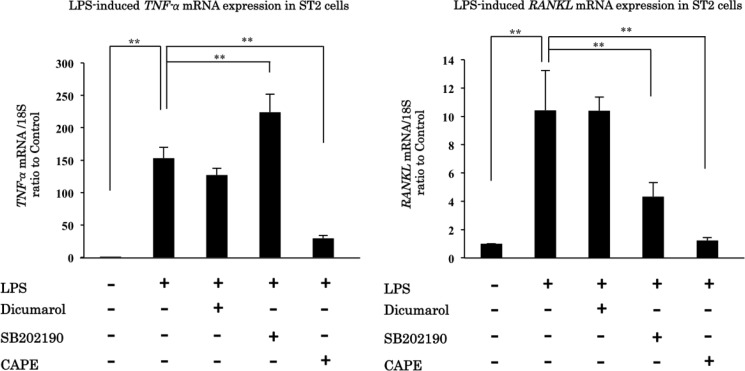
It is well known that LPS can induce NFκB and MAP kinase activation. We used the inhibitors for each pathway to clarify the role of each pathway in LPS-induced up-regulation of TNF-α and RANKL mRNA expression. In this experiment, we used dicumarol as a specific inhibitor for JNK, SB202190 as a specific inhibitor for p38, and CAPE as a specific inhibitor for NFκB (15). CAPE, a NFκB inhibitor, pretreatment eliminated the up-regulation of TNF-α and RANKL mRNA expression induced by LPS stimulation. Our results show that LPS up-regulates TNF-α and RANKL expression mainly through the NFκB pathway (Fig. 2).
FIGURE 2.
LPS up-regulates TNF-α and RANKL mRNA expression mainly through NFκB pathway. ST2 cells were pretreated with dicumarol, SB202190, CAPE, or medium and treated with medium or LPS for 2 or 12 h. TNF-α and RANKL mRNA expression were analyzed 2 h and 12 h after LPS stimulation, respectively. Dicumarol is a JNK MAPK inhibitor, SB202190 is a p38 MAPK inhibitor, and CAPE is an NFκB inhibitor. **, p < 0.01.
bLF Inhibits the Activation of the NFκB Pathway Induced by LPS Stimulation
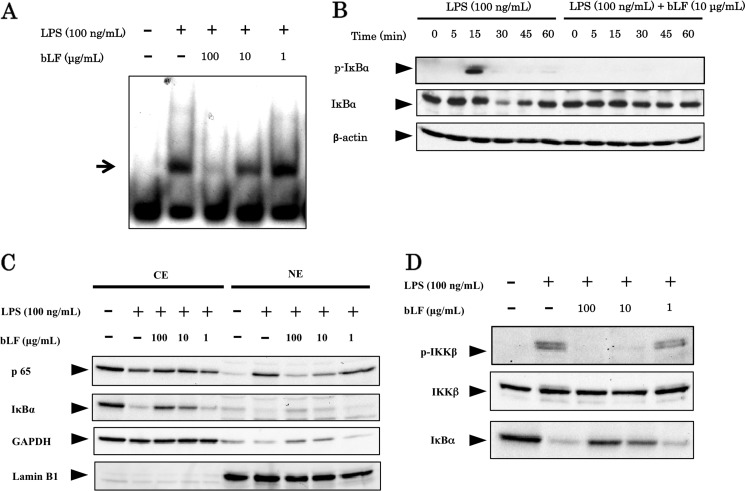
Next, we checked on the effects of bLF on the NFκB signaling pathway activated by LPS stimulation. Here, we demonstrate that bLF inhibits DNA binding by NFκB induced by LPS stimulation in ST2 cells (Fig. 3A). Among the NFκB family members, the p65:p50 dimer is considered part of the canonical pathway. This classical NFκB dimer is activated by IκBα degradation, which occurs subsequent to IκBα phosphorylation. We also show that pretreatment with bLF (100 μg/ml) completely blocks LPS-induced phosphorylation of IκBα and the degradation of IκBα (Fig. 3B). As shown in Fig. 3C, nuclear translocation of p65 was detected in LPS-stimulated cells, but this process was inhibited by bLF pretreatment in a dose-dependent manner. In addition, we clearly demonstrate that activation of IKKβ, which is responsible for the phosphorylation and degradation of IκBα, was inhibited by bLF pretreatment in a dose-dependent manner (Fig. 3D). Taken together, these results clearly indicate that bLF inhibits the activation of the NFκB pathway induced by LPS stimulation.
FIGURE 3.
bLF inhibits the activation of the NFκB pathway induced by LPS stimulation. A, ST2 cells were pretreated for 4 h with 0, 1, 10, or 100 μg/ml bLF. Subsequently, ST2 cells were carefully washed twice with PBS and stimulated with LPS (100 ng/ml). Nuclear extracts from each group were subjected to the EMSA assay for analyzing the DNA binding activity of NFκB. B, ST2 cells were preincubated with bLF (100 μg/ml), washed, and stimulated with LPS (100 ng/ml) for the indicated periods. Cell lysates from each group were examined by immunoblotting analysis using p-IκBα and IκBα antibodies. C, the nuclear and cytoplasmic extracts from each group were immunoblotted with an anti-p65, anti-IκBα, anti-GAPDH, or anti-lamin B1 antibody. CE, cytoplasm extracts; NE, nuclear extracts. D, ST2 cells were preincubated for 4 h with 0, 1, 10 or 100 μg/ml bLF, and then washed with PBS and stimulated with LPS for 30 min in the presence of okadaic acid. Cell lysates from each group were examined by immunoblotting analysis using p-IKKβ and IκBα antibodies. Immunoblotting of ΙKKβ was performed to control for protein loading. The experiments were performed at least twice, with similar results.
bLF Inhibits the Activation of the MAP Kinase Pathway but Not IRAK1 Degradation
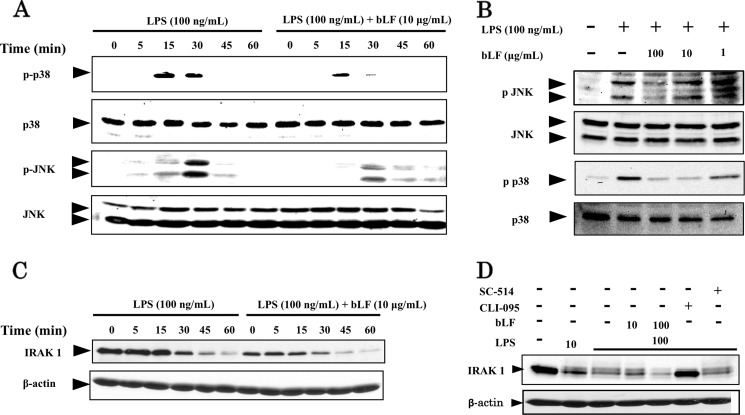
It is well known that LPS activates not only the NFκB pathway but also the MAP kinase cascade as well. We confirmed that LPS induces the phosphorylation of p38 and JNK in ST2 cells. bLF also inhibited the phosphorylation of p38 and JNK induced by LPS stimulation in a time- and dose-dependent manner (Fig. 4, A and B).
FIGURE 4.
bLF inhibits the activation of the MAP kinase pathway but not IRAK1 degradation. A, ST2 cells were preincubated for 4 h with 10 μg/ml bLF and then carefully washed twice with PBS and stimulated with LPS (100 ng/ml) for the indicated periods. Whole cell lysates from each group were examined by immunoblotting analysis using p-p38 and p-JNK antibodies. Immunoblotting of p38 and JNK were performed to control for protein loading. B, ST2 cells were pretreated with the indicated concentrations of bLF for 4 h before LPS (100 ng/ml) stimulation. The cells were then carefully washed twice with PBS and treated with or without LPS for 30 min. Whole cell lysates were subjected to immunoblot analysis with p-p38 and p-JNK antibodies. Total p38 and JNK protein expression was evaluated by immunoblotting with p38 and JNK antibodies. C, ST2 cells were pretreated with 100 μg/ml bLF for 4 h, washed, and treated for the indicated period with medium or LPS (100 ng/ml). Cell lysates were immunoblotted with IRAK1 antibody. D, ST2 cells were preincubated with bLF (10 or 100 μg/ml), CLI-095, SC-514, or medium for 4 h and were briefly washed twice with PBS and treated with medium or LPS for 60 min. Whole cell lysates were examined by immunoblotting with IRAK1 antibody. Immunoblotting for β-actin was performed to control for protein loading. The experiments were performed at least twice, with similar results.
We next examined the effects of bLF on targets upstream of the NFκB and MAP kinase signaling pathways activated by LPS stimulation. Among the molecules upstream of NFκB and MAP kinase, IRAK1 was ubiquitinated and subsequently degraded after its phosphorylation was induced by LPS stimulation. Others have shown that by inhibiting IRAK1 kinase activity, the protein is no longer subject to autophosphorylation or subsequent degradation (16, 17). We observed IRAK1 degradation at 30, 45, and 60 min after LPS stimulation. However, bLF had no effect on LPS-induced IRAK1 degradation (Fig. 4C). To confirm our results, we used 2 different inhibitors, CLI-095 and SC-514, both of which inhibit LPS-mediated NFκB activation. CLI-095, also known as TAK-242, is a novel cyclohexene derivative that specifically suppresses TLR4 signaling mediated by the intracellular domain of the receptor but not that mediated by TLR4 adaptors (18). SC-514 is a cell-permeable, ATP-competitive, and highly selective inhibitor of IKKβ (19). Only CLI-095 inhibited the degradation of IRAK1 (Fig. 4D). As mentioned above, IRAK1 degradation requires IRAK1 kinase activity and autophosphorylation. These data suggest that the targets of bLF are downstream of IRAK1.
bLF Inhibits TRAF6 Autoubiquitination
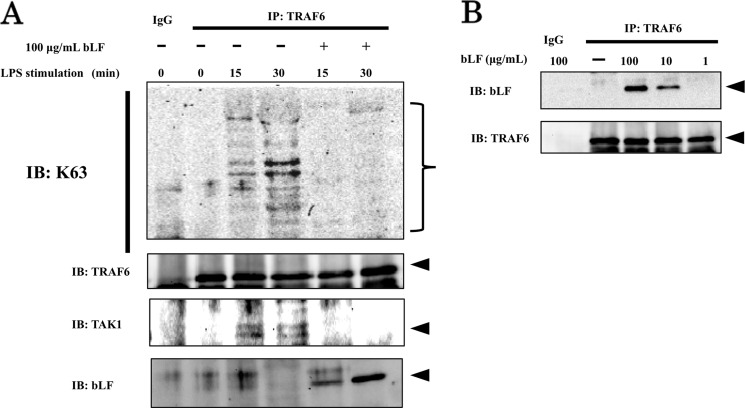
Subsequently, we focused on the activation of TRAF6, for which autoubiquitination is necessary for participation in the NFκB and MAP kinase signaling pathways. As shown in Fig. 5A, endogenous TRAF6 immunoprecipitated from LPS-stimulated ST2 cells displayed strong Lys-63 ubiquitination in a time-dependent manner, whereas immunoprecipitates from bLF-pretreated ST2 cells were not ubiquitinated after LPS stimulation. In addition, we demonstrated that the formation of the TRAF6-TAK1 (TGF β-activated kinase 1) complex was inhibited in bLF-pretreated ST2 cells, whereas LPS stimulation recruited the TRAF6-TAK1 complex (Fig. 5A). Furthermore, we examined whether bLF directly bound TRAF6 or not. Strikingly, Western blotting of immunoprecipitates from bLF-pretreated ST2 cells revealed that bLF directly bound endogenous TRAF6 (Fig. 5B).
FIGURE 5.
bLF interrupts TRAF6 Lys-63-linked autoubiquitination. A, ST2 cells were treated with 100 μg/ml bLF for 4 h before stimulation with LPS. Then, ST2 cells were briefly washed 2 times with PBS and exposed to medium with or without LPS (100 ng/ml) for 15 or 30 min, respectively. Endogenous TRAF6 protein was immunoprecipitated (IP) from whole cell lysates and subjected to immunoblot (IB) analysis with anti-Lys-63-linked ubiquitin-specific antibody as well as anti-TAK1 and anti-bLF antibodies. Immunoblotting of TRAF6 was performed to control for protein loading. B, ST2 cells were treated with 1, 10, or 100 μg/ml bLF for 4 h. Whole cell lysates were immunoprecipitated with anti-TRAF6 and examined by immunoblotting with bLF. Immunoblotting of TRAF6 was performed to control for protein loading. The experiments were performed at least twice with similar results.
bLF Inhibits the IL-1β and Flagellin-mediated NFκB Pathway
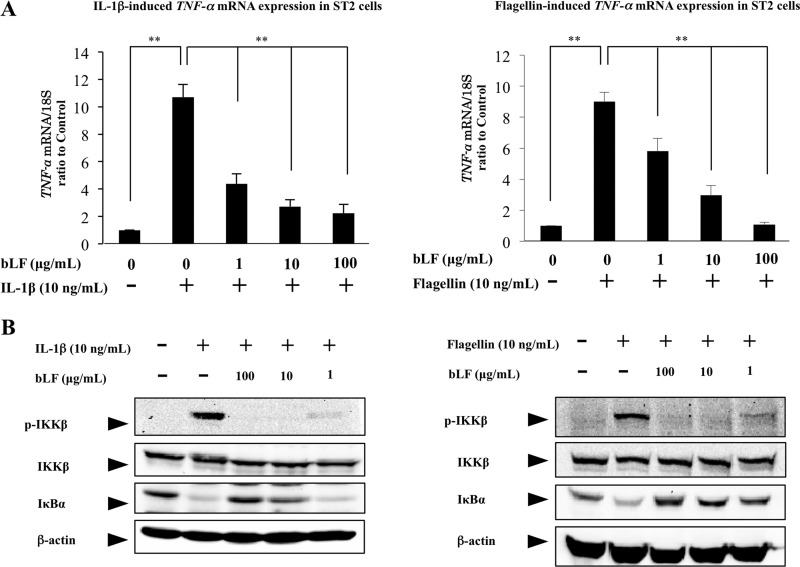
Based on the finding that bLF blocks LPS-induced NFκB activation by inhibiting TRAF6 autoubiquitination, we conjectured that bLF directly interacts with TRAF6 and inhibits the NFκB pathway. The mechanism whereby bLF inhibits LPS-induced NFκB activation appears not to be due to the suppression of LPS binding to CD14, a co-receptor, along with TLR4, which binds LPS. To clarify the inhibitory effects of bLF on the TRAF6-mediated pathway, we further examined the effects of bLF pretreatment on the IL-1β signaling pathway and TLR signaling pathway, where NFκB pathway is activated by TRAF6 autoubiquitination. As the results show, bLF blocked the TNF-α mRNA up-regulation induced by IL-1β and Flagellin stimulation in a dose-dependent manner (Fig. 6A). Furthermore, these results clearly demonstrate that pretreatment with bLF inhibits IκBα degradation and IKKβ phosphorylation in a dose-dependent manner (Fig. 6B). Collectively, these data strongly suggest that bLF inhibits the TRAF6-dependent NFκB pathway.
FIGURE 6.
bLF inhibits the IL-1β and flagellin-mediated NFκB pathway. ST2 cells were pretreated with the indicated concentration of bLF or vehicle before IL-1β (10 ng/ml) (A) or flagellin (100 ng/ml) (B) stimulation, respectively. After each stimulation cells were collected for examining the expression of TNF-α and for immunoblotting with antibodies against p-IKKβ, IKKβ, IκBα, and β-actin. The experiments were performed at least twice, with similar results. **, p < 0.01.
Intracellular Distribution of bLF in Osteoblasts
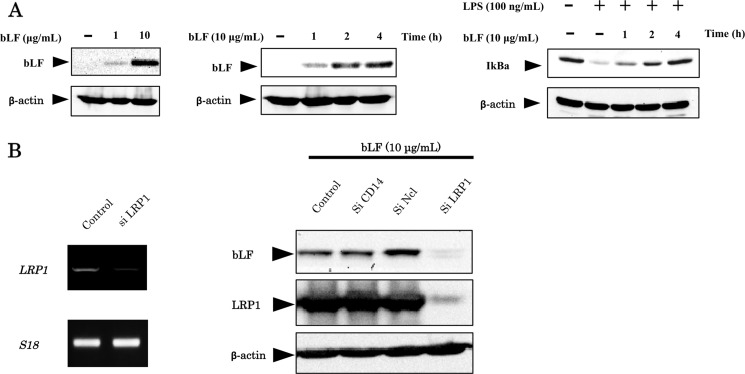
We previously demonstrated that bLF is endocytosed by osteoblasts (3). Here, we examined the intracellular distribution of bLF in a time- and dose-dependent manner (Fig. 7A). The intracellular localization of full-length bLF was detected by Western blot analysis. The intracellular localization of bLF was increased in a time- and dose-dependent fashion (Fig. 7A). In addition, the inhibition on the degradation of IκBα, a inhibitory protein for NFκB activation, was clearly correlated with the amount of intracellular bLF (Fig. 7A). We focused on three candidate receptors for bLF endocytosis. We performed RNA interference to reduce the expression of these potential bLF receptors and examined the intracellular levels of bLF. The cellular accumulation of bLF was inhibited only by LRP1 knockdown in ST2 cells (Fig. 7B). These findings taken together with the previous studies on TLR signaling (20, 21) indicate that bLF is mainly endocytosed through LRP1 and intracellular distributed bLF binds to TRAF6, resulting in the inhibition of TLR4/NFκB signaling (Fig. 8).
FIGURE 7.
bLF is endocytosed by LRP1. A, ST2 cells were incubated with the indicated concentration of bLF or PBS for 4 h. Whole cell lysates were immunoblotted with the anti-bLF antibody (left panel). ST2 cells were pretreated with 10 μg/ml bLF or PBS for 1, 2, or 4 h (middle panel). The inhibitory effects of bLF on the NFκB pathway at different incubation periods were examined by immunoblotting with an anti-IkBa antibody (right panel). B, ST2 cells were transfected with control, CD14, nucleolin (Ncl), or LRP1 siRNA. Thereafter, cells were incubated with 10 μg/ml bLF for 4 h. Whole cell lysates were immunoblotted with the anti-bLF antibody. Immunoblotting of β-actin was performed to control for protein loading. The experiments were performed at least twice, with similar results.
FIGURE 8.
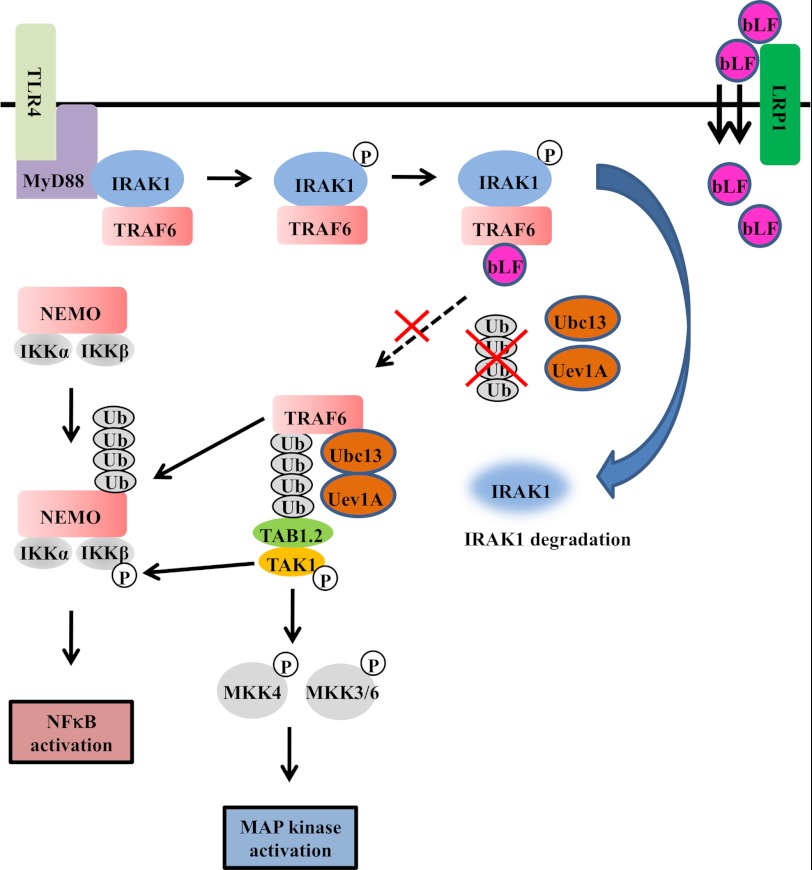
The molecular mechanisms behind the effects of bLF on LPS-mediated osteoclastogenesis. The binding of LPS to TLR4 initiates the formation of a complex that includes MyD88 and IRAK1. IRAK1 undergoes autophosphorylation shortly after LPS stimulation. Hyperphosphorylated IRAK1 then disengages from MyD88 and forms a cytosolic IRAK1-TRAF6 complex. TRAF6 is then autoubiquitinated and interacts with TAK and TAB. Phosphorylated IRAK1 also undergoes ubiquitin-mediated degradation. Once TAK1 is phosphorylated subsequent to TRAF6 activation, it activates the NFκB pathway by phosphorylating IKKβ, for which TRAF6-mediated polyubiquitination of NEMO (NFκB essential modulator) is necessary. Active TAK1 also phosphorylates the MAPK kinases MKK4 and MKK3/6, which activate p38 and JNK, respectively. Endocytosed bLF through LRP1 binds to TRAF6 after bLF pretreatment. In bLF-pretreated cells, LPS stimulation induces IRAK1 degradation subsequent to its kinase activation. In contrast, bLF inhibits TRAF6 autoubiquitination. NEMO, NFκB essential modulator.
DISCUSSION
Excessive bone resorption is a major pathological feature of chronic inflammatory diseases, such as arthritis, osteoporosis, and periodontitis. It is now clear that the dysregulation of immune and inflammatory responses is a critical factor in the initiation of the bone destruction associated with these conditions (22). A great deal of research indicates that numerous osteotropic factors, including cytokines and hormones, exert their primary osteoclastogenic effects by inducing RANKL expression in cells of the osteoblast lineage (23, 24). RANKL, which is a member of the TNF family of cytokines, is a ligand for RANK and functions as a key factor for osteoclast differentiation and activation. The overproduction of RANKL has been implicated in a variety of degenerative bone diseases, such as osteoporosis, rheumatoid arthritis, and psoriatic arthritis.
Periodontitis-associated bone destruction, a chronic inflammatory disease, is due to an excessive host defense response against plaque-associated bacteria and LPS (25, 26). LPS is the major component of the outer membrane of Gram-negative bacteria. It acts as an endotoxin and strongly induces proinflammatory cytokines, including TNF-α. TNF-α is one of the key cytokines involved in the pathogenesis of periodontitis; it stimulates the production of other proinflammatory cytokines, such as IL-1β, chemokines, prostaglandin E2, and RANKL, which is an essential mediator of osteoclast differentiation. In addition, similar to RANKL, TNF-α is a potent osteoclastogenic factor that directly enhances the proliferation and differentiation of osteoclasts (27). bLF inhibits LPS-induced osteoclastogenesis, which is mediated through osteoblasts, by suppressing the production of TNF-α by osteoblasts.
It has been firmly established that LPS activates the NFκB and MAP kinase pathways through its essential receptor, TLR4 (20). In this study we demonstrate that bLF pretreatment blocks LPS-induced NFκB activation, IκBα degradation, and IKKβ phosphorylation (Fig. 8). IRAK is a serine/threonine-specific kinase that is associated with the IL-1 and TLR receptors (29, 30). The mammalian family of IRAK kinases contains four members, IRAK1, IRAK-2, IRAK-3/IRAK-M, and IRAK-4. The binding of LPS to TLR4 initiates the formation of a complex that includes MyD88 and IRAK1 (31). IRAK1 undergoes autophosphorylation shortly after LPS stimulation. Hyperphosphorylated IRAK1 then disengages from myeloid differentiation primary response gene 88 (MyD88) and forms a cytosolic IRAK1-TRAF6 complex (31, 32). TRAF6 is then autoubiquitinated and interacts with TAK and TAB, resulting in the activation of the NFκB and MAP kinase pathways (Fig. 8). Phosphorylated IRAK1 also undergoes ubiquitin-mediated degradation (Fig. 8). Once TAK1 is phosphorylated as a consequence of TRAF6 activation, it activates the NFκB pathway by phosphorylating IKKβ. Active TAK1 also phosphorylates the MAPK kinases, MKK4 and MKK3/6, which activate p38 and JNK, respectively.
As shown in Fig. 8, we found that bLF effectively blocked IKKβ phosphorylation as well as JNK and p38 phosphorylation. In addition, the degradation of IRAK1, which occurs subsequent to phosphorylation, was observed in bLF-pretreated cells. Furthermore, TRAF6 autoubiquitination was inhibited by bLF pretreatment. Stimulating cells with IL-1β or LPS leads to the oligomerization and subsequent self-ubiquitination of TRAF6 (12). The Lys-63-linked TRAF6 ubiquitin conjugates are subsequently recognized by the highly conserved carboxyl-terminal Npl4 zinc finger (NZF) domain of either TAB2 or TAB3 (33), which function as scaffold proteins that recruit and activate TAK1, the kinase that is thought to directly activate IKK (34). Recently, it was shown that Lys-63-linked TRAF6 autoubiquitination is site-specific and that mutation of this site results in the lack of TAK1 and IKK activation, which is necessary for NFκB activation (35). Significantly, we found that bLF binds to endogenous TRAF6 4 h after bLF treatment (Fig. 5B). We also show that bLF was primarily internalized by the endocytic receptor LRP1, which is a ubiquitously expressed type 1 membrane protein and a member of the low density lipoprotein receptor family (36) (Fig. 7). Furthermore, the inhibitory effects of bLF were clearly correlated with the amount of intracellular bLF. In addition, we showed that bLF also showed the inhibitory effects against other TRAF6-dependent components, IL-1β and flagellin (Fig. 6). Collectively, our results indicate that not extracellular, but internalized bLF inhibits the NFκB pathway by interfering with the autoubiquitination of TRAF6 (Fig. 8).
TRAF6 has been shown to be involved in not only TLR4 but also other TLRs-mediated MyD88-dependent signaling pathways (20, 21). Each TLR has been shown to recognize specific components of pathogen, thus demonstrating that the immune system detects pathogen-associated molecular pattern challenge by TLRs and induce NFκB activation leading to the inflammatory responses. Therefore, our results suggest that bLF administration has beneficial impacts on control of excessive inflammatory responses caused by innate immunity against a wide range of pathogen-associated molecular patterns, including LPS and flagellin, through interfering TRAF6 autoubiquitination.
Very high concentrations of LF are found in the first milk produced by the breastfeeding mother, which has for long been acknowledged as the ideal first food for babies and the perfect vehicle for transferring immunity from mother to child. The concentration of LF, which is secreted by exocrine glands, as well as neutrophils increases during inflammation and viral infections. The highest levels of LF have been detected in the nidus of inflammation (37). In animal studies it has been reported that locally administered bLF suppresses local inflammation mediated by collagen-induced arthritis (38). Thus, effective administration of bLF appears to play important roles in minimizing tissue destruction associated with an excessive inflammatory response and defending against viral infection. bLF has not yet been clinically used for the prevention or therapeutic treatment of inflammatory disease despite its ability to robustly inhibit the production of proinflammatory cytokines, in large part because of its instability in the stomach and a lack of mechanistic information on its mode of action.
In infants, LF is not degraded in the stomach, but in adults most of the orally ingested protein is destroyed by digestive enzymes, such as pepsin or trypsin. This is because infants have low pepsin secretion, and the pH of the infant stomach is usually around pH 4–5 (28), a pH too high for significant pepsin activity. In addition, secretion of pancreatic enzymes is minor, limiting the proteolytic activity in the small intestine. We previously reported that oral administration of liposomal bLF, which has enhanced resistance to gastric digestion, was able to deliver the protein to all tissues via the circulation, including joints and periodontal tissue, where it exerted its anti-inflammatory effects (3).
Our present results provide evidence for the anti-inflammatory effects of LF as well as insight into its mechanism of action. Notably, TRAF6 is one of the key components of the signal transduction pathway of members of the TNF receptor family and the IL-1 receptor/Toll-like receptor superfamily. In addition, TRAF6 is the only member of the TRAF family that plays a critical role in osteoclastogenesis, a process in which the autoubiquitination of TRAF6 is required for the induction of NFATc1 and the terminal differentiation of osteoclasts (35).
In conclusion, our results suggest that bLF can be an effective therapeutic agent for several degenerative bone diseases associated with dysregulated and prolonged NFκB activation. Our research is the first to demonstrate the inhibitory effects of bLF on osteoblast-mediated osteoclastogenesis using a molecular biological approach. We found that bLF targets TRAF6 autoubiquitination in the NFκB pathway that is induced not only by LPS but also by other TRAF6-dependent components, IL-1β and flagellin. Our investigation raises new questions on how bLF directly interrupts TRAF6 site-specific autoubiquitination. Thus, we recognize that further studies are necessary to fully elucidate the mechanisms of bLF anti-inflammatory effects. We anticipate that bLF will be a valuable therapeutic agent for inflammatory diseases associated with bone destruction, such as periodontitis and rheumatoid arthritis.
Acknowledgments
We thank professors Kazuo Tanne (Hiroshima University, Japan) and Eiji Tanaka (Tokushima University, Japan) for helpful suggestions. We thank Azuma Kosai for help with figure preparation.
Footnotes
- LF
- lactoferrin
- bLF
- bovine lactoferrin
- TRAF6
- TNF receptor-associated factor 6
- IRAK1
- interleukin-1 receptor-associated kinase 1
- LPS
- lipopolysaccharide
- RANKL
- receptor activator of nuclear factor κB ligand
- NFκB
- nuclear factor κB
- IKK
- IκB kinase
- OB
- osteoblast
- TAK1
- TGF β-activated kinase 1
- MyD88
- myeloid differentiation primary response gene 88
- LRP1
- lipoprotein receptor-related protein-1
- CAPE
- caffeic acid phenethyl ester
- TLR
- Toll-like receptor.
REFERENCES
- 1. Håversen L., Ohlsson B. G., Hahn-Zoric M., Hanson L. A., Mattsby-Baltzer I. (2002) Lactoferrin down-regulates the LPS-induced cytokine production in monocytic cells via NF-κB. Cell Immunol. 220, 83–95 [DOI] [PubMed] [Google Scholar]
- 2. Machnicki M., Zimecki M., Zagulski T. (1993) Lactoferrin regulates the release of tumor necrosis factor α and interleukin 6 in vivo. Int. J. Exp. Pathol. 74, 433–439 [PMC free article] [PubMed] [Google Scholar]
- 3. Yamano E., Miyauchi M., Furusyo H., Kawazoe A., Ishikado A., Makino T., Tanne K., Tanaka E., Takata T. (2010) Inhibitory effects of orally administrated liposomal bovine lactoferrin on the LPS-induced osteoclastogenesis. Lab. Invest. 90, 1236–1246 [DOI] [PubMed] [Google Scholar]
- 4. Appelmelk B. J., An Y. Q., Geerts M., Thijs B. G., de Boer H. A., MacLaren D. M., de Graaff J., Nuijens J. H. (1994) Lactoferrin is a lipid A-binding protein. Infect. Immun. 62, 2628–2632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Elass-Rochard E., Legrand D., Salmon V., Roseanu A., Trif M., Tobias P. S., Mazurier J., Spik G. (1998) Lactoferrin inhibits the endotoxin interaction with CD14 by competition with the lipopolysaccharide-binding protein. Infect. Immun. 66, 486–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hayden M. S., Ghosh S. (2008) Shared principles in NF-κB signaling. Cell 132, 344–362 [DOI] [PubMed] [Google Scholar]
- 7. Tak P. P., Firestein G. S. (2001) NF-κB. A key role in inflammatory diseases. J. Clin. Invest. 107, 7–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jimi E., Aoki K., Saito H., D'Acquisto F., May M. J., Nakamura I., Sudo T., Kojima T., Okamoto F., Fukushima H., Okabe K., Ohya K., Ghosh S. (2004) Selective inhibition of NF-κ B blocks osteoclastogenesis and prevents inflammatory bone destruction in vivo. Nat. Med. 10, 617–624 [DOI] [PubMed] [Google Scholar]
- 9. Karin M., Ben-Neriah Y. (2000) Phosphorylation meets ubiquitination. The control of NF-κB activity. Annu. Rev. Immunol. 18, 621–663 [DOI] [PubMed] [Google Scholar]
- 10. Bonizzi G., Karin M. (2004) The two NF-κB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 25, 280–288 [DOI] [PubMed] [Google Scholar]
- 11. Sun L., Deng L., Ea C. K., Xia Z. P., Chen Z. J. (2004) The TRAF6 ubiquitin ligase and TAK1 kinase mediate IKK activation by BCL10 and MALT1 in T lymphocytes. Mol. Cell 14, 289–301 [DOI] [PubMed] [Google Scholar]
- 12. Deng L., Wang C., Spencer E., Yang L., Braun A., You J., Slaughter C., Pickart C., Chen Z. J. (2000) Activation of the IκB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell 103, 351–361 [DOI] [PubMed] [Google Scholar]
- 13. Qi G., Kudo Y., Ando T., Tsunematsu T., Shimizu N., Siriwardena S. B., Yoshida M., Keikhaee M. R., Ogawa I., Takata T. (2010) Nuclear Survivin expression is correlated with malignant behaviors of head and neck cancer together with Aurora-B. Oral Oncol. 46, 263–270 [DOI] [PubMed] [Google Scholar]
- 14. Iizuka S., Kudo Y., Yoshida M., Tsunematsu T., Yoshiko Y., Uchida T., Ogawa I., Miyauchi M., Takata T. (2011) Ameloblastin regulates osteogenic differentiation by inhibiting Src kinase via cross-talk between integrin β1 and CD63. Mol. Cell. Biol. 31, 783–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Song J. J., Cho J. G., Hwang S. J., Cho C. G., Park S. W., Chae S. W. (2008) Inhibitory effect of caffeic acid phenethyl ester (CAPE) on LPS-induced inflammation of human middle ear epithelial cells. Acta Otolaryngol. 128, 1303–1307 [DOI] [PubMed] [Google Scholar]
- 16. Jensen L. E., Whitehead A. S. (2001) IRAK1b, a novel alternative splice variant of interleukin-1 receptor-associated kinase (IRAK), mediates interleukin-1 signaling and has prolonged stability. J. Biol. Chem. 276, 29037–29044 [DOI] [PubMed] [Google Scholar]
- 17. Rao N., Nguyen S., Ngo K., Fung-Leung W. P. (2005) A novel splice variant of interleukin-1 receptor (IL-1R)-associated kinase 1 plays a negative regulatory role in Toll/IL-1R-induced inflammatory signaling. Mol. Cell. Biol. 25, 6521–6532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kawamoto T., Ii M., Kitazaki T., Iizawa Y., Kimura H. (2008) TAK-242 selectively suppresses Toll-like receptor 4-signaling mediated by the intracellular domain. Eur. J. Pharmacol. 584, 40–48 [DOI] [PubMed] [Google Scholar]
- 19. Kishore N., Sommers C., Mathialagan S., Guzova J., Yao M., Hauser S., Huynh K., Bonar S., Mielke C., Albee L., Weier R., Graneto M., Hanau C., Perry T., Tripp C. S. (2003) A selective IKK-2 inhibitor blocks NF-κB-dependent gene expression in interleukin-1 β-stimulated synovial fibroblasts. J. Biol. Chem. 278, 32861–32871 [DOI] [PubMed] [Google Scholar]
- 20. Takeda K., Akira S. (2004) TLR signaling pathways. Semin. Immunol. 16, 3–9 [DOI] [PubMed] [Google Scholar]
- 21. Gottipati S., Rao N. L., Fung-Leung W. P. (2008) IRAK1. A critical signaling mediator of innate immunity. Cell. Signal. 20, 269–276 [DOI] [PubMed] [Google Scholar]
- 22. Roodman G. D. (1999) Cell biology of the osteoclast. Exp. Hematol. 27, 1229–1241 [DOI] [PubMed] [Google Scholar]
- 23. Suda T., Takahashi N., Udagawa N., Jimi E., Gillespie M. T., Martin T. J. (1999) Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr. Rev. 20, 345–357 [DOI] [PubMed] [Google Scholar]
- 24. Boyle W. J., Simonet W. S., vLacey D. L. (2003) Osteoclast differentiation and activation. Nature 423, 337–342 [DOI] [PubMed] [Google Scholar]
- 25. Socransky S. S., Haffajee A. D. (1992) The bacterial etiology of destructive periodontal disease. Current concepts. J. Periodontol. 63, 322–331 [DOI] [PubMed] [Google Scholar]
- 26. Wilson M., Reddi K., Henderson B. (1996) Cytokine-inducing components of periodontopathogenic bacteria. J. Periodont. Res. 31, 393–407 [DOI] [PubMed] [Google Scholar]
- 27. Bertolini D. R., Nedwin G. E., Bringman T. S., Smith D. D., Mundy G. R. (1986) Stimulation of bone resorption and inhibition of bone formation in vitro by human tumornecrosis factors. Nature 319, 516–518 [DOI] [PubMed] [Google Scholar]
- 28. Agunod M., Yamaguchi N., Lopez R., Luhby A. L., vGlass G. B. J. (1969) Correlative study of hydrochloric acid, pepsin, and intrinsic factor secretion in newborns and infants. Am. J. Dig. Dis. 14, 401–414 [DOI] [PubMed] [Google Scholar]
- 29. Dinarello C. A. (1996) Biologic basis for interleukin-1 in disease. Blood 87, 2095–2147 [PubMed] [Google Scholar]
- 30. Swantek J. L., Tsen M. F., Cobb M. H., Thomas J. A. (2000) IL-1 receptor-associated kinase modulates host responsiveness to endotoxin. J. Immunol. 164, 4301–4306 [DOI] [PubMed] [Google Scholar]
- 31. Wesche H., Henzel W. J., Shillinglaw W., Li S., Cao Z. (1997) MyD88. An adapter that recruits IRAK to the IL-1 receptor complex. Immunity 7, 837–847 [DOI] [PubMed] [Google Scholar]
- 32. Cao Z., Xiong J., Takeuchi M., Kurama T., Goeddel D. V. (1996) TRAF6 is a signal transducer for interleukin-1. Nature 383, 443–446 [DOI] [PubMed] [Google Scholar]
- 33. Alam S. L., Sun J., Payne M., Welch B. D., Blake B. K., Davis D. R., Meyer H. H., Emr S. D., Sundquist W. I. (2004) Ubiquitin interactions of NZF zinc fingers. EMBO J. 23, 1411–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kanayama A., Seth B. R., Sun L., Ea C., Hong M., Shaito A., Chiu Y., Deng L., Chen Z. (2004) TAB2 and TAB3 activate the NF-kappa B pathway through binding to polyubiquitin chains. Cell 15, 535–548 [DOI] [PubMed] [Google Scholar]
- 35. Lamothe B., Besse A., Campos A. D., Webster W. K., Wu H., Darnay B. G. (2007) Site-specific Lys-63-linked tumor necrosis factor receptor-associated factor 6 auto-ubiquitination is a critical determinant of I κ B kinase activation. J. Biol. Chem. 282, 4102–4112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Herz J., Hamann U., Rogne S., Myklebost O., Gausepohl H., Stanley K. K. (1988) Surface location and high affinity for calcium of a 500-kDa liver membrane protein closely related to the LDL receptor suggest a physiological role as lipoprotein receptor. EMBO J. 7, 4119–4127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Brines R. D., Brock J. H. (1983) The effect of trypsin and chymotrypsin on the in vitro antimicrobial and iron binding properties of lactoferrin in human milk and bovine colostrum. Unusual resistance of human apolactoferrin to proteolytic digestion. Biochim. Biophys. Acta 759, 229–235 [DOI] [PubMed] [Google Scholar]
- 38. Guillen C., McInnes I. B., Vaughan D., Speekenbrink A. B., Brock J. H. (2000) The effects of local administration of lactoferrin on inflammation in murine autoimmune and infectious arthritis. Arthritis Rheum. 43, 2073–2080 [DOI] [PubMed] [Google Scholar]
- 39. Boabaid F., Berry J. E., Koh A. J., Somerman M. J., McCauley L. K., (2004) The role of parathyroid hormone-related protein in the regulation of osteoclastogenesis by cementoblasts. J. Periodontol. 75, 1247–1254 [DOI] [PubMed] [Google Scholar]